Abstract
Background and aims
Long-term kinetics of anti-RBD IgG and neutralizing antibodies were analyzed in a cohort of COVID-19 naïve health care workers (HCW) undergoing SARS-CoV-2 vaccination.
Methods
An anti-RBD IgG immunoassay and a surrogate virus neutralization test (sVNT) were performed at different time points up to 6 months after vaccination in 57 HCWs. Values of anti-RBD IgG predicting an high neutralizing bioactivity (>60%) were also calculated.
Results
Mean (range) values of anti-RBD IgG were 294.7 (11.6–1554), 2583 (398–8391), 320.4 (42.3–1134) BAU/mL at T1 (21 days after the 1st dose [T0]), T2 (30 days after the 2nd dose) and T3 (+180 days after T0), respectively. Mean (range) percentages of neutralization (NS%) were 24 (0–76), 86 (59–96) and 82 (52–99) at T1, T2 and T3, respectively. Anti-RBD IgG values and NS% were positively correlated at T2 and T3 while anti-RBD IgG value predicting a NS% > 60 markedly differed at T2 and T3 (594 vs. 108 BAU/mL, respectively).
Conclusion
While a high neutralizing bioactivity was maintained at least 6 months after vaccination in almost all individuals, the mean values of anti-RBD-IgG showed a marked decline at 6 months. The absolute value of anti-RBD IgG is a poor marker of neutralizing bioactivity.
Keywords: SARS-CoV-2, COVID-19, Neutralizing antibody, Immunogenicity, Anti-S-RBD, BNT162b2
Abbreviations: Abs, Antibodies; BAU, Binding Antibody Unit; COP, Correlate of Protection; COVID-19, Coronavirus disease 2019; ELISA, Enzyme-linked Immunosorbent assay; hACE2, human Angiotensin Converting Enzyme 2; HCW, Health Care Worker; nAbs, neutralizing Antibodies; IH%, Percentage of Inhibition; S-RBD, Spike-Receptor-Binding Domain; S, Spike; SARS-CoV-2, Severe Acute Respiratory Syndrome CoronaVirus type 2; VNT, Virus Neutralization Test; sVNT, Surrogate Virus Neutralization Test
1. Introduction
Waning of humoral and cellular immune responses against SARS-CoV-2 after infection or vaccination [1] makes the identification of correlates of protection (COP) for monitoring immune status and driving risk-adapted vaccination schedules a priority [2]. Evidence from real-world data suggests that neutralizing antibodies (nAbs) to SARS-CoV-2 positively correlate with protection against SARS-CoV-2 infection and COVID-19 [3], [4], [5], [6] by blocking the interaction of the Spike Receptor-Binding Domain (S-RBD) with the human cell receptor angiotensin converting enzyme 2 (hACE2) [7]. Thus, a number of binding immune assays and virus neutralization test (VNT) platforms have been implemented, cross-validated and eventually traced to a WHO International Standard [8], in order to establish the usefulness of anti-SARS-CoV-2 antibodies as diagnostic tools or COP [9], [10], [11], [12], [13]. While quantitative binding assays only identify physical interactions and are mainly used for diagnostic purposes, functional neutralizing activity can only be directly investigated by means of VNTs platforms, that are grossly divided into direct VNTs, which require viable virions of SARS-CoV-2 and are considered the gold standard, and surrogate (s)VNTs, based on binding competition assays between antibodies and target receptors that mediate viral attachment and entry. Studies available so far have shown a significant degree of correlation between anti-S-RBD Abs levels and serum neutralizing bioactivity as assessed by VNTs [14], [15], [16], [17], [18], [19], [20], [21], nonetheless some other studies have remarked that this correlation might change over time due to affinity maturation of humoral response, thus limiting the absolute quantitative value of anti-S-RBD Abs as a reliable COP [15], [16], [22], [23], [24], [25], [26]. In fact, waning of binding Abs either after natural infection or vaccination might be countered by the maintenance of neutralizing capacity by ongoing affinity maturation of Spike-specific B lymphocytes [27]. Failure of affinity maturation has been previously associated with vaccine failure [28] and some Authors have advocated that the development of high affinity antibodies targeting multiple epitopes of RBD are needed for effective long-term protection against SARS-CoV-2 [29], [30], [31], [32]. Available studies [30], [33], [34] have shown parallel increases in avidity values, neutralizing activity and quantitative anti-RBD antibody titres up to 8 weeks from the second dose of vaccine but these short-term analyses were not designed to prove whether quantitative RBD-antibody level would predict neutralizing bioactivity and clinical protection on the long-term, when divergent kinetics of immunogenicity markers may unmask functional mechanistic associations [16], [22].
In this study, an anti-S-RBD IgG quantitative immunoassay and a sVNT based on antibody-mediated competitive blockage of hACE2-RBD interaction were performed in parallel at four prefixed time points across a 6-month period in a cohort of COVID-19-naïve health care workers (HCWs) vaccinated with the Pfizer/BioNTech BNT162b2 vaccine. Temporal trends and quantitative correlations between anti-S-RBD IgG values and functional neutralizing bioactivity were reported.
2. Materials and Methods
2.1. Study population
Fifty-seven HCWs, without clinical history of COVID-19 infection and persistently negative virus detection by PCR on nasal swab monitoring, were consecutively enrolled. No information was provided on comorbidities and concomitant drug therapy. Serum samples from each subject were obtained and analyzed for anti-SARS-CoV2 antibodies at 4 prefixed time points: T0 (first shot of vaccine), T1 (second shot of vaccine, i.e. + 21 days from T0), T2 (51 days after T0) and T3 (6 months after T0). HCWs were monitored monthly with antigenic tests on nasopharyngeal swab for SARS-CoV-2 infection and additional on-demand antigenic tests were performed in case of clinical suspicion of COVID-19. The study was conducted in accordance with the ethical standards as formulated in the Helsinki Declaration and written informed consent was obtained from all the participants. The study was approved by the local Ethical Committee and assigned the internal protocol number 034 2020H EPIDEMIOLOGIA COVID-19.
2.2. Anti-S-RBD antibody assays
Anti-S-RBD IgG Abs were detected by a quantitative chemiluminescence immunoassay (sCOVG, Siemens Healthineers, Erlangen, Germany) using the RBD of the Spike protein as capture antigen. All samples were processed according to the manufacturer’s instruction using the Advia Centaur XPT, CLIA automated platform with a cutoff level of 1.0 U/mL for a positive result (sensitivity 96.4%, C.I.95% 92.7–98.5%; specificity 99.9%, C.I.95% 99.6–100% for a previous infection). Results of the assay were given in Binding Antibody Units (BAU) per milliliter (BAU/mL) by applying the conversion factor 21.8, determined by the manufacturers based on the measurement of the first WHO International Standard Anti-SARS-CoV-2 Immunoglobulin (NIBSC-Code 20–136) [8].
2.3. Antibody-mediated RBD-ACE2 neutralization assay
The ACE2-RBD Neutralization assay (Dia.Pro Diagnotic Bioprobes, Milano, Italy) (REF. ACE2-RBDNEUTR.CE 96 Test) is a CE marked ELISA using a recombinant RBD of the SARS-CoV-2 spike protein to detect antibodies of any isotype that block the RBD from binding to the hACE2 receptor. Briefly, the samples and the positive/negative controls provided by the manufacturer are incubated 60′ at + 37 °C in microplates coated with recombinant glycosilated RBD (traditional strain sequence) to allow the interaction and binding of antibodies, if present, with the RBD. After washing, recombinant biotinilated hACE2 and streptavidin-horseradish peroxidase are added to each well and incubated for 45′ at 37 °C. After a second washing, the binding of hACE2 to the RBD-plate is visualized by adding tetramethylbenzidine followed by stop solution to all wells and measuring the OD450nm intensity. The colour intensity is directly proportional to the degree of RBD unbound to specific antibodies present in patients’ sera, while a strong inhibition on the colour development will be observed in case antibodies to RBD have blocked the binding of the biotinylated ACE2 to it. Data are expressed as percentage of inhibition of the sample (IH%), calculated as follows: IH% = 100 – [(OD450nm value of sample/OD450nm value of Negative Control) × 100]. Values of IH% < 20 are considered negative; values of IH% between 20 and 29 are considered to have moderate neutralizing activity, between 30 and 59, good, and > 60 excellent neutralizing activity.
2.4. Statistical analysis
Statistical analysis was performed by using MedCalc Software (Mariakerke; Belgium) and Graph Pad (GraphPad Prism 8 XML ProjecT). Comparison of continuous variable was performed using Mann-Whitney U test. Correlation between continuous variables was performed by using Spearman’s rho test. A p-value <0.05 was considered statistically significant. Levels of anti-S-RBD Abs able to identify an excellent neutralizing activity (IH% > 60) was calculated by using Youden’s index (i.e. cutoff at the highest sum of specificity and sensitivity) from a Receiver Operating Characteristic (ROC) Curve analysis.
3. Results
A total of 57 HCWs were enrolled. Mean age was 51 yrs (range: 23–69), female/male ratio was 42/15. All patients had available serological data at T0, T1 and T2, and 42 patients at T3. No subject had signs or symptoms of breakthrough COVID-19 during the observation period and monthly antigenic tests resulted negative in all HCWs.
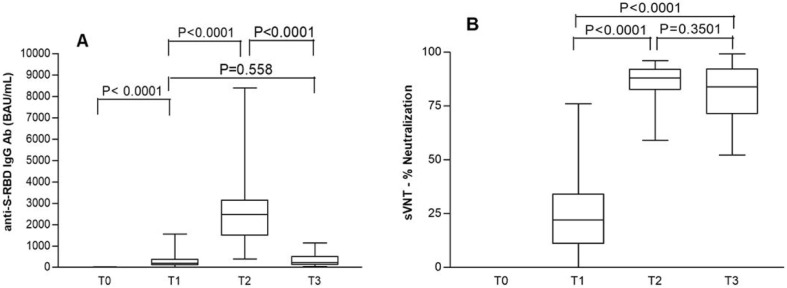
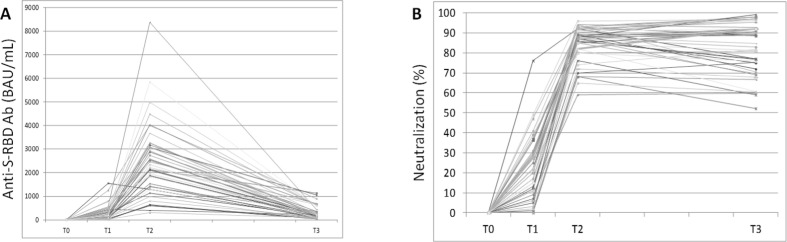
At T0 no patient had serological evidence of previous SARS-CoV-2 infection (Table 1 ). Mean values of anti-RBD IgG significantly increased from 294.7 BAU/mL (range: 11.6–1554) at T1 to 2583 BAU/mL (range: 398–8391) at T2 and then decreased to 320.4 BAU/mL (range: 42.3–1134) at T3, levels similar to T1: in fact, no significant difference was noted between T1 and T3 (Table 1; Fig. 1 , panel A). Mean values of IH% significantly increased from 24 (range: 0–76) at T1 to 86 (range: 59–96) at T2 and then remained at a mean value of 82 (range: 52–99) at T3 (Fig. 1, Panel B). Proportions of patients stratified according to the degree of neutralization are reported in Table 2 and show that all patients exhibited medium–high neutralizing serum bioactivity at both T2 and T3. Temporal trajectories of individual patients’ values are illustrated in Fig. 2 for both quantitative anti-RBD IgG and IH%.
Table 1.
Mean, median and range of anti-RBD IgG values at T0 (day of first dose), T1 (day of second dose, i.e. + 21 days from the first dose), T2 (+51 days from T0), T3 (+6 months from T0) in a cohort of 57 HCWs vaccinated with the BNT162b2 vaccine.
| Mean (BAU/mL) | Min-Max (BAU/mL) | Median (BAU/mL) | |
|---|---|---|---|
| T0 | 3.23 | 0.0–27.2 | 0.0 |
| T1 | 294.7 | 11.6–1554 | 196 |
| T2 | 2583 | 398–8391 | 2472 |
| T3 | 320.4 | 42.3–1134 | 227.2 |
Fig. 1.
Mean values of anti-RBD IgG (panel A) and neutralization bioactivity, expressed as percentage of inhibition (IH%) of the sample (panel B), at T0 (day of first dose), T1 (day of second dose, i.e. + 21 days from the first dose), T2 (+51 days from T0), T3 (+6 months from T0) in a cohort of 57 HCWs vaccinated with the BNT162b2 vaccine.
Table 2.
Percentages of inhibition (IH%) at T0 (day of first dose), T1 (day of second dose, i.e. + 21 days from the first dose), T2 (+51 days from T0), T3 (+6 months from T0) in a cohort of 57 HCWs vaccinated with the BNT162b2 vaccine. All patients had available functional data at T0, T1 and T2, and 42 patients at T3.
| NEG (<20%) | LOW POS (20–29%) | MEDIUM POS (30–59%) | HIGH POS (>60%) | |
|---|---|---|---|---|
| T0 | 57/57 (100%) | 0/57 (0%) | 0/57 (0%) | 0/57 (0%) |
| T1 | 24/57 (42.1%) | 14/57 (24.6%) | 17/57 (29.8%) | 2/57 (3.5%) |
| T2 | 0/57 (0%) | 0/57 (0%) | 1/57 (1.8%) | 56/57 (98.2%) |
| T3 | 0/42 (0%) | 0/42 (0%) | 3/42 (7.1%) | 39/42 (92.9%) |
Fig. 2.
Individual trajectories of anti-RBD IgG values (panel A) and IH% (panel B) in 42 patients with serological data available at all time points (from T0 to T3).
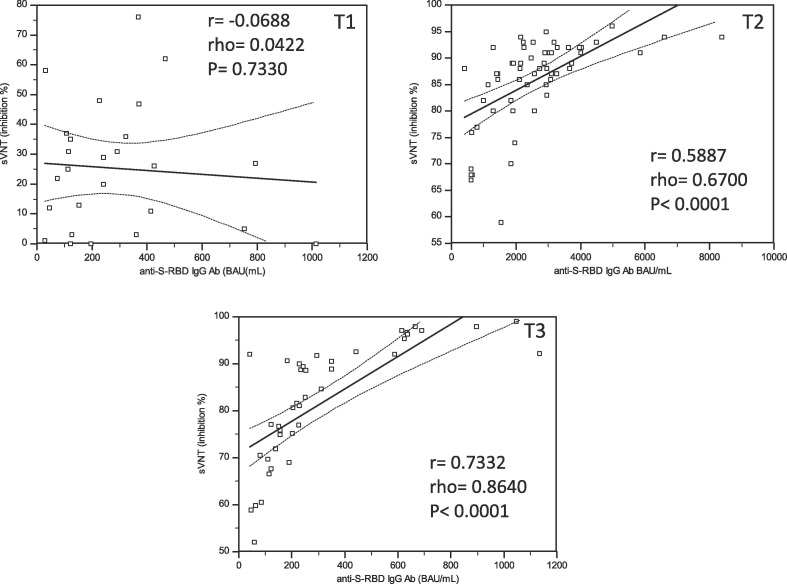
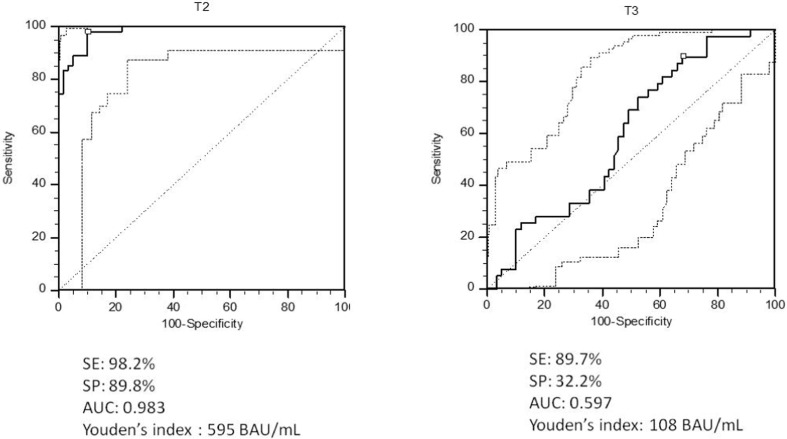
Quantitative correlations between RBD-Abs and IH% at T1, T2 and T3 are reported in Fig. 3 : while at T1 no correlation could be demonstrated (r = −0.0688; rho = 0.0422; p = 0.7330), a significant one emerged at T2 (r = 0.5887, rho = 0.6700, p = 0.0001) and further increased at T3 (r = 0.7332; rho = 0.8640; p = 0.0001). Only a few people at T2 and T3 showed a clear discrepancy between antibody level and IH%, confirming the individual variability of the immune response. The best levels of anti-RBD Abs able to discriminate a IH% > 60%, calculated by ROC curves analysis, were different between T2 and T3. Indeed, the Youden’s index was 595 BAU/mL (sensitivity 98.2% and specificity 89.5%; AUC 0.939- good accuracy) at T2 and 108 BAU/mL (sensitivity 89.7%, specificity 32.2%; AUC 0.597 – very low accuracy) at T3 (Fig. 4 ).
Fig. 3.
Correlations between anti-RBD IgG values and percentage of inhibition (IH%) at T1, T2 and T3.
Fig. 4.
ROC curves analysis used to define the best level of anti-RBD Abs able to discriminate an IH% > 60% at T2 and T3.
4. Discussion
Immunogenicity of vaccines can be evaluated through different serum and cellular markers that exhibit a range of kinetics and evolutions over time. It became evident early on through the pandemic that neutralizing activity is highly correlated with anti-RBD IgG values [14], [15], [16], [17], [18], [19], [20], [21] but an absolute conversion factor could not be established. That may in part be due to major inter-studies heterogeneity as to the kind of patients (COVID-19 experienced vs. COVID-19 naive), immune binding assays and neutralization assays that were implemented, making comparisons unreliable, in part to apparent divergent kinetics of neutralizing and anti-RBD Abs that some studies highlighted [16], [22], [35], [36]. In particular, studies are concordant that the initial immune response to the vaccine is characterized by a parallel increase in quantitative anti-S-RBD antibodies, neutralizing serum activity and antigen-specific B lymphocytes and T lymphocytes clonal expansion. Nonetheless, as long as the immune response evolves, studies have provided somewhat conflicting results, with some studies showing a parallel decline in anti-RBD Ig and neutralizing activity [37] and others not [16], [22]. This study showed that the anti-S-RBD response elicited by the BNT162b2 vaccine was already detectable at the second BNT162b2 dose administration (T1), increased after the booster dose (T2) and was still detectable after 6 months (T3), although at markedly reduced levels. Conversely, the serum neutralizing capacity paralleled that of anti-S-RBD-Abs in the first weeks after vaccination (T1-T2) but then evolved separately by maintaining medium–high mean values (T3). These data are consistent with those of Terpos and Trougakos [16], [22], who showed different kinetics of decline of nAbs and anti-RBD Abs, namely that anti-RBD levels decreased much faster than nAbs levels.
An important finding of this study was the evidence of the lack of a consistent threshold value of anti-RBD Abs able to predict a high neutralizing bioactivity at different time points during a 6-month follow-up period. When longitudinally analyzed, the correlation between anti-S-RBD values and neutralizing bioactivity was not present at T1 but progressively increased at T2 and further at T3. Actually, neutralizing bioactivity was still high at 6 months and likely protective (which would be also confirmed by the fact that none of the patients had clinical or laboratory evidence of SARS-CoV-2 infection at T3), while most subjects would be considered at risk if the anti-RBD IgG level estimated by other authors as protective [38] was the only marker evaluated. This particular kinetic is highly suggestive of increased avidity of the serum, which, in turn, is the overall result of germinal centres-driven affinity maturation of nAbs each targeting a specific epitope on the Spike protein through somatic mutation in Spike-specific B cells and implies that avidity is a variable to factor in when predicting functional serum neutralizing activity from purely quantitative antibody values [39]. In COVID-19 patients, affinity maturation is potentially impaired by the dysfunction of germinal centres associated with severe disease [40], [41]. Actually, longitudinal studies on functional affinity maturation after natural infection have produced heterogeneous results [21], [30], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53] with gender, age and COVID-19 severity likely playing a role in driving divergent pathways [42], [43], [48]. On the contrary, in vaccine recipients, affinity maturation seems to be guaranteed by the uploading and persistence of viral Spike antigen on dendritic cells of germinal centers up to 12 weeks after liposomal mRNA inoculation [54], which in turn provide a solid background for long-term antigen presentation in germinal centres [55] and eventual achievement of high avidity values [30], [35], [36], [56]. Notably, a study from Struck et al. [30], which directly assessed avidity of anti-SARS-CoV-2 sera from vaccinated subject using the chaotropic agent urea, have provided strong evidence that avidity significantly increases in the first two months after vaccination, which may also explains the persistence of high neutralizing bioactivity in the face of declining anti-RBD IgG over a longer period of time, as this study showed. While these findings discharge the use of quantitative assays by themselves for predicting neutralizing bioactivity, it is clear that a high-throughput automated sVNT would avoid the shortcomings of a combined evaluation of quantitative and affinity features of antibodies.
A number of relevant limitations should be discussed. First, the sVNT we implemented captures all nAbs in a serum sample and not only S-RBD specific IgG isotype antibodies. Consequently, anti-RBD Abs are not fully representative of the whole spectrum of possible neutralizing antibodies. While anti-RBD Abs of the IgG class seem to progressively gain the most relevant role in neutralization, as suggested by the increase in correlation at T3, we could not exclude that other more precocious immunoglobulin classes, such as IgA or IgM, which were not directly measured in our quantitative binding assay, could explain the lack of correlation at earlier time points (T1) [57]. Next, it should be pointed out that this sVNT was not directly compared with a cell-based VNT, which is considered the gold-standard in neutralization assays [13], [58]. Finally, from a strictly theoretical point of view, the detection of neutralizing antibodies should not be viewed as unequivocal evidence of sterilizing immunity, which might require mucosal priming of antigen-specific cells, mucosal IgA elicitation, etc. [2]. Breakthrough SARS-CoV-2 infections have been documented in vaccinated individuals [59], even in the presence of neutralizing immunity [60], [61], [62]. Moreover, since anti-RBD IgG assays are necessarily carried out using a strain-specific Spike, either traditional or variant, results cannot directly define neutralizing activity of a serum sample to other variants [63], [64] and cross-neutralization assays should be continuously carried on as long as new variants emerge [65]. Our assays, using the traditional Spike protein as capture antigen, are fit for investigating the immunogenicity of BNT12b, which uses the traditional Spike mRNA sequence but, for the above reasons, could prove misleading if used to predict neutralizing immunity to SARS-CoV-2 variants.
In summary, the results of the present study suggest that anti-RBD IgG value should not be considered by itself to predict neutralizing serum bioactivity, the latter which, in this cohort of COVID-19 naïve vaccine recipients without detected breakthrough infections, could be easily detected and quantified by means of a hand-friendly, cheap and high-throughput automated antibody-mediated neutralization assay. The collection of multidimensional data about the long-term kinetics of multiple immunogenicity markers in vaccines cohorts, including RBD-specific memory B-cells, IgG quantitative values, neutralizing serum potency and avidity as well as markers of T-cell responsiveness, should be prioritized in order to identify the best COP for clinical practice and surveillance studies.
CRediT authorship contribution statement
Giacomo Malipiero: Methodology, Writing – original draft. Pierlanfranco D'Agaro: Conceptualization, Data curation, Supervision, Resources. Ludovica Segat: Data curation, Resources. Anna Moratto: Investigation, Resources. Danilo Villalta: Software, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank doctor Desrè E. Fontana for English language revision, doctors Barbara Miglietta, Simona Cofano, Daniela Turchetto and Stefania Lussi for technical support, and DIA.PRO Diagnostic Bioprobes (Milan) for kindly providing the reagents for sVNT free of cost.
References
- 1.Cromer D., Juno J.A., Khoury D., Reynaldi A., Wheatley A.K., Kent S.J., Davenport M.P. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat. Rev. Immunol. 2021;21(6):395–404. doi: 10.1038/s41577-021-00550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krammer F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat. Med. 2021;27(7):1147–1148. doi: 10.1038/s41591-021-01432-4. [DOI] [PubMed] [Google Scholar]
- 3.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 4.Addetia A., Crawford K.H.D., Dingens A., Zhu H., Roychoudhury P., Huang M.-L., Jerome K.R., Bloom J.D., Greninger A.L., McAdam A.J. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J. Clin. Microbiol. 2020;58(11) doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., Dull P., Plotkin S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., Mandelboim M., Levin E.G., Rubin C., Indenbaum V., Tal I., Zavitan M., Zuckerman N., Bar-Chaim A., Kreiss Y., Regev-Yochay G. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y., Du L. SARS-CoV-2 spike protein: a key target for eliciting persistent neutralizing antibodies. Signal. Transduct. Target Ther. 2021;6:95. doi: 10.1038/s41392-021-00523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristiansen P.A., Page M., Bernasconi V., Mattiuzzo G., Dull P., Makar K., Plotkin S., Knezevic I. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397(10282):1347–1348. doi: 10.1016/S0140-6736(21)00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian L., Elsheikh E.B., Patrone P.N., et al. Towards quantitative and standardized serological and neutralization assays for COVID-19. Int. J. Mol. Sci. 2021;22:2723. doi: 10.3390/ijms22052723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.-C., Tiu C., Hu Z., Chen V.-W., Young B.E., Sia W.R., Tan Y.-J., Foo R., Yi Y., Lye D.C., Anderson D.E., Wang L.-F. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 11.Meyer B., Reimerink J., Torriani G., et al. Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT) Emerg. Microbes. Infect. 2020;9:2394–2403. doi: 10.1080/22221751.2020.1835448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller K., Girl P., von Buttlar H., Dobler G., Wölfel R. Comparison of two commercial surrogate ELISAs to detect a neutralising antibody response to SARS-CoV-2. J. Virol. Methods. 2021;292 doi: 10.1016/j.jviromet.2021.114122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohmer N., Rühl C., Ciesek S., Rabenau H.F. Utility of different surrogate enzyme-linked immunosorbent assays (sELISAs) for detection of SARS-CoV-2 neutralizing antibodies. J. Clin. Med. 2021;10:2128. doi: 10.3390/jcm10102128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savage H.R., Santos V.S., Edwards T., Giorgi E., Krishna S., Planche T.D., Staines H.M., Fitchett J.R.A., Kirwan D.E., Cubas Atienzar A.I., Clark D.J., Adams E.R., Cuevas L.E., Lin T. Prevalence of neutralising antibodies against SARS-CoV-2 in acute infection and convalescence: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2021;15(7):e0009551. doi: 10.1371/journal.pntd.0009551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terpos E., Trougakos I.P., Apostolakou F., et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am. J. Hematol. 2021;96:E257–E259. doi: 10.1002/ajh.26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trougakos I.P., Terpos E., Zirou C., et al. Comparative kinetics of SARS-CoV-2 anti-spike protein RBD IgGs and neutralizing antibodies in convalescent and naïve recipients of the BNT162b2 mRNA vaccine versus COVID-19 patients. BMC Med. 2021;19:208. doi: 10.1186/s12916-021-02090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterhoff D., Glück V., Vogel M., Schuster P., Schütz A., Neubert P., Albert V., Frisch S., Kiessling M., Pervan P., Werner M., Ritter N., Babl L., Deichner M., Hanses F., Lubnow M., Müller T., Lunz D., Hitzenbichler F., Audebert F., Hähnel V., Offner R., Müller M., Schmid S., Burkhardt R., Glück T., Koller M., Niller H.H., Graf B., Salzberger B., Wenzel J.J., Jantsch J., Gessner A., Schmidt B., Wagner R. A highly specific and sensitive serological assay detects SARS-CoV-2 antibody levels in COVID-19 patients that correlate with neutralization. Infection. 2021;49(1):75–82. doi: 10.1007/s15010-020-01503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.J. Papenburg, M.P. Cheng, R. Corsini, et al., Evaluation of a commercial culture-free neutralization antibody detection kit for severe acute respiratory syndrome-related coronavirus-2 and comparison with an antireceptor-binding domain enzyme-linked immunosorbent assay, Open Forum Infect. Dis. 8 (2021) ofab220. [DOI] [PMC free article] [PubMed]
- 19.Michos A., Tatsi E.-B., Filippatos F., Dellis C., Koukou D., Efthymiou V., Kastrinelli E., Mantzou A., Syriopoulou V. Association of total and neutralizing SARS-CoV-2 spike-receptor binding domain antibodies with epidemiological and clinical characteristics after immunization with the 1st and 2nd doses of the BNT162b2 vaccine. Vaccine. 2021;39(40):5963–5967. doi: 10.1016/j.vaccine.2021.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favresse J., Gillot C., Di Chiaro L., et al. Neutralizing antibodies in COVID-19 patients and vaccine recipients after two doses of BNT162b2. Viruses. 2021;13:1364. doi: 10.3390/v13071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowntree L.C., Chua B.Y., Nicholson S., Koutsakos M., Hensen L., Douros C., Selva K., Mordant F.L., Wong C.Y., Habel J.R., Zhang W., Jia X., Allen L., Doolan D.L., Jackson D.C., Wheatley A.K., Kent S.J., Amanat F., Krammer F., Subbarao K., Cheng A.C., Chung A.W., Catton M., Nguyen T.HO., Sandt C.E., Kedzierska K. Robust correlations across six SARS-CoV-2 serology assays detecting distinct antibody features. Clin. Transl. Immunology. 2021;10(3) doi: 10.1002/cti2.v10.310.1002/cti2.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.E. Terpos, I.P. Trougakos, V. Karalis, et al., Kinetics of anti-SARS-CoV-2 antibody responses 3 months post complete vaccination with BNT162b2; a prospective study in 283 health workers, Cells 10 (2021) 1942. [DOI] [PMC free article] [PubMed]
- 23.Douxfils J., Gillot C., Mullier F., Favresse J. Post-SARS-CoV-2 vaccination specific antibody decrease - Thresholds for determining seroprevalence and seroneutralization differ. J. Infect. 2021;83(4):e4–e5. doi: 10.1016/j.jinf.2021.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.B.T. Bradley, A. Bryan, S.L. Fink, et al., Anti-SARS-CoV-2 antibody levels measured by the AdviseDx SARS-CoV-2 assay are concordant with previously available serologic assays but are not fully predictive of sterilizing immunity, J. Clin. Microbiol. 59 (2021) e0098921. [DOI] [PMC free article] [PubMed]
- 25.Marot S., Malet I., Leducq V., et al. Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers. Nat. Commun. 2021;12:844. doi: 10.1038/s41467-021-21111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu L., Xu X., Zhu B., Guo X., Xu K.e., Song C.i., Fu J., Yu H., Kong X., Peng J., Huang H., Zou X., Ding Y., Bao C., Zhu F., Hu Z., Wu M., Shen H., Jhaveri T. Kinetics of SARS-CoV-2 specific and neutralizing antibodies over seven months after symptom onset in COVID-19 patients. Microbiol. Spectr. 2021;9(2) doi: 10.1128/Spectrum.00590-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radbruch A., Chang H.-D. A long-term perspective on immunity to COVID. Nature. 2021;595(7867):359–360. doi: 10.1038/d41586-021-01557-z. [DOI] [PubMed] [Google Scholar]
- 28.Bauer G. The variability of the serological response to SARS-coronavirus-2: Potential resolution of ambiguity through determination of avidity (functional affinity) J. Med. Virol. 2021;93:311–322. doi: 10.1002/jmv.26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauer G. The potential significance of high avidity immunoglobulin G (IgG) for protective immunity towards SARS-CoV-2. Int. J. Infect. Dis. 2021;106:61–64. doi: 10.1016/j.ijid.2021.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Struck F., Schreiner P., Staschik E., Wochinz‐Richter K., Schulz S., Soutschek E., Motz M., Bauer G. Vaccination versus infection with SARS-CoV-2: Establishment of a high avidity IgG response versus incomplete avidity maturation. J. Med. Virol. 2021;93(12):6765–6777. doi: 10.1002/jmv.v93.1210.1002/jmv.27270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khatri I., Staal F.J.T., van Dongen J.J.M. Blocking of the high-affinity interaction-synapse between SARS-CoV-2 spike and human ACE2 proteins likely requires multiple high-affinity antibodies: an immune perspective. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.570018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H., Wu N.C., Yuan M., Bangaru S., Torres J.L., Caniels T.G., van Schooten J., Zhu X., Lee C.-C., Brouwer P.J.M., van Gils M.J., Sanders R.W., Ward A.B., Wilson I.A. Cross-neutralization of a SARS-CoV-2 antibody to a functionally conserved site is mediated by avidity. Immunity. 2020;53(6):1272–1280.e5. doi: 10.1016/j.immuni.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pratesi F., Caruso T., Testa D., et al. BNT162b2 mRNA SARS-CoV-2 vaccine elicits high avidity and neutralizing antibodies in healthcare workers. Vaccines. 2021;9:672. doi: 10.3390/vaccines9060672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D. Hillus, T. Schwarz, P. Tober-Lau, et al., Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study, Lancet Respir. Med. (2021) S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed]
- 35.Goel R.R., Apostolidis S.A., Painter M.M., et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021;6:eabi6950. doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goel R.R., Apostolidis S.A., Painter M.M., et al. Longitudinal analysis reveals distinct antibody and memory B cell responses in SARS-CoV2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021;6 doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bayart J.L., Douxfils J., Gillot C., et al. Waning of IgG, total and neutralizing antibodies 6 months post-vaccination with BNT162b2 in healthcare workers. Vaccines (Basel). 2021;9:1092. doi: 10.3390/vaccines9101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., Dold C., Fuskova M., Gilbert S.C., Hirsch I., Humphries H.E., Jepson B., Kelly E.J., Plested E., Shoemaker K., Thomas K.M., Vekemans J., Villafana T.L., Lambe T., Pollard A.J., Voysey M., Adlou S., Allen L., Angus B., Anslow R., Asselin M.-C., Baker N., Baker P., Barlow T., Beveridge A., Bewley K.R., Brown P., Brunt E., Buttigieg K.R., Camara S., Charlton S., Chiplin E., Cicconi P., Clutterbuck E.A., Collins A.M., Coombes N.S., Clemens S.A.C., Davison M., Demissie T., Dinesh T., Douglas A.D., Duncan C.J.A., Emary K.R.W., Ewer K.J., Felle S., Ferreira D.M., Finn A., Folegatti P.M., Fothergill R., Fraser S., Garlant H., Gatcombe L., Godwin K.J., Goodman A.L., Green C.A., Hallis B., Hart T.C., Heath P.T., Hill H., Hill A.V.S., Jenkin D., Kasanyinga M., Kerridge S., Knight C., Leung S., Libri V., Lillie P.J., Marinou S., McGlashan J., McGregor A.C., McInroy L., Minassian A.M., Mujadidi Y.F., Penn E.J., Petropoulos C.J., Pollock K.M., Proud P.C., Provstgaard-Morys S., Rajapaska D., Ramasamy M.N., Sanders K., Shaik I., Singh N., Smith A., Snape M.D., Song R., Shrestha S., Sutherland R.K., Thomson E.C., Turner D.P.J., Webb-Bridges A., Wrin T., Williams C.J. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021;27(11):2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.C.A. Sariol, P. Pantoja, C. Serrano-Collazo, et al., Function is more reliable than quantity to follow up the humoral response to the Receptor Binding Domain of SARS- CoV-2 Spike protein after natural infection or COVID-19 vaccination, medRxiv [Preprint]. 2021 Aug 10:2021.06.02.21257975. doi: 10.1101/2021.06.02.21257975. PMID: 34100029; PMCID: PMC8183028. [DOI] [PMC free article] [PubMed]
- 40.Kaneko N., Kuo H.H., Boucau J., et al. Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell. 2020;183:143–157. doi: 10.1016/j.cell.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pérez-Gómez A., Vitallé J., Gasca-Capote C., et al. Dendritic cell deficiencies persist seven months after SARS-CoV-2 infection. Cell. Mol. Immunol. 2021;1–12 doi: 10.1038/s41423-021-00728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo Y.R., Chakraborty I., Yun C., Wu A.H.B., Lynch K.L. Kinetics of SARS-CoV-2 antibody avidity maturation and association with disease severity. Clin. Infect. Dis. 2020:ciaa1389. doi: 10.1093/cid/ciaa1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pichler D., Baumgartner M., Kimpel J., et al. Marked increase in avidity of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) antibodies 7–8 months after infection is not diminished in old age. J. Infect. Dis. 2021:jiab300. doi: 10.1093/infdis/jiab300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muecksch F., Weisblum Y., Barnes C.O., Schmidt F., Schaefer-Babajew D., Wang Z., C. Lorenzi J.C., Flyak A.I., DeLaitsch A.T., Huey-Tubman K.E., Hou S., Schiffer C.A., Gaebler C., Da Silva J., Poston D., Finkin S., Cho A., Cipolla M., Oliveira T.Y., Millard K.G., Ramos V., Gazumyan A., Rutkowska M., Caskey M., Nussenzweig M.C., Bjorkman P.J., Hatziioannou T., Bieniasz P.D. Affinity maturation of SARS-CoV-2 neutralizing antibodies confers potency, breadth, and resilience to viral escape mutations. Immunity. 2021;54(8):1853–1868.e7. doi: 10.1016/j.immuni.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moriyama S., Adachi Y., Sato T., et al. Temporal maturation of neutralizing antibodies in COVID-19 convalescent individuals improves potency and breadth to circulating SARS-CoV-2 variants. Immunity. 2021;54:1841–1852. doi: 10.1016/j.immuni.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strömer A., Rose R., Grobe O., et al. Kinetics of nucleo- and spike protein-specific immunoglobulin G and of virus-neutralizing antibodies after SARS-CoV-2 infection. Microorganisms. 2020;8:1572. doi: 10.3390/microorganisms8101572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moura A.D., da Costa H.H.M., Correa V.A., et al. Assessment of avidity related to IgG subclasses in SARS-CoV-2 Brazilian infected patients. Sci. Rep. 2021;11:17642. doi: 10.1038/s41598-021-95045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.S.E. Benner, E.U. Patel, O. Laeyendecker, et al., SARS-CoV-2 antibody avidity responses in COVID-19 patients and convalescent plasma donors, J. Infect. Dis. 222 (2020) 1974-1984. [DOI] [PMC free article] [PubMed]
- 49.Neumann F., Rose R., Römpke J., et al. Development of SARS-CoV-2 specific IgG and virus-neutralizing antibodies after infection with variants of concern or vaccination. Vaccines. 2021;9:700. doi: 10.3390/vaccines9070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z., Muecksch F., Schaefer-Babajew D., Finkin S., Viant C., Gaebler C., Hoffmann H.-H., Barnes C.O., Cipolla M., Ramos V., Oliveira T.Y., Cho A., Schmidt F., Da Silva J., Bednarski E., Aguado L., Yee J., Daga M., Turroja M., Millard K.G., Jankovic M., Gazumyan A., Zhao Z., Rice C.M., Bieniasz P.D., Caskey M., Hatziioannou T., Nussenzweig M.C. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595(7867):426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chia W.N., Zhu F., Ong S.W.X., Young B.E., Fong S.-W., Le Bert N., Tan C.W., Tiu C., Zhang J., Tan S.Y., Pada S., Chan Y.-H., Tham C.Y.L., Kunasegaran K., Chen M.-C., Low J.G.H., Leo Y.-S., Renia L., Bertoletti A., Ng L.F.P., Lye D.C., Wang L.-F. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2(6):e240–e249. doi: 10.1016/S2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.den Hartog G., Vos E.R.A., van den Hoogen L.L., et al. Persistence of antibodies to SARS-CoV-2 in relation to symptoms in a nationwide prospective study. Clin. Infect. Dis. 2021:ciab172. doi: 10.1093/cid/ciab172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piccoli L., Park Y.-J., Tortorici M.A., Czudnochowski N., Walls A.C., Beltramello M., Silacci-Fregni C., Pinto D., Rosen L.E., Bowen J.E., Acton O.J., Jaconi S., Guarino B., Minola A., Zatta F., Sprugasci N., Bassi J., Peter A., De Marco A., Nix J.C., Mele F., Jovic S., Rodriguez B.F., Gupta S.V., Jin F., Piumatti G., Lo Presti G., Pellanda A.F., Biggiogero M., Tarkowski M., Pizzuto M.S., Cameroni E., Havenar-Daughton C., Smithey M., Hong D., Lepori V., Albanese E., Ceschi A., Bernasconi E., Elzi L., Ferrari P., Garzoni C., Riva A., Snell G., Sallusto F., Fink K., Virgin H.W., Lanzavecchia A., Corti D., Veesler D. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020;183(4):1024–1042.e21. doi: 10.1016/j.cell.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner J.S., O’Halloran J.A., Kalaidina E., Kim W., Schmitz A.J., Zhou J.Q., Lei T., Thapa M., Chen R.E., Case J.B., Amanat F., Rauseo A.M., Haile A., Xie X., Klebert M.K., Suessen T., Middleton W.D., Shi P.-Y., Krammer F., Teefey S.A., Diamond M.S., Presti R.M., Ellebedy A.H. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596(7870):109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Painter M.M., Mathew D., Goel R.R., et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity. 2021;S1074–7613(21):00308–313. doi: 10.1016/j.immuni.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greaney A.J., Loes A.N., Gentles L.E., Crawford K.H.D., Starr T.N., Malone K.D., Chu H.Y., Bloom J.D. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci. Transl. Med. 2021;13(600) doi: 10.1126/scitranslmed.abi9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.G.L. Salvagno, B.M. Henry, G. Lippi, Anti-SARS-CoV-2 IgA Response in Baseline Seronegative and Seropositive Recipients of BNT162b2 mRNA COVID-19 Vaccine, J. Occup. Environ. Med. (2021) doi: 10.1097/JOM.0000000000002362. [DOI] [PMC free article] [PubMed]
- 58.A.E. Muruato, C.R. Fontes-Garfias, P. Ren, e al., A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation, Nat. Commun. 11 (2020) 4059. [DOI] [PMC free article] [PubMed]
- 59.Schieffelin J.S., Norton E.B., Kolls J.K. What should define a SARS-CoV-2 “breakthrough” infection? J. Clin. Invest. 2021;131 doi: 10.1172/JCI151186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keehner Jocelyn, Horton Lucy E., Pfeffer Michael A., Longhurst Christopher A., Schooley Robert T., Currier Judith S., Abeles Shira R., Torriani Francesca J. SARS-CoV-2 infection after vaccination in health care workers in california. N. Engl. J. Med. 2021;384(18):1774–1775. doi: 10.1056/NEJMc2101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angel Yoel, Spitzer Avishay, Henig Oryan, Saiag Esther, Sprecher Eli, Padova Hagit, Ben-Ami Ronen. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325(24):2457. doi: 10.1001/jama.2021.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.R.A. Teran, K.A. Walblay, E.L. Shane, S. Xydis, et al., Postvaccination SARS-CoV-2 infections among skilled nursing facility residents and staff members - Chicago, Illinois, December 2020-March 2021, MMWR Morb. Mortal. Wkly. Rep. 70 (2021) 632-638. [DOI] [PMC free article] [PubMed]
- 63.Lu L., Chu A.W., Zhang R.R., et al. The impact of spike N501Y mutation on neutralizing activity and RBD binding of SARS-CoV-2 convalescent serum. EBioMedicine. 2021;71 doi: 10.1016/j.ebiom.2021.103544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Planas Delphine, Veyer David, Baidaliuk Artem, Staropoli Isabelle, Guivel-Benhassine Florence, Rajah Maaran Michael, Planchais Cyril, Porrot Françoise, Robillard Nicolas, Puech Julien, Prot Matthieu, Gallais Floriane, Gantner Pierre, Velay Aurélie, Le Guen Julien, Kassis-Chikhani Najiby, Edriss Dhiaeddine, Belec Laurent, Seve Aymeric, Courtellemont Laura, Péré Hélène, Hocqueloux Laurent, Fafi-Kremer Samira, Prazuck Thierry, Mouquet Hugo, Bruel Timothée, Simon-Lorière Etienne, Rey Felix A., Schwartz Olivier. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 65.F. Muecksch, H. Wise, K. Templeton, et al., Longitudinal variation in SARS-CoV-2 antibody levels and emergence of viral variants: implications for the ability of serological assays to predict immunity. medRxiv [Preprint]. 2021 Jul 7:2021.07.02.21259939. doi: 10.1101/2021.07.02.21259939. PMID: 34268524; PMCID: PMC8282113. [DOI] [PMC free article] [PubMed]