Abstract
Purpose
Breast cancer (BRCA) is the second most common malignancy in the world and the most common in women. Here, we utilized publicly available BRCA dataset to investigate potential prognosis-related genes through integrated bioinformatics analysis.
Materials and Methods
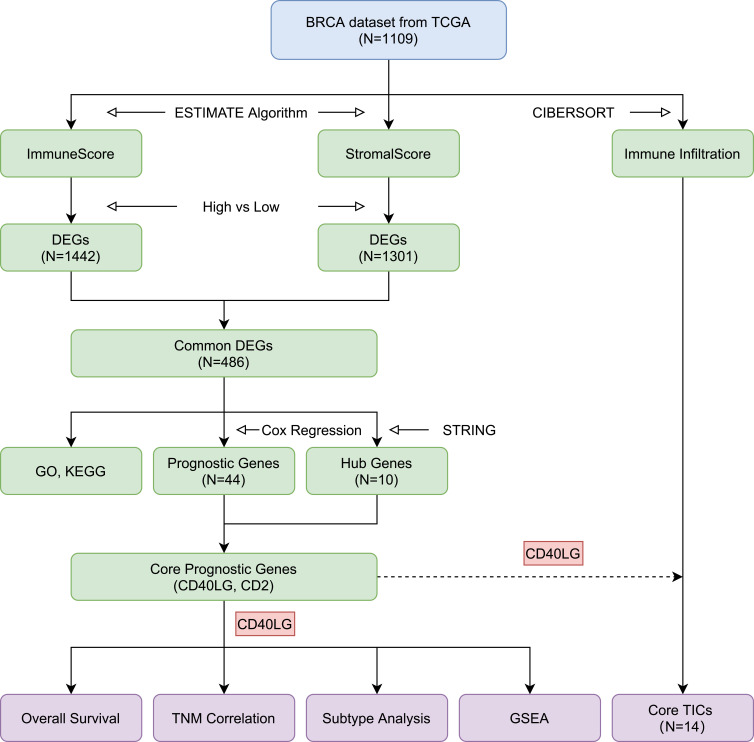
BRCA dataset was obtained from the Cancer Genome Atlas (TCGA) database. The ESTIMATE algorithm was used to calculate the ImmuneScores and StromalScores of the samples and then divided them into high- and low-score groups based on the median score. Common differentially expressed genes (DEGs) were identified through differential expression analysis. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed. The core prognostic genes were the intersection of hub genes from PPI network and prognostic genes from univariate Cox proportional hazard regression analysis. Finally, the CIBERSORT algorithm was used to calculate proportions of 22 tumor-infiltrating immune cells (TICs) in BRCA samples.
Results
A total of 486 DEGs were identified. These genes were mainly enriched in immune-related pathways. Crossover genes between the hub genes and the prognostic genes were CD2 and CD40LG. CD40LG was further investigated in this study. CD40LG was downregulated in BRCA samples compared with normal samples, and a lower CD40LG expression was associated with advanced tumor stages and a poor prognosis. CD40LG was shown to be involved in immune-related pathways of BRCA by Gene Set Enrichment Analysis. Finally, 14 TICs were found to have a close relationship with CD40LG.
Conclusion
CD40LG was found to be a core prognostic gene related to tumor microenvironment and deeply involved in immune-related pathways in BRCA. Our findings may provide new insights for exploring the molecular mechanisms of BRCA and developing new immunotherapies for the disease.
Keywords: bioinformatics, TCGA, DEGs, TICs, ESTIMATE, CIBERSORT
Graphical Abstract
Introduction
Breast cancer (BRCA) is the second most common cancer in the world after lung cancer and the most common in women. It is projected that the incidence of BRCA will increase to 85 cases per 100,000 women by 2022.1 Owing to substantial heterogeneity, treatment and prognosis of breast cancer vary, even if clinicopathological characteristics are the same. Therefore, it is important to find new prognostic molecular markers to explore individualized treatment for patients.
Tumor microenvironment (TME) is the internal environment where tumor cells are produced and live. It is a complex network composed of multiple cell types, extracellular matrix and signaling molecules.2 An abundance of studies have suggested that the tumor microenvironment plays a significant role during breast cancer development, progression, metastasis and in determining the therapeutic response. However, the TME is also on a dynamic change with tumor progression and treatment. In primary breast cancer, surrounding stroma with immune cells, extracellular matrix, cancer-associated fibroblasts, blood vessels support the develop of tumor. Then, primary tumor cells entering circulation are facilitated by interactions with platelets and may be supported by neutrophils and macrophages, resulting in distal metastasis.3,4 However, the interactions between tumor cells and TME can be complex with various cell types and molecules involved. Therefore, more knowledge is needed to better understand the exact mechanisms in order to guide precise treatment for this heterogeneous disease.
The Estimation of Stromal and Immune Cells in Malignant Tumor Tissues Using Expression Data (ESTIMATE) algorithm is introduced to estimate stromal and immune components in malignant tumor tissues. It utilizes gene expression data to output the estimated levels of infiltrating stromal and immune cells and estimated tumor purity and therefore represent the status of TME.5 The Cell Type Identification by Estimating Relative Subsets of Known RNA Transcripts (CIBERSORT) algorithm is another bioinformatic tools that is able to calculate the proportions of 22 types of immune cells based on transcriptome data.6 Recently, studies have applied ESTIMATE and CIBERSORT algorithm to describe immune infiltration landscapes of gastric cancer,7 colon cancer,8 hepatocellular carcinoma9 and lung cancer.10 Also, several studies have utilized the two algorithms to identify immune-related pathogenesis of BRCA. In the Liu et al investigation, ATP2C2 was found to have a pivotal role in the maintenance of immune-dominant status for TME.11 In addition, Chen et al and Wang et al respectively revealed that CD2 and CD52 were two immune-related prognostic factor in BRCA via regulating TME.12,13 With similar approach, we identified another prognostic gene that was deeply involved in the immune and TME regulation of BRCA through an integrated bioinformatic analysis.
Materials and Methods
Breast Cancer Data Source
The BRCA dataset, comprising RNA-sequence data and corresponding clinical information of 1109 tumor samples and 113 normal samples, were obtained from the TCGA database (https://portal.gdc.cancer.gov/). There is no need for approval from the Ethics committee or written informed consent from patients since this study is based on a publicly available database.
Estimation of Immune and Stromal Component and Its Associations with Clinical Features
The ESTIMATE algorithm was used to calculated the ImmuneScore, the StromalScore and the ESTIMATEScore of each of the BRCA samples through the “estimate” R package. And based on these scores, the samples were divided into high or low ImmuneScore/StromalScore/EstimateScore groups based on the median scores. Survival comparisons between high and low ImmuneScore/StromalScore/ESTIMATEScore groups were visualized by Kaplan–Meier survival curves and examined by Log rank test using the “survival” and “survminer” R-packages. Kruskal–Wallis rank sum test was utilized to analyze the correlations between the high or low ImmuneScore/StromalScore/EstimateScore groups and BRCA clinicopathological characteristics through the “ggpubr” R package.
Identification of Differentially Expressed Genes
Differentially expressed genes (DEGs) in high and low ImmuneScore/StromalScore groups were screened and identified with “limma” R package. The thresholds for DEGs were: |log2-fold change| > 1 and false discovery rate (fdr) < 0.05. Heatmaps of DEGs were drawn using the “pheatmap” R package. The common upregulated and downregulated DEGs were seen as TME-related genes.
Enrichment Analysis and the Protein–Protein Interaction Network Construction of the Intersected DEGs
The “clusterProfiler” R package was used to identify biological processes (BP), cell components (CC), and molecular functions (MF) of the common DEGs with Gene Ontology (GO) functional enrichment, and pathway enrichment with Kyoto Encyclopedia of Genes and Genomes (KEGG). In addition, the protein–protein interaction (PPI) network was constructed using the Search Tool for the Retrieval of Interacting Genes (STRING, https://string-db.org/) database to further explore the relationships between DEGs at the protein level. Cytoscape software was used to visualized that network. Ten genes with the maximum numbers of adjacent nodes in the PPI network were selected as hub genes.
Identification of Core Prognostic Genes
Based on the gene data and clinical information of the BRCA dataset, genes related to overall survival were identified through univariate Cox proportional hazard regression analysis (P value < 0.05). Intersecting genes of the hub genes from PPI network and the prognostic genes related to overall survival were considered as core prognostic genes. Then, the expression levels of the core prognostic genes we compared in tumor samples and normal samples. Furthermore, the correlations between the core prognosis genes and overall survival, and tumor clinicopathological characteristics were analyzed.
Core Prognostic Genes and Molecular Subtypes
The molecular subtype information of TCGA BRCA dataset was obtained from UCSC Xena (https://xenabrowser.net/). Molecular subtype information was available for 956 samples including luminal A (n = 434), luminal B (n = 194), HER2-enriched (n = 67), basal-like (n = 142) and normal-like (n = 119). Kruskal–Wallis test were used to compare the expressions of CD40LG among different molecular subtypes. Then, after removing cases with a 0-day survival, Kaplan–Meier survival curves with Log rank test were performed for each subtype stratified by CD40LG expression.
Gene Set Enrichment Analysis (GSEA) of Core Prognostic Genes
The GSEA software (https://www.gsea-msigdb.org/gsea/index.jsp) was used to explore the enriched KEGG pathways associated with high or low expression of the core prognostic genes in BRCA. The pathways with a NOM p value < 0.05 and FDR q value < 0.25 were selected.
Analysis of TME Immune Infiltration Through CIBERSORT Algorithm
The CIBERSORT algorithm was utilized to evaluate the relative proportions of 22 TICs in each of the tumor samples and the bar charts of each sample were drawn by the “barplot” R package. Meanwhile, the correlations between these TICs were evaluated through the “corrplot” R package.
Correlation Analysis of Core Prognostic Genes with TICs
First, Pearson’s correlation analysis was used to identify the correlation between the expression of core prognostic genes and each TIC. Second, the proportions of each TIC in high and low gene groups were compared using Wilcoxon rank sum test. Finally, a Venn diagram was drawn to obtain the intersection of TICs from the above two steps. The R packages used for calculation and visualization were “ggplot2”, “ggpubr”, “vioplot” and “VennDiagram”.
Statistical Analysis
All the statistical analyses were performed with R software (version 4.0.3). A P value less than 0.05 was considered statistically significant unless otherwise specified.
Results
High ImmuneScores Was Associated with Better Survival
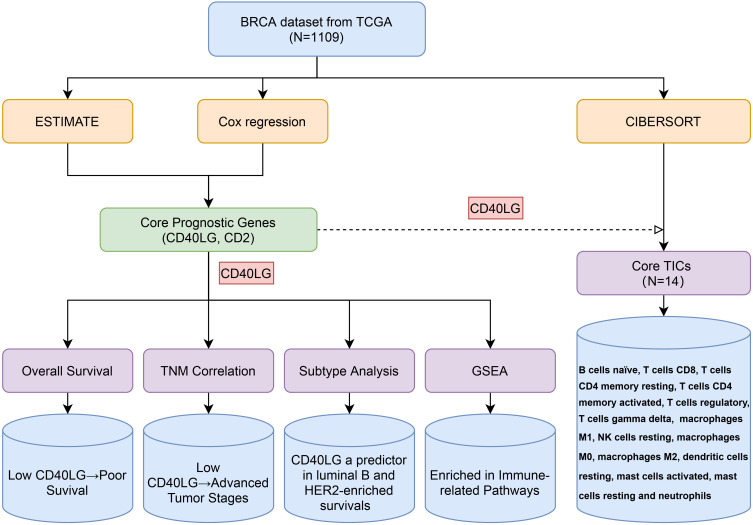
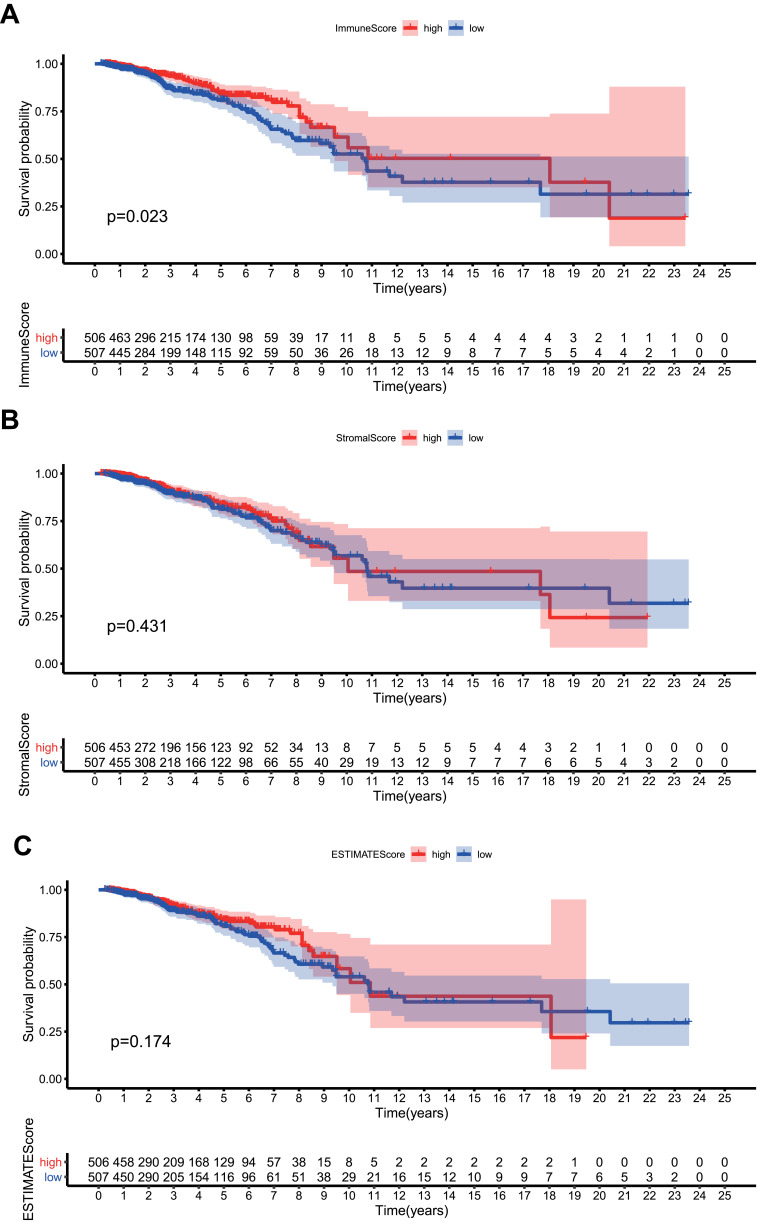
The overall design of the study was shown in Figure 1. The ImmuneScores, StromalScores, and ESTIMATEScores of all samples were calculated by the ESTIMATE algorithm. Samples with a score higher than the median score were divided into the high score group, and the rest samples were divided into the low score group. Survival analysis suggested that higher ImmuneScores were associated with better overall survival (Figure 2A, P < 0.023). However, no such association was found for StromalScores (Figure 2B, P = 0.431) or ESTIMATEScores (Figure 2C, P = 0.174). Though lack of statistical confidence, ImmuneScore seemed to be negatively correlated with tumor T stage (Figure S1). From these data, it can be inferred that the immune component of the TME tend to impact survival probability of BRCA.
Figure 1.
Flow diagram of the study design and main results.
Abbreviations: BRCA, breast cancer; TCGA, the Cancer Genome Atlas; ESTIMATE, Estimation of Stromal and Immune Cells in Malignant Tumor Tissues Using Expression Data; CIBERSORT, Cell Type Identification by Estimating Relative Subsets of Known RNA Transcripts; DEG, differentially expressed gene; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; GSEA, Gene Set Enrichment Analysis; TIC, tumor-infiltrating cell.
Figure 2.
ImmunScore/StromalScore/ESTIMATEScore and overall survival. (A) ImmuneScore and overall survival. (B) StromalScore and overall survival. (C) ESTIMATEScore and overall survival.
DEGs Based on the Analysis of ImmuneScore and StromalScore
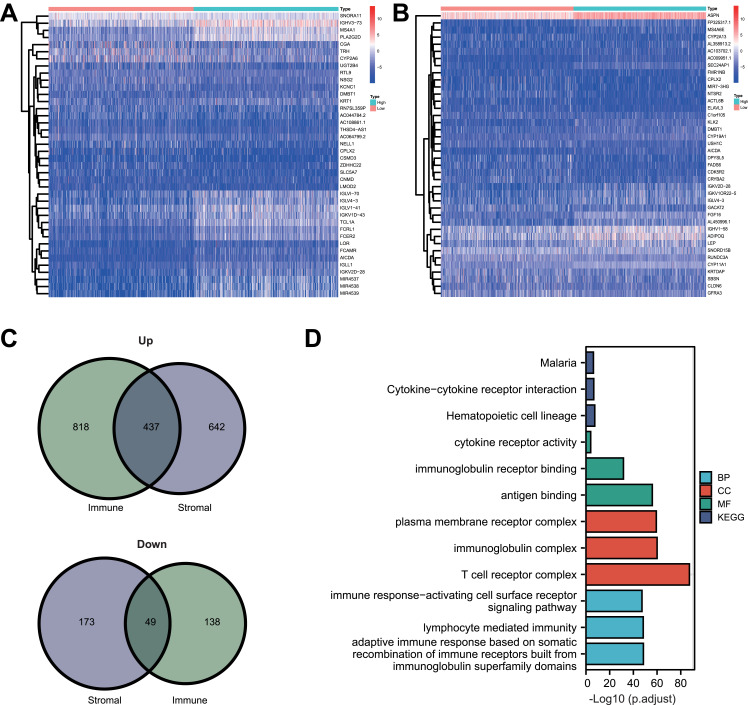
With the “limma” R package, we found 1255 upregulated genes and 187 downregulated genes were differentially expressed between the high and low ImmuneScore groups. Additional 1079 up-regulated and 222 down-regulated DEGs were found between the high and low StromalScore groups. The top 20 upregulated and downregulated DEGs for the ImmuneScore groups and the StromalScore groups were visualized as heatmaps (Figure 3A and Figure 3B). The numbers commonly upregulated and downregulated DEGs between the ImmuneScore groups and the StromalScore groups were 437 and 49, respectively (Figure 3C). These 486 intersecting DEGs combined were therefore seen as crucial genes in the TME of BRCA.
Figure 3.
DEGs, GO and KEGG analysis. (A) Heatmap of top 20 upregulated and downregulated DEGs in high and low ImmuneScore groups. (B) Heatmap of top 20 upregulated and downregulated DEGs in high and low StromalScore groups. (C) Venn diagrams of commonly upregulated and downregulated genes. (D) Biological process, cellular component, molecular function and KEGG pathway enrichment analysis.
Abbreviations: BP, biological process; CC, cellular component; MF, molecular function; KEGG, Kyoto Encyclopedia of Genes and Genomes.
DEGs Were Enriched in Immune-Related GO Annotations
Through the GO analysis, we obtained the enrichment annotations of the DEGs in biological processes (BP), molecular function (MF) and cell component (CC). The top 3 enriched annotations for BP are immune response-activating cell surface receptor signaling pathway, lymphocyte mediated immunity, and adaptive immune response based on somatic recombination of immune receptors built from immunoglobulin superfamily domains (Figure 3D). DEGs in the molecular function (MF) category were mainly involved in antigen binding, immunoglobulin receptor binding, immune receptor activity (Figure 3D). In the cell component (CC), DEGs primarily clustered in the T cell receptor complex, immunoglobulin complex, external side of plasma membrane and immunoglobulin complex (Figure 3D). In the KEGG pathways, the DEGs were enriched in malaria, cytokine–cytokine receptor interaction and hematopoietic cell lineage (Figure 3D). These results showed that the top enrichment pathways are all related to immunologic process, suggesting theses DEGs closely participated in immune-related pathologies.
CD40LG and CD2 Were Core Prognostic Genes
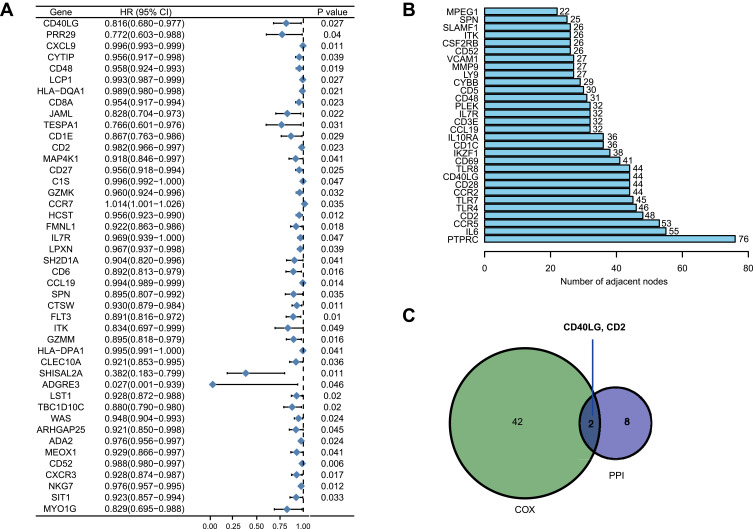
Univariate Cox proportional hazard regression analysis revealed that the intersecting DEGs were associated with overall survival of BRCA (Figure 4A). The PPI network was constructed by STRING tool with a confidence score > 0.9 and visualized by Cytoscape software (Figure S2). The top 10 hub genes with the maximum numbers of adjacent nodes extracted from the PPI network were: PTPRC, IL6, CCR5, CD2, TLR4, TLR7, CCR2, CD28, CD40LG, TLR8 (Figure 4B). The intersecting genes between the prognostic genes and the hub genes were CD2 and CD40LG (Figure 4C). Because CD2 had been a known prognostic genes in BRCA,12,14 we herein focused on CD40LG for further investigation.
Figure 4.
Core prognostic genes analysis. (A) Forest plot of prognostic genes for overall survival using univariate Cox proportional hazard analysis. (B) Genes with the maximum numbers of adjacent nodes in the PPI network. (C) Venn diagram of core prognostic genes.
The Expression of CD40LG Was Lower in Tumor Samples and Higher CD40LG Predicted Better Survival
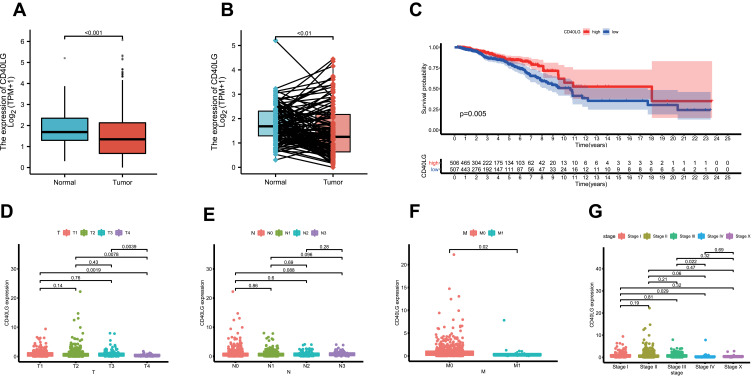
The expression of CD40LG in tumor samples was significantly lower than that in normal samples (Figure 5A, P < 0.001). This was validated after paired analysis (Figure 5B, P < 0.01). The tumor samples were divided into high and low CD40LG expression groups based on the median expression level. The Kaplan–Meier survival curve showed that patients with a higher expression level of CD40LG had a longer overall survival as compared with those with a lower expression of CD40LG (Figure 5C, p = 0.005).
Figure 5.
CD40LG expression level and its association with tumor clinicopathological characteristics. (A) Comparison of CD40LG expression between normal samples and tumor samples. (B) Comparison of CD40LG expression between normal samples and paired tumor samples. (C) Kaplan–Meier survival curve of high and low CD40LG expression groups. (D) CD40LG expression and tumor T stage. (E) CD40LG expression and tumor N stage. (F) CD40LG expression and tumor distal metastasis. (G) CD40LG expression and tumor overall TNM stage.
CD40LG Expression Negatively Correlated with BRCA Progression
Compared with T1, T2, and T3, T4 (tumor size over 5cm) BRCA had a significantly lower expression of CD40LG, though difference was not significant within T1, T2, and T3 (Figure 5D). In the lymph node staging (N), the expression of CD40LG in N0 was the highest, and the order for their expression levels were: N0 > N1 > N2 > N3, however the difference between them is not significant (Figure 5E). In addition, tumors with distant metastasis were also associated with lower CD40LG expression (Figure 5F). Given these results, lower CD40LG expression was found to be associated with more advanced BRCA (Figure 5G) and that may partially explain why it predicts a poor overall survival.
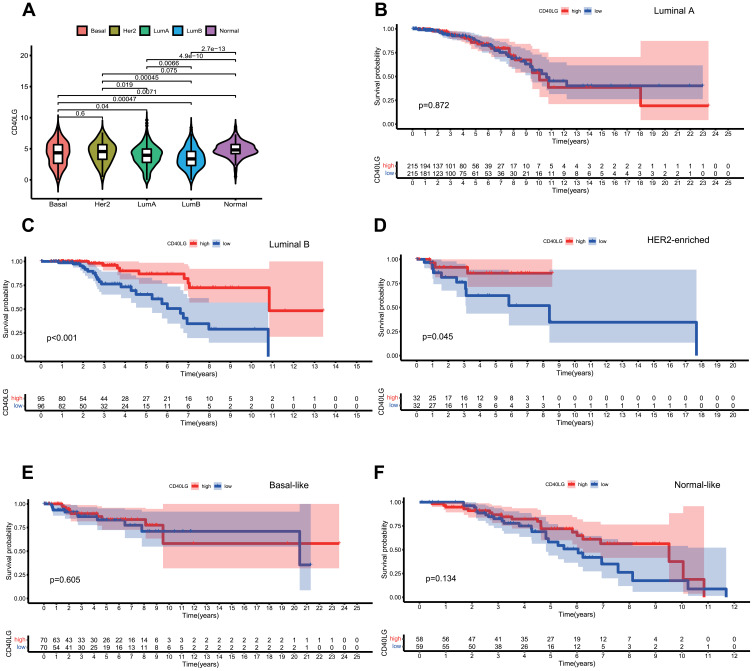
The Expression of CD40LG Differed in BRCA Molecular Subtypes
According to the box plot (Figure 6A), the expression of CD40LG was different among the five molecular subtypes except the comparison between luminal A and luminal B. The Kaplan–Meier survival curve of each molecular subtype stratified for CD40LG expression were shown in Figure 6. These curves suggested that the predictive value of CD40LG was significant in type luminal B (Figure 6C) and type HER2-enriched (Figure 6D) but might not be for the others.
Figure 6.
Analysis of PAM50 molecular subtypes and CD40LG. (A) The expression of CD40LG in each molecular subtype. (B) Survival curve of luminal A stratified by CD40LG expression. (C) Survival curves of luminal B stratified by CD40LG expression. (D) Survival curves of HER2-enriched stratified by CD40LG expression. (E) Survival curves of basal-like stratified by CD40LG expression. (F) Survival curves of normal-like stratified by CD40LG expression.
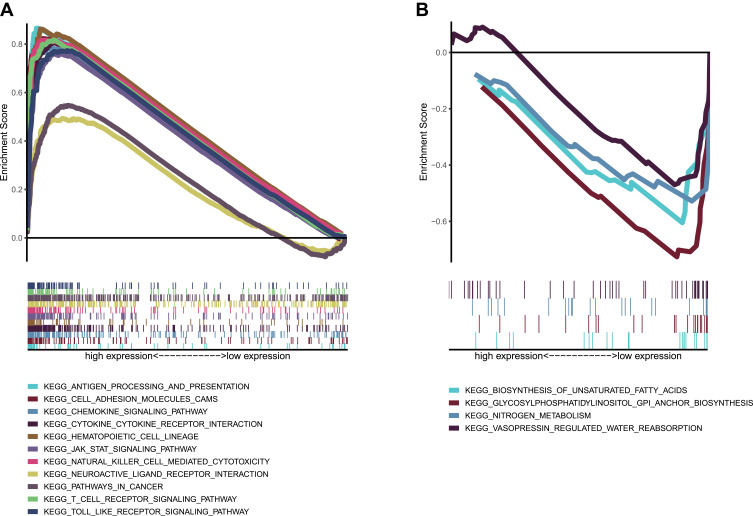
Genes in the High CD40LG Expression Group are Enriched in Immune-Related Pathways
The top ten pathways with the highest enrichment scores for the high CD40LG expression group were shown in Figure 7A. Many of these pathways were immunologically relevant, such as antigen processing and presentation, natural killer cell mediated cytotoxicity and T cell receptor signaling. The other pathways were associated with inflammation, which can also trigger immune response, such as cytokine-cytokine receptor interaction, chemokine signaling pathway. Therefore, we inferred that CD40LG had an important role in the immune process of BRCA. As for the low CD40LG expression group, the genes were mainly enriched in metabolic pathways Figure 7B
Figure 7.
GSEA analysis of CD40LG expression. (A) KEGG pathway enrichment in high CD40LG expression group. (B) KEGG pathway enrichment in low CD40LG expression group.
Correlation Between CD40LG Expression and TICs
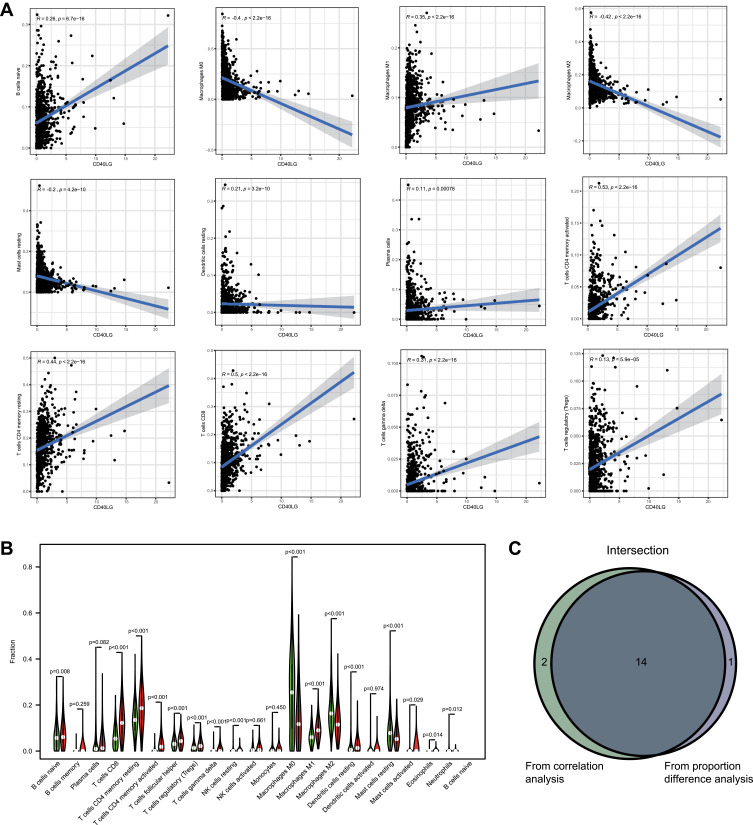
The relative proportions of the 22 TICs were estimated by the CIBERSORT algorithm, and their correlations with each other were shown Figure S3. The expression of CD40LG was significantly correlated with 16 TICs (nine were positively correlated and seven were negatively correlated). Among them, 12 TICs with the highest correlation coefficients were shown in Figure 8A. In addition, 15 TICs had significant different proportions between high and low CD40LG expression groups (Figure 8B). And finally, there were 14 intersecting TICs according to the results of correlation analysis and proportion difference analysis (Figure 8C). Those intersecting TICs with a positive correlation with CD40LG were B cells naïve, T cells CD8, T cells CD4 memory resting, T cells CD4 memory activated, T cells regulatory, T cells gamma delta, and macrophages M1. And those with a negative correlation were NK cells resting, macrophages M0, macrophages M2, dendritic cells resting, mast cells activated, mast cells resting and neutrophils.
Figure 8.
Associations between CD40LG expression and TICs. (A) Correlations between CD40LG expression and TICs. (B) Violin diagram showing proportions of TICs in high and low CD40LG expression groups. (C) Venn diagram of showing intersecting TICs in correlation analysis and difference proportion analysis.
Discussion
The mortality of BRCA has been steadily decreasing due to advancements of treatment modalities, which significantly improved the long-term survival. However, it is not enough since these treatments are still ineffective in refractory cases.15–17 Recent immunotherapies provide a new basis for the treatment of patients with BRCA, but the underlying mechanisms are still under investigation. Further studies on prognostic molecular markers and therapeutic targets are therefore needed.18,19
In this integrated bioinformatic study, we found that immune-related pathways played a vital role in TME and might determine the prognosis of BRCA patients. We also revealed that CD40LG was a core prognostic gene associated with BRCA overall survival and a lower CD40LG expression predicted worse clinicopathological characteristics. With GSEA analysis, we revealed that CD40LG was deeply involved in immunological modulations of BRCA. These results suggest that CD40LG has the potential to serve as a target for immunotherapies.
Human CD40LG or CD40L, also known as CD154, is a transmembrane protein and the ligand of CD40. It belongs to the tumor necrosis factor (TNF) gene superfamily. CD40LG is mainly expressed in activated T cells and effects critical roles in the regulation of activation and differentiation of B cell and maturation of dendritic cells.20,21 Alex W. Tong et al revealed that almost all infiltrating breast carcinomas expressed CD40, whereas CD40L were less expressed.22 In another study, CD40(+) human BRCA cell lines and tumor-bearing mice treated with CD40L stimulation showed increased tumor cell apoptosis and longer survival, respectively.23 The interaction between CD40L and CD40 can directly inhibit growth of CD40-positive carcinoma cells and may indirectly inhibit tumor growth through coordination of immune responses.24 However, in this early investigation, the specific molecular mechanism of CD40L in BRCA was unclear. In the following study, Loskog et al suggested that CD40L played an antitumor role through inducing autocrine and paracrine death ligands like TNF-α, Fas ligand (FasL) and TNF-associated apoptosis-inducing ligand.21 In the Purwanti's investigation, systemic injection of the CD40L-expression EPCs stimulated the secretion of both tumor necrosis factor-α and interferon-γ and led to prolonged survival of the tumor-bearing mice.25 However, Kim et al showed that CD40LG on the surface of T cells directly interacts with CD40 on the surface of tumor cells, promoting the proliferation of breast tumor cells through accelerating the production of TGF-β and the differentiation of Th17, which might be closely related to the immune escape of tumors.26 Therefore, further study of the interaction between CD40 and CD40LG in TME of BRCA is of great significance for immunotherapy strategies.
In 2000, Perou et al firstly identify four subtypes of breast cancer in relation to different molecular features of mammary epithelial biology (ie, ER+/luminal-like, basal-like, Erb-B2+ and normal breast), based on complementary microarrays representing 8102 human genes.27 In 2009, Parker et al constructed an array of 50 genes to rapidly distinguish 5 molecular types of breast cancer (ie, luminal A, luminal B, basal-like, normal-like, HER-2 enriched), and called that PAM50 subtype,28 which was investigated in this studies with respect to CD40LG. Our results showed that the expression of CD40LG were different among these subtypes and the prognostic value of CD40LG in BRCA could be largely attributed to type luminal B and type HER2-enriched. However, related studies are lacking and more data are need to examine whether there is a type-specific effect of CD40LG on BRCA.
We also noticed that CD40LG high-expression group had significantly higher proportions of B cells naïve, Plasma cells, T cells CD8, T cells CD4, T cells follicular helper, Tregs and T cells gamma delta than low-expression group. Based on these results, we hypothesized that CD40LG might participate in both cell-mediated immunity and humoral immunity in BRCA. In the group with high CD40LG expression, the proportion of M1 macrophages was significantly higher than that in the group with low CD40LG expression, while the proportion distributions of M2 macrophages were the opposite. M1 macrophages secrete cytokines, including TNF-A, IL-6, and IL-12, that kill tumor cells in the TME. Whereas M2 macrophages promote tumor growth and metastasis by secreting anti-inflammatory factors such as TGF-β and IL-10.29,30 In a recent study, recombinant vaccinia virus expressing CD40L promoted the induction of TNF-α-dependent antitumor activity of M1-like macrophages.31 These results suggest that CD40LG may play an anti-tumor role by regulating macrophages in the TME of BRCA.
The generation of anti-tumor immunity depends on the nature of dendritic cell (DC)-tumor interactions.32 CD40LG may rescue dendritic cells from apoptosis induced by BRCA products and functionally improves immature antigen-presenting cells and CD40L is also the key in overturning tumor-induced DC dysfunction.33 In addition, stimulation of CD40L/CD40 by endogenous expression of (m)CD40L has been shown to increase the immunomodulatory capacity of DC in cholangiocellular, pancreatic, and colorectal tumor cells.34 These results corroborate with our finding that CD40LG is enriched in antigen processing and presentation pathway (Figure 7A), but more in-depth researches for exact mechanism are needed to guide new therapies.
In prostate cancer, mast cells have an immunoregulatory effect on polymorphonuclear myeloid-derived suppressor cells (PNM-MDSC) activity through CD40L-CD40 interaction, favoring immunosuppression and tumor onset.35 Also, mast cells boost MDSC activity and contribute to the development of tumor-favoring microenvironment via CD40:CD40L cross-talk in colon carcinoma.36 However, the role of mast cell in BRCA is poorly understood and even controversial.17,37,38 In our results, the expression of CD40LG was negatively correlated with mast cells. We therefore speculate that regulations of mast cells by targeting CD40L may be one of the anti-tumor pathways for BRCA.
Conclusions
With a series of bioinformatic tools to obtain immune-related core prognostic genes of BRCA, CD40LG emerges as a valuable prognostic molecular marker. CD40LG is downregulated in BRCA patients, and a lower CD40LG expression is associated with advanced tumor stages and a poor prognosis. We also reveal that CD40LG involves in a series of immune-related pathways and is associated with multiple TICs in the TME, which coincides with recent studies. Therefore, CD40LG may serve as a promising therapeutic target, but more studies are need to better explore the exact mechanisms of CD40LG in modulating TME in BRCA.
Acknowledgments
We thank TCGA team for generously sharing their data. We also appreciate the R system developers for their hard work.
Funding Statement
There is no funding to report.
Abbreviations
BRCA, breast cancer; PAM50, Prediction Analysis of Microarray 50; TME, tumor microenvironment; TCGA, The Cancer Genome Atlas; ESTIMATE, Estimation of Stromal and Immune Cells in Malignant Tumor Tissues Using Expression Data; DEGs, differentially expressed genes; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; CIBERSORT, The Cell Type Identification by Estimating Relative Subsets of Known RNA Transcripts; TICs, tumor-infiltrating immune cells; GSEA, Gene Set Enrichment Analysis; PNM-MDSC, polymorphonuclear myeloid-derived suppressor cells.
Data Sharing Statement
Our data is based on the TCGA database and available at https://portal.gdc.cancer.gov/.
Ethics Approval and Informed Consent
There is no need for approval from the Ethics committee or written informed consent from patients since this study is based on a publicly available database.
Consent for Publication
Final approval was obtained from all authors.
Author Contributions
Conception and design: Manqiu Yuan, Jianying Pei
Collection and assembly of data: Ruihao Li, Xin He, Lirong Tian
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittal S, Brown NJ, Holen I. The breast tumor microenvironment: role in cancer development, progression and response to therapy. Expert Rev Mol Diagn. 2018;18(3):227–243. doi: 10.1080/14737159.2018.1439382 [DOI] [PubMed] [Google Scholar]
- 4.Houthuijzen JM, Jonkers J. Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment. Cancer Metastasis Rev. 2018;37(4):577–597. doi: 10.1007/s10555-018-9768-3 [DOI] [PubMed] [Google Scholar]
- 5.Yoshihara K, Shahmoradgoli M, Martínez E, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4(1):2612. doi: 10.1038/ncomms3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–945. doi: 10.1038/nm.3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Wang JS, Zhang T, Wang HC, Li LP. Identification of new therapeutic targets for gastric cancer with bioinformatics. Front Genet. 2020;11:865. doi: 10.3389/fgene.2020.00865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong Y, Wang K, Zhou H, Peng L, You W, Fu Z. Profiles of immune infiltration in colorectal cancer and their clinical significant: a gene expression-based study. Cancer Med. 2018;7(9):4496–4508. doi: 10.1002/cam4.1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohr-Udilova N, Klinglmüller F, Schulte-Hermann R, et al. Deviations of the immune cell landscape between healthy liver and hepatocellular carcinoma. Sci Rep. 2018;8(1):6220. doi: 10.1038/s41598-018-24437-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Wu S, Yang Y, Zhao M, Zhu G, Hou Z. The prognostic landscape of tumor-infiltrating immune cell and immunomodulators in lung cancer. Biomed Pharmacother. 2017;95:55–61. doi: 10.1016/j.biopha.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Wei Y, Wu Y, et al. ATP2C2 has potential to define tumor microenvironment in breast cancer. Front Immunol. 2021;12:657950. doi: 10.3389/fimmu.2021.657950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Meng Z, Zhang L, Liu F. CD2 is a novel immune-related prognostic biomarker of invasive breast carcinoma that modulates the tumor microenvironment. Front Immunol. 2021;12:664845. doi: 10.3389/fimmu.2021.664845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Zhang G, Sui Y, et al. CD52 is a prognostic biomarker and associated with tumor microenvironment in breast cancer. Front Genet. 2020;11:578002. doi: 10.3389/fgene.2020.578002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.THE HUMAN PROTEIN ATLAS. Expression of CD2 in breast cancer. Available from: https://www.proteinatlas.org/ENSG00000116824-CD2/pathology/breast+cancer. Accessed July 20, 2021.
- 15.Baik SH, Gallo LC, Wells KJ. Patient navigation in breast cancer treatment and survivorship: a systematic review. J Clin Oncol. 2016;34(30):3686–3696. doi: 10.1200/JCO.2016.67.5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D, Si M, Xue HY, Wong HL. Nanomedicine applications in the treatment of breast cancer: current state of the art. Int J Nanomedicine. 2017;12:5879–5892. doi: 10.2147/IJN.S123437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bense RD, Sotiriou C, Piccart-Gebhart MJ, et al. Relevance of tumor-infiltrating immune cell composition and functionality for disease outcome in breast cancer. J Natl Cancer Inst. 2017;109(1). doi: 10.1093/jnci/djw192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Aranda M, Redondo M. Immunotherapy: a challenge of breast cancer treatment. Cancers. 2019;11(12):1822. doi: 10.3390/cancers11121822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Law AM, Lim E, Ormandy CJ, Gallego-Ortega D. The innate and adaptive infiltrating immune systems as targets for breast cancer immunotherapy. Endocr Relat Cancer. 2017;24(4):R123–R144. doi: 10.1530/ERC-16-0404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laman JD, Claassen E, Noelle RJ. Functions of CD40 and its ligand, gp39 (CD40L). Crit Rev Immunol. 2017;37(2–6):371–420. doi: 10.1615/CritRevImmunol.v37.i2-6.100 [DOI] [PubMed] [Google Scholar]
- 21.Loskog A, Tötterman TH. CD40L - A multipotent molecule for tumor therapy. Endocr Metab Immune Disord Drug Targets. 2007;7(1):23–28. doi: 10.2174/187153007780059432 [DOI] [PubMed] [Google Scholar]
- 22.Tong AW, Papayoti MH, Netto G, et al. Growth-inhibitory effects of CD40 ligand (CD154) and its endogenous expression in human breast cancer. Clin Cancer Res. 2001;7(3):691–703. [PubMed] [Google Scholar]
- 23.Hirano A, Longo DL, Taub DD, et al. Inhibition of human breast carcinoma growth by a soluble recombinant human CD40 ligand. Blood. 1999;93(9):2999–3007. doi: 10.1182/blood.V93.9.2999 [DOI] [PubMed] [Google Scholar]
- 24.Zhu D, Chen C, Purwanti YI, et al. Induced pluripotent stem cell-derived neural stem cells transduced with baculovirus encoding CD40 ligand for immunogene therapy in mouse models of breast cancer. Hum Gene Ther. 2014;25(8):747–758. doi: 10.1089/hum.2013.160 [DOI] [PubMed] [Google Scholar]
- 25.Purwanti YI, Chen C, Lam DH, et al. Antitumor effects of CD40 ligand-expressing endothelial progenitor cells derived from human induced pluripotent stem cells in a metastatic breast cancer model. Stem Cells Transl Med. 2014;3(8):923–935. doi: 10.5966/sctm.2013-0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H, Kim Y, Bae S, et al. Direct interaction of CD40 on tumor cells with CD40L on T cells increases the proliferation of tumor cells by enhancing TGF-β production and Th17 differentiation. PLoS One. 2015;10(5):e0125742. doi: 10.1371/journal.pone.0125742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 28.Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–1167. doi: 10.1200/JCO.2008.18.1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linde N, Casanova-Acebes M, Sosa MS, et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat Commun. 2018;9(1):21. doi: 10.1038/s41467-017-02481-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu SQ, Waaijer SJH, Zwager MC, de Vries EGE, van der Vegt B, Schröder CP. Tumor-associated macrophages in breast cancer: innocent bystander or important player? Cancer Treat Rev. 2018;70:178–189. doi: 10.1016/j.ctrv.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 31.Governa V, Brittoli A, Mele V, et al. A replication-incompetent CD154/40L recombinant vaccinia virus induces direct and macrophage-mediated antitumor effects in vitro and in vivo. Oncoimmunology. 2019;8(5):e1568162. doi: 10.1080/2162402X.2019.1568162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinzon-Charry A, Ho CS, Maxwell T, et al. Numerical and functional defects of blood dendritic cells in early- and late-stage breast cancer. Br J Cancer. 2007;97(9):1251–1259. doi: 10.1038/sj.bjc.6604018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinzon-Charry A, Schmidt CW, López JA. The key role of CD40 ligand in overcoming tumor-induced dendritic cell dysfunction. Breast Cancer Res. 2006;8(1):402. doi: 10.1186/bcr1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadeghlar F, Vogt A, Mohr RU, et al. Induction of cytotoxic effector cells towards cholangiocellular, pancreatic, and colorectal tumor cells by activation of the immune checkpoint CD40/CD40L on dendritic cells. Cancer Immunol Immunother. 2021;70(5):1451–1464. doi: 10.1007/s00262-020-02746-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jachetti E, Cancila V, Rigoni A, et al. Cross-talk between myeloid-derived suppressor cells and mast cells mediates tumor-specific immunosuppression in prostate cancer. Cancer Immunol Res. 2018;6(5):552–565. doi: 10.1158/2326-6066.CIR-17-0385 [DOI] [PubMed] [Google Scholar]
- 36.Danelli L, Frossi B, Gri G, et al. Mast cells boost myeloid-derived suppressor cell activity and contribute to the development of tumor-favoring microenvironment. Cancer Immunol Res. 2015;3(1):85–95. doi: 10.1158/2326-6066.CIR-14-0102 [DOI] [PubMed] [Google Scholar]
- 37.Samoszuk M, Corwin MA. Mast cell inhibitor cromolyn increases blood clotting and hypoxia in murine breast cancer. Int J Cancer. 2003;107(1):159–163. doi: 10.1002/ijc.11340 [DOI] [PubMed] [Google Scholar]
- 38.Kankkunen JP, Harvima IT, Naukkarinen A. Quantitative analysis of tryptase and chymase containing mast cells in benign and malignant breast lesions. Int J Cancer. 1997;72(3):385–388. doi: [DOI] [PubMed] [Google Scholar]