Abstract
Basal cell carcinoma (BCC) is one of the most common malignant tumors worldwide, involving the skin. It is also part of keratinocyte carcinomas, alongside its squamous counterpart. It has low mortality and extremely low metastatic rates (although when present, it indicates a poor patient prognosis); it also has a high morbidity rate through local destruction and recurrence, particularly when perineural invasion is observed, clinically or histopathologically. BCC development is the result of environmental and patient factors, with genetics and ultraviolet radiation playing major roles. The clinical and histopathological aspects vary according to tumor subtype, being classified as high-risk tumors (nodular, superficial, pigmented and infundibulocystic BCC with adnexal differentiation) and fibroepithelial subtypes, or as high-risk tumors (micronodular, infiltrating, sclerosing/morphoeic and basosquamous subtype or the type with sarcomatoid differentiation). Dermoscopy is now complimented by novel in vivo diagnostic tools (optical coherence tomography, reflectance confocal microscopy, high-resolution ultrasonography, Raman spectroscopy or terahertz pulse imaging), improving the diagnostic accuracy and providing tumor depth and lateral margins without the use of invasive techniques. Novel treatment approaches for the treatment of BCC have recently been investigated with the use of hedgehog pathway inhibitors, such as Vismodegib. These approaches aim for complete resolution, minimal side-effects, high patient satisfaction with the optimal cosmetic results, particularly in key areas, such as the face. The present review article summarizes and discusses the comprehensive clinical and histopathological aspects of BCC, and presents novel imaging tools and therapeutic approaches that have been identified.
Keywords: basal cell carcinoma, subtypes, dermoscopy, pathology, optical coherence tomography, reflectance confocal microscopy
1. Introduction
The skin is the largest organ of the human body and it is the site from which various types of tumors may arise, from benign ones, such as seborrheic keratosis, nevi and spiradenomas (some of which are considered even precursor lesions for their malignant counterparts) to malignant masses, such as basal cell carcinomas (BCCs) or squamous cell carcinomas (SCCs), melanomas or spiradenocarcinomas.
BCCs are one of the most common malignant tumors worldwide with skin involvement, being part of non-melanoma skin cancers, alongside SCCs, with which it can coexist in the same lesion, evolving at the site of a burn scar, or even from a benign tumor-like lesion, such as a verruca vulgaris (1-4). According to the World Health Organization (WHO), BCCs represent malignant tumors which have originated from basal cells found in the inter-follicular epidermis or in the hair follicle. BCC cases further increase the burden to global cancer cases which are constantly on the rise; it is estimated that 1 in 5 males and 1 in 6 females will suffer from one form of cancer during their lifetime (5,6). Although the reporting of BCC cases is not precise (data are rarely collected), its global incidence is increasing, affecting approximately one million new patients each year. It is one of the most common types of skin cancer in certain populations such as among individuals of Asian, African or Hispanic origin (7).
BCC has low mortality and extremely low metastatic rates (although when present, it indicates a poor prognosis). However, it has a high morbidity rate, exemplified through local destruction and recurrence, particularly when perineural invasion (PNI) is present, allowing BCC to spread in a contiguous manner with secondary neuropathy. Extremely rare cases from the literature (11 in total) have reported BCC with intravascular invasion, having higher metastatic rates than BCC cases without this aggressive trait (8-11).
2. Pathogenesis and risk factors
BCC pathogenesis is the direct result of interactions between environmental factors and patient characteristics, as well as genetic factors. As regards genetics, the aberrant activation of the Hedgehog signaling pathway has been shown to be pathognomonic for BCC development and it can interact with other oncogenic pathways, such as EGFR, TGF-β, PI3K, NF-κB and atypical protein kinase C (aPKC) (9,12).
The risk factors incriminated in BCC development are classified as follows: Modifiable risk factors, such as ultraviolet (UV) radiation and behavioral factors associated with an increased exposure to this type of radiation (multiple sunburns at an early age and occupational sun exposure), being the most incriminating one; and non-modifiable risk factors, such as the elderly/aging population (with cumulative sun exposure), a positive family history of BCC and genetic factors, such as mutations of the patched tumor suppressor genes 1 and 2 (PTCH1 and PTCH2), which can be inherited as an autosomal dominant trait or can appear de novo, in the basal cell nevus syndrome/nevoid BCC syndrome/Gorlin-Goltz syndrome; other genetic syndromes associated with BCC development, such as xeroderma pigmentosum and Bazex-Dupré-Christol syndrome. The non-modifiable factors which are mainly incriminated in BCC development in the Caucasian population are the following: A fair skin (Fitzpatrick skin types I and II), a light eye color, blonde/red hair, freckles, photosensitizing medication (tetracyclins, hydrochlorothiazide and statins) and exposure to carcinogenic substances (such as arsenic). Along with other skin pathologies (Kaposi's sarcoma, herpes zoster, oral candidiasis or hairy leucoplakia), BCC has been observed to develop with a 2- and 5-fold increased frequency in patients who are human immunodeficiency virus (HIV)-positive and, respectively, in those who have received organ transplants (7,9,13-18).
As the main factor responsible for the development of BCC, UV radiation exposure has been found to be responsible for the release of interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) from keratinocytes. In some benign and malignant tumors or in certain conditions, IL-6 is known to function as a growth factor [acquired immunodeficiency syndrome (AIDS), Kaposi's sarcoma, multiple myeloma, renal cell carcinoma, some T-cell or B-cell lymphomas], while also preventing apoptosis and inducing the expression of the anti-apoptotic protein, Bcl-xL. At the same time, IL-6 stimulates vascular endothelial growth factor (VEGF) expression in various cells, functioning as a pro-angiogenic factor by enhancing angiogenesis. Thus, IL-6 is incriminated in the pathogenesis of BCC (19,20). Neuroactive factors seem to also play a role not only in BCC, but also in SCC development; the interactions between the peripheral nervous system and skin cells are mediated by several locally secreted, possibly stress-induced neuroendocrine factors, such as substance P, catecholamines, somatostatin, calcitonin gene-related peptide or neurohormones (proopiomelanocortin, adrenocorticotropin and α-melanocyte-stimulating hormone). These substances which are dysregulated by chronic stress have been found to be involved in the development and progression of BCC and SCC, by their involvement in immune system suppression and by ensuring a favorable tumor microenvironment (21).
BCC arising in uncommon locations, such as the oral cavity or the vulva is not associated with UV radiation exposure as a primary risk factor, as these are sun-protected areas. In such rare primary site cases, other risk factors are involved, such as chronic inflammation, immune suppression, Paget's disease of the vulva or lichen sclerosus, addressing the genital location; as regards the oral cavity, risk factors for BCC development remain unknown (22-24). Although vitiligo may be a predisposing factor for sunburn, and it may potentiate the effects of UV radiation and subsequent consequences, the available data reveal the contrary, a protective effect; the association between vitiligo and BCC is rare, as it is also in the case of melanoma (25-28).
3. Clinical aspects
BCC may have a wide array of clinical presentations; its medico-social importance resides in its prevalence, its detrimental effects on health and associated complications, as well as in its psychological burden, as with other pathologies (such as diabetes or other tumors). Oftentimes, patients suffering from BCC may present with a lesion which has enlarged with time, is non-healing and which may bleed or ulcerate; these aspects may also be accompanied by pruritus. The classical clinical description of such a tumor is that of a shiny, pearly, smooth papule/nodule with conspicuous, dilated and arborizing blood vessels, often with erosions or ulcerations and rolled margins. The clinical aspects vary with the histological type of the tumor (discussed below); thus, the classical clinical description may correspond to the nodular type of BCC, while the discovery of a circumscribed, annular, thin patch or plaque with/without scales, central clearing and rolled borders can be observed in superficial BCC; scar-like lesions or poorly defined, infiltrative, shiny plaques (which can be flat or depressed) can indicate the presence of an infiltrative or morphoeic/sclerosing BCC, and the pigmented variant of BCC can be clinically confused with melanoma or Spitz-Reed nevus. The fibroepithelial variant of BCC is frequently mistaken for an acrochordon or a seborrheic keratosis, presenting as sessile plaque or pedunculated papule or nodule, which can be flesh-colored or erythematous. Infundibulocystic BCCs present as pearly papules that are well circumscribed, being frequently mistaken for benign follicular processes (1,5,29-31).
4. Histopathology
The histopathology of BCC is largely characterized by aggregates of basal cells with a small cytoplasm and large, hyperchromatic nuclei, apoptotic cells, all included in a fibromyxoid stroma, with tumor retraction spaces. Angiogenesis is an indicator of tumor development and its progression; the stroma surrounding BCC also reveals increased numbers of microvessels, being associated with local aggressive behavior (1,5,32).
The BCC histopathologic subtypes are classified according to the risk of tumor recurrence; BCCs with a low risk of recurrence are nodular, superficial, pigmented and infundibulocystic (BCC with adnexal differentiation) and fibroepithelial. However, those with a high risk of are recurrence are micronodular, infiltrating, sclerosing/morphoeic and basosquamous BCCs, and BCCs with sarcomatoid differentiation.
Nodular BCC presents microscopically as large nests or islands of malignant basaloid cells with central, haphazard cell arrangement and peripheral palisading, tumor-stroma clefting, mucoid/myxoid stroma with spindle cells, with/without amyloid deposits; sometimes the tumor stroma has a collagenous, keloidal-type aspect. The malignant tumor nests extend deep into the dermis and apoptotic cells can be found centrally. Nodular BCC has several subtypes, according to the secondary findings that characterize such tumors, keratotic (with mature keratin deposits found central in the tumor islands, Fig. 1), cystic/nodulocystic (cystic degeneration) and adenoid (with cribriform arrangement of tumor nests) (1,5,33).
Figure 1.
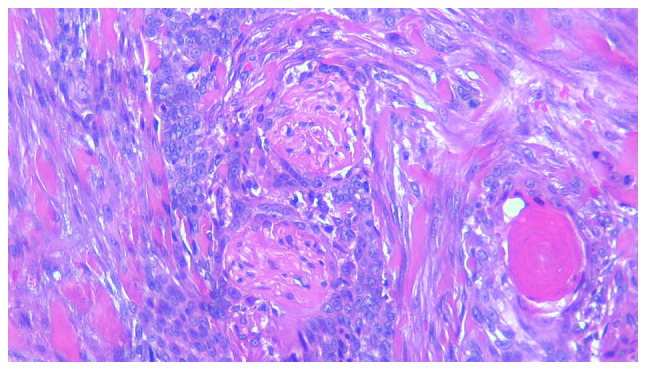
Keratotic basal cell carcinoma with perineural invasion. Hematoxylin and eosin staining of tissue was from an elderly Caucasian female, with a lesion on the face. Magnification, x200.
Superficial BCC (Fig. 2) develops as small islands or lobules of malignant basaloid cells with peripheral palisading, localized in the superficial dermis, with a connection to the epidermis and within a myxoid stroma, associated with a lichenoid, band-like, inflammatory infiltrate. It can appear as a multicentric tumor and may sometimes be part of a mixed-pattern tumor, with micronodular, nodular, or infiltrating components (1,5,34).
Figure 2.
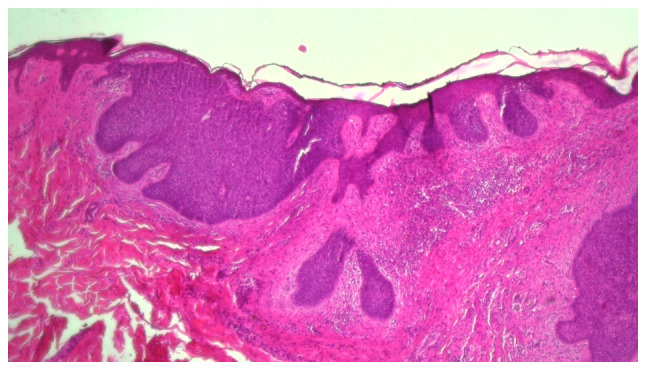
Mixed basal cell carcinoma with superficial multicentric and nodular components, associated with dense inflammatory infiltrate. Hematoxylin and eosin staining of tissue from elderly Caucasian male, with a lesion on the head and neck region. Magnification, x40.
Micronodular BCC is characterized by small islands or nests of malignant tumor cells which infiltrate deep into the dermis, sometimes even into the subcutaneous tissue; the tumor has a satellite-like arrangement of discrete nodules with irregular contours, lined by a thin margin of stroma and separated by normal dermal collagen (1,5,35).
Infiltrating BCC is a subtype composed mainly of chords or thin nests of tumor cells (with a thickness of >5-8 cells) which infiltrate deeply, with angulated edges and have an irregular, permeating invasion pattern at the tumor edge. It frequently overlaps with morphoeic/sclerosing BCC and can be found with a nodular component (1,5,35).
Sclerosing/morphoeic BCC is comprised of very thin strands/chords of tumor cells (with a thickness of 1-5 cells, and also with angulated ends) found in a collagenous type of stroma, with seldom tumor-stroma clefting. It infiltrates deeply and differs from the infiltrating subtype of BCC by the stromal characteristics, the latter lacking the highly collagenous stroma (1,5,34).
Basosquamous carcinoma (metatypical BCC, Fig. 3) is a subtype characterized by the presence of both BCC and SCC tumor features, with transition areas between the two. The tumor nests are comprised of basaloid cells, which are intermingled with atypical squamous cells with eosinophilic cytoplasm which are dispersed or have a focal distribution; the stroma is oftentimes highly cellular, with a fibrotic appearance (1,5,36).
Figure 3.
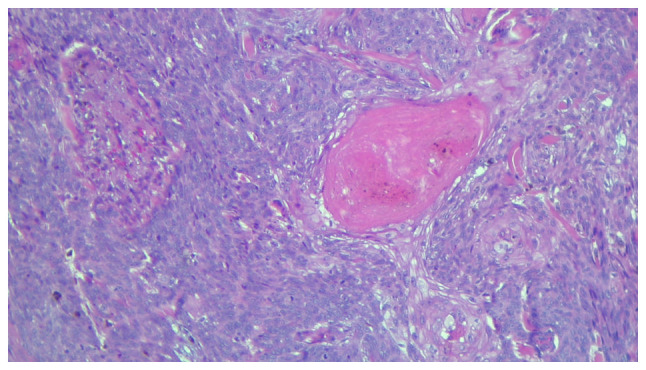
Partial view of a basosquamous carcinoma. Hematoxylin and eosin staining of elderly Caucasian male, with a lesion on the face. Magnification, x100.
Pigmented BCC is a variant of nodular or superficial BCC, which contains melanin pigment derived from an increased number of dendritic melanocytes within the malignant tumor nests, being found within the malignant basaloid cells or the macrophages which surround the malignant proliferation (1,5,37).
BCC with sarcomatoid differentiation (metaplastic carcinoma) is characterized by a malignant proliferation of basaloid cells found within a sarcomatous stroma with variable histology. The malignant mesenchymal stromal component can take the form of an osteosarcoma, chondrosarcoma, leiomyosarcoma, pleomorphic undifferentiated sarcoma, or rhabdomyosarcoma (1,5).
BCC with adnexal differentiation defines a subtype of BCC which is frequently found in the skin around the eyes, exhibiting differentiation towards follicular, eccrine, apocrine or sebaceous glands. Matrical differentiation in BCC is revealed by the presence of shadow cells; the infundibulocystic variant is characterized by the presence of small infundibular cyst-like spaces inside the tumor nodules; mature sebocytes can be found in BCC with sebaceous differentiation, while ductal structures, such as those found in eccrine and apocrine glands (and decapitation secretion in apocrine differentiation) are found in BCC with differentiation towards eccrine or apocrine sweat glands (1,5,38).
Fibroepithelial BCC (fibroepithelioma of Pinkus or Pinkus tumor) is a distinct variant composed of thin strands of anastomosing basaloid cells with reticular pattern of development, linked to the epidermis and within a fibroblastic stroma. Sometimes rare basaloid islands can be found (1,5,39).
5. Dermoscopy
Dermoscopy is a non-invasive skin examination procedure which allows the clinician to observe certain lesional characteristics which are not visible to the naked eye. The BCC examination and its positive diagnosis rely on three main features: Vascular structures (arborizing vessels and pigmented structures), nests with a blue-gray coloration and ovoid shape and the presence of ulcerations; these three features, along with features of melanocytic lesions (network areas) indicate the positive clinical diagnosis of BCC. This in vivo technique has a sensitivity of 89-91.2% and a specificity of 95% (34,40-42).
The dermoscopic features indicative of BCC are the following: Arborizing vessels (the most prevalent feature), a dotted or corkscrew appearance, or glomerular vascularization, short and fine telangiectasia; shiny and white structures which alternate with shiny white and red areas without a certain structure; nests with blue-gray coloration and ovoid shape, multiple blue and gray globules/dots (Table I). These features vary in prevalence and distribution among BCC subtypes, with certain other characteristics being found in those subtypes, such as concentric, spoke wheel structures or leaf-like areas in nodular BCC, while morpheaform BCC rarely presents with pigmentation.
Table I.
Dermoscopic features of basal cell carcinoma.
| Vascular structures | Pigmented structures | Other features |
|---|---|---|
| Arborizing vessels | Ovoid nests with blue-gray coloration | Ulceration |
| Short and fine telangiectasia | Shiny, white structures alternating with shiny white-red areas (no structure) | Erosion/erosions |
| Dotted vascularization | Multiple blue and gray globules/dots | |
| ‘Corkscrew’ vascularization | ||
| Glomerular vascularization | ||
| Concentric, spoke wheel structures | ||
| Leaf-like areas |
The overall dermoscopic aspect of BCC is represented by the mixture of the abovementioned features, with variation according to the patient's age, sex, race, BCC subtype, tumor location or the presence or absence of pigmentation (40-42).
6. Novel diagnostic approaches
In recent years, novel technologies have been developed for the diagnosis of BCC; some of these technologies are non-invasive, such as optical coherence tomography (OCT) and reflectance confocal microscopy (RCM), which are both in vivo diagnostic tools.
RCM uses a near-infrared laser in order to obtain images of thin sections of the skin; the light is reflected back from the selected focal point and enters the detector through a special pinhole. It is a highly sensitive (sensitivity varying between 100 and 91.7%) and specific (specificity varying between 91.3 and 88.5%) technique. This technique has the capacity for high resolution imaging, which is equivalent to 30-fold the magnification of an optical microscope; however, it can only penetrate to depths of 250 µm and does not have the capacity to evaluate tumor depth margins and invasion (12,43-48).
RCM criteria indicative of a BCC diagnosis can be found in the superficial dermis (or at the dermo-epidermal junction) and these include dark silhouettes (hyporeflective zones surrounded by bright collagen bundles, bright tumor islands which are often limited by a dark cleft, dendritic cells, plump, bright cells and canalicular vessels). As regards superficial BCC, features which are indicative of its diagnosis are epithelial chords connected to the epidermis; nodular BCC has an additional increased vascular density, while aggressive BCC subtypes are evidenced by hyporeflective areas (12,43-49).
OCT uses infrared light in order to obtain a real-time image of the examined skin; the technique is based on the sum of light refractions of different skin components, with various optical properties; it is capable of examining the skin at depths of 500 µm (although at a depth >400 µm the image increases in noise and decreases in resolution). Its sensitivity is 87% and its specificity is 80%. This technique has the capacity for deeper examination and is capable of en face and cross-sectional imaging; however, it has limited use for pigmented lesions (12,43-48,50).
When identifying BCC with the help of OCT, the following images have been found to be positively associated with the diagnosis: Oval structures (with/without bright colored centers), dark areas bordering the dermis (hyporeflective area as the lateral tumor border), black zones or cones protruding into the adjacent dermis and disruption of the epidermal layering. For the nodular subtype of BCC, the most characteristic image is that of oval structures, with the presence of dark or black zones or cysts. For the superficial subtype, the presence of a dark area bordering the dermis and the bulges or cones extending from the epidermis to the dermis are the most characteristic and positively associated images. The image compared to ‘a shoal of fish’ which translates as elongated and narrow structures found in the dermis, is indicative of the infiltrative subtype of BCC (12,43-48,50,51).
Both techniques can be combined for a more accurate diagnosis (resulting in line-field confocal OCT, combining the advantages of OCT, with vertical in-depth penetration and RCM with its high resolution) and can be accompanied by the classic dermoscopic examination, thus improving the accuracy of diagnosis (12,43-48).
Other techniques that can be used in the diagnosis of BCC are high-resolution ultrasonography, Raman spectroscopy or terahertz pulse imaging.
High resolution ultrasonography is a diagnostic tool using frequencies between 20-100 MHz, and it can measure the extent of the tumor, including its depth or thickness, while evaluating deep structure involvement. As regards the subtypes of BCC, the nodular one is described as dermal or hypodermal hypo-echoic nodules with various shapes (oval, irregular or ribbon-like shapes), with variably defined margins (well or ill-defined), some with or without an internal echo (which can be homogenous or non-homogenous) or with hyper-echoic spots. The superficial subtype has a less variable appearance, presenting as a hypo-echoic ribbon-like area, which is homogenous in appearance, without any internal echoes, posterior acoustic artifacts or hyper-echoic spots. The micronodular subtype of BCC is characterized by the presence of ill-defined, hypo-echoic tumor nodules located in the dermis, having internal echoes, some with hyper-echoic spots, with small, anechoic areas (cystic) or with posterior acoustic artifact. Infiltrative BCC has similar characteristics, also having ill-defined, hypo-echoic tumor nodules, but which were located both in the dermis and hypodermis, with a number of internal hyper-echoic spots and without a posterior reinforcement artifact. Basosquamous BCC is described as having dermal and hypodermal ill-defined, hypo-echoic nodules with multiple internal, hyper-echoic spots, some internal anechoic zones and without posterior acoustic artifact. When using this diagnostic tool, careful attention should be paid as tumor thickness is frequently overestimated, and the inflammatory infiltrate which is found at the base of the tumor nodules or islands may be confused as part of the tumor. However, this technique yields promising results in the pre-operative diagnosis of BCC, revealing important tumor traits, such as tumor depth, associated lesions or margins (52,53).
Raman spectroscopy is an optical technique which analyzes the vibrational molecule modes, and, apart from the other techniques used for BCC diagnosis, it offers a special focus on tumor vascularization.
Terahertz pulse imaging is a portable ex vivo or in vivo diagnostic tool which uses terahertz radiation, an electromagnetic spectrum found between the infrared and microwave regions, having the ability to evaluate the low-frequency type, vibrational and torsional motions in molecular systems and registering its absorption. Studies have registered spectroscopic differences between the normal tissue and that which is affected by a tumor process, meaning that that the absorption coefficient and refractive index are higher in areas affected by BCC, which are consistent with a higher water content, providing information regarding the extent of the tumor (54-56).
In vivo confocal laser scanning microscopy (CLSM) is also an innovative technique which is currently used in studying the morphological course of a disease by repeated, high magnification examination, revealing its dynamic treatment response. It is currently used in the study of inflammatory skin diseases; however, it is a promising diagnostic and monitoring tool for skin tumors, allowing the close inspection of the body's immune response to the tumor and the response to the applied treatment. However, further studies are required in order to evaluate BCC from this perspective (57).
7. Treatment
The surgical treatment of BCC is the main approach through which the entire tumor mass can be excised, and the cosmetic and functional aspects can be preserved, providing optimal results for the patient. The surgical excision needs to be made with at least 4-mm margins in low-risk BCC, whereas for high-risk ones, margins of at least 6 mm should be ensured.
Mohs micrographic surgery involves the complete excision of the BCC with a microscopic examination of the surgical margins. It is most frequently used in high-risk tumors, with higher rates for long-term cure. This approach has a high success rate, with dermatopathologists and Mohs surgeons agreeing on surgical excision margin clearance (12,58).
Curettage or electrodessication are older techniques, recommended in superficial BCC or low-risk tumors, having the downside of not allowing a histopathological examination of surgical margins. It is recommended that these two techniques should not be used in parts of the body with terminal hair growth, such as the scalp, beard area, axillae, or pubis, due to the risk of tumor extension in the hair follicle (12,59).
Cryosurgery uses freeze-thaw cycles in order to destroy the malignant tumor cells, and, as with curettage and electrodessication, does not allow the microscopic examination of tumor margins and is mostly recommended in low-risk cases, such as superficial BCC (12).
Photodynamic therapy is a two-step therapeutic approach; it involves the initial local application of a photosensitizing chemical substance (methyl aminolevulinate or aminolevulinic acid), followed by irradiation with the help of a light source. It determines oxidative damage in the tumor mass with the apoptosis and necrosis of tumor cells, along with vascular damage, and without having major effects on the surrounding normal tissue; it may be recommended in cases of periocular BCC, having a good function preservation and cosmetic outcome. However, this technique has high recurrence rates of up to 30.7% within 5 years, and is mainly recommended for superficial BCC (12,60).
Radiotherapy is a less frequent treatment approach, being used for unresectable tumors or in cases where surgery is not recommended; brachytherapy and external beam radiotherapy/teletherapy have been used in the treatment of BCC, having lower recurrence rates as compared with cryosurgery (12).
Topical treatments, such as 5-fluorouracil or imiquimod 5% applications, are recommended in superficial BCC subtypes and have registered histologic clearances ranging from 76-100%, in 6 to 12 weeks of treatment. The study by Williams et al (61) reported the higher effectiveness of surgical treatment over imiquimod 5% topical applications, with recurrences being observed as early as 1 year post-treatment concerning the local treatment. The 5-year success rates were higher for the cases where surgical treatment was applied (97.7%), as compared with those treated with imiquimod 5% (82.5%). However, this type of treatment may be associated with side-effects, such as erythema, erosions or swelling, affecting patient compliance and treatment effectiveness (12,61).
Intralesional chemotherapy with 5-fluorouracil, bleomycin, IL-2 or interferons have registered variable results and can have local side-effects or even general ones, such as flu-like manifestations.
Laser therapy, such as superpulsed carbon dioxide laser therapy or pulsed neodymium-based laser therapy, can be used alone or in combination with other therapeutic approaches. It has proven to have small recurrence rates of up to 1.8% in 5 years, although it presents with local adverse effects, such as soreness, edema, hyperemia and scarring.
Hedgehog pathway inhibitors, such as Vismodegib, are used in surgically advanced tumors as neoadjuvant therapy, prior to Mohs micrographic surgery or radiotherapy, allowing the tumor to decrease in size. As is the case with other treatment agents, its long-term use is associated with adverse effects and in order to combat such effects, treatment interruptions of up to 8 weeks have been practiced (12,62,63).
8. Conclusions
The diagnosis and characterization of BCC needs to be based on the clinical, imaging and histopathology features of the tumor mass, combined with the patient's characteristics, in order to select the most effective treatment option and yield optimal results with the highest disease-free intervals and lowest recurrence rates. BCC treatment aims for a complete resolution, with minimal side-effects and high patient satisfaction, aiming for optimal cosmetic results, particularly in key areas such as the face.
Acknowledgements
The authors wish to acknowledge that the present study was supported by the 'Dunarea de Jos' University of Galati, Romania, through the research center - Multidisciplinary Integrated Center of Dermatological Interface Research MIC-DIR (Centrul Integrat Multidisciplinar de Cercetare de Interfata Dermatologica - CIM-CID).
Funding Statement
Funding: The present study was supported by the 'Dunarea de Jos' University of Galati through an internal grant (grant no. RF3668/01.10.2021).
Availability of data and materials
Not applicable.
Authors' contributions
EN, MC, CB, FN, DJS, ML and ALT were major contributors to the writing of the manuscript and the literature search. EN, MC, CB and FN were involved in all the stages of the study (conception, design, revision, data collection, critical analysis and language editing). DJS, ML and ALT contributed to the conception and design of the study, as well as in the study revision. EN, ALT, DJS and FN assisted in the collection of the data for the review article. LR, GL, VC and LA revised the study for important intellectual content. All authors have read and approved the final version of the manuscript to be published. EN and DJS confirm the authenticity of all the raw data. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work. All authors have had equal participation, contribution and equal rights to this article.
Ethics approval and consent to participate
The present study was approved by the ‘Sf. Apostol Andrei’ Emergency Clinical Hospital Ethics Committee (decision no. 19758). The patients provided written informed consent for the publication of any associated data and accompanying image.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cameron MC, Lee E, Hibler BP, Barker CA, Mori S, Cordova M, Nehal KS, Rossi AM. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303–317. doi: 10.1016/j.jaad.2018.03.060. [DOI] [PubMed] [Google Scholar]
- 2.Matsui Y, Makino T, Takemoto K, Kagoyama K, Shimizu T. Co-existence of basal cell carcinoma and squamous cell carcinoma in a single burn scar region. Burns Open. 2020;4:64–66. [Google Scholar]
- 3.Lai K, Chan E, Ko SC. Combination of squamous cell carcinoma and basal cell carcinoma arising from a giant verruca vulgaris involving the eyelid. Am J Ophthalmol Case Rep. 2020;21(100858) doi: 10.1016/j.ajoc.2020.100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rebegea LF, Firescu D, Dumitru M, Patrascu A. Skin spiradenocarcinoma-case presentation. Rom J Morphol Embryol. 2016;57:327–330. [PubMed] [Google Scholar]
- 5.Messina J, Epstein EH Jr, Kossard S, McKenzie C, Patel RM, Patterson JW, Scolyer RA. Chapter 1-Keratinocytic/epidermal tumors. Basal cell carcinoma. In: WHO Classification of skin tumors. Elder DE, Massi D, Scolyer RA and Willemze R (eds). 4th edition. International Agency for Research on Cancer, Lyon, pp26-24, 2017. [Google Scholar]
- 6.Rebegea L, Firescu D, Baciu G, Ciubara A. Psycho-oncology support. Brain. 2019;10:77–88. [Google Scholar]
- 7.Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519–526. doi: 10.1016/j.det.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Poignet B, Gardrat S, Dendale R, Lemaitre S, Lumbroso-Le Rouic L, Desjardins L, Cassoux N, Levy Gabriel C. Basal cell carcinomas of the eyelid: Results of an initial surgical management. J Fr Ophtalmol. 2019;42:1094–1099. doi: 10.1016/j.jfo.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13–24. doi: 10.1016/j.hoc.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Ashraf DC, Kalin-Hajdu E, Levin MH, Kersten RC. Mixed cranial neuropathies due to occult perineural invasion of basal cell carcinoma. Am J Ophthalmol Case Rep. 2018;13:136–139. doi: 10.1016/j.ajoc.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muzumdar S, Stewart CL, Feng H. Rare case of a basal cell carcinoma with intravascular invasion. Int J Womens Dermatol. 2020;6:334–335. doi: 10.1016/j.ijwd.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fania L, Didona D, Morese R, Campana I, Coco V, Di Pietro FR, Ricci F, Pallotta S, Candi E, Abeni D, Dellambra E. Basal cell carcinoma: From pathophysiology to novel therapeutic approaches. Biomedicines. 2020;8(449) doi: 10.3390/biomedicines8110449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicoara M, Bain K, Patel R, Jaikaran O, Hingorani A, Asher E. Malignant transformation of nonhealing ulcer-basal cell carcinoma. Ann Vasc Surg. 2021;70:565.e7–565.e10. doi: 10.1016/j.avsg.2020.01.100. [DOI] [PubMed] [Google Scholar]
- 14.Coulombe C, Gagnon LP, Larouche V, Dionne MC. Infantile-onset palmo-plantar basal cell carcinomas and pits in Gorlin syndrome. JAAD Case Rep. 2018;4:662–664. doi: 10.1016/j.jdcr.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nwabudike LC, Tatu AL. Response to-Chronic exposure to tetracyclines and subsequent diagnosis for non-melanoma skin cancer in a large Mid-Western US population. J Eur Acad Dermatol Venereol. 2018;32(e159) doi: 10.1111/jdv.14657. [DOI] [PubMed] [Google Scholar]
- 16.Tatu AL, Ciobotaru OR, Miulescu M, Buzia OD, Elisei AM, Mardare N, Diaconu C, Robu S, Nwabudike LC. Hydrochlorothiazide: Chemical structure, therapeutic, phototoxic and carcinogenetic effects in dermatology. Rev Chim (Bucharest) 2018;69:2110–2114. [Google Scholar]
- 17.Nwabudike LC, Elisei AM, Buzia OD, Miulescu M, Tatu AL. Statins. A review on structural perspectives, adverse reactions and relations with non-melanoma skin cancer Rev Chim. (Bucharest) 2018;69:2557–2562. [Google Scholar]
- 18.Draganescu M, Baroiu L, Iancu A, Dumitru C, Radaschin D, Polea ED, Bobeica C, Tatu AL, Niculet E, Fekete GL. Perspectives on skin disorder diagnosis among people living with HIV in southeastern Romania. Exp Ther Med. 2021;21(97) doi: 10.3892/etm.2020.9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niculet E, Chioncel V, Elisei AM, Miulescu M, Buzia OD, Nwabudike LC, Craescu M, Draganescu M, Bujoreanu F, Marinescu E, et al. Multifactorial expression of IL-6 with update on COVID-19 and the therapeutic strategies of its blockade (Review) Exp Ther Med. 2021;21(263) doi: 10.3892/etm.2021.9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jee SH, Shen SC, Chiu HC, Tsai WL, Kuo ML. Overexpression of interleukin-6 in human basal cell carcinoma cell lines increases anti-apoptotic activity and tumorigenic potency. Oncogene. 2001;20:198–208. doi: 10.1038/sj.onc.1204076. [DOI] [PubMed] [Google Scholar]
- 21.Lupu M, Caruntu A, Caruntu C, Papagheorghe LML, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki M, et al. Neuroendocrine factors: The missing link in non melanoma skin cancer (Review) Oncol Rep. 2017;38:1327–1340. doi: 10.3892/or.2017.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEnery-Stonelake ME, Clark MA, Vidimos AT. Vulvar basal cell carcinoma arising in the setting of repeated perilamp exposure. JAAD Case Rep. 2020;6:103–105. doi: 10.1016/j.jdcr.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varadarajan VV, Nasri E, Dziegielewski PT. Basal cell carcinoma of the oral cavity: A case report. Otolaryngol Case Rep. 2020;15(100159) [Google Scholar]
- 24.Tatu AL, Nwabudike LC. The treatment options of male genital lichen sclerosus et atrophicus short title for a running head: Treatments of genital lichen sclerosus. In: Proceedings of the 14th National Congress of Urogynecology and the National Conference of the Romanian Association for the Study of Pain, pp262-264, 2017. [Google Scholar]
- 25.Rustemeyer J, Günther L, Deichert L. A rare association: Basal cell carcinoma in a vitiliginous macula. Oral Maxillofac Surg. 2011;15:175–177. doi: 10.1007/s10006-010-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mihăilă B, Dinică RM, Tatu AL, Buzia OD. New insights in vitiligo treatments using bioactive compounds from Piper nigrum. Exp Ther Med. 2019;17:1039–1044. doi: 10.3892/etm.2018.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiszon-Cerqueira L, Ramos-E-Silva M, Guerreiro FB, Cistaro-Serrano M, Carneiro AHC, Gomes MK. Giant basal cell carcinoma associated with vitiligo. Clin Case Rep. 2019;7:1782–1786. doi: 10.1002/ccr3.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrne KT, Turk MJ. New perspectives on the role of vitiligo in immune responses to melanoma. Oncotarget. 2011;2:684–694. doi: 10.18632/oncotarget.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nwabudike LC, Tebeica T, Tatu AL. Nodular, ulcerated seborrheic keratosis. Clin Exp Dermatol. 2020;45:602–604. doi: 10.1111/ced.14172. [DOI] [PubMed] [Google Scholar]
- 30.Tatu AL. Umbilicated blue black lesion on the lateral thorax. J Cutan Med Surg. 2017;21(252) doi: 10.1177/1203475417694859. [DOI] [PubMed] [Google Scholar]
- 31.Earar K, Sirbu I, Onisor C, Luca E. Oral rehabilitation on implants and introduction of pathogenic mechanisms in relation to oral implants-sugar diabetes. Rev Chim (Bucharest) 2019;70:3750–3752. [Google Scholar]
- 32.Lupu M, Caruntu C, Popa MI, Voiculescu VM, Zurac S, Boda D. Vascular patterns in basal cell carcinoma: Dermoscopic, confocal and histopathological perspectives. Oncol Lett. 2019;17:4112–4125. doi: 10.3892/ol.2019.10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Căruntu C, Boda D, Guţu DE, Căruntu A. In vivo reflectance confocal microscopy of basal cell carcinoma with cystic degeneration. Rom J Morphol Embryol. 2014;55:1437–1441. [PubMed] [Google Scholar]
- 34.Mackiewicz-Wysocka M, Bowszyc-Dmochowska M, Strzelecka-Węklar D, Dańczak-Pazdrowska A, Adamski Z. Basal cell carcinoma-diagnosis. Contemp Oncol (Pozn) 2013;17:337–342. doi: 10.5114/wo.2013.35684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dourmishev LA, Rusinova D, Botev I. Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol Online J. 2013;4:12–17. doi: 10.4103/2229-5178.105456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lima NL, Verli FD, de Miranda JL, Marinho SA. Basosquamous carcinoma: Histopathological features. Indian J Dermatol. 2012;57:382–383. doi: 10.4103/0019-5154.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abudu B, Cohen PR. Pigmented basal cell carcinoma masquerading as a melanoma. Cureus. 2019;11(e4369) doi: 10.7759/cureus.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shash HA, Almarzouq SF, Alghamdi AA, Alratroot JA. Basal cell carcinoma with sebaceous differentiation: A case report and review of literature. Plast Reconstr Surg Glob Open. 2020;8(e3234) doi: 10.1097/GOX.0000000000003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haddock ES, Cohen PR. Fibroepithelioma of pinkus revisited. Dermatol Ther (Heidelb) 2016;6:347–362. doi: 10.1007/s13555-016-0123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiter O, Mimouni I, Gdalevich M, Marghoob AA, Levi A, Hodak E, Leshem YA. The diagnostic accuracy of dermoscopy for basal cell carcinoma: A systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1380–1388. doi: 10.1016/j.jaad.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 41.Reiter O, Mimouni I, Dusza S, Halpern AC, Leshem YA, Marghoob AA. Dermoscopic features of basal cell carcinoma and its subtypes: A systematic review. J Am Acad Dermatol. 2019;85:653–664. doi: 10.1016/j.jaad.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Álvarez-Salafranca M, Ara M, Zaballos P. Dermoscopy in basal cell carcinoma: An updated review. Actas Dermosifiliogr. 2021;112:330–338. doi: 10.1016/j.ad.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Cameron MC, Lee E, Hibler BP, Giordano CN, Barker CA, Mori S, Cordova M, Nehal KS, Rossi AM. Basal cell carcinoma: Contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321–339. doi: 10.1016/j.jaad.2018.02.083. [DOI] [PubMed] [Google Scholar]
- 44.Suppa M, Fontaine M, Dejonckheere G, Cinotti E, Yélamos O, Diet G, Tognetti L, Miyamoto M, Orte Cano C, Perez-Anker J, et al. Line-field confocal optical coherence tomography of basal cell carcinoma: A descriptive study. J Eur Acad Dermatol Venereol. 2021;35:1099–1110. doi: 10.1111/jdv.17078. [DOI] [PubMed] [Google Scholar]
- 45.Holmes J, von Braunmühl T, Berking C, Sattler E, Ulrich M, Reinhold U, Kurzen H, Dirschka T, Kellner C, Schuh S, Welzel J. Optical coherence tomography of basal cell carcinoma: Influence of location, subtype, observer variability and image quality on diagnostic performance. Br J Dermatol. 2018;178:1102–1110. doi: 10.1111/bjd.16154. [DOI] [PubMed] [Google Scholar]
- 46.Hussain AA, Themstrup L, Jemec GB. Optical coherence tomography in the diagnosis of basal cell carcinoma. Arch Dermatol Res. 2015;307:1–10. doi: 10.1007/s00403-014-1498-y. [DOI] [PubMed] [Google Scholar]
- 47.Lupu M, Popa IM, Voiculescu VM, Boda D, Caruntu C, Zurac S, Giurcaneanu C. A retrospective study of the diagnostic accuracy of in vivo reflectance confocal microscopy for basal cell carcinoma diagnosis and subtyping. J Clin Med. 2019;8(449) doi: 10.3390/jcm8040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghita MA, Caruntu C, Rosca AE, Kaleshi H, Caruntu A, Moraru L, Docea AO, Zurac S, Boda D, Neagu M, et al. Reflectance confocal microscopy and dermoscopy for in vivo, non-invasive skin imaging of superficial basal cell carcinoma. Oncol Lett. 2016;11:3019–3024. doi: 10.3892/ol.2016.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sahu A, Yélamos O, Iftimia N, Cordova M, Alessi-Fox C, Gill M, Maguluri G, Dusza SW, Navarrete-Dechent C, González S, et al. Evaluation of a combined reflectance confocal microscopy-optical coherence tomography device for detection and depth assessment of basal cell carcinoma. JAMA Dermatol. 2018;154:1175–1183. doi: 10.1001/jamadermatol.2018.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reddy N, Nguyen BT. The utility of optical coherence tomography for diagnosis of basal cell carcinoma: A quantitative review. Br J Dermatol. 2019;180:475–483. doi: 10.1111/bjd.17201. [DOI] [PubMed] [Google Scholar]
- 51.Navarrete-Dechent C, Rajadhyaksha M, Nehal KS. Can optical coherence tomography improve the management of basal cell carcinoma? Br J Dermatol. 2019;180:448–449. doi: 10.1111/bjd.17522. [DOI] [PubMed] [Google Scholar]
- 52.Wang SQ, Liu J, Zhu QL, Zhao CY, Qu T, Li F, Wortsman X, Jin HZ. High-frequency ultrasound features of basal cell carcinoma and its association with histological recurrence risk. Chin Med J (Engl) 2019;132:2021–2026. doi: 10.1097/CM9.0000000000000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bobadilla F, Wortsman X, Muñoz C, Segovia L, Espinoza M, Jemec GB. Pre-surgical high resolution ultrasound of facial basal cell carcinoma: Correlation with histology. Cancer Imaging. 2008;8:163–172. doi: 10.1102/1470-7330.2008.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallace VP, Fitzgerald AJ, Pickwell E, Pye RJ, Taday PF, Flanagan N, Ha T. Terahertz pulsed spectroscopy of human Basal cell carcinoma. Appl Spectrosc. 2006;60:1127–1133. doi: 10.1366/000370206778664635. [DOI] [PubMed] [Google Scholar]
- 55.Woodward RM, Cole B, Wallace VP, Arnone DD, Pye R, Linfield EH, Pepper M, Davies AG. Terahertz pulse imaging of in-vitro basal cell carcinoma samples. Technical Digest. Summaries of papers presented at the Conference on Lasers and Electro-Optics. Postconference Technical Digest (IEEE Cat. No. 01CH37170), 329-330, 2001. [Google Scholar]
- 56.Zhang J, Fan Y, Song Y, Xu J. Accuracy of Raman spectroscopy for differentiating skin cancer from normal tissue. Medicine (Baltimore) 2018;97(e12022) doi: 10.1097/MD.0000000000012022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ilie MA, Caruntu C, Lixandru D, Tampa M, Georgescu SR, Constantin MM, Constantin C, Neagu M, Zurac SA, Boda D. In vivo confocal laser scanning microscopy imaging of skin inflammation: Clinical applications and research directions (Review) Exp Ther Med. 2019;17:1004–1011. doi: 10.3892/etm.2018.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cerci FB, Kubo EM, Werner B. Comparison of basal cell carcinoma subtypes observed in preoperative biopsy and Mohs micrographic surgery. An Bras Dermatol. 2020;95:594–601. doi: 10.1016/j.abd.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kopf AW, Bart RS, Schrager D, Lazar M, Popkin GL. Curettage-electrodesiccation treatment of basal cell carcinomas. Arch Dermatol. 1977;113:439–443. [PubMed] [Google Scholar]
- 60.Li X, Tan L, Kou H, Zhang J, Wang Y, Li G, Lu Y. Ocular preservation through limited tumor excision combined with ALA-PDT in patients with periocular basal cell carcinoma. Photodiagnosis Photodyn Ther. 2019;27:291–294. doi: 10.1016/j.pdpdt.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 61.Williams HC, Bath-Hextall F, Ozolins M, Armstrong SJ, Colver GB, Perkins W, Miller PSJ. Surgery versus imiquimod for nodular and superficial basal cell carcinoma (SINS) study group. surgery versus 5% imiquimod for nodular and superficial basal cell carcinoma: 5-year results of the SINS randomized controlled trial. J Invest Dermatol. 2017;137:614–619. doi: 10.1016/j.jid.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 62.Sabu DM, Kroes J, Gilham C, Fleming A, Kelleher FC. doi: 10.1016/j.currproblcancer.2021.100736. Neo-adjuvant Vismodegib followed by radiation in locally advanced basal cell carcinoma. Curr Probl Cancer: Apr 1, 2021 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 63.Chanu P, Musib L, Wang X, Cheeti S, Girish S, Bruno R, Lu T, Reddy J, Jin JY, Caro I. Vismodegib efficacy in advanced basal cell carcinoma maintained with 8-week dose interruptions: A model-based evaluation. J Invest Dermatol. 2021;141:930–933. doi: 10.1016/j.jid.2020.07.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


