Abstract
Purpose
The aim of this updated systematic review and meta-analysis was the association between hyperglycemia and periodontitis.
Methods
We searched PubMed/MEDLINE, Web of Science, and Scopus until March 2021. The key search words were based on "periodontitis" and "hyperglycemia." We included cohort, case–control, and cross-sectional studies, restricted to publications in English. The quality assessment of included studies and data extraction were done by two independent reviewers. Meta-analysis was performed for cross-sectional studies using the random-effects model.
Results
The literature search yielded 340 studies, and finally, 19 and 11 studies were included in systematic review and meta-analysis, respectively. The total sample size of the eligible studies in the meta-analysis was 38,896 participants, of whom 33% were male with a mean age of 51.20 ± 14.0 years. According to a random-effect meta-analysis in cross-sectional studies, the pooled odds ratio (OR) for the association between hyperglycemia and periodontal indices was statistically significant (OR: 1.50, 95%CI: 1.11, 1.90). There was evidence of publication bias (coefficient: − 3.53, p-value = 0.014) which, after imputing missing studies, the pooled OR of the association between hyperglycemia and periodontitis change to 1.55 (95%CI: 1.20, 1.90).
Conclusion
Results of the present study show that hyperglycemia was positively associated with periodontitis. However, more cohort and prospective longitudinal studies should be conducted to find the exact association. Overall, it seems the management of hyperglycemia could be considered as a preventive strategy for periodontitis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-021-00861-9.
Keywords: Hyperglycemic, Periodontitis, Metabolic Syndrome, Systematic Review, Meta-Analysis
Introduction
Periodontitis is a chronic inflammatory disease that can accumulate during a lifetime. There is an increased prevalence of periodontitis in the aging population as they keep their teeth [1, 2]. In 2010, 10.8% (743 million) people worldwide were affected by severe periodontitis, with the maximum prevalence at the age of 40 [3]. National Health and Nutrition Examination Survey in 2009–2014 reported a higher prevalence of periodontitis among dentate US adults with the age of 30–79 years than previous studies. Also, it was established that 42.2% of dentate US individuals older than 30 years had some category of periodontitis, including 7.9% with severe periodontitis and 34.4% with non-severe periodontitis [2]. Periodontitis is also the sixth major complication of diabetes [4]. It is generally accepted that the cause of most chronic diseases such as diabetes and metabolic syndrome is a pro-inflammatory state derived from excessive calorie intake, over nutrition, and chronic inflammatory dysfunctions [5]. This pro-inflammatory state leads to an increase in inflammatory mediators such as tumor necrosis factor-alpha (TNF-α) and oxidative stress, which causes impairment in several crucial biological mechanisms [6]. It seems that hyperglycemia has the most common relationship to periodontal disease. The chronic hyperglycemia state resulting in increased oxidative stress in the periodontium, and it causes elevated levels of inflammatory mediators. These mediators finally lead to the destruction of the crestal alveolar bone and cause periodontitis [7, 8]. Due to the prevalence of periodontitis and the importance of monitoring, this study aims to collect and summarize all evidence of the association between hyperglycemia and periodontitis based on clinical periodontal examinations.
Methods
This systematic review and meta-analysis were done according to the PRISMA guideline [9]. To assess the association between hyperglycemia with periodontitis, we conducted a systematic review in all related studies which were searched from comprehensive international databases of PubMed and NLM Gateway (for MEDLINE), Web of Science, and Scopus up to March 2021.
Search strategy
The main bases for the development of search strategies were extracted from “periodontitis” or “hyperglycemia,” and all related terms were included according to this main strategy. The databases were searched by two reviewers independently (Supplementary 1).
Eligibility criteria and selection study
All observational studies (cross-sectional, case–control, and cohort) that investigated the association between hyperglycemia with periodontitis were considered eligible. Studies that were conducted on non-human-subjects, those with duplicate citations, and the non-generalized studies that were limited to sub-group populations were excluded. In the case of multiple publications from the same study, the study with the most comprehensive sample size was included.
After removing duplicate studies, two independent reviewers examined titles and abstracts as well as full texts for relevance. Disagreement between these reviewers was resolved by a third reviewer.
The data was extracted through a checklist which included the following items; information of study; demographic and bibliographic characteristics; methodological information; definition of hyperglycemia and periodontitis, and results of each study.
Quality assessment
Quality assessment was assessed using the Newcastle–Ottawa quality assessment scale by study design (cross-sectional, case–control, and cohort study) [10]. Two reviewers independently evaluated quality assessment and any disagreement resolved by a third reviewer.
Statistical analysis
Meta-analysis was performed for studies that reported odds ratio (OR) and 95% confidence interval (CI) as a measure of the association between hyperglycemia and periodontitis. Due to the small number of cohort and case–control studies in the healthy population, meta-analysis was performed only in cross-sectional studies. Chi-square-based Q test and I-square statistics were used to assess the heterogeneity among studies. I-squared values, 0%, 25%, and 75% showed low, moderate, and high heterogeneity, respectively. If heterogeneity were statistically significant (P-value < 0.1) [11], the random-effect meta-analysis model was used (using the Der-Simonian and Laird method) to find the association between hyperglycemia and periodontitis. Subgroup analyses were done with quality assessment, hyperglycemia definition (fasting blood sugar (FBS) and Glycated hemoglobin (HbA1c)), and dental indices (periodontal disease (PD), and clinical attachment loss (CAL)). Publication bias was estimated by Egger’s test and the result of this test was considered to be statistically significant at P < 0.1. Publication bias was presented schematically using a funnel plot. When potential publication bias existed, the trim and fill correction method was used to impute missing studies and correct publication bias. Sensitivity analysis was performed to identify the effectiveness of exclusion of each study on the pooled effect size. We undertook a meta-regression analysis to detect the source of heterogeneity. All analyses were conducted using Stata 11 (version 11; StataCorp, College Station, Texas).
Results
Search strategy
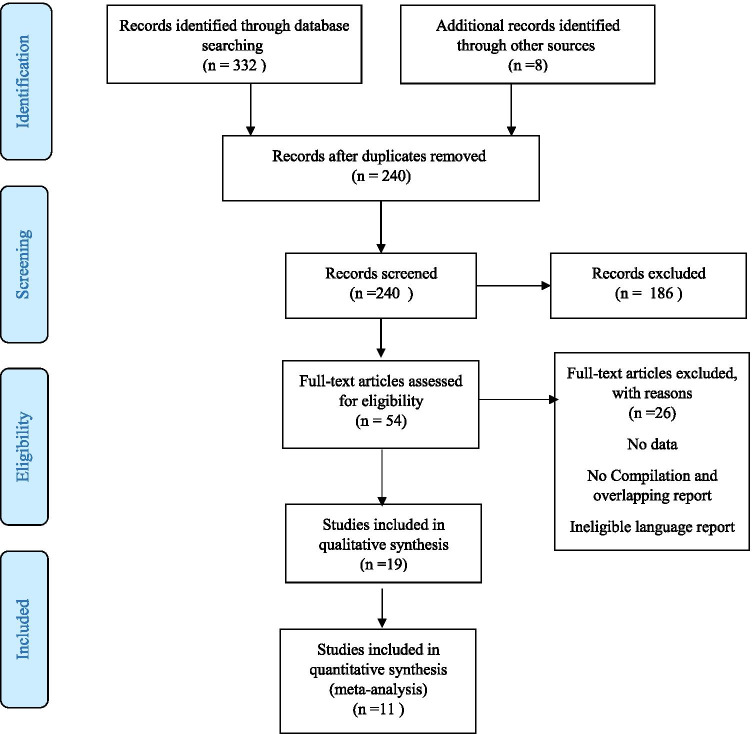
The literature search yielded 340 studies, and after the screening, 19 and 11 studies were included in systematic review and meta-analysis, respectively. Figure 1 summarizes the screening process of the studies. No studies were added during the examination references list of relevant studies.
Fig. 1.
PRISMA flowchart of the study selection process
Qualitative synthesis
The main characteristics of included studies and their quality score are shown in Table 1. Of 19 studies that met the eligibility criteria in the systematic review, 11 were cross-sectional study, 4 case–control, and 2 cohorts which one study reported beta coefficient and one other had not eligible dental indices.
Table 1.
Characteristics of the included studies
| ID | Study characteristics | Outcome and exposure characteristics | Findings | Quality scores (9) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First authors, year, country | Study subject | Age Mean ± SD |
Study design | Sample size |
Male% | Definition of hyperglycemia | Definition of periodontitis | Effect size (95% CI) | Confounders | ||
| 1 | Hendri Susanto, 2012, Indonesia [27] |
Diabetic patient Healthy control |
50.71 ± 10.3 | Case–control |
Case group: 101 Control group: 132 |
31.75% | HbA1c > 6.5% | PD (≥ 4 mm) and AL (≥ 3 mm) | OR: 3.58 (1.77–7.24) | _ | 7 |
| PD (≥ 5 mm) AL (≥ 2 mm) | OR: 4.20 (2.41–7.30) | ||||||||||
| 2 | Shu-Fen Chuang, 2005, Taiwa [17] |
Diabetic patient Healthy control |
57.5 | Case–control |
Case group: 85 Control group: 43 |
29.68% | HbA1c > 9% | CPI ≥ 4 mm; (PD ≥ 4 to 5 mm) | OR: 1.86 ( 0.98- 3.50) | _ | 6 |
| 3 | Katz J, 2001, Israel [16] |
Normal serum glucose levels Abnormal serum glucose levels |
– | Cross-sectional | 10,590 | – | FBS > 120 mg/dl | CPI > 4.5 | OR: 2.46 ( 1.86- 3.20) | _ | 6 |
| 4 | Farzeen Tanwir, 2010, Karachi [29] |
Controlled diabetic patients Uncontrolled diabetic patients |
49.23 ± 9.49 | Cross-sectional |
Case group: 141 Control group: 143 |
38.10% | HbA1c > 7.5% | GI > 2 mm | OR: 2.49 (1.50–4.13) | Age, and gender | 6 |
| PI > 2 mm | OR: 1.80 (1.11–2.93) | ||||||||||
| PI > 2 mm | OR: 2.35 (1.37–4.02) | ||||||||||
| CI > 0.75 mm | OR: 1.65 (0.98–2.78) | ||||||||||
| 5 | Shimazaki, 2007, Japan [25] | Women population | 55.7 ± 18.8 | Cross-sectional | 584 | 0% | FBS > 110 | PD ≥ 2 mm | OR: 2.20 (1.30–3.90) | Age, smoking status, lipid-lowering medication, and total cholesterol | 9 |
| CAL ≥ 3 mm | OR: 1.70 (0.70–4.00) | ||||||||||
| 6 | Khader, 2008, Jordan [30] |
Patients with metabolic syndrome Persons without metabolic syndrome |
48.3 ± 13.0 46.1 ± 10.9 |
Cross-sectional | 156 | 35.9% | FBS > 110 mg/dl | CAL > 3 mm | β = 8.0, SE = 3.5 | Age, gender, education, income, smoking, frequency of tooth brushing | 8 |
| PD > 3 mm | β = 7.9, SE = 3.6 | ||||||||||
| Average CAL | β = 2.3, SE = 0.14 | ||||||||||
| Average PD | β = 0.74, SE = 0.17 | ||||||||||
| 7 |
Kushiyama, 2009, Japan [12] |
General population | 40–70 | Cross-sectional | 1,070 | 26.7% | FBS > 110 | CPI ≥ 3 | OR: 1.27 (0.83 to 1.96) | Age, gender, and smoking habits | 8 |
| 8 | Benguigui, 2010, France [19] | General population | 35–74 | Cross-sectional | 255 | 54.9% | FBS < 126 mg/dl | CAL ≥ 4 mm, with PD ≥ 5 mm, | OR: 0.88 (0.27–2.81) | Age, gender, educational level, smoking habits, alcohol consumption, CRP, and dental plaque | 9 |
|
CAL ≥ 6 mm, with PD ≥ 5 mm |
OR: 1.55 (0.46–5.25) | ||||||||||
| 9 | Kwon, 2011, Korea [15] | General population |
No periodontitis 37.79 Periodontitis 55.00 |
Cross-sectional | 7,178 | 37.5% | FBS < 100 mg/dl | CPI ≥ 3(3.5 mm > pocket) | OR: 2.45 (2.20–2.74) | Age, smoking habits, alcohol intake, tooth brushing frequency and present number of teeth | 9 |
| 10 | Minagawa, 2015, Japan [22] | Elderly population | > 80 | Cross-sectional | 234 | 47.4% | HbA1c ≥ 6.0% | PD ≥ 5 mm or CAL ≥ 6 mm | OR: 1.42 (0.71–2.84) | Gender, income, education, smoking status, brushing frequency, exercise food intake, visit dentistry | 9 |
| 11 | Timonen, 2010, Finland [24] | General population | 30 to 64 | Cross-sectional | 2,050 | 39.2% | FBS ≥ 6.1 mmol/L | PD ≥ 4 mm | RR: 0.91 (0.70–1.18) | Gender, age, education, plaque level, tooth brushing frequency, dental attendance pattern, and alcohol | 8 |
| FBS ≥ 6.1 mmol/L | PD ≥ 6 mm | RR: 0.60 (0.32–1.13) | |||||||||
| 12 | Thanakun, 2014, Taiwan [20] |
Patients with metabolic syndrome Persons without metabolic syndrome |
35–76 | Cross-sectional | 125 | 42.4% | FPG* levels ≥ 100 mg/dL | Severe Periodontitis CAL > 4 mm | OR: 1.81 (0.55 to 5.96) | Age, sex, alcohol consumption, education level, and frequency of tooth brushing | 8 |
| FPG levels ≥ 100 mg/dL | Moderate/Severe Periodontitis (PD ≥ 4 mm and BOP > 10%) | OR: 0.88 (0.12 to 6.49) | |||||||||
| 13 | Masanori Iwasaki, 2015, Japan [28] | Elderly population | 75 | Cohort | 125 | 89.55% | HbA1c ≥ 6% | CAL ≥ 3 mm | RR: 0.86 (0.36–2.06) | Gender, income, education, smoking status, number of the teeth | 7 |
| No. of teeth | RR: 1.88 (0.86- 4.10) | ||||||||||
| 14 | Wijnand J, 2017, Netherlands [23] |
Periodontitis patient Without periodontitis patient |
– | Case–control | 313 | 48.27% | HbA1c > 6.5% |
PD ≥ 5 mm CAL ≥ 6 mm |
OR: 2.73) 1.60–4.68 ( | Sex, age, ethnicity, education, smoking, history of periodontal treatment | 7 |
|
PD ≥ 4 mm CAL ≥ 3 mm |
OR: 1.76 (1.03–3.00) | ||||||||||
| 15 | D´Aiuto, 2008, USA [18] | General population |
Severe periodontitis 52.3 Moderate periodontitis 54.2 Mild or no periodontitis 38.7 |
Cross-sectional | 13,677 | 62.0% | FBS > 110 g/dl |
CAL > 6 mm (two sites not on the same tooth or greater) PD > 4 mm (one site or greater) |
OR: 1.71 (1.16 –2.54) | Age, sex, years of education, poverty to income ratio, ethnicity, general conditions, and smoking | 9 |
|
CAL > 4 mm (two sites not on the same tooth) PD > 4 mm(one site) |
OR: 1.13 (0.84–1.53) | Age, sex, years of education, poverty to income ratio, ethnicity, general conditions, and smoking | |||||||||
| 16 | Morita, 2010, Japan [14] | Adult employees | 37.3 | Retrospective Cohort | 1,023 | 71.0% | FBS ≥ 110 mg/dl | CPI > 3 (PD > 4 mm) | OR: 1.4 (1.0 to 2.1) | Age, gender, smoking habit, exercise, eating between meals, and healthy body weight | 8 |
| Missing teeth: One or more | OR: 1.0 (0.6 to 1.5) | ||||||||||
| 17 | Morita, 2009, Japan [13] | Adult employees | 43.3 | Cross-sectional | 2,478 | 81.8% | FBS ≥ 110 mg/dL | CPI > 3 (PD > 4 mm) | OR: 1.9 (1.4–2.7) | Age, gender, and smoking habit. CI, confidence interval | |
| HbA1c ≥ 5.5 | CPI > 3 (PD > 4 mm) | OR: 2.0 (1.5–2.6) | |||||||||
| 18 | LaMonte, 2014, USA [21] | Postmenopausal women | 50 to 79 years | Cross-sectional | 657 | 0% | FBS ≥ 100 mg/dL |
tooth loss; ACH ≥ 3 mm or 2 sites CAL ≥ 5 mm or ≥ 1 tooth loss |
OR: 0.96 (0.54 to 1.71) | Age, smoking, hormone therapy, history of diagnosed heart disease, tooth brushing, dental visits, | 8 |
| ≥ 2 interproximal sites with CAL ≥ 6 mm or ≥ 1 interproximal site with PD ≥ 5 mm | OR: 0.86 (0.44 to 1.70) | ||||||||||
| PI ≥ 50% of sites | OR: 1.40 (0.83 to 2.32) | ||||||||||
| 19 | Lei Chen, 2010, China [26] |
Periodontitis patient Without periodontitis patient |
36–85 | Case–control | 140 | 63.04% | HbA1c > 8.0% | PD) 2.76 to 4.95 mm( | OR: 3.83 (1.78–8.26) | Age, gender, BMI, and smoking | 7 |
| F-glucose | PD) 2.76 to 4.95 mm( | OR: 2.38(1.12–5.06) | |||||||||
| HbA1c > 8.0% | PD (2.26 to 2.75 mm) | OR: 2.96 (1.40–6.28) | |||||||||
| F-glucose | PD (2.26 to 2.75 mm) | OR: 2.05(0.97–4.30) | |||||||||
FBG Fasting plasma glucose test, MetS metabolic syndrome, PD Probing depth, CAL Clinical attachment loss, CPI Community Periodontal Indices, ACH alveolar crest height, PI plaque indices;
The total sample size of the eligible studies in the systematic review was 41,300, of whom 25% of participants were male with a mean age of 50.0 ± 13.0 years. The total sample size of the eligible studies in the meta-analysis was 38,896 participants, of whom 33% were male with a mean age of 51.20 ± 14.0 years. Periodontal indices and hyperglycemia were measured by using different methods. By type of Community Periodontal Index (CPI), four studies [12–15] used CPI ≥ 3 mm, and two studies used CPI ≥ 4 mm [16, 17]. By type of CAL periodontal index, 4 studies used CAL ≥ 3 mm [12–15], 3 used CAL ≥ 4 mm [18–20], one studies used CAL ≥ 5 mm [21] and 5 studies used CAL ≥ 6 mm [18, 19, 21–23]. By type of PD periodontal index, 6 studies used PD ≥ 4 mm [14, 17, 18, 20, 23, 24], 4 used PD ≥ 5 mm [19, 21–23], 2 studies used PD ≥ 2 mm [25, 26], and 1 study PD ≥ 6 mm [24]. Also, by type of hyperglycemia based on HbA1c, 4 studies used HbA1c ≥ 6% [22, 23, 27, 28], one study used HbA1c ≥ 7.5% [29] and one study used HbA1c ≥ 8% [26]. By type of hyperglycemia based on high FBS, 6 studies used FBS ≥ 110 mg/d [12–14, 18, 25, 30] and one studies used FBS ≥ 126 mg/dl [19]. The studies were published between 2001 and 2017 years, and most were conducted in Asian countries. Sixteen studies reported adjusted OR at least for two confounders; the most commonly confounding factors were age, gender and smoking status. In 2 cohort studies, effect measure were ranged between 0.86 (95%CI: 0.36–2.06) and 1.88 (95%CI: 0.86- 4.10). In 4 case–control studies, OR and confidence interval was ranged between 1.76 (1.03–3.00) and 4.20 (2.41–7.30).
Quality assessment
The results of the qualitative assessment showed that 15 studies had a high quality, and the remains had a moderate quality. Also, no low-quality studies were observed. The quality scores ranged from 6 to 9.
Quantitative synthesis
The results of the meta-analyses on the association between hyperglycemia and periodontitis according to quality assessment, hyperglycemia, assessed periodontal indices, and hyperglycemia-periodontal indices in cross-sectional studies are shown in Table 2 and Fig. 2. Significant heterogeneity was observed among the included studies (I-squared = 84.1%, P < 0.001). There was a significant association between hyperglycemia and periodontal indices (OR: 1.50; 95% CI: 1.11, 1.90). By type of periodontal index, hyperglycemia has a significant association with PD (OR: 1.66; 95% CI: 1.06, 2.26) but not with CAL (OR: 1.07; 95% CI: 0.53, 1.61) and PD/CAL (OR: 1.19; 95% CI: 0.92, 1.45).
Table 2.
Meta-analysis of the association between hyperglycemia and periodontitis in cross-sectional studies according to study characteristics
| Overall periodontitis risk | No point | Sample size | Pooled OR (95% CI) | Heterogeneity assessment | |||
|---|---|---|---|---|---|---|---|
| Model | I-squared % | Q test | P-value of heterogeneity | ||||
| By Quality assessment | |||||||
| Moderate | 2 | 11,660 | 1.85 (0.68, 3.00) | Random | 85.90 | 7.08 | 0.008 |
| High | 15 | 46,800 | 1.44 (1.02, 1.87) | Random | 86.50 | 106.81 | < 0.001 |
| By hyperglycemia definition | |||||||
| FBS | 15 | 55,755 | 1.47 (1.04, 1.89) | Random | 87.60 | 112.06 | < 0.001 |
| HbA1c | 2 | 2,712 | 1.87 (1.38, 2.36) | Fixed | 0.00 | 0.90 | 0.343 |
| By dental indices | |||||||
| PD | 9 | 28,602 | 1.66 (1.06, 2.26) | Random | 92.00 | 104.76 | < 0.001 |
| CAL | 3 | 1,365 | 1.07 (0.53, 1.61) | Fixed | 0.00 | 0.98 | 0.612 |
| PD/CAL | 5 | 28,500 | 1.19 (0.92, 1.45) | Fixed | 0.00 | 3.62 | 0.460 |
| By hyperglycemia—dental indices | |||||||
| FBS-PD | 8 | 26,120 | 1.62 (0.95, 2.28) | Random | 93.10 | 101.85 | < 0.001 |
| FBS-CAL | 3 | 1,365 | 1.07 (0.53, 1.61) | Fixed | 0.00 | 0.98 | 0.612 |
| FBS-PD/CAL | 4 | 28,264 | 1.18 (0.86, 1.50) | Fixed | 12.50 | 3.43 | 0.330 |
| HbA1c-PD | 2 | 2,712 | 1.87 (1.39, 2.37) | Fixed | 0.00 | 0.90 | 0.343 |
Fig. 2.
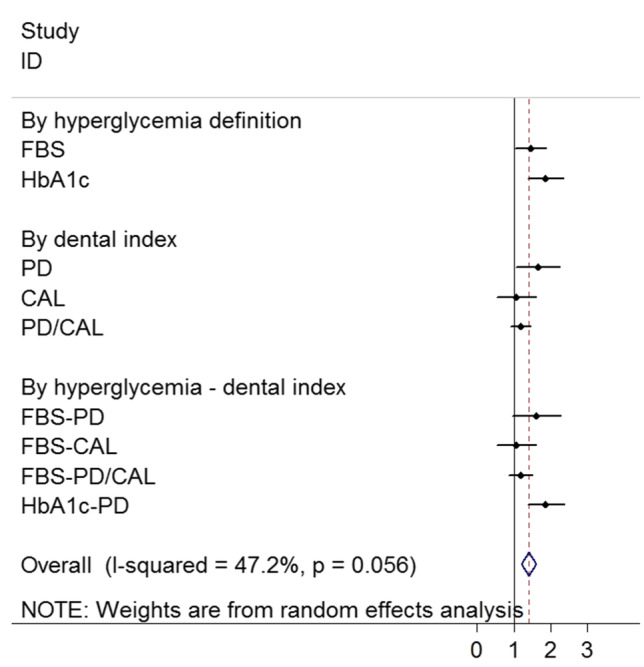
Forest plot of the association between hyperglycemia and periodontitis according to the definition of hyperglycemia, dental indices, and hyperglycemia & dental indices in the cross-sectional studies
According to hyperglycemia definition, we don't found any significant association between FBS with PD (OR: 1.62; 95% CI: 0.95, 2.28), CAL (OR: 1.07; 95% CI: 0.53, 1.61), and PD/CAL (OR: 1.18; 95% CI: 0.86, 1.50). There was also a significant association between HbA1c and PD (OR: 1.87; 95% CI: 1.39, 2.37). By quality assessment, studies with high quality had a significant association (OR: 1.44; 95% CI: 1.02, 1.87) than those with moderate quality (OR: 1.85; 95% CI: 0.68, 3.00).
Publication bias
There was evidence of publication bias (coefficient = -3.53, p-value = 0.014) (Fig. 3). Trim-and-fill analysis indicated that if missing studies are included in the analysis, the pooled OR of the association between hyperglycemia and periodontitis change from 1.50 (95%CI: 1.11, 1.90) to 1.55 (95%CI: 1.20, 1.90).
Fig. 3.
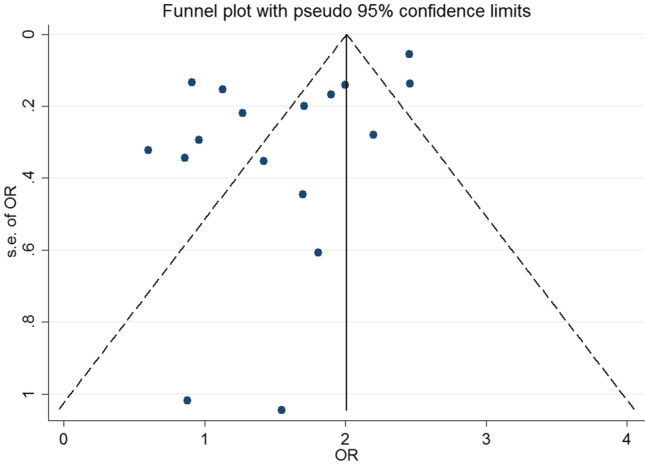
Funnel plot of the association between hyperglycemia and periodontitis in cross-sectional studies
Meta-regression
Based on the results of the meta-regression analysis, none of the covariates, including sample size, quality score, hyperglycemia definition, and dental indices affect the observed heterogeneity (p-value > 0.10).
Sensitivity analysis
The sensitivity analysis result was demonstrated that the pooled results were robust, and excluding each study couldn't be affected on the pooled estimate.
Discussion
In the present systematic review and meta-analysis, we aimed to assess the association between hyperglycemia and periodontitis indices. To the best of our knowledge, up to now, it is the first comprehensive systematic review run on this topic. All observational studies included in this systematic review revealed the associations between high levels of plasma glucose and periodontitis. Based on findings, hyperglycemia was associated with periodontitis. This association was significant regardless of the type of indicators, and definition of diabetes.
Considering the increasing trends of diabetes, MetS, and other metabolic risk factors, attention to the role of these potential risk factors in the incidence or progression of other comorbidities has become an interesting field of research [8, 31]. In this regard, there is some evidence on the association between hyperglycemia and periodontal diseases. This assumption explains that increased levels of plasma glucose could be a risk factor for periodontitis [32]. Our results are consistent with the previous evidence; that reveals a potential association between hyperglycemia and periodontitis [8, 16, 31–33]. Previous study revealed that regarding the chronic immune system activation in patients with diabetes, leukocytes and pro-inflammatory markers may be increased [34]. Moreover, the advanced glycated end products (AGEs) as a consequence of hyperglycemia, can increase the inflammatory processes which induce apoptosis [35]. The chronically increased level of plasma glucose and inflammation could be increasing oxidative stress in the periodontium that could lead to elevated levels of inflammatory mediators. Following these procedures destruction of the crestal alveolar bone lead to periodontitis [8, 16, 36].
The methodological quality of included papers was moderate and high. In cross-sectional studies, the sample size was mostly representative of the general population. The exposure was assessed through a defined standard measurement tool. In case–control studies, the same procedures for setting the case and controls were conducted, and exposure was evaluated through the secure data. The criteria for the detection of periodontitis is the critical determinant. The clinical assessment is the gold-standard method of detection of periodontitis progression [31]. The variation of findings may be rooted in different methodological and practical approaches in the selection of periodontal indices, sampling frames, and technical procedures of detection [37, 38].
Scientific experiments have shown that the promotion of general health and detection and treatment of many risk factors such as high plasma glucose could increase oral health and decrease oral diseases [31, 39]. Some of them even emphasized the bidirectional association between diabetes and inflammatory periodontal disease [40]. Considering the discussed results, as a practical implication of research; increasing the awareness about the importance of the control of plasma glucose and management of diabetes must be added to health programs to reduce adverse health outcomes of many oral diseases [31, 39].
Compare with the parallel studies, the present study benefited from many estrangements. It is the first comprehensive systematic review and meta-analysis on the association of hyperglycemia and periodontitis which all available data were searched from international databases. We revealed the gaps of evidence in this field for future complementary researches.
There is some limitation in this study that should be considered. First, it is a secondary study and the quality and representativeness of our data were dependent on the accuracy of the data extracted from the primary studies. Second, according to the cross-sectional nature, it is difficult to establish whether PD causes hyperglycemia or hyperglycemia favors the incidence of PD. Third, because there were few case–control and cohort studies and heterogeneity of presented results, we could not analyze the sub-groups of sex, age, ethnicity, and other practical specifications. Forth, the adjustments of confounders were different in included primary studies; therefore, many other factors, such as the type of treatment and medication or supplement intakes that could influence the association between hyperglycemia and PD, have not been adjusted. Fifth, many potential co-factors of such as oral health, smoking, alcohol consumption, job description, and socio-economic class were not controlled.
Conclusion
The practical findings of the present study suggest an association between hyperglycemia and periodontitis. Thus, prevention and control of hyperglycemia could be considered as a preventive strategy for periodontitis. This approach also could be a helpful method in usual tactic protocols in patients with periodontitis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Research reported in this publication was supported by Elite Researcher Grant Committee under award number [971188] from the National Institute for Medical Research Development (NIMAD), Tehran, Iran.
Author’s contribution
AM: contributed to the conception, design, interpretation, did a search strategy, analyzed data, drafted the manuscript, and critically revised the manuscript; SD: contributed to the conception, investigated quality assessment of studies, and drafted the manuscript. ES: contributed to the conception, extracted, and extracted and analyzed data; ED: contributed to the conception, assessed the studies, and critically revised the manuscript; JH: contributed to the conception, assessed the studies, investigated quality assessment; AM-G: contributed to the conception, assessed the studies, and investigated quality assessment; RH, contributed to the conception, assessed the studies, drafted the manuscript, and critically revised the manuscript; MQ, contributed to conception, design, interpretation, assessed and investigated studies, extracted data, drafted the manuscript critically, and revised the manuscript.
Funding
Not applicable.
Declarations
Conflict of interest
Not applicable.
Ethical approval
The protocol study and the proposal approved by the ethical committee of Alborz University of Medical Sciences. All of the included studies would be cited in all reports and all future publications.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ramin Heshmat, Email: rheshmat@tums.ac.ir.
Mostafa Qorbani, Email: mqorbani1379@yahoo.com.
References
- 1.Slots J. Periodontitis: facts, fallacies and the future. Periodontology 2000. 2017;75(1):7–23. doi: 10.1111/prd.12221. [DOI] [PubMed] [Google Scholar]
- 2.Eke PI, Borgnakke WS, Genco RJ. Recent epidemiologic trends in periodontitis in the USA. Periodontology 2000. 2020;82(1):257–267. doi: 10.1111/prd.12323. [DOI] [PubMed] [Google Scholar]
- 3.Frencken JE, et al. Global epidemiology of dental caries and severe periodontitis–a comprehensive review. J Clin Periodontol. 2017;44:S94–S105. doi: 10.1111/jcpe.12677. [DOI] [PubMed] [Google Scholar]
- 4.Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16(1):329–34. doi: 10.2337/diacare.16.1.329. [DOI] [PubMed] [Google Scholar]
- 5.Bullon P, et al. Metabolic syndrome and periodontitis: is oxidative stress a common link? J Dent Res. 2009;88:503–518. doi: 10.1177/0022034509337479. [DOI] [PubMed] [Google Scholar]
- 6.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Deshpande K, et al. Diabetes and periodontitis. J Indian Soc Periodontol. 2010;14:207. doi: 10.4103/0972-124X.76917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y-Y, Xiao E, Graves DT. Diabetes mellitus related bone metabolism and periodontal disease. I J Oral Sci. 2015;7:63–72. doi: 10.1038/ijos.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JPT, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kushiyama M, Shimazaki Y, Yamashita Y. Relationship between metabolic syndrome and periodontal disease in Japanese adults. J Periodontol. 2009;80(10):1610–1615. doi: 10.1902/jop.2009.090218. [DOI] [PubMed] [Google Scholar]
- 13.Morita T, et al. Association between periodontal disease and metabolic syndrome. J Public Health Dent. 2009;69(4):248–253. doi: 10.1111/j.1752-7325.2009.00130.x. [DOI] [PubMed] [Google Scholar]
- 14.Morita T, et al. A cohort study on the association between periodontal disease and the development of metabolic syndrome. J Periodontol. 2010;81(4):512–519. doi: 10.1902/jop.2010.090594. [DOI] [PubMed] [Google Scholar]
- 15.Kwon YE, et al. The relationship between periodontitis and metabolic syndrome among a Korean nationally representative sample of adults. J Clin Periodontol. 2011;38:781–786. doi: 10.1111/j.1600-051X.2011.01756.x. [DOI] [PubMed] [Google Scholar]
- 16.Katz J. Elevated blood glucose levels in patients with severe periodontal disease. J Clin Periodontol. 2001;28(7):710–712. doi: 10.1034/j.1600-051x.2001.028007710.x. [DOI] [PubMed] [Google Scholar]
- 17.Chuang S-F, et al. Oral and dental manifestations in diabetic and nondiabetic uremic patients receiving hemodialysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2005;99(6):689–695. doi: 10.1016/j.tripleo.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 18.D'Aiuto F, et al. Association of the metabolic syndrome with severe periodontitis in a large US population-based survey. J Clin Endocrinol Metabol. 2008;93(10):3989–3994. doi: 10.1210/jc.2007-2522. [DOI] [PubMed] [Google Scholar]
- 19.Benguigui C, et al. Metabolic syndrome, insulin resistance, and periodontitis: a cross-sectional study in a middle-aged French population. J Clin Periodontol. 2010;37(7):601–608. doi: 10.1111/j.1600-051X.2010.01571.x. [DOI] [PubMed] [Google Scholar]
- 20.Thanakun S, et al. Association of untreated metabolic syndrome with moderate to severe periodontitis in Thai population. J Periodontol. 2014;85(11):1502–1514. doi: 10.1902/jop.2014.140105. [DOI] [PubMed] [Google Scholar]
- 21.LaMonte MJ, et al. Association between metabolic syndrome and periodontal disease measures in postmenopausal women: the Buffalo OsteoPerio study. J Periodontol. 2014;85(11):1489–1501. doi: 10.1902/jop.2014.140185. [DOI] [PubMed] [Google Scholar]
- 22.Minagawa K, et al. Relationship between metabolic syndrome and periodontitis in 80-year-old Japanese subjects. J Periodontal Res. 2015;50(2):173–179. doi: 10.1111/jre.12190. [DOI] [PubMed] [Google Scholar]
- 23.Teeuw WJ, et al. Periodontitis as a possible early sign of diabetes mellitus. BMJ Open Diab Res Care. 2017;5(1):e000326. doi: 10.1136/bmjdrc-2016-000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timonen P, et al. Metabolic syndrome, periodontal infection, and dental caries. J Dent Res. 2010;89(10):1068–1073. doi: 10.1177/0022034510376542. [DOI] [PubMed] [Google Scholar]
- 25.Shimazaki Y, et al. Relationship of metabolic syndrome to periodontal disease in Japanese women: the Hisayama Study. J Dent Res. 2007;86(3):271–275. doi: 10.1177/154405910708600314. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, et al. Association of periodontal parameters with metabolic level and systemic inflammatory markers in patients with type 2 diabetes. J Periodontol. 2010;81(3):364–371. doi: 10.1902/jop.2009.090544. [DOI] [PubMed] [Google Scholar]
- 27.Susanto H, et al. Periodontal inflamed surface area and C-reactive protein as predictors of HbA1c: a study in Indonesia. Clin Oral Invest. 2012;16(4):1237–1242. doi: 10.1007/s00784-011-0621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwasaki M, et al. Longitudinal relationship between metabolic syndrome and periodontal disease among Japanese adults aged≥ 70 years: the Niigata Study. J Periodontol. 2015;86(4):491–498. doi: 10.1902/jop.2015.140398. [DOI] [PubMed] [Google Scholar]
- 29.Tanwir F, Tariq A. Effect of glycemic conrol on periodontal status. J Coll Physicians Surg Pak. 2012;22(6):371–374. [PubMed] [Google Scholar]
- 30.Khader Y, et al. Periodontal status of patients with metabolic syndrome compared to those without metabolic syndrome. J Periodontol. 2008;79(11):2048–2053. doi: 10.1902/jop.2008.080022. [DOI] [PubMed] [Google Scholar]
- 31.Rocchietta I, Nisand D. A review assessing the quality of reporting of risk factor research in implant dentistry using smoking, diabetes and periodontitis and implant loss as an outcome: critical aspects in design and outcome assessment. J Clin Periodontol. 2012;39:114–121. doi: 10.1111/j.1600-051X.2011.01829.x. [DOI] [PubMed] [Google Scholar]
- 32.Nascimento GG, et al. Does diabetes increase the risk of periodontitis? A systematic review and meta-regression analysis of longitudinal prospective studies. Acta Diabetol. 2018;55(7):653–667. doi: 10.1007/s00592-018-1120-4. [DOI] [PubMed] [Google Scholar]
- 33.Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77(8):1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 34.Corbella S, et al. Effect of periodontal treatment on glycemic control of patients with diabetes: A systematic review and meta-analysis. J Diabetes Invest. 2013;4(5):502–9. doi: 10.1111/jdi.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graves DT, et al. Diabetes-enhanced inflammation and apoptosis–impact on periodontal pathology. J Dent Res. 2006;85(1):15–21. doi: 10.1177/154405910608500103. [DOI] [PubMed] [Google Scholar]
- 36.Pradhan S, Goel K. Interrelationship between diabetes and periodontitis: a review. JNMA. 2011;51(183):144–153. [PubMed] [Google Scholar]
- 37.Hardo PG, et al. Helicobacter pylori infection and dental care. Gut. 1995;37(1):44–46. doi: 10.1136/gut.37.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarić S, et al. Periodontal therapy improves gastric Helicobacter pylori eradication. J Dent Res. 2009;88(10):946–950. doi: 10.1177/0022034509344559. [DOI] [PubMed] [Google Scholar]
- 39.Bascones-Martinez A, Gonzalez-Febles J, Sanz-Esporrin J. Diabetes and periodontal disease. Review of the literature. Am J Dent. 2014;27(2):63–67. [PubMed] [Google Scholar]
- 40.Stanko P, Holla LI. Bidirectional association between diabetes mellitus and inflammatory periodontal disease. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(1):35–38. doi: 10.5507/bp.2014.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.