Abstract
This study aimed to investigate the effects of thermal conditioning and folic acid on the methylation levels of the avian brain-derived neurotrophic factor (BDNF) promoter region at the M3 and M9 positions in the early life of broiler chicks. In Experiment 1, male broiler chicks (day 3 of life) were orally injected with methyl cellulose solution with or without folic acid (25 mg). The chicks in the heat-treatment groups were immediately exposed to a high ambient temperature (40±0.5°C) for 12 h, while chicks in the non-heat treatment groups were left in the thermoneutral zone (30±0.5°C). The groups were as follows: 1) no thermal conditioning group without folic acid (control), 2) thermal conditioning group without folic acid, 3) no thermal conditioning group with folic acid, and 4) thermal conditioning group with folic acid. In Experiment 2, treatments were similar to those in Experiment 1, except for the usage of female chicks. After the treatments, the methylation levels of the BDNF promoter in chicks were determined using semiquantitative PCR. There were no significant differences between groups in the levels of methylation at the M3 position in both males and females as a result of thermal conditioning and folic acid treatment. Interestingly, significant effects of thermal conditioning and folic acid treatment on methylation at the M9 position were found. BDNF methylation levels at M9 significantly decreased following thermal conditioning, while folic acid suppressed demethylation in both male and female chicks. These data suggest that folic acid and thermal conditioning affects DNA methylation patterns in the central nervous system of chicks, regardless of sex.
Keywords: brain-derived neurotrophic factor, chicks, DNA methylation, folic acid, thermal conditioning
Introduction
Early life experiences affect the structural and functional development of the nervous system in animals, leading to the development of physiological abilities. Several studies provide evidence indicating that epigenetic modifications of the genome exert effects later in life (Sweatt, 2009; Szyf, 2009). DNA methylation is an epigenetic modification that causes gene expression or phenotypic changes without changing the primary DNA sequence (Berry et al., 2010; Crider et al., 2012).
Early thermal conditioning to improve the ability of chickens to survive heat stress involves the exposure of young chicks to a high ambient temperature for half a day or a whole day. This increases heat tolerance, weight gain, and mortality (Yahav and Hurwitz, 1996; Yahav and McMurtry, 2001). These changes have been related to DNA methylation or histone modification (Yossifoff et al., 2008; Kisliouk and Meiri, 2009). As for a candidate of the epidemic episode, Yossifoff et al. (2008) suggested that the acquisition of heat tolerance through thermal conditioning occurred through the methylation of the brain-derived neurotropic factor (BDNF) promoter region in the hypothalamus of chicks. The methylation levels in some cytosine-guanine dinucleotide (CpG) sites of the promoter region were analyzed, and significant changes at positions M1, M3, and M9 were found after heat exposure of Cobb male chicks (Yossifoff et al., 2008). Several reports (e.g., Tanizawa et al., 2014; Ouchi et al., 2020) have revealed the acquisition of heat tolerance by thermal conditioning using various breeds or lines of chickens; however, there have been few investigations regarding line or sex differences in the methylation levels of the BDNF promoter region. Information regarding these differences should help understand the mechanism underlying the acquisition of thermotolerance by thermal conditioning and enable the utilization of thermal conditioning in poultry production.
It is necessary to accelerate thermal conditioning-induced heat tolerance in order to improve poultry production, such as through nutritional supplementation. Methylation of DNA requires a methyl donor. Folic acid is an important source of methyl moieties, which are used to synthesize S-adenosyl methionine, the methyl donor for DNA methylation (Fig. 1). There are several studies on the interactions between folate and folic acid status and DNA methylation (Crider et al., 2012). However, there is no information about the effect of folic acid on the methylation of the BDNF promoter region in chicks.
Fig. 1.
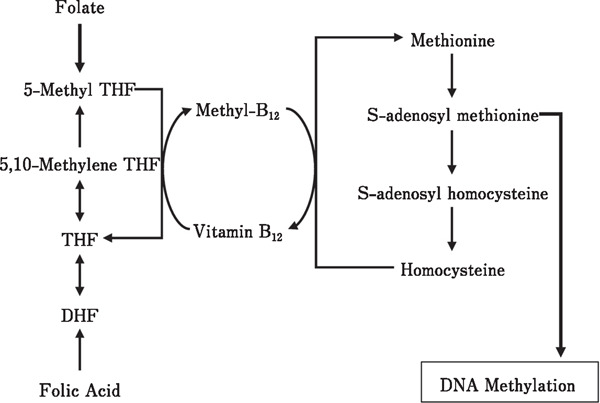
Schematic chart of the relationship between folic acid and DNA methylation. DHF: dihydrofolate; THF: tetrahydrofolate
This study aimed to investigate the effects of early thermal conditioning and folic acid treatment on the methylation levels of the avian BDNF promoter regions M3 and M9 in Chunky chicks. In addition, we compared the effects of sex on methylation levels after thermal conditioning in the chicks.
Materials and Methods
Birds were handled in accordance with the Animal Experiment Committee of Hiroshima University (authorization No. C19-15) regulations and Law No. 105 and Notification No. 6 of the Japanese government.
Animals
Day-old male and female broiler chicks (Chunky: Ross 308) were obtained from local hatcheries (Fukuda Hatchery, Okayama, Japan). The chicks were maintained in a room with 24-h lighting, at a temperature of 30 ±0.2°C, in polypropylene boxes with sawdust litter (36×40×30 cm), and at a population density of six chicks per box during the experimental period. The chicks were given free access to a commercial starter diet (Nichiwa Sangyo Co. Ltd., Kobe, Japan) and water until the end of the experiment.
Preparations of Drugs
Folic acid (pteroylglutamic acid) was obtained from Wako Pure Chemical Industries (Osaka, Japan) and suspended in a 0.25% methyl cellulose solution. The suspension was settled on the stirrer with the heater kept at 38±0.5°C during treatment administration.
Experimental Design
In Experiment 1, male chicks (3 days old) were distributed into four groups (n=8 per group) based on their body weight, so that the average body weight was similar in all groups. The groups were as follows: 1) no thermal conditioning group without folic acid (control), 2) thermal conditioning group without folic acid, 3) no thermal conditioning group with folic acid, and 4) thermal conditioning group with folic acid. Chicks were orally administered 0.1 mL of a 0.25% methyl cellulose solution with or without folic acid (50 mg/kg) using a syringe with a silicone tube. The dose of folic acid was determined based on a previous report (Gao et al., 2017), and the average body weight. Immediately after folic acid administration, the chicks in the treatment group were exposed to a high ambient temperature (40±0. 5°C; inner size of heat chamber: 90×90×115 cm; Ouchi et al., 2020) for 12 h, and those in the control group were kept in the thermoneutral zone (30±0.5°C). The duration of heat exposure (12 h) was based on the report by Yossifoff et al. (2008). In Experiment 2, female chicks (3 days old) grouped similarly to those in Experiment 1 were orally administered folic acid and transferred to the heat chamber for 12 h (n=8 per group). After these treatments, chicks in all groups were anesthetized with isoflurane (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) and decapitated. Immediately after decapitation, the diencephalic tissues of the chicks were removed and stored at −80°C till DNA extraction was performed.
DNA Methylation Analysis by Bisulfite Modification
DNA methylation analysis was performed for the BDNF promoter region at positions M3 and M9, and a schematic chart is presented in Fig. 2. DNA was extracted from diencephalic tissues using a commercial DNA isolation kit (Takara Bio Inc., Shiga, Japan), and purified DNA was measured using a spectrophotometer (NanoDrop ND-2000c, Thermo Scientific, Inc.) at 260 nm. The purity of the DNA was also analyzed by measuring absorbance at 260 and 280 nm. Bisulfite analysis of BDNF promoter methylation was performed using the MethylEasy Xceed Rapid DNA Bisulfite Modification Kit (Human Genetic Signatures Pty. Ltd., New South Wales, Australia), according to the manufacturer's instructions. The methylation levels of the BDNF promoter were determined by semiquantitative PCR. The primers used for methylation-specific PCR are presented in Table 1.
Fig. 2.
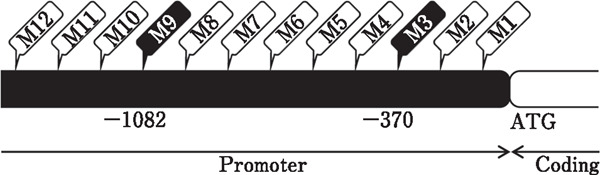
Schematic diagram of the CpG sites upstream of the translational start site (ATG) of the BDNF coding region. White characters with black backgrounds show the sites analyzed for methylation level.
Table 1. Primer sequences for methylation-specific PCR.
| CpG site | F/R | Sequence (5′ - 3′) |
|---|---|---|
| M3 | ||
| Methylated | F | GTTGAAACGTTGTGTTGTTAAATAG |
| Unmethylated | F | GTTGAAATGTTGTGTTGTTAAATAG |
| M + U | R | AAATCAAATACTACATAAACTCCT |
| M9 | ||
| Methylated | F | GTGTTGGTAGGAATGACGTTTTG |
| Unmethylated | F | GTGTTGGTAGGAATGATGTTTTG |
| M + U | R | AACACCAACTAACAACATCAATAAA |
F, forward primer; R, reverse primer; M + U, methylated and unmethylated
Statistical Analysis
Data were analyzed using the commercially available package, StatView (Version 5, SAS Institute, Cary, USA, 1998); two-way ANOVA was used to analyze the effects of thermal conditioning and folate treatment. When effects were found to be significant, a post-hoc test was performed using the Tukey–Kramer test. Statistical significance was considered at P<0.05. All data are expressed as mean±standard error of the mean (SEM).
Results
DNA Methylation by Thermal Conditioning and Oral Administration of Folic Acid in Male Chicks
Figure 3 shows the methylation levels of the avian BDNF promoter region at the M3 and M9 positions in male chicks. There were no significant differences in methylation levels at M3 among groups (left panel; P>0.05). At M9, the main effects of treatments and interaction between thermal conditioning and folic acid treatment were significant (right panel; P<0.05), and the methylation level in the thermal conditioned group without folic acid was significantly lower than those in the other groups.
Fig. 3.
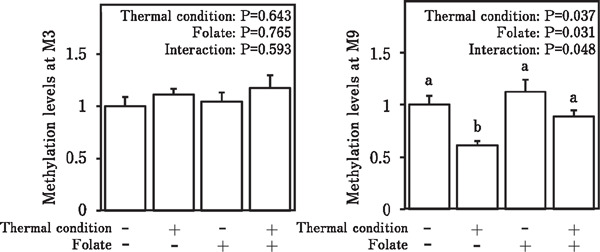
Methylation levels at the M3 (left panel) and M9 (right panel) positions of the avian BDNF promotor region in 3-day-old male chicks. Data are expressed as mean±SEM. Means with different letters are significantly different at P<0.05.
DNA Methylation by Thermal Conditioning and Oral Administration of Folic Acid in Female Chicks
The methylation levels of the avian BDNF promoter region at the M3 and M9 positions in female chicks are shown in Fig. 4. Although no significant changes in the BDNF methylation levels at M3 in chicks after thermal conditioning and folic acid treatment were detected (left panel; P>0.05), there were significant effects of both treatments and interaction on the levels of BDNF methylation at M9 (right panel; P<0.05). Similar to male chicks, thermal conditioning without folic acid decreased the methylation level of the BDNF promoter region in female chicks.
Fig. 4.
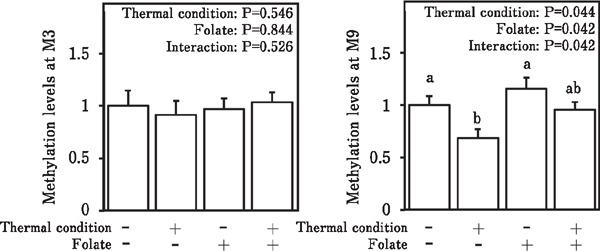
Methylation levels at the M3 (left panel) or M9 (right panel) position of the avian BDNF promotor region in 3-day-old female chicks. Data are expressed as mean±SEM. Means with different letters are significantly different at P<0.05.
Discussion
In this study, we measured the methylation levels of the BDNF promoter region in the central nervous system of chicks subjected to thermal conditioning and orally administered folic acid. We also compared the effects of these treatments between males and females. There were no significant differences between groups in the level of methylation at the M3 position in both males and females, and no effects of thermal conditioning and folic acid were found. Interestingly, there were significant differences between groups in response to thermal conditioning, folic acid treatment, and the interaction between thermal conditioning and folic acid treatment at the M9 position. Heat exposure significantly decreased BDNF methylation levels at M9, while folic acid treatment suppressed demethylation in both male and female chicks.
An epigenetic change is a change in the gene expression or cell phenotype that is inherited by progeny cells without any change in the DNA sequence; epigenetic changes include histone modifications and DNA methylation (Kelly et al., 2010; Meissner, 2010; Portela and Esteller, 2010). DNA methylation, the focus of this study, regulates gene expression through the binding of methyl groups to the CpG islands of the genome (Moore et al., 2013). In chickens, DNA methylation levels of the BDNF promoter regions are changed by thermal conditioning at an early age (Yossifoff et al., 2008). Here, the methylation level of the M3 position of the BDNF promoter region (Fig. 2) was not affected by thermal conditioning; however, thermal conditioning downregulated the methylation level of the M9 position of the BDNF promoter region (Fig. 2, 3, and 4). In a previous report, thermal conditioning of Cobb chicken upregulated the DNA methylation level of M3 and downregulated that of M9 (Yossifoff et al., 2008). The results of this study did not match those of this previous report. In this study, Chunky (Ross 308) chicks were used as the experimental model. The results of the present study suggested that differences in DNA methylation patterns and susceptibility to DNA methylation depend on the line of chickens. In humans, DNA methylation patterns differ between Caucasians, African Americans, and Hispanics (Adkins et al., 2011; Zhang et al., 2011).
The effects of environmental factors, such as immune response and thermoregulation (Cryan and Wolf, 2003; Kelein and Flanagan, 2016), differ among genders, similar to DNA methylation. In humans, the effects of prenatal fasting on DNA methylation levels in metabolism-related genes, such as the leptin and insulin growth factor 2 receptor genes, vary between males and females (Tobi et al., 2009). In addition, the effects of environmental factors on DNA methylation differ between males and females in mammals, including humans (Davegårdh et al., 2019; Maschietto et al., 2017; Kippler et al., 2013; Gallou-Kabani et al., 2010; Liu et al., 2010). In this study, changes in the DNA methylation levels of the BDNF promoter region due to thermal conditioning did not differ between male and female chicks (Fig. 3 and 4). DNA methylation is affected by gender; however, some genes were affected, while others were not (Tobi et al., 2009). Global DNA methylation patterns may differ among genders in chickens. Thermal conditioning did not cause changes in the methylation patterns of the BDNF promoter region among genders.
Folic acid acts as a methyl group donor in the process of DNA methylation (Fig. 1). Dietary folate is metabolized to 5-methyltetrahydrofolate (5-methyl THF) and is converted to tetrahydrofolate (THF) in the gut and liver, where methyl groups are simultaneously released to the methionine synthesis reaction (Fig. 1). S-adenosyl methionine (SAM) is a metabolite of methionine that is converted to S-adenosyl-homocysteine (SAH). In this conversion, methyl groups are released from SAM and are used for DNA methylation (Fig. 1). A relationship has been found between folate and global DNA methylation (Cravo et al., 1994, 1998; Pufulete et al., 2005). In general, demethylation has been confirmed in the tumor tissues and blood of patients with cancer. Dietary and supplementary folate have been reported to suppress the demethylation of tumor-related genes (Levine et al., 2003; Christensen et al., 2010; Wallace et al., 2010; Kim et al., 2011); thus, folate affects, not only the global DNA methylation patterns but also the level of DNA methylation in specific genes. In this study, the methylation level at M3 of BDNF was not affected by folate; however, demethylation was found at M9 due to the attenuation of the effect of thermal conditioning by orally administered folate (Fig. 3 and 4). Folate has been used in patients with cancer and suppresses DNA demethylation (Wallace et al., 2010; Kim et al., 2011); these findings are consistent with those of the present study. DNA methylation occurs through two mechanisms: de novo methylation, which involves the methylation of unmethylated genes, and inheritance methylation, which involves the maintenance of methylation during DNA replication. DNA methyltransferases (DNMTs) bind a methyl group to the cytosine in the base of SAM (Hervouet et al., 2018). However, the mechanism of DNMT action during thermal conditioning in chicks remains unclear. Future studies on this topic are needed.
It has been suggested that the methylation of the BDNF promoter region is involved in heat tolerance induction by thermal conditioning in chicks (Yossifoff et al., 2008). BDNF plays a pivotal role in neuronal development and neurogenesis (Poo, 2001; Lee et al., 2002; Scharfman et al., 2005) and in feeding regulation and energy metabolism in animals (Nakagawa et al., 2000; Xu et al., 2003). Therefore, thermotolerance in thermally-conditioned chickens can be attributed to the epidemic regulation of BDNF gene transcription. However, the present results regarding changes in DNA methylation in response to thermal conditioning and folic acid treatment did not coincide with the findings of Yossifoff et al. (2008). It is unlikely that the methylation levels of the avian BDNF promoter region are critical for the acquisition of thermotolerance by early thermal conditioning in chicks. Further study of epigenetic changes related to thermal conditioning is necessary to elucidate thermotolerance acquisition in chicks.
In conclusion, our findings indicate that the oral administration of folic acid partially affected DNA methylation patterns in the central nervous system of chicks, regardless of sex. Additionally, our findings suggest that changes in BDNF promoter methylation at M3 may differ depending on the genetic line of chicks.
Acknowledgment
This work was supported by a Grant-in-Aid for Scientific Research from JSPS (No. 17KT0077 to TB). Financial support was partially received from the Ministry of the Environment, Japan (Regional Adaptation Consortium Project [Chugoku-Shikoku region]). The authors would like to acknowledge the staff of the Laboratory of Animal Behavior and Physiology, Hiroshima University, for their technical support in maintaining the animals.
Conflict of Interest
The authors declare no conflict of interest.
References
- Adkins RM, Krushlal J, Tylavsky FA and Thomas F. Racial differences in gene-specific DNA methylation levels are present at birth. Birth Defects Research Part A- Clinical and Molecular Teratology, 91: 728-736. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry RJ, Bailey L, Mulinare J and Bower C. Fortification of flour with folic acid. Food and Nutrition Bulletin, 31: S22-35. 2010. [DOI] [PubMed] [Google Scholar]
- Cravo M, Fidalgo P, Pereia AD, Gouveia-Oliveria A, Chaves P, Selhub J, Mason JB, Mira FC and Leitao CN. DNA methylation as an intermediate biomarker in colorectal cancer: modulation by folic acid supplementation. European Journal of Cancer Prevention, 3: 473-479. 1994. [DOI] [PubMed] [Google Scholar]
- Carvo ML, Pinto AG, Chaves P, Cruz JA, Lage P, Leitao CN and Mira FC. Effect of folate supplementation on DNA methylation of rectal mucosa in patients with colonic adenomas: correlation with nutrient intake. Clinical Nutrition, 17: 45-49. 1998. [DOI] [PubMed] [Google Scholar]
- Christensen BC, Kelsey KT, Zheng S, Houseman EA, Wrensch MR, Wiemels JL, Nelson HH, Karagas MR, Kushi LH, Kwan ML and Wiencke JK. Breast cancer DNA methylation profiles are associated with tumor size and alcohol and folate intake. PLoS Genetics, 7: 1-10. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider KS, Yang TP, Berry RJ and Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate's role. Advances in Nutrition, 3: 21-38. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan PM and Wolf BO. Sex differences in the thermoregulation and evaporative water loss of a heterothermic bat, Lasiurus cinereus, during its spring migration. Journal of Experimental Biology, 206: 3381-3390. 2003. [DOI] [PubMed] [Google Scholar]
- Davegårdh C, Wedin EH, Broholm C, Henriksen TI, Pedersen M, Pedersen BH, Scheele C and Ling C. Sex influences DNA methylation and gene expression in human skeletal muscle myoblasts and myotubes. Stem Cell Research and Therapy, 10: 1-17. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallou-Kabani C, Gabory A, Tost J, Karimi M, Msayeur S, Lesage J, Boudadi E, Gross MS, Taurelle J, Vigé A, Breton C, Reusens B, Remacle C, Vieau D, Ekström TJ, Jais JP and Junien C. Sex- and diet-specific changes of imprinted gene expression and DNA methylation in mouse placenta under a high-fat diet. PLoS ONE, 5: e14398. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Liu X, Yu L, Wu J, Xu M and Liu Y. Folic acid exerts antidepressant effects by upregulating brain-derived neurotrophic factor and glutamate receptor 1 expression in brain. Neuro Report, 28; 1078-1084. 2017. [DOI] [PubMed] [Google Scholar]
- Hervouet E, Peixoto P, Delage-Mourroux R, Boyer-Guittaut M and Cartron PF. Specific or not specific recruitment of DNMTs for DNA methylation, an epigenetic dilemma. Clinical Epigenetics, 10: 17. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelein LK and Flanagan L. Sex differences in immune responses. Nature Reviews Immunology, 16: 626-638. 2016. [DOI] [PubMed] [Google Scholar]
- Kelly TK, De Carvalho DD and Jones PA. Epigenetic modifications as therapeutic targets. Nature Biotechnology, 28: 1069-1078. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Park HM, Choi YK, Chong SY, Oh D and Kim NK. Polymorphisms in gene involved in folate metabolism and plasma DNA methylation in colorectal cancer patients. Oncology Reports, 25: 167-172. 2011. [PubMed] [Google Scholar]
- Kippler M, Engström K, Mlakar SJ, Bottai M, Ahmed S, Hossain MB, Raqid R, Vahter M and Broberg K. Sex-specific effects of early life cadmium exposure on DNA methylation and implications for birth weight. Epigenetics, 8: 494-503. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisliouk T and Meiri N. A critical role for dynamic changes in histone H3 methylation and the Bdnf-promoter during postnatal thermotolerance acquisition. European Journal of Neuroscience, 30: 1909-1922. 2009. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W and Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. Journal of Neurochemistry, 82: 1367-1375. 2002. [DOI] [PubMed] [Google Scholar]
- Levine JJ, Stimson-Crider KM and Vertino PM. Effects of methylation on expression of TMS1/ ASC in human breast cancer cells. Oncogene, 22: 3475-3488. 2003. [DOI] [PubMed] [Google Scholar]
- Liu J, Morgan M, Hutchison K and Calhoun VD. A study of the influence of sex on genome wide methylation. PLoS ONE, 5: e10028. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschietto M, Bastos LC, Tahira AA, Bastos EP, Euclydes VLV, Brentani A, Fink G, Baumont DA, Felipe-Silva A, Francisco RPV, Gouveia G, Grisi SJFE, Escobar AMU, Moreira-Filho GA, Polanczyk GV, Miguel EC and Brentani H. Sex differences in DNA methylation of the cord blood are related to sex-bias psychiatric diseases. Scientific Reports, 7: 44547. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner A. Epigenetic modifications in pluripotent and differentiated cells. Nature Biotechnology, 28: 1079-1088. 2010. [DOI] [PubMed] [Google Scholar]
- Moore L, Le T and Fan G. DNA methylation and its basic function. Neuropsychopharmacology, 38: 23-38. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Tsuchida A, Itakura Y, Nonomura T, Ono M, Hirota F, Inoue T, Nakayama C, Taiji M and Noguchi H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes, 49: 436-444. 2000. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Tanizawa H, Shiraishi J, Cockrem JF, Chowdhury VS and Bungo T. Repeated thermal conditioning during the neonatal period affects behavioral and physiological responses to acute heat stress in chicks. Journal of Thermal Biology, 94: 102759. 2020. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nature Reviews Neuroscience, 2: 24-32. 2001. [DOI] [PubMed] [Google Scholar]
- Portela A and Esteller M. Epigenetic modifications and human disease. Nature Biotechnology, 28: 1057-1068. 2010. [DOI] [PubMed] [Google Scholar]
- Pufulete M, Al-Ghnaniem R, Khushal A, Appleby P, Harris N, Gout S, Emery PW and Sanders TAB. Effect of folic acid supplementation on genomic DNA methylation in patients with colorectal adenoma. Gut, 54: 648-653. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C and Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Experimental Neurology, 192: 348-356. 2005. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biological Psychiatry, 65: 191-197. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M. The early life environment and the epigenome. Biochimica et Bioohysica Acta (BBA) - General Subjects, 1790: 878-885. 2009. [DOI] [PubMed] [Google Scholar]
- Tanizawa H, Shiraishi J, Kawakami S, Tsudzuki M and Bungo T. Effect of short, early thermal conditioning on physiological and behavioral responses to acute heat exposure in chicks. Journal of Poultry Science, 51: 80-86. 2014. [Google Scholar]
- Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, Slagboom PE and Heijmans BE. DNA methylation differences after exposure to prenatal famine are common and timing- and sex- specific. Human Molecular Genetics, 18: 4046-4053. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace K, Grau MV, Levine AJ, Shen L, Hamdan R, Chen X, Gui J, Haile RW, Barry EL, Ahnen D, McKeown-Eyssem G, Baron JA and Issa PJ. Association between folate levels and CpG island hypermethylation in normal colorectal mucosa. Cancer Prevention Research, 3: 1552-1564. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Goulding EH, Zang K, Cepoi D, Cone D, Jones KR, Tecott LH and Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nature Neuroscience, 6: 736-742. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahav S and Hurwitz S. Induction of thermotolerance in male broiler chickens by temperature conditioning at an early age. Poultry Science, 75: 402-406. 1996. [DOI] [PubMed] [Google Scholar]
- Yahav S and McMurtry J. Thermotolerance acquisition in broiler chickens by temperature conditioning early in life – the effect of timing and ambient temperature. Poultry Science, 80: 1662-1666. 2001. [DOI] [PubMed] [Google Scholar]
- Yossifoff M, Kisliouk T and Meiri N. Dynamic changes in DNA methylation during thermal control establishment affect CREB binding to the brain-derived neurotrophic factor promoter. European Journal of Neuroscience, 28: 2267-2277. 2008. [DOI] [PubMed] [Google Scholar]
- Zhang FF, Cardarelli R, Carroll J, Fulda KG, Kaur M, Gonzalez K, Vishwanatha JK, Sentella RM and Morabia A. Significant differences in global genomic DNA methylation by gender and race/ ethnicity in peripheral blood. Epigenetics, 6: 623-629. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


