Abstract
Objective
Emergency department‐initiated buprenorphine (BUP) for opioid use disorder is an evidence‐based practice, but limited data exist on BUP initiation practices in real‐world settings. We sought to characterize protocols for BUP initiation among a geographically diverse sample of emergency departments (EDs).
Methods
In December 2020, we reviewed prestudy clinical BUP initiation protocols from all EDs participating in CTN0099 Emergency Department‐INitiated bupreNOrphine VAlidaTION (ED‐INNOVATION). We abstracted information on processes for identification of treatment‐eligible patients, BUP administration, and discharge care.
Results
All participating ED‐INNOVATION sites across 22 states submitted protocols; 31 protocols were analyzed. Identification of treatment‐eligible patients: Most EDs 22 (71%) relied on clinician judgment to determine appropriateness of BUP treatment with only 7 (23%) requiring decision support tools or diagnosis checklists. Before BUP initiation, 27 (87%) protocols required a documented Clinical Opiate Withdrawal Scale (COWS) score; 4 (13%) required a clinical diagnosis of withdrawal with optional COWS score. Twenty‐seven (87%) recommended a minimum COWS score of 8 for ED‐initiated BUP. BUP administration: Initial BUP dose ranged from 2–16 mg (mode = 4). For continued withdrawal symptoms, 27 (87%) protocols recommended an interval of 30–60 minutes between first and second BUP dose. Total BUP dose in the ED ranged from 8 to 32 mg. Discharge care: Twenty‐eight (90%) protocols recommended a BUP prescription (mode 16 mg daily) at discharge. Naloxone prescription and/or provision was suggested in 23 (74%) protocols.
Conclusions
In this geographically diverse sample of EDs, protocols for ED‐initiated BUP differed between sites. Future work should evaluate the association between this variation and patient outcomes.
Keywords: buprenorphine protocol, emergency department, opioid use disorder, opioid withdrawal
1. INTRODUCTION
1.1. Background
From 1999 to 2018, the opioid epidemic claimed over 446,000 lives in the United States. 1 In 2020, there was a 21% increase in fatal overdoses, two thirds of which were opioid related. 2 Medications for opioid use disorder (MOUD), particularly methadone and buprenorphine, reduce mortality, increase treatment retention, decrease opioid misuse, and reduce craving and withdrawal. 3 , 4 , 5 Despite benefit, the majority of patients with opioid use disorder (OUD) do not receive MOUD. 6 , 7 , 8
The emergency department (ED) is an important setting to identify patients with OUD and initiate buprenorphine (BUP). 6 , 9 , 10 From 2005 to 2014, opioid‐related visits in the United States to EDs doubled. 11 In 2015, a randomized controlled trial demonstrated that ED‐initiated BUP increased engagement in treatment at 30 days, reduced opioid misuse, and was cost effective compared to brief intervention with a referral or referral alone. 10 , 12 Although there has been a modest increase in ED BUP prescribing, few ED clinicians indicate high readiness to initiate BUP. 13 , 14
When experience is lacking, clinical protocols may help facilitate adoption of ED‐initiated BUP. 14 , 15 , 16 Most research on BUP has not been conducted in EDs, and existing protocols for BUP initiation in other settings may not reflect the unique needs of the ED and/or its patient population. The recently released guideline published by the Substance Abuse and Mental Health Services Administration (SAMHSA) on ED‐initiated BUP suggests dosing that is lower and less frequent than the emergency medicine literature. 17 , 18 , 19 , 20 , 21 , 22 , 23 Instead, the SAMHSA guideline mirrors dosing regimens from clinical trials and observational studies in office‐based settings. 3 , 24 , 25
Although patients presenting for office‐based BUP initiation are not physiologically different from those in the ED, context matters. For example, often the individuals with OUD who use the ED represent vulnerable populations, lacking other sources of healthcare access. They are more often uninsured or underinsured, and many have coexisting mental health issues and unstable housing. 17 , 26 , 27 ED patients often have more acute illnesses compared to those in office‐based settings and may require more intense treatment and monitoring. In addition to these patient‐related differences, EDs are faced with mounting pressures caused by unprecedented crowding; thus, enhancing throughput has become a priority. Minimizing repeat visits is essential to reduce unnecessary volume and to eliminate any potential additional insurance copays. Given the differences between the office and ED settings, it is not surprising that BUP protocols for office‐based inductions may not translate well to the ED. To date, EDs have developed their own protocols but only few have made them publicly available. 18 , 19
1.2. Importance
The generation of site‐specific evidence‐based protocols may be an important strategy to streamline implementation and enhance the adoption of ED‐initiated BUP. 14 Understanding variations and similarities in BUP initiation protocols across a diverse sample of EDs can help guide recommendations for protocol development and future research on best practices.
1.3. Goals of this investigation
We sought to characterize a national sample of ED‐initiated BUP clinical protocols on (1) identification of treatment‐eligible patients, (2) ED BUP administration, and (3) discharge care with the goals of providing recommendations for key components of an ED‐initiated BUP protocol based on similarities across protocols analyzed, as well as lay the groundwork for research to evaluate best practices.
2. METHODS
2.1. Study design
We analyzed ED BUP initiation protocols for all EDs enrolled in the National Institute on Drug Abuse Clinical Trials Network (NIDA CTN) 0099 Emergency Department‐INitiated BupreNOrphine VAlidaTION Trial Network (ED‐INNOVATION) study (ClinicalTrials.gov: NCT04225598). 28 The ED‐INNOVATION protocol has been published elsewhere. 28 Briefly, the study has 2 components: (1) an implementation phase using implementation facilitation to enhance adoption of ED‐initiated BUP and (2) a randomized controlled trial comparing the effectiveness of 2 BUP formulations, sublingual (SL‐BUP) and a 7‐day extended‐release injectable (CAM2038, XR‐BUP) among patients with OUD on the primary outcome of engagement in formal addiction treatment at 7 days. 28 , 29 As part of the implementation facilitation phase but before initiation of any associated support efforts, all sites were required to submit their clinical BUP initiation protocols for review by the lead research team before October 31, 2020.
2.2. Site selection
ED‐INNOVATION sites were chosen based on a formal application process that took place in June 2019. Site selection was based on (1) the prevalence of patients with International Classification of Diseases, 10th revision codes for the prior 12 months related to overdose, OUD, and other opioid‐related diagnoses using a predetermined list of codes including those in the F11 (opioid related disorders) and T40 (poisoning by, adverse effects of and underdosing of narcotics and psychodysleptics [hallucinogens]) categories; (2) presence of ED investigators with demonstrated experience conducting research; (3) an electronic health record that can be queried routinely to assess opioid‐related diagnoses and provision of ED‐initiated BUP; (4) ability to have SL‐BUP and store extended release BUP under investigational drug status in the ED; and (5) availability of community clinicians and programs for referral for ongoing MOUD (methadone or BUP) treatment. Sites were chosen independently of whether they had a completed protocol for ED‐initiated BUP. They were selected to reflect a mix of rural/urban/suburban; community/academic; geographic location; and size. Thirty sites were chosen from 69 applications. After initial site selection, 1 site dropped out and 4 were added, resulting in 33 sites, all of which submitted clinical BUP protocols for review. All sites were approved for participation in ED‐INNOVATION by the Western Institutional Review Board.
2.3. Procedures
Two investigators independently reviewed all submitted clinical BUP protocols using a standardized data abstraction survey created in Qualtrics (Qualtrics, Provo, UT) that collected data on (1) identification of treatment eligible patients, (2) ED BUP administration, and (3) discharge care. These 3 elements were chosen a priori based on a review of the literature on BUP initiation both in the ED and other clinical settings. 18 , 19 , 30 , 31 Specific variables of interest under each section were identified, and questions were drafted and used to review each protocol (Web Appendix 1). After independent review of 5 protocols, data abstractors met to clarify and revise the questions, and all previously reviewed protocols were re‐reviewed. Chart abstractors were not blinded to the study objective. Survey results were exported to Excel and reviewed for disagreements then reexamined to see if consensus could be reached. When consensus could not be achieved, a third investigator acted as the final arbitrator.
2.4. Analysis
This study is descriptive with no formal hypothesis being tested. Descriptive measures used to describe the data include frequencies and percentages for nominal and ordinal categorical variables and mean, mode, minimum, maximum, and total range where appropriate for continuous variables. Interrater reliability after initial data abstraction was calculated for categorical and ordinal variables using either unweighted or weighted Cohen's kappa, respectively.
The Bottom Line.
This study demonstrates that variability exists across emergency department‐initiated buprenorphine protocols across a sample of 31 geographically diverse sites; however, most protocols used a similar framework of identifying eligible patients, further assessing eligibility, providing buprenorphine, and discharging patients with harm reduction and referral to care. Future research could best test and define the specific steps within that framework.
3. RESULTS
3.1. Site characteristics
All sites (n = 33) EDs participating in ED‐INNOVATION submitted protocols for lead team review. Among the 4 new sites added to the study, 2 were from the same health system, had the same study staff, and used the same protocol as their original sites. Therefore, 31 protocols were analyzed, representing EDs from 22 states (Figure 1) and a variety of practice settings.
FIGURE 1.
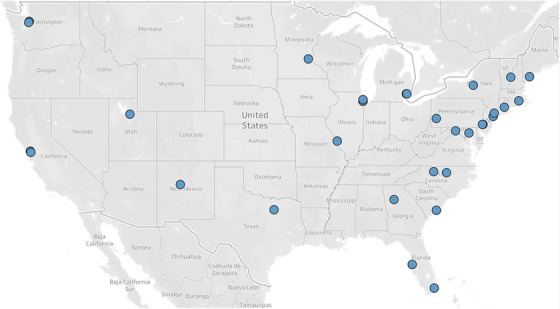
Location of emergency department sites that were selected to participate in CTN 0099 ED‐INNOVATION (n = 33). Sites that submitted protocols and were selected to participate in the National Institute on Drug Abuse Clinical Trials Network (NIDA CTN) 0099 Emergency Department‐INitiated BupreNOrphine VAlidaTION Trial Network (ED‐INNOVATION) study are indicated by blue circles (n = 33). The 33 sites represented 22 states; overlapping circles include 2 in California, 2 in Illinois, 2 in Michigan, 2 in New Mexico, 3 in Pennsylvania, and 2 in Washington. Abbreviations: BUP, buprenorphine; COWS, Clinical Opiate Withdrawal Scale; ED, emergency department; OUD, opioid use disorder
Two sites had previously participated in a clinical trial with the lead research team on ED‐initiated BUP (CTN‐0069, NCT03023930), during which feedback was provided on their ED‐initiated BUP protocols. 29 The lead research team, as an ED participating in clinical trial enrollment, also submitted a clinical protocol for review. The remaining 28 sites submitted protocols with no prior lead team feedback, although all had access to the lead team's protocol for reference.
3.2. Identification of treatment‐eligible patients
Six (19%) protocols had guidelines for universal screening for substance use disorders; 2 (6%) recommended using chief complaints or triage screening and 4 (31%) used either a site‐specific assessment or other screening tools such as the NIDA Quick Screen or Screening and Brief Intervention and Referral to Treatment screening tool. 32 , 33 (Table 1).
TABLE 1.
Identification of treatment‐eligible patients before ED BUP administration (n = 31 site protocols)
| # of site protocols | % of site protocols | |
|---|---|---|
| a) Inclusion criteria for ED BUP administration: | 31 | 100% |
| OUD determination | 22 | 71% |
| Formal OUD screen using DSM‐5 | 7 | 23% |
| Active withdrawal | 31 | 100% |
| COWS score | 27 | 87% |
| Clinical diagnosis of withdrawal, optional COWS score | 4 | 13% |
| Time elapsed since last opioid use: | 19 | 61% |
| Methadone | 16 | 52% |
| Long‐acting opioids excluding methadone | 9 | 29% |
| Short‐acting opioids | 9 | 29% |
| Heroin | 5 | 16% |
| b) Minimum COWS score required before ED BUP initiation | 30 | 97% |
| Minimum COWS score of 8 | 27 | 87% |
| c) Contraindications to ED BUP administration: | 21 | 68% |
| Recent methadone use | 16 | 52% |
| Severe medical illness including liver disease | 11 | 35% |
| Altered mental status and/or intoxication | 9 | 29% |
| Pain, trauma, and/or planned surgeries | 6 | 19% |
| Alcohol and/or benzodiazepine withdrawal or use | 5 | 16% |
| Other | 9 | 29% |
| d) Other evaluations before BUP administration: | ||
| Pregnancy determination | 21 | 68% |
| Other labs required or when clinically indicated | 9 | 29% |
| e) Guidelines for identifying patients for ED buprenorphine | 6 | 19% |
| f) Ancillary staff for ED BUP | 21 | 68% |
Number of site protocols that provided guidelines related to identification of treatment‐eligible patients: (a) inclusion criteria, (b) minimum COWS score, (c) absolute contraindications to ED BUP (“other” contraindications included, but were not limited to, naloxone induced withdrawal, BUP allergy, lack of patient willingness to initiate BUP, and exacerbation of psychiatric illness or active psychosis), (d) other evaluations (other labs included urine toxicology, liver function tests, complete blood count (CBC), basic/complete metabolic panel [BMP/CMP], and otherwise not specified), (e) guidelines to identify patients, and (f) ancillary staff.
Abbreviations: BUP, buprenorphine; COWS, Clinical Opiate Withdrawal Scale; DSM‐5, Diagnostic and Statistical Manual of Mental Disorders, 5th edition; ED, emergency department; OUD, opioid use disorder
In 22 of 31 (71%) protocols, identification of patients with OUD was performed based on clinical judgment; only 7 (23%) expected documentation of the specific Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM‐5) criteria to diagnose OUD. 34 All 31 protocols required determination of a patient's severity of withdrawal: 27 (87%) required a documented Clinical Opiate Withdrawal Scale (COWS) score, and 4 (13%) required a clinical diagnosis of withdrawal with optional COWS score. Among the protocols with required or optional COWS score: 30 (97%) recommended or required a minimum COWS score before ED BUP initiation; 27 (87%) recommended a minimum COWS score of 8 with 3 (10%) or without 24 (77%) objective signs; and the remaining 3 protocols recommended a minimum COWS score of 5, 11, or 12.
Nineteen (61%) protocols listed the minimum number of hours since last opioid use to help inform eligibility for ED BUP initiation: most commonly 8 (26%) requiring 12 hours since short‐acting opioid use, 7 (23%) requiring 24 hours since long‐acting opioids, and 13 (42%) requiring 48–72 hours since last methadone use. Twenty‐one (68%) protocols listed contraindications to ED BUP initiation: 16 (52%) included recent (24 hours to 2‐week) methadone use; 11 (35%) included severe medical illness including liver disease; 9 (29%) included altered mental status and/or intoxication; 6 (19%) included pain, trauma, and/or planned surgeries; and 5 (16%) included alcohol and/or benzodiazepine withdrawal or use.
Pregnancy determination before ED BUP initiation was required or recommended in 21 (68%) protocols. In 9 (29%) protocols, other labs such as urine toxicology, liver function tests, complete blood count, and basic/complete metabolic panel were required or recommended if clinically indicated.
Twenty‐one (68%) protocols mentioned ancillary staff involvement, including 12 (39%) that used social workers, 11 (35%) that used peer counselors/advocates, and 7 (23%) that used care managers.
3.3. ED BUP administration
Twenty‐eight (90%) protocols specified using SL‐BUP in the ED; among those, 3 (10%) also suggested the use of intramuscular or intravenous routes of administration in certain circumstances. The remaining 3 (10%) protocols did not specify the mode of BUP administration. Fifteen (48%) protocols specified using the buprenorphine/naloxone combination product; among those, 6 (19%) additionally allowed buprenorphine monotherapy use. The remaining 16 (52%) protocols did not specify formulation. (Table 2).
TABLE 2.
Details of ED BUP administration (n = 31 site protocols)
| # of site protocols | % of site protocols | |
|---|---|---|
| a) Variable initial buprenorphine dose based on COWS score | 14 | 45% |
| Dose based on COWS score 8–12, 13+ | 12 | 39% |
| b) Time frame between buprenorphine dose 1 and 2 | 29 | 94% |
| 30–60 min | 27 | 87% |
| <30 or >60 min | 2 | 6% |
| c) Maximum total buprenorphine dose in ED | 29 | 94% |
| 8 mg | 5 | 16% |
| 12 mg | 5 | 16% |
| 16 mg | 11 | 35% |
| 24 mg | 2 | 6% |
| 32 mg | 6 | 19% |
| d) Precipitated withdrawal guidelines | 10 | 32% |
| e) Ancillary medications for symptoms of: | 9 | 29% |
| Muscle aches and pains | 8 | 26% |
| Nausea | 9 | 29% |
| Abdominal cramps and diarrhea | 8 | 26% |
| Other | 7 | 23% |
Number of site protocols that provided details related to administration of BUP in the ED: (a) initial ED BUP dose dependent on or independent of the patient's COWS score, (b) time frame between the initial BUP dose and a second BUP dose, (c) maximum total BUP dose, (d) precipitated withdrawal guidelines, and (e) ancillary medications for symptomatic management of withdrawal–“other” ancillary medications included clonidine (23%), antihistamines (16%), gabapentin (3%), antipsychotics (3%), and methadone (3%).
Abbreviations: BUP, buprenorphine; COWS, Clinical Opiate Withdrawal Scale; ED, emergency department.
Initial recommended BUP dose ranged from 2–16 mg (mode = 4). In 14 (45%) protocols, first BUP dose varied by COWS score: 11 (35%) protocols specified 4 mg BUP for COWS score 8–12 and 8 mg for COWS score 13+.
Additional recommendations for BUP dosing were specified in 28 (90%) protocols ranging from 4 to 24 mg. For continued withdrawal symptoms, 27 (87%) protocols recommended an interval of 30–60 minutes between the first and second BUP dose (range 20–360 minutes). The maximum total BUP dose in the ED was specified in 29 (94%) protocols and ranged from 8 mg to 32 mg (mode 16 mg).
In terms of worsening or perceived precipitated withdrawal, 9 (29%) protocols described ancillary medications for continued withdrawal symptoms. The most frequently listed medications were ondansetron, loperamide, clonidine and nonsteroid anti‐inflammatory drugs. Ten (32%) protocols included specific guidelines for precipitated withdrawal; among those, 5 (16%) recommended additional BUP for precipitated withdrawal, and 5 (16%) recommended ancillary medications without additional BUP.
3.4. Discharge care
Treatment for patients presenting with low COWS scores varied. Twenty‐two (71%) protocols included recommendations for management of patients with low COWS scores. Among those, 12 (39%) recommended discharging the patient from the ED with a prescription for home initiation of BUP; 7 (23%) provided the option of either discharging or holding in the ED; and 3 (10%) recommended holding the patient in the ED until withdrawal worsened. (Table 3).
TABLE 3.
Discharge care after ED BUP administration (n = 31 site protocols)
| # of site protocols | % of site protocols | |
|---|---|---|
| a) Policy for low COWS score | 22 | 71% |
| Discharge from ED for home induction | 12 | 39% |
| Hold in ED until COWS score increases | 3 | 10% |
| Both discharge and hold in ED | 7 | 23% |
| b) Home induction instructions provided | 12 | 39% |
| c) Buprenorphine prescription at discharge | 28 | 90% |
| 4 mg | 2 | 6% |
| 8 mg | 4 | 13% |
| 12 mg | 2 | 6% |
| 16 mg | 21 | 68% |
| 24 mg | 3 | 10% |
| 32 mg | 3 | 10% |
| Depends on ED dose/Other | 4 | 13% |
| d) Naloxone provided and/or prescribed | 23 | 74% |
| e) Harm reduction education | 5 | 16% |
Number of sites with guidelines related to discharge care instructions: (a) policy for low COWS score, (b) provision of home BUP induction instructions, (c) BUP prescription at discharge (note that some site protocols allowed several dosages to be prescribed at discharge), (d) naloxone for overdose prevention, and (e) harm reduction education.
Referral to outpatient treatment was addressed by all but one (97%) protocol, although only 2 (6%) explicitly mentioned a warm handoff. A BUP prescription provided at discharge was recommended by most, 28 (90%), ranging from 4–32 mg daily; the remaining 3 (10%) protocols did not include information on a discharge prescription of BUP. When prescribed, the most frequent dose (mode) of BUP was 16 mg daily (recommended in 13 [42%] protocols) and 8 mg twice a day (recommended in 8 [26%]). Seventeen (55%) protocols advised a prescription sufficient until the patient's follow‐up appointment; 4 (13%) specified a range between 3 and 14 days; and 7 (23%) did not mention a set length of time for the prescription at discharge. If no DATA2000 waivered clinicians were present to prescribe BUP, 14 (45%) protocols recommended having the patient return to the ED daily for subsequent doses for up to 3 days, 3 (10%) recommended a loading dose in the ED up to 32 mg, and 5 (16%) mentioned finding a waivered clinician.
Naloxone prescription and/or provision was suggested in 23 (74%) protocols. Other specific harm reduction education, such as explicit overdose education, was mentioned in only 5 (16%) protocols.
3.5. Interrater reliability
Kappa values for categorical and ordinal survey questions ranged from 0.37 to 1.0. Only 1 out of 40 survey question had a kappa value <0.41 and only 5 had a kappa <0.61. In general, observers agreed on data abstraction conclusions; agreement between observers was moderate to near perfect. Only 3 disagreements between the data abstractors could not be resolved and required adjudication by a third reviewer.
3.6. LIMITATIONS
Our findings may be subject to selection bias. Although the ED‐INNOVATION sites represented a geographically diverse sample, they may not accurately reflect the spectrum of ED BUP initiation practices across the country. Sites had to apply for inclusion in the CTN study, implying that prior work had been completed on ED‐initiated BUP or at least contemplated. Furthermore, ED‐INNOVATION sites had access to the lead research team's ED BUP initiation protocol during the implementation facilitation phase and were encouraged to review that protocol when creating or modifying their own site‐specific clinical protocols. Additionally, the lead research team also submitted a protocol for review, and 2 sites had participated in a prior CTN study during which feedback was given on their respective ED‐initiated BUP protocols; thus, at least 3 protocols were not in early protocol development. Despite this limitation, the presence of significant variability in the analyzed protocols suggests that sites developed BUP initiation procedures that were adapted to their unique settings.
Beyond the generalizability of sites themselves, individual protocols varied in structure and level of detail, potentially precluding consistent interpretation by our investigative team.
Finally, the protocols themselves may not accurately reflect clinical practice, but prior qualitative research suggests that clinicians less experienced at BUP initiation would likely lean heavily on protocols if they existed and that the absence of protocols is a major barrier to adoption of ED‐initiated BUP. 14
4. DISCUSSION
Our study demonstrated considerable variability in protocols related to all 3 steps of ED‐initiated BUP: (1) identification of treatment‐eligible patients, (2) BUP administration, and (3) discharge care.
4.1. Identification of treatment‐eligible patients
In most protocols, clinical judgment rather than formal diagnostic criteria was used to determine the presence of OUD in treatment‐eligible ED patients. Furthermore, protocols often did not note the involvement of other multidisciplinary team members, such as social workers, health advocates, or other peer counselors/coaches or what training in psychosocial interventions they used to motivate patients with OUD to engage in treatment. The most consistent finding across protocols was the evaluation of withdrawal, either by clinical gestalt or a formal structured instrument such as the COWS, which grades withdrawal severity on a scale of 0–36 based on signs and symptoms such as heart rate, pupil size, restlessness, and yawning. 35 A clinical diagnosis of OUD and formal evaluation of withdrawal is consistent with SAMHSA Treatment Improvement Protocol Series 63 (TIP 63), the American Society of Addiction Medicine (ASAM) and American College of Emergency Physicians (ACEP) guidelines that recommend initiating BUP in patients experiencing opioid withdrawal. 3 , 24 , 31
Most protocols recommended a minimum COWS score of 8 before starting BUP, consistent with ACEP guidelines but lower than SAMHSA Treatment Improvement Protocol (TIP) 63′s recommendation of COWS score ≥12 and ASAM National Practice Guidelines recommendation of COWS score 11–12. 3 , 24 , 31 Several individual ED protocols also used a lower COWS score of 5 or 6 for BUP initiation. Initiating BUP in ED patients with lower COWS score is appealing because it leverages the “teachable moment” of the ED experience when patients may have a heightened interest in behavior change while avoiding the need to have patients return to the ED later in worse withdrawal, potentially exposing them to increased costs and long ED wait times.
Overall, protocols varied in their listed contraindications to ED‐initiated BUP. The most common contraindication was recent methadone use, likely due to the risk of BUP‐precipitated withdrawal. 3 , 24 Other listed contraindications, such as alcohol and/or benzodiazepine withdrawal or use, may be viewed as complicating factors of withdrawal or a misunderstanding of risk. 36 Although SAMHSA listed BUP allergy as its only contraindication, only 4 sites did so in their protocols. 24
Pregnancy was not a contraindication to ED‐initiated BUP in any protocol; however, pregnancy was mentioned in most protocols as a special consideration or as a required lab test before initiation, likely because of prior recommendations to use BUP monotherapy in pregnancy to avoid prenatal exposure to naloxone and to provide appropriate, rapid follow‐up. 37 Recent guidelines from the American College of Obstetricians and Gynecologists, SAMHSA, ASAM, and ACEP recommend the use of both BUP monotherapy and combination product in pregnancy given similar outcomes and safety profiles. 3 , 24 , 31 , 37
4.2. ED BUP administration
Protocols varied in the initial and total dose of ED BUP administered in the ED to patients in opioid withdrawal. For patients with clinical signs of opioid withdrawal, most protocols suggest administering 4–8 mg of BUP independent of initial COWS score. Most protocols that used tiered scoring used a COWS vof <13 as a proxy for mild withdrawal and a COWS score of 13 or higher as a proxy for moderate or severe withdrawal. Among those protocols, most would administer 4 mg for a lower COWS range and 8 mg for a higher COWS range. These initiation doses differ from what is suggested by both SAMHSA and ASAM, which both recommend a lower initial BUP dose of 2–4 mg. 3 , 24 More recent ED literature has also shown that higher doses of BUP (>12 mg during an ED visit) in patients experiencing opioid withdrawal can be tolerated in select patients. 27 , 38 , 39
Although the lower limit of the COWS for ED initiation differed between protocols, ranging from 5 to 12, most (61%) recommended unobserved (home) initiation for those not in adequate withdrawal for ED BUP initiation. Unobserved BUP initiation has several advantages. 40 It is less resource intensive, requires shorter ED lengths of stay, and adds individual patient flexibility as to how and when to start treatment. 40 One barrier to unobserved initiation for ED patients is the need for a DATA2000 X‐waivered clinician to prescribe BUP that may not be available 24/7 in many EDs; however, the new Practice Guideline by the Department of Health and Human Services decreases this barrier substantially by allowing those who treat up to 30 patients to submit an alternative notification of intent in place of the required waiver training. 41 Patient‐level barriers are also a factor including the need for transportation to the pharmacy to pick up the medications, lack of valid identification, medication preauthorization, or lack of insurance altogether. The ED INNOVATION randomized controlled trial seeks to test if these patient‐ and clinician‐level barriers to follow‐up care are affected by different formulation of buprenorphine such as a long acting 7‐day injectable BUP compared to the standard sublingual preparation. 28
For patients initiated on BUP in the ED with continued withdrawal symptoms after the first BUP dose, most protocols recommended an additional 4–8 mg of BUP, with only a few suggesting higher additional doses of 12 mg or greater. In total, most protocols set the maximum ED BUP dose to 16 mg, although the range was wide at 8 mg to 32 mg. Although 8 mg of BUP for withdrawal was the day 1 limit set by the Food and Drug Administration label for Suboxone (buprenorphine/naloxone), studies have ended day 1 of BUP on a higher total dose. 27 , 38 , 39 , 42 Although the ideal indications for and safety of higher day 1 doses of BUP in patients coming to the ED is under investigation, a higher maximum BUP dose may be beneficial not only for withdrawal relief but also for patients with barriers to follow‐up care such as prolonged wait time for postdischarge follow‐up or prior authorizations. 27 , 30 On the whole, variability in initial and maximum ED doses of BUP and the timing between doses in ED BUP initiation protocols underscore both the flexibility of BUP initiation across different EDs settings and a lack of consensus on best practices.
Lastly, although precipitated withdrawal is a rare event, only 10 (32%) protocols provided guidelines on how to manage these occurrences. 21 Half of those protocols recommended additional BUP and the other half supportive medications only. Neither SAMHSA TIP 63 or ASAM guidelines contain information on managing precipitated withdrawal. 3 , 24 ACEP recommends both additional BUP and ancillary medications for targeted symptoms but acknowledges the limited published data on the rapid effectiveness of additional BUP. 31 , 43
4.3. Discharge care
For patients presenting to the ED in mild or no opioid withdrawal, most protocols recommended discharging the patient for unobserved initiation with BUP. 40 , 44 , 45 A few protocols suggested holding a patient with a low COWS score in the ED until withdrawal worsens. Given that EDs have been faced with increased crowding and boarding times, this strategy could compound existing logistical challenges that have already been shown to result in worse objective clinical end points. 46 , 47
For patients initiated on BUP in the ED, most protocols recommended prescribing 16 mg of BUP per day on subsequent days regardless of the dose the patient received in the ED. This dose recommendation is consistent with ASAM recommendations of a daily treatment dose of at least 16 mg and SAMHSA recommendations of 4–24 mg. 3 , 24 It is concerning that some protocols neither mentioned providing a BUP prescription at discharge nor specified a treatment duration (ie, days of coverage with the prescription). Post‐ED discharge is a tenuous time for many patients with OUD, especially those who were treated for a non‐fatal opioid overdose. 48 , 49 During this time, it is critically important that patients amenable to treatment are provided with sufficient medication until they can be linked to comprehensive addiction treatment. Providing BUP for these patients can both treat their OUD and help prevent recurrent overdose. 50 In combination with BUP, the provision of naloxone and harm reduction education are essential program components to provide at discharge to improve patient safety, reduce overdose death, and prevent injection and drug use complications such as hepatitis and HIV. 51 , 52 Most protocols did mention the provision or prescribing of naloxone, a recommended best practice. 53 , 54 Conversely, very few protocols mentioned other risk reduction measures such as education on safe injection practices.
4.4. Summary and recommendations for protocol development
In summary, the ED is a unique setting in which to treat patients with OUD. In fact, some have argued that withholding care for those with substance use disorders in the ED is a violation of federal law. 55
Our study aimed to describe the landscape of ED‐initiated BUP protocols in a geographically diverse sample of EDs in the United States. Protocols differed regarding identification of treatment‐eligible patients, ED BUP administration, and discharge care, demonstrating both the flexibility of BUP initiation in the ED setting and the realities of carving out a lifesaving strategy with little previous guidance.
Although variation existed across the 31 protocols, most used a similar flow of patients from ED presentation to discharge. We therefore recommend the following evidence‐based framework (Figure 2), consistent with the recently released ACEP Consensus Recommendations on the Treatment of Opioid Use Disorder in the Emergency Department 56 : (1) identification of patients; (2) Assessment of OUD, withdrawal severity, and pregnancy; (3) treatment with buprenorphine initiation or instructions for unobserved (home) induction; and (4) discharge with overdose education and naloxone distribution, buprenorphine prescription, and referral for follow‐up care.
FIGURE 2.
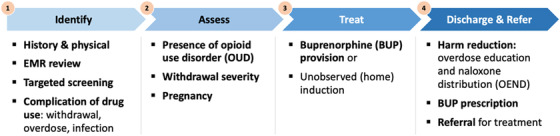
Framework for ED‐initiated buprenorphine
Abbreviation: EMR, electronic medical record
Different EDs may tailor the specifics of each step of the framework to the unique needs of their ED, which is reflected in the variability demonstrated across the 31 protocols. Current and future research should evaluate how variations in screening techniques, BUP dosing, and harm reduction strategies affect patient outcomes and support dissemination of ED‐BUP initiation practices to continue to drive innovation that improves patient outcomes.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Ethan Cowan and Clara Zhang Guo conceived and designed the study methods with input from all authors. Clara Zhang Guo and Ethan Cowan reviewed all protocols, David Fiellin arbitrated discrepancies, and Clara Zhang Guo analyzed the data once aligned upon. Clara Zhang Guo and Ethan Cowan drafted the manuscript, and all authors contributed to revisions. Clara Zhang Guo takes responsibility for the paper.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health through the NIH HEAL Initiative under award number 3UG1DA015831. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its NIH HEAL Initiative.
Biography
Clara Guo, BA, is a MD Candidate at Yale School of Medicine.

WEB APPENDIX A.
| Extraction Elements for ED Buprenorphine Initiation Protocol Survey | |
| Identification of treatment‐eligible patients |
|
| Buprenorphine administration |
|
| Discharge care |
|
Guo CZ, D'Onofrio G, Fiellin DA, et al. Emergency department‐initiated buprenorphine protocols: A national evaluation. JACEP Open. 2021;2:e12606. 10.1002/emp2.12606
Meetings: The College on Problems of Drug Dependence 2021 Annual Meeting, June 2021, virtual.
Clinical trial: NCT04225598 – National Institute on Drug Abuse Clinical Trials Network (NIDA CTN) 0099 Emergency Department‐INitiated BupreNOrphine VAlidaTION Trial Network (ED‐INNOVATION).
Supervising Editor: Marna Rayl Greenberg, DO, MPH
REFERENCES
- 1. Wilson N, Kariisa M, Seth P, Ht Smith, Davis NL. Drug and opioid‐involved overdose deaths ‐ United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 2020;69:290‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad FB, Rossen LM, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics; 2021. [Google Scholar]
- 3. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14:1‐91. [DOI] [PubMed] [Google Scholar]
- 4. Substance Abuse and Mental Health Service Administration . FAQs: Provision of methadone and buprenorphine for the treatment of opioid use disorder in the COVID‐19 emergency. National Mental Health and Substance Use Policy Laboratory. Substance Abuse and Mental Health Services Administration, 2020. https://www.samhsa.gov/sites/default/files/faqs‐for‐oudprescribing‐and‐dispensing.pdf [Google Scholar]
- 5. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta‐analysis of cohort studies. BMJ. 2017:357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D'Onofrio G, Venkatesh A, Hawk K. The adverse impact of COVID‐19 on individuals with OUD s the urgent need for reform to leverage emergency department–based treatment. NEJM Catalyst. 2020. [Google Scholar]
- 7. Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication‐assisted therapies—tackling the opioid‐overdose epidemic. N Engl J Med. 2014;370:2063‐2066. [DOI] [PubMed] [Google Scholar]
- 8. Olfson M, Zhang VS, Schoenbaum M, King M. Trends in buprenorphine treatment in the United States, 2009–2018. JAMA. 2020;323:276‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Onofrio G, McCormack RP, Hawk K. Emergency departments‐a 24/7/365 option for combating the opioid crisis. N Engl J Med. 2018;379:2487‐2490. [DOI] [PubMed] [Google Scholar]
- 10. D'Onofrio G, O'Connor PG, Pantalon MV, et al. Emergency department–initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313:1636‐1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weiss AJ, Bailey MK, O'Malley L, Barrett ML, Elixhauser A, Steiner CA. Patient characteristics of opioid‐related inpatient stays and emergency department visits nationally and by state, 2014: statistical brief# 224. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2017. [PubMed] [Google Scholar]
- 12. Busch SH, Fiellin DA, Chawarski MC, et al. Cost‐effectiveness of emergency department‐initiated treatment for opioid dependence. Addiction. 2017;112:2002‐2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rhee TG, D'Onofrio G, Fiellin DA. Trends in the use of buprenorphine in US emergency departments, 2002–2017. JAMA Network Open. 2020;3:e2021209‐e2021209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hawk KF, D'Onofrio G, Chawarski MC, et al. Barriers and facilitators to clinician readiness to provide emergency department–initiated buprenorphine. JAMA network open. 2020;3:e204561‐e204561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: a physician survey. Am J Emerg Med. 2019;37:1787‐1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Im DD, Chary A, Condella AL, et al. Emergency department clinicians’ attitudes toward opioid use disorder and emergency department‐initiated buprenorphine treatment: a mixed‐methods study. West J Emerg Med. 2020;21:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Substance Abuse and Mental Health Services Administration: Use of Medication‐Assisted Treatment in Emergency Departments. HHS Publication No. PEP21‐PL‐Guide‐5 National Mental Health and Substance Use Policy Laboratory. Substance Abuse and Mental Health Services Administration, 2021. [Google Scholar]
- 18. Yale School of Medicine Emergency Medicine . ED‐initiated buprenorphine. Available at https://medicine.yale.edu/edbup/Algorithm_338052_5_v2.pdf. Accessed February 7, 2021.
- 19. California Bridge . Buprenorphine (BUP) hospital quick start. Available at https://static1.squarespace.com/static/5c412ab755b02cec3b4ed998/t/5dc255df2d46c2731a7b366c/1573017059129/CA+Bridge+‐+Protocol+‐+Bup+Hospital+Quick+Start+‐+NOV+2019.pdf. Accessed February 7, 2021.
- 20. Ray JM, Ahmed OM, Solad Y, et al. Computerized clinical decision support system for emergency department–initiated buprenorphine for opioid use disorder: user‐centered design. JMIR human factors. 2019;6:e13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. LeSaint KT, Klapthor B, Wang RC, Geier C. Buprenorphine for opioid use disorder in the Emergency Department: a retrospective chart review. West J Emerg Med. 2020;21:1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu T, Snider‐Adler M, Nijmeh L, Pyle A. Buprenorphine/naloxone induction in a Canadian emergency department with rapid access to community‐based addictions providers. CJEM: Journal of the Canadian Association of Emergency Physicians. 2019;21:492‐498. [DOI] [PubMed] [Google Scholar]
- 23. Edwards FJ, Wicelinski R, Gallagher N, McKinzie A, White R, Domingos A. Treating opioid withdrawal with buprenorphine in a community hospital emergency department: an outreach program. Ann Emerg Med. 2020;75:49‐56. [DOI] [PubMed] [Google Scholar]
- 24. Substance Abuse and Mental Health Services Administration . Medications for opioid use disorder, SAMHSA Treatment Improvement Protocol (TIP63): For healthcare and addiction professionals, policymakers, patients, and families. HHS Publication No. PEP20‐02‐01‐006. National Mental Health and Substance Use Policy Laboratory. Substance Abuse and Mental Health Services Administration, 2020. [Google Scholar]
- 25. Fiellin DA, Kleber H, Trumble‐Hejduk JG, McLellan AT, Kosten TR. Consensus statement on office‐based treatment of opioid dependence using buprenorphine. J Subst Abuse Treat. 2004;27:153‐159. [DOI] [PubMed] [Google Scholar]
- 26. Weiss AJ, Heslin KC. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2018. payers of opioid‐related inpatient stays and emergency department visits nationally and by state, 2010 and 2015: statistical brief# 239. [PubMed] [Google Scholar]
- 27. Herring AA, Vosooghi AA, Luftig J, et al. High‐dose buprenorphine induction in the Emergency Department for treatment of opioid use disorder. JAMA Network Open. 2021;4(7):e2117128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. D'Onofrio G, Hawk KF, Herring A, et al. The design and conduct of a randomized clinical trial comparing emergency department initiation of sublingual versus a 7‐day extended‐release injection formulation of buprenorphine for opioid use disorder: project ED Innovation. Contemporary Clinical Trials. 2021:106359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. D'Onofrio G, Edelman EJ, Hawk KF, et al. Implementation facilitation to promote emergency department‐initiated buprenorphine for opioid use disorder: protocol for a hybrid type III effectiveness‐implementation study (Project ED HEALTH). Implementation Science. 2019;14:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herring AA, Perrone J, Nelson LS. Managing opioid withdrawal in the emergency department with buprenorphine. Ann Emerg Med. 2019;73:481‐487. [DOI] [PubMed] [Google Scholar]
- 31. American College of Emergency Physicians . BUPE: buprenorphine use in the emergency department tool. Available at: https://www.acep.org/patient‐care/bupe/ Accessed February 8, 2021.
- 32. NIDA . Resource Guide: Screening for Drug Use in General Medical Settings. 2012. Available at https://archives.drugabuse.gov/publications/resource‐guide‐screening‐drug‐use‐in‐general‐medical‐settings on 2021, April 23.
- 33. Substance Abuse and Mental Health Services Administration . White paper on screening, brief intervention and referral to treatment (SBIRT) in behavioral healthcare. 2011. Available at: https://www.samhsa.gov/sites/default/files/sbirtwhitepaper_0.pdf Accessed April 23, 2021.
- 34. American Psychiatric Association . Diagnostic and statistical manual of mental disorders (DSM‐5): American Psychiatric Pub; 2013. https://dsm.psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596 [Google Scholar]
- 35. Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs. 2003;35:253‐259. [DOI] [PubMed] [Google Scholar]
- 36. Mahmoud S, Anderson E, Vosooghi A, Herring AA. Treatment of opioid and alcohol withdrawal in a cohort of emergency department patients. Am J Emerg Med. 2021;43:17‐20. [DOI] [PubMed] [Google Scholar]
- 37. American College of Obstetricians and Gynecologists. Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 524. Obstet Gynecol. 2012;119:1070‐1076. [DOI] [PubMed] [Google Scholar]
- 38. Carroll GG, Wasserman DD, Shah AA, et al. Buprenorphine field initiation of ReScue treatment by emergency medical services (Bupe FIRST EMS): a case series. Prehosp Emerg Care. 2021;25(2):289‐293. [DOI] [PubMed] [Google Scholar]
- 39. Ang‐Lee K, Oreskovich MR, Saxon AJ, et al. Single dose of 24 milligrams of buprenorphine for heroin detoxification: an open‐label study of five inpatients. J Psychoactive Drugs. 2006;38:505‐512. [DOI] [PubMed] [Google Scholar]
- 40. Lee JD, Vocci F, Fiellin DA. Unobserved “home” induction onto buprenorphine. J Addict Med. 2014;8:299‐308. [DOI] [PubMed] [Google Scholar]
- 41. Office of the Secretary, Department of Health and Human Services . Practice guidelines for the administration of buprenorphine for treating opioid use disorder. Document citation 86 FR 22439. Fed Regist. 2021;86:22439‐22440. [Google Scholar]
- 42. Indivior . Suboxone (buprenorphine and naloxone) sublingual film: full prescribing information. 2019. Available at: https://www.suboxone.com/pdfs/prescribing‐information.pdf Accessed February 8, 2021
- 43. Oakley B, Wilson H, Hayes V, Lintzeris N. Managing opioid withdrawal precipitated by buprenorphine with buprenorphine. Drug Alcohol Rev. 2021;40(4):567‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cunningham CO, Giovanniello A, Li X, Kunins HV, Roose RJ, Sohler NL. A comparison of buprenorphine induction strategies: patient‐centered home‐based inductions versus standard‐of‐care office‐based inductions. J Subst Abuse Treat. 2011;40:349‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gunderson EW, Wang X‐Q, Fiellin DA, Bryan B, Levin FR. Unobserved versus observed office buprenorphine/naloxone induction: a pilot randomized clinical trial. Addict Behav. 2010;35:537‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoot NR, Aronsky D. Systematic review of emergency department crowding: causes, effects, and solutions. Ann Emerg Med. 2008;52:126‐136. e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1‐10. [DOI] [PubMed] [Google Scholar]
- 48. Leece P, Chen C, Manson H, et al. One‐year mortality after emergency department visit for nonfatal opioid poisoning: a population‐based analysis. Ann Emerg Med. 2020;75:20‐28. [DOI] [PubMed] [Google Scholar]
- 49. Weiner SG, Baker O, Bernson D, Schuur JD. One‐year mortality of patients after emergency department treatment for nonfatal opioid overdose. Ann Emerg Med. 2020;75:13‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169:137‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Strang J, Volkow ND, Degenhardt L, et al. Opioid use disorder. Nature reviews Disease primers. 2020;6:1‐28. [DOI] [PubMed] [Google Scholar]
- 52. Saloner B, McGinty EE, Beletsky L, et al. A public health strategy for the opioid crisis. Public Health Rep. 2018;133:24S‐34S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Substance Abuse and Mental Health Services Administration . Opioid overdose prevention toolkit. HHS Publication No. SMA18‐4742. National Mental Health and Substance Use Policy Laboratory. Substance Abuse and Mental Health Services Administration, 2018. [Google Scholar]
- 54. American College of Emergency Physicians . Naloxone prescriptions for emergency physicians. Policy statement. 2015. Available at https://www.acep.org/patient‐care/policy‐statements/naloxone‐prescriptions‐by‐emergency‐physicians/ Accessed February 8, 2021.
- 55. Yeboah‐Sampong S, Weber E, Friedman S. EMERGENCY: hospitals are violating federal law by denying required care for substance use disorders in emergency departments. Legal Action Center. 2021. [Google Scholar]
- 56. Hawk K, Hoppe J, Ketcham E, et al. Consensus recommendations on the treatment of opioid use disorder in the emergency department. Ann Emerg Med. 2021;78(3):434‐442. [DOI] [PubMed] [Google Scholar]


