Abstract
Background
Recent country surveys have shown an unacceptably high prevalence of confirmed tuberculosis (TB) even among those with a low duration of cough, and more than 50% of those with bacteriologically confirmed pulmonary tuberculosis (PTB) do not report symptoms that correspond to presumptive TB. Furthermore, there has been an increase in the incidence of smear-negative PTB patients who can serve as a source of infection. We investigated whether screening people who sought healthcare for cough of any duration can increase TB case detection in Ethiopia, and compiled the lessons learned and recommendations.
Methods
We carried out a facility-based study in Ethiopia. All consenting participants who sought any healthcare at the outpatients department, and healthcare facilities for reproductive and child health, anti-retroviral therapy, and diabetes were screened for cough of any duration, and those with cough underwent further investigations using chest radiography (CXR) (except for pregnant women, patients on anti-retroviral therapy, and diabetic patients) and microbiological tests. Confirmed cases were linked to TB treatment following the country’s standard guidelines.
Results
We screened 195,713 people who sought healthcare for cough of any duration. Of these, 2647 reported cough symptom of any duration, of whom 1853 underwent further diagnostic tests as they fulfilled the criteria for presumptive TB. Overall, 309/1853 (16.7%) were diagnosed with PTB and linked to TB treatment. Screening by cough of any duration and/or CXR improved TB case finding, and engaging all health teams (administrative and supportive staff, as well as healthcare providers) in the TB screening and diagnosis significantly improved the process.
Conclusion
Screening for TB using cough of any duration and/or CXR for any patient who sought healthcare has the potential to increase both the number of presumptive TB cases and the number of patients diagnosed with and treated for TB in Ethiopia. Such initiatives require strong engagement of facility staff, regular maintenance and calibration of TB diagnostic equipment, and uninterrupted reagent supplies.
Keywords: cough, chest X-ray, CXR, tuberculosis, screening, diagnosis, health facility, Ethiopia
Introduction
The World Health Organization (WHO) African Region disproportionally contributed 25% of global tuberculosis (TB) cases, even though it covered as low as 13% of the world’s population, in 2019. Ethiopia is one of 30 countries with a high TB burden that together contributed 87% of the incidence of TB in 2019.1 The country has made efforts to combat the burden of TB and has achieved remarkable results. However, TB continues to be a major cause of morbidity and mortality in the country. Ethiopia is among the low-income countries, with a universal health coverage service index of 39, and 4.9% of the general population experience catastrophic health expenditure, with a low rate of domestically funded TB programs, at 11%, and 56% of programs remaining unfunded.1
The United Nations Sustainable Development Goals (SDGs) are aimed at reducing TB incidence and death rates by 80% and 90%, respectively, by 2030,2 but progress has been more sluggish than expected. For instance, between 2015 and 2020, the TB incidence rate and TB death rate were planned to be reduced by 20% and 35%, respectively, but both moved less than halfway towards these milestones, by 9% and 14%, respectively in 2019.1 Ethiopia has endorsed SDGs to achieve the global targets by improving the capacity of the healthcare system and TB case finding, in which screening has been a priority, as stipulated in the country’s Health Sector Transformation Plan (HSTP). However, Ethiopia is still with a high TB burden, with TB incidence, HIV-negative and -positive TB death rates, of 140, 19, and 2.5, per 100,000 population, respectively, in 2019.1
Ethiopian national TB guidelines endorse the investigation of TB among people with cough for ≥2 weeks, except for HIV/AIDS patients, who are screened for cough of any duration.3 However, in Ethiopia, a high proportion of health centers, 30%, do not have a TB screening service, which is a missed opportunity to find and treat infectious TB cases. TB care capacity is poorer in health centers and private facilities compared to hospitals, and is lower in rural than in urban settings.4 Evidence has shown that trained staff are critically required for TB control, but in Ethiopia, less than 10% of health centers have trained and qualified staff to diagnose and treat TB cases.4 TB care quality has been affected by a lack of trained staff,5 high patient load, suboptimal screening and diagnosis of TB,6 and poor TB screening systems, which have contributed to presumptive TB cases being missed. The system fails to screen eligible individuals at the earliest points, missing up to 40%,7,8 and one-third of smear microscopy tests were not requested,9 given that finding of a systemetic review revealed that the pooled healthcare seeking among presumptive TB in Ethiopia was 65.5%.10 Training of all nurses and laboratory technicians in TB control has been used to improve TB case finding11 and TB notification rate,12 with a focus on those working in the directly observed treatment, short course (DOTS) clinics.13
In Ethiopia, analysis of the patient pathway at the national level indicated that the proportion of TB care initiated at public facilities was 76%14 and the care pathway was long.6 More than one-third of patients initiated care with health extension workers (HEWs), where TB diagnostic technologies were not available; then, HEWs referred patients to a higher level.14 This contributed to the outcomes, as the majority, 82%, of health centers in Ethiopia used smear microscopy,14 which has poor sensitivity relative to other methods such as GeneXpert MTB/RIF.15,16 The implementation of GeneXpert has been restricted to some at-risk populations, such as people with HIV/AIDS, since 2014,17 for budgetary reasons.15,18
In TB case finding, many challenges have been identified, including resources, accessibility, and stakeholders’ participation.19,20 TB screening in targeted groups can be implemented in resource-limited settings if the support and stakeholder engagement are ensured before the actual screening is launched, and active follow-up to start treatment for diagnosed TB cases is established.20,21 We performed targeted TB screening in selected health facilities in Ethiopia and investigated whether screening people seeking healthcare for cough of any duration at outpatients departments (OPDs) and reproductive and child health (RCH) units, and anti-retroviral therapy (ART) and diabetic patients who report a cough, irrespective of duration, could increase TB case detection and enrollment into care. Here, we summarize the findings and the lessons learned, with recommendations for consideration in the national TB program and similar settings.
Methods
The study was conducted from July 2019 to March 2020 and from August 2020 to December 2020. We did not enroll participants from April 2020 to July 2020 because of the COVID-19 pandemic.13 The study was conducted in four health facilities, located in two regions (Oromia Regional State and Harari Regional State) and two city administrations (Addis Ababa and Dire Dawa). Two referral hospitals were located in urban settings (Hiwot Fana Specialized University Hospital and Zewditu Memorial Hospital), while one hospital (Chelenko Primary Hospital) and one health center (Melka Jebdu Health Center) were located in rural settings.
We screened people who sought healthcare for a cough of any duration, and then further screened symptom-positive patients by chest X-ray (CXR), among those visiting OPDs and RCH units. Pregnant mothers, and diabetic and ART patients, were screened only by symptom. For the eligible study participants, the investigations were performed either by GeneXpert or smear microscopy, as per the national TB guidelines.3 Collected data were analyzed using Stata version 14.0 (StataCorp, College Station, Texas, USA). Data were summarized as frequencies.
Results and Discussion
TB Case Finding
We screened 195,713 study participants with TB symptoms out of 241,052 people who sought healthcare at four health facilities in Ethiopia (Table 1). Of these, 2647 had a cough symptom and agreed to participate in the study. In total, 2142 (92.7%) of the eligible study participants (2312) were screened by CXR of those visited at the OPD and RCH units. Moreover, 335 individuals, comprising pregnant women, diabetic patients, and patients on ART, were screened only by symptom. A total of 1853 patients fulfilled the eligibility criteria for presumptive TB (Table 1).
Table 1.
Number of PTB Cases Diagnosed Among People Screened for Cough of Any Duration at Health Facilities, Ethiopia
| Month, Year | People Who Sought Healthcare, n (%) | TB Symptoms Screened, n (%) | Cough Symptom Positive, n (%) | Presumptive TB Cases, n (%) | PTB Cases Diagnosed, n (%) |
|---|---|---|---|---|---|
| July, 2019 | 14,744 (6.1) | 13,850 (7) | 64 (2) | 39 (2.1) | 12 (3.9) |
| August, 2019 | 15,430 (6.4) | 12,913 (6.6) | 259 (10) | 157 (8.5) | 38 (12.4) |
| September, 2019 | 17,365 (7.2) | 14,147 (7.2) | 243 (9) | 152 (8.2) | 40 (13) |
| October, 2019 | 19,358 (8.0) | 16,969 (8.7) | 275 (10) | 177 (9.5) | 34 (11) |
| November, 2019 | 21,161 (8.8) | 19,001 (9.7) | 210 (8) | 158 (8.5) | 29 (9.4) |
| December, 2019 | 21,846 (9.1) | 18,599 (9.5) | 257 (10) | 169 (9) | 24 (7.8) |
| January, 2020 | 19,831 (8.2) | 14,806 (7.6) | 221 (8) | 140 (7.5) | 12 (3.9) |
| February, 2020 | 23,663 (9.8) | 20,278 (10.4) | 327 (12) | 239 (13) | 38 (12.3) |
| March, 2020 | 12,157 (5) | 10,737 (5.5) | 257 (10) | 195 (10.5) | 16 (5.2) |
| August, 2020 | 5872 (2.4) | 3284 (1.7) | 27 (1) | 23 (1.2) | 6 (2) |
| September, 2020 | 13,903 (5.8) | 7668 (3.9) | 58 (2) | 57 (3.1) | 11 (3) |
| October, 2020 | 15,644 (6.5) | 10,998 (5.6) | 80 (3) | 67 (3.6) | 10 (3.2) |
| November, 2020 | 19,585 (8.1) | 15,207 (7.8) | 204 (8) | 158 (8.5) | 25 (8) |
| December, 2020 | 20,493 (8.5) | 17,256 (8.8) | 165 (6) | 122 (6.6) | 14 (4.5) |
| Total | 241,052 (100) | 195,713 (100) | 2647 (100) | 1853 (100) | 309 (100) |
Abbreviation: PTB, pulmonary tuberculosis.
Overall, 309/1853 (16.7%) pulmonary tuberculosis (PTB) cases were diagnosed, of which 185 (62.7%) were bacteriologically confirmed PTB cases whereas 110 (37.3%) were GeneXpert MTB/RIF or smear-negative but clinically diagnosed PTB cases based on clinical and CXR results. Fourteen clinically diagnosed PTB cases were diagnosed by healthcare providers based on clinical symptoms and CXR results.
Lesson Learned: Who to Involve in the Screening and Diagnosis of PTB
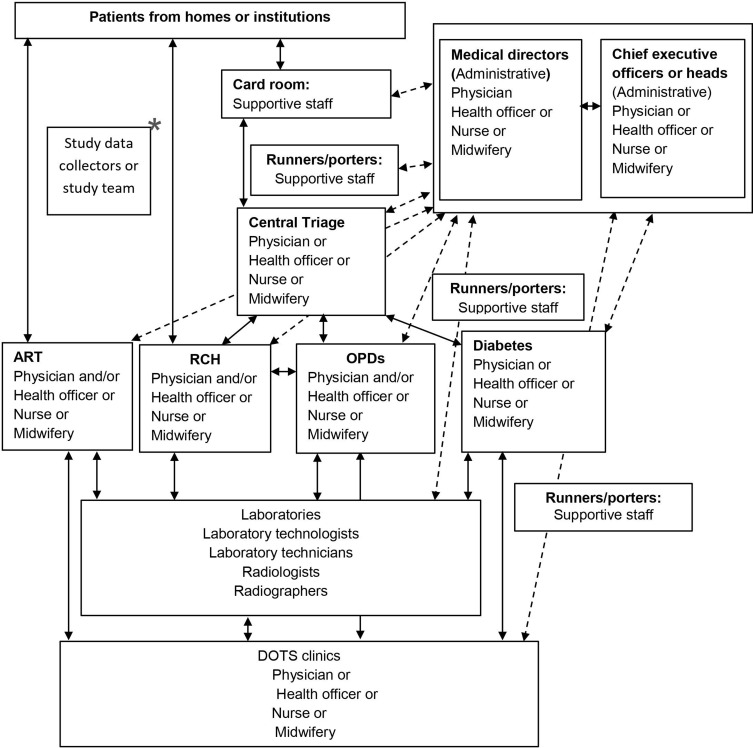
As Figure 1 shows, the people who were working as healthcare providers at different units varied in their specialties, including nurses, midwives, health officers, physicians, radiographers, and laboratory technologists. The administrative staff included the chief executive officers or heads, medical directors, or coordinators of the units. The supportive staff who participated worked in the card rooms and as runners or porters who helped to guide patients regarding where to go next and who to contact, or took patients’ folders from place to place within the health facilities of hospitals while maintaining the confidentiality of patients’ data as per ethical standards.
Figure 1.
People involved in TB screening and diagnosis at a given study health facility in Ethiopia.
Notes:  indicates the study flow;
indicates the study flow;  indicates communication and follow-up between HCPs and supportive and administrative staff; *indicates communication follow-up between the research team and staff.
indicates communication and follow-up between HCPs and supportive and administrative staff; *indicates communication follow-up between the research team and staff.
Abbreviations: ART, anti-retroviral therapy; DOTS, directly observed treatment, short course; HCP, healthcare provider; OPD, outpatients department; RCH, reproductive and child health.
The screening and diagnosis were performed in the health facilities among healthcare-seeking people. Systematic screening for the target groups was performed at four health facilities. The study involved two days’ training for healthcare providers who were working in triage, OPDs, RCH units, ART, diabetic clinics, DOTS, TB laboratories, and X-ray; administrative staff working at the respective facilities; and supportive staff working in the card rooms.
The training included TB burden, national TB guideline updates,3 the importance of screening and diagnosis for TB case finding, and how to link the eligible clients to be screened at the study health facilities using a clinical algorithm, as follows. In the card rooms, the patients’ folders were stamped with a TB screening stamp (TB symptoms were written on the stamp, with “Yes” or “No”), when they arrived at the central triage rooms they were taken by runners/porters at the hospitals, healthcare providers who were working in triage filled in the symptom(s) screening stamp on the patients’ folders, and if they were symptom positive (cough), the clients were linked to the data collectors. After checking the eligibility, the data collectors obtained informed consent or parental consent and/or assent, and put the CXR request and/or smear microscopy or GeneXpert requests in the patients’ folders, which were later filled and sent to the laboratories by healthcare providers working in the respective units.
The healthcare providers working in the aforementioned units assessed the people seeking healthcare, filled in the laboratory request forms, and sent for the tests according to the national TB guidelines.3 When laboratory results were received, if bacteriologically confirmed (either GeneXpert MTB/RIF or smear positive), patients were started on anti-TB treatment as per the national TB guidelines.3 TB patients with positive screening but negative sputum results were evaluated as per the national TB guidelines for clinical diagnosis based on symptoms and/or CXR screening results.3 When the healthcare providers received the required investigations, they decided on the diagnosis and linked them to DOTS clinics and the study data collectors.
The study results also revealed that engaging all health teams (administrative and supportive staff, as well as healthcare providers) during TB screening and diagnosis contributed significantly. This highlights that the screening and diagnosis of TB at health facilities in Ethiopia were operationally feasible when all healthcare providers, supported by administrative and support staff, are engaged. Figure 1 summarizes the detail of the screening and diagnosis pathway in a given health facility, showing the working communication flow and follow-up of health teams among themselves and with the study team.
Lesson Learned: Gaps in the TB Care Cascade
Patients who visited a health center that had no X-ray facilities were taken to a public health hospital. Transport was provided, to resolve physical accessibility barriers. Technical barriers to reading the CXR were resolved by transporting the CXR films or CDs to another facility that had a radiologist. The costs of CXR imaging, reading by the radiologist, and transportation were covered by the research project.
We observed that the contribution of CXR to screening and diagnosis was high, but the costs were difficult to meet for the people seeking healthcare.6 We recommend that the national TB program should consider making the CXR free, as for other tests such as acid-fast bacillus (AFB) smear microscopy, GeneXpert MTB/RIF, and anti-TB treatment, as the guideline already includes CXR for triage, screening, and aiding diagnosis, but this is not yet practical.3 Besides, the CXR has been included in the diagnostic algorithm, but only for smear-negative cases after a two-week course of antibiotics, and it also recommends anti-TB treatment for those with suggestive results on the CXR.3
People seeking healthcare were screened for a cough of any duration and linked to the data collectors in the health facilities for further study procedures such as CXR screening and sputum examinations. A system created for linking started in the card room (registration), where patients visited first, and was then sent to the central triage, where the decision was made on where/who patients would be contacted for further investigations. This system could help the national TB program to improve TB case finding by ensuring that all people visiting health facilities are screened for cough regardless of their compliance. Thus, screening for cough of any duration and/or CXR among people who seek healthcare could improve TB case finding in Ethiopia, improve detection of missing cases, and significantly reduce the delay among PTB cases, in whom the diagnostic delay was as low as 2.6% in this study.
The availability of GeneXpert cartridges was improved by communicating with stakeholders from health facilities to the Federal Ministry of Health (FMoH) through the existing system. Maintenance of diagnostic laboratory equipment was assisted by communication to the concerned bodies at early as possible and by searching for replacements on the market, by ourselves or with the facility. Early dropout among TB patients who were diagnosed at study facilities in referral-out or transferred-out processes was prevented by contacting the receiving facility through the health system or, in some cases, by calling the patients on their telephone number, which was recorded securely in a separate document for research purposes (Table 2).
Table 2.
Core TB Care Gaps Observed During Screening and Diagnosis in Ethiopia, and Suggested Solutions
| Organizations | Core TB Activity Gaps Observed | Suggested Solutions |
|---|---|---|
| Federal Ministry of Health, Ethiopia (FMoH) | A health center has no GeneXpert machine | FMoH/RHB should provide GeneXpert for health center |
| What was requested and what was provided for a facility were very far apart | The target people have to be reported by the facilities, and equipment and supplies provided by EPSA/FMoH | |
| Cartridges were frequently interrupted and restricted to use for prioritized groups such as people with HIV/AIDS | ||
| The maintenance of GeneXpert was done by the EPHI. Some GeneXpert modules had not worked for a long time | Planned maintenance of equipment required | |
| The registers of some units of a given health facility had no dedicated columns to record the TB screening, eg ANC, FP, PNC, EPI | Integration of TB screening column in existing registers | |
| Screening present at OPDs, ART, PMCT, and diabetes, but there is no mechanism to confirm whether an individual has been screened or not. A patient could screen negative upon registration in the OPD (yes/no) but could be diagnosed and on treatment at DOTS | Putting a screening log in patients’ folders or preparing a stamp for TB screening to put on patients’ folders can help to ensure screening | |
| Regional Health Bureaus (RHBs) | When chest X-ray equipment was bought or obtained through aid and installed in a given facility, the materials and human resources required for its maintenance were not ready in advance at the level expected for sustainability; if damaged, repairs took a long time. | Full documentation of all equipment and training in ongoing maintenance required |
| Zonal Health Bureaus and facilities | FMoH was sending the GeneXpert cartridges directly from the center to the facility through EPSA, but sometimes the machines were damaged; then, either the facility did not take the cartridges or they took and put them at their facility, while other nearby health facilities had a shortage of cartridges | Follow-up is necessary for equipment functionality, and supply required by zonal, regional, and FMoH |
| Facilities (hospitals/health centers) | Lack of staff at DOTS clinics when HCPs were not on job owing to a competing priority, eg training | Formal delegation by facilities required |
| Some HCPs were not interested in performing AFB for diagnosis when GeneXpert was interrupted at a hospital level | Training, close follow-up, and timely provision of supplies |
Abbreviations: AFB, acid-fast bacillus; ANC, antenatal care; ART, anti-retroviral therapy; DOTS, directly observed treatment, short course; EPHI, Ethiopian Public Health Institute; EPI, expanded program of immunization; EPSA, Ethiopian Pharmaceuticals Supply Agency; FP, family planning; HCP, healthcare provider; OPD, outpatients department; PMCT, prevention of mother-to-child transmission; PNC, postnatal care.
To prevent missed opportunities, healthcare providers initiated screening for a cough of any duration rather than screening for a cough of ≥2 weeks, as we observed that we obtained 45/309 (14.6%) PTB cases among those reporting a cough for <2 weeks. Study participants accessed the primary healthcare facilities easily and early but were diagnosed less owing to low capacity of health facilities in terms of skilled workers and laboratory equipment, which resulted in either patients or sputum samples having to be sent to higher health facilities, as we did for the CXR screening. Consequently, this added burdens on the patients in terms of costs and delays in the diagnosis, which further created disease transmission in the communities. Therefore, empowering the primary health facilities, where many presumptive TB cases initiated their healthcare seeking, is crucially important.
Conclusion
Screening for TB using cough of any duration and/or CXR for any patient who seeks healthcare has the potential to increase the number of presumptive TB cases, and therefore to increase the number of patients diagnosed with and treated for TB in Ethiopia. Such initiatives require strong engagement of facility staff, regular maintenance and calibration of TB diagnostic equipment, and uninterrupted supplies of reagents. The primary health facilities should be empowered to strengthen the screening and diagnosis of TB to achieve the WHO End TB Strategy.
Funding Statement
This study was helped by the EXIT-TB project, which is part of the European & Developing Countries Clinical Trials Partnership 2 (EDCTP2) program supported by the European Union (grant number CSA2016S-1608). The funder had no role in the conception, data collection, analysis, or write-up of the study.
Abbreviations
ANC, antenatal care; ART, anti-retroviral therapy; CXR, chest X-ray; DOTS, directly observed treatment, short course; EPHI, Ethiopian Public Health Institute; EPI, expanded program of immunization; EPSA, Ethiopian Pharmaceuticals Supply Agency; FP, family planning; OPD, outpatients department; PMCT, prevention of mother-to-child transmission; PNC, postnatal care; RCH, reproductive and child health.
Data Sharing Statement
Datasets used in this article can be obtained upon reasonable request from the corresponding author.
Ethical Approval and Informed Consent
The study was approved by the Institutional Review Board (IRB) of the College of Health Sciences, Addis Ababa University (Ref. No. AAUMF 03-008), and Haramaya University, College of Health and Medical Sciences, Institutional Health Research Ethics Review Committee (IHRERC) (Ref. No. IHRERC/004/2019). Letters of permission were obtained from the respective health facilities. After enough time had been given to respond to any question raised by study participants, interviews and procedures were performed. Informed and written consent or parental consent and/or assent was obtained after information was provided for study participants. For illiterate study participants, a witness attended the process during which the form was read for them.
Acknowledgments
The authors are thankful to the Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), Addis Ababa University, for providing technical support and managing the grant. The authors also thank Haramaya University for assisting with the project, and acknowledge all study participants and the study facilities for permitting us to collect the data, and all staff of the study health facilities.
Author Contributions
HM: conceptualization, study conduct, administration, formal analysis, investigation, visualization, writing (original draft and rewriting). LO, KTR, EN, TM: supervision, protocol review, data collection, and critical review of the manuscript. RM: coordination of data collection, and critical review of the manuscript. GY: supervision, protocol review, data collection, writing (review and editing), administration, critical review of the manuscript, and funding acquisition. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest.
References
- 1.WHO. Global TB Report Final. Geneva, Switzerland: World Health Organization; 2020. [Google Scholar]
- 2.World Health Organization. Implementing the END TB Strategy: The Essentials. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 3.FMoH. Ethiopia National guideline for TB Leprosy and DR TB 6th ed. Addis Ababa; 2018.
- 4.Deribew A, Dejene T, Defar A, et al. Health system capacity for tuberculosis care in Ethiopia: evidence from national representative survey. Int J Qual Health Care. 2020;32:306–312. doi: 10.1093/intqhc/mzaa024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gebreegziabher SB, Yimer SA, Bjune GA. Qualitative assessment of challenges in tuberculosis control in West Gojjam Zone, Northwest Ethiopia: health workers’ and tuberculosis control program coordinators’ perspectives. Tuberc Res Treat. 2016:2036234. doi: 10.1155/2016/2036234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammed H, Oljira L, Roba KT, et al. Burden of tuberculosis and challenges related to screening and diagnosis in Ethiopia. J Clin Tuberc Other Mycobact Dis. 2020;19:100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abayneh M, HaileMariam S, Asres A. Low tuberculosis (TB) case detection: a health facility-based study of possible obstacles in Kaffa Zone, Southwest District of Ethiopia. Can J Infect Dis Med Microbiol. 2020:1–9. doi: 10.1155/2020/7029458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assefa D, Belachew F, Wondimagegn G, Klinkenberg E. Missed pulmonary tuberculosis: a cross sectional study in the general medical inpatient wards of a large referral hospital in Ethiopia. BMC Infect Dis. 2019;19(1):60. doi: 10.1186/s12879-019-3716-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dabaro D. Factors affecting tuberculosis case detection in Kersa district, south west Ethiopia. J Clin Tuberc Other Mycobact Dis. 2017;9:1–4. doi: 10.1016/j.jctube.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gamtesa DF, Tola HH, Mehamed Z, Tesfaye E, Alemu A. Health care seeking behavior among presumptive tuberculosis patients in Ethiopia: a systematic review and meta-analysis. BMC Health Serv Res. 2020;20(1):445. doi: 10.1186/s12913-020-05284-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahyuni CU, Rahariyani LD, Sulistyowati M, Rachmawati T, Yuliwati S, van der Werf MJ. Obstacles for optimal tuberculosis case detection in primary health centers (PHC) in Sidoarjo district, East Java, Indonesia. BMC Health Serv Res. 2007;7(1):135. doi: 10.1186/1472-6963-7-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon T, Shiferaw M, Abreham W, Tayu B, Klinkenberg E, Loha E. Assigning focal persons to notify more tuberculosis patients: lessons learned in southern Ethiopia. Public Health Action. 2014;4(3):S18–S24. doi: 10.5588/pha.14.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammed H, Oljira L, Roba KT, Yimer G, Fekadu A, Manyazewal T. Containment of COVID-19 in Ethiopia and implications for tuberculosis care and research. Infect Dis Poverty. 2020;9(1):131. doi: 10.1186/s40249-020-00753-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fekadu L, Hanson C, Osberg M, Makayova J, Mingkwan P, Chin D. Increasing access to tuberculosis services in Ethiopia: findings from a patient-pathway analysis. Int J Infect Dis. 2017;216(suppl_7):S696–S701. doi: 10.1093/infdis/jix378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin ZZ, Pai M, Van Gemert W, Sahu S, Ghiasi M, Creswell J. How is Xpert MTB/RIF being implemented in 22 high tuberculosis burden countries? Eur Respir J. 2015;45(2):549–554. doi: 10.1183/09031936.00147714 [DOI] [PubMed] [Google Scholar]
- 16.Subbaraman R, Jhaveri T, Nathavitharana RR. Closing gaps in the tuberculosis care cascade: an action-oriented research agenda. J Clin Tuberc Other Mycobact Dis. 2020;19:100144. doi: 10.1016/j.jctube.2020.100144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FMoH. Implementation Guideline for GeneXpert MTB/RIF Assay in Ethiopia, Addis Ababa; 2014.
- 18.Ereso BA-O, Yimer SA, Gradmann C, Sagbakken M, Torpey K. Barriers for tuberculosis case finding in Southwest Ethiopia: a qualitative study. PLoS One. 2020;15(1):e0226307. doi: 10.1371/journal.pone.0226307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pande T, Vasquez NA, Cazabon D, et al. Finding the missing millions: lessons from 10 active case finding interventions in high tuberculosis burden countries. BMJ Glob Health. 2020;5(12):e003835. doi: 10.1136/bmjgh-2020-003835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oshi DC, Omeje JC, Oshi SN, et al. An evaluation of innovative community-based approaches and systematic tuberculosis screening to improve tuberculosis case detection in Ebonyi state, Nigeria. Int J Mycobacteriol. 2017;6(3):246. doi: 10.4103/ijmy.ijmy_91_17 [DOI] [PubMed] [Google Scholar]
- 21.Kranzer K, Afnan-Holmes H, Tomlin K. The benefits to communities and individuals of screening for active tuberculosis disease: a systematic review. Int J Tuberc Lung Dis. 2013;17(4):432–446. doi: 10.5588/ijtld.12.0743 [DOI] [PubMed] [Google Scholar]