Abstract
Patient: Female, 29-year-old
Final Diagnosis: Restrictive cardiomyopathy with isolated endomyocardial fibrosis of the right ventricle • tricuspid valve insufficiency
Symptoms: Reduced exercise tolerance • dyspnea and heart rhythm disorders
Medication: —
Clinical Procedure: —
Specialty: Cardiac Surgery • Cardiology
Objective:
Rare disease
Background:
The cardiotoxic effects of chemotherapy in cancer treatment can damage cardiomyocytes. A common link in the pathogenesis is the proliferation of fibroblasts and the increase of collagen synthesis, leading to development of common endomyocardial fibrosis. The walls of ventricles become rigid and their inability to relax prevents them from carrying the required amount of blood. The myocardial contractility gradually decreases and leads to ventricular dysfunction and signs of heart failure.
Case Report:
A 29-year-old woman with reduced exercise tolerance, dyspnea, and heart rhythm disorders was admitted to our hospital. Lymphoblastic leukemia had been diagnosed at the age of 8 years, and she underwent 8 courses of polychemotherapy. She had normal heart anatomy. At the current admission, the diagnostic protocol included echocardiography, computed tomography, cardiac catheterization, and angiocardiography. She was diagnosed with restrictive cardiomyopathy with isolated endomyocardial fibrosis of the right ventricle, and moderate tricuspid valve insufficiency NYHA class III. The patient underwent a right-sided bidirectional cavopulmonary connection with tricuspid valve repair. The early postoperative period was uneventful, and SVCp decreased to 14 mmHg. At discharge, the patient’s clinical condition had improved and tricuspid regurgitation was minimal.
Conclusions:
The one-and-a-half ventricular correction, commonly used in patients with Ebstein’s anomaly and RV dysfunction or in patients with congenital heart defects associated with RV hypoplasia, is proposed as the method of choice for cardiomyopathy type RV dysfunction.
Keywords: Cardiomyopathies, Endomyocardial Fibrosis, Fontan Procedure, Heart Failure
Background
Restrictive cardiomyopathy (CMP) is a rare type of the pathology (3% of all cases of CMP). Hypertrophic, dilated, and arrhythmogenic CMP are presented in 61%, 31%, and 5% of cases, respectively [1,2]. The annual incidence of CMP is 4–12 per 100 000 population in Europe and 2–5 in Russia [3,4].
One of the etiological factors of CMP is cardiotoxic effects of certain drugs used in cancer chemotherapy (eg, anthracycline antibiotics and busulfan) [3,5,6].
A common link in pathogenesis is the proliferation of fibro-blasts and the increase of collagen synthesis, leading to development of common endomyocardial fibrosis. This process can be accompanied by formation of thrombotic or calcium deposits as a source of embolism [3,6,7]. Endomyocardial damage can be located in the right, left, or both ventricles of the heart [8].
The goal of this report is to present a case of successful oneand-a-half ventricular correction of right ventricular dysfunction in a patient with restrictive cardiomyopathy due to lymphoblastic leukemia chemotherapy.
Case Report
A 29-year-old woman with reduced exercise tolerance, dyspnea, and heart rhythm disorders was admitted for surgical treatment to Bakulev Cardiovascular Surgery Center in April 2020.
At the age of 8 years, lymphoblastic leukemia was diagnosed, and she underwent 8 courses of polychemotherapy according to the ALL-BFM-90 program and was treated with infusions of cardiotoxic drugs (eg, cyclophosphamide and daunorubicin) and was removed from the cancer register in 2004, as a stable remission was achieved. In 2018, during pregnancy, the patient developed dyspnea and pedal edema, which worsened after childbirth. Tricuspid valve regurgitation of 2–3 degree and signs of exudative pericarditis were noted on echo-cardiography. However, 3D echocardiography revealed that left ventricular systolic and diastolic function was preserved: E/A 1.2, E/e 7.4, DT (deceleration time) 228 ms, IVRT (isovolumic relaxation time) 87 ms, LV EF (left ventricular ejection fraction) 69%, ESD (end-systolic diameter) 2.3 cm, EDD (end-diastolic diameter) 3.7 cm, EDV (end-diastolic volume) 58 ml, ESV (end-systolic volume) 18 ml, fibrous annulus mitral valve 25 mm, no regurgitation, and MAPSE 21 mm. The right atrium (RA) was increased in size to 59x61 mm, with fibrous obliteration and calcification of the right ventricle (RV) apex, spreading to its trabecular part free wall and partially to the ventricular septum (VS), with involvement of the tricuspid valve (TV) subvalvular apparatus (Figure 1). The calculated RV pressure was 37 mmHg. Systolic and diastolic function of the ventricle myocardium was reduced: EF RV 24%, EDV 82 ml, ESV 61 ml, EF RV 25%, TAPSE 9 mm, E/A 2.6, E/e 15, and Vs 5.6 cm/s. TV leaflets were thin and mobile. The fibrous annulus TV was 45 mm (Z-score= +2.1), regurgitation of 3–4 degrees (vena contracta 0.9 cm, ERO 0.48 cm2, MR FR 81%) (Figure 1). IVS and AS were intact, with no shunt. There was fluid (4–5 mm) in the pericardial cavity.
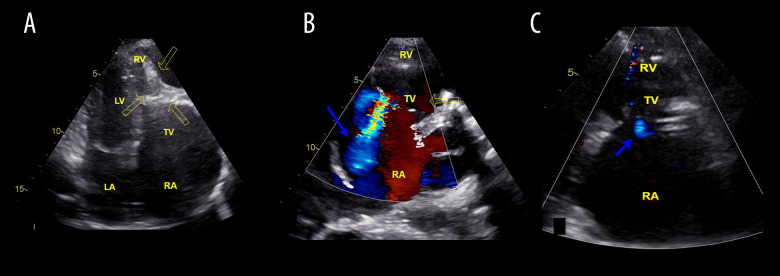
Figure 1.
Echocardiograms of the patient before (A, B) and after surgery (C). (A) Echocardiography, 4-chamber plane. Arrows indicate endomyocardial fibrosis of the ventricle with areas of calcification. (B) Illustration of pronounced regurgitation of the TC (the blue arrow). (C) Shows minimal regurgitation on the TV after valvulo-annuloplasty (the blue arrow). LA – left atrium; RA – right atrium; TV – tricuspid valve; LV – left ventricle; RV – right ventricle.
Cardiac catheterization and angiocardiography showed the absence of the trabecular part due to pronounced calcification in the RV, with tricuspid valve regurgitation of 3–4 degrees and right atriomegaly. RV pressure was 35 mmHg, PA 23/14 (mean 17), LV 137/0–9, and aorta 135/80 mmHg. The Nakata Index was 273 mm/m2 and the McGoon Index was 2.07.
The multi-spiral computed tomography, cardiac catheterization, angiocardiography, and MRI images showed right atriomegaly, multiple areas of severe calcification in the right ventricle (RV) of the heart, located from the base of the anterior cusp of the tricuspid valve to the outflow part of the RV with a decrease of its cavity (Figure 2). There were no episodes of pulmonary embolism (Figure 2).
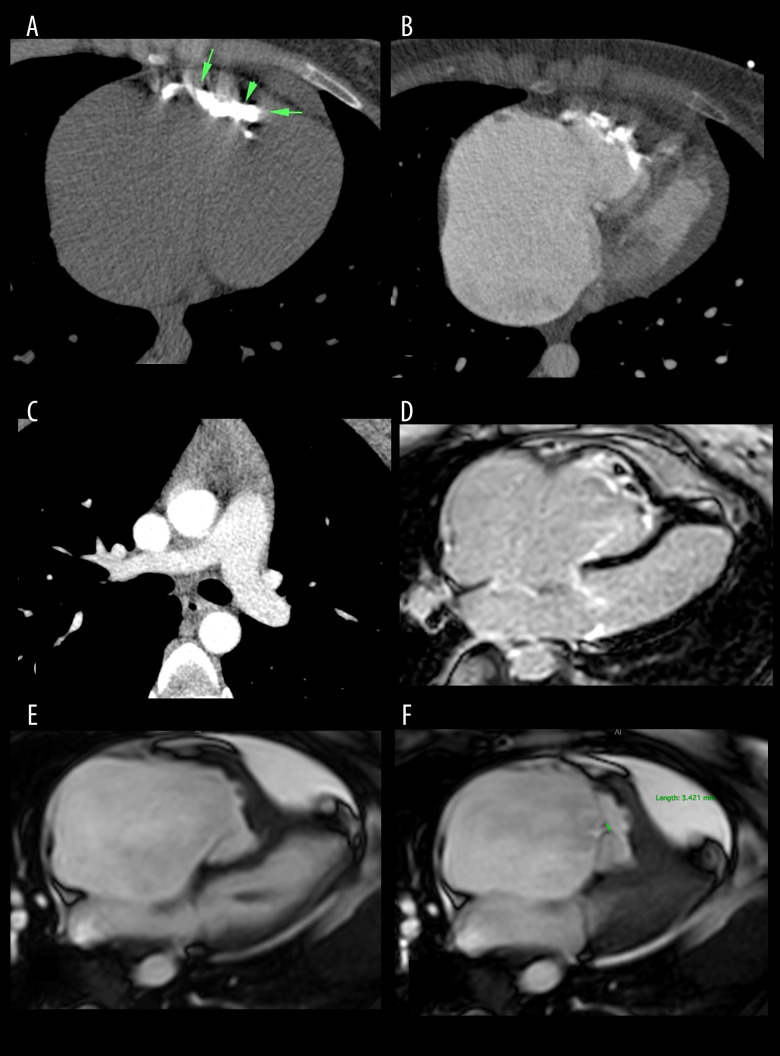
Figure 2.
Multi-spiral computed tomography of the chest organs (axial plane) (A–C). Magnetic resonance imaging of the heart (4-chamber plane) (D–F). (A) “Native” series: arrows indicate massive calcification in the ventricular cavity; (B, C) – series with contrast enhancement: (B) The cavity of the ventricle is reduced in size due to obliteration of the trabecular part; (C) No signs of thromboembolism and areas of calcification at the site of pulmonary. (D) Delayed contrasting (LGE). (E) Phase of diastole; (F) Phase of systole.
The diagnosis was determined to be restrictive cardiomyopathy with isolated endomyocardial fibrosis of the right ventricle, tricuspid valve insufficiency 3 degrees, and III FC by NYHA after polychemotherapy for lymphoblastic leukemia (remission stage).
Surgery was performed on 25 March 2020. The patient underwent a right-sided bidirectional cavopulmonary connection (BCPC) with tricuspid valve reconstruction on a soft supporting PTFE half-ring on hypothermic bypass and cold cardioplegia (Figure 3). The early postoperative period was uneventful, and SVCp decreased to 14 mmHg. The patient was discharged on the 11th day after surgery in satisfactory condition. The trabecular part of the RV cavity was represented by a coral-shaped calcified conglomerate, which partially extended to the interventricular septum, to the inflow and out-flow parts of the RV (Figure 3). Due to the massiveness of the lesion and the high risk of RV structure damage, it was decided not to perform decalcification of the walls and cavity of the RV. The main substrate of TV regurgitation was the incomplete leaflets coaptation due to TV fibrous annulus dilatation. The posterior leaflet was partially joined to the inflow part of the RV, and partial calcification of the base of the papillary muscles was obvious. The TV reconstruction included the soft supporting PTFE half-ring placement fixed by 10 U-shaped sutures and 1 U-shaped Prolene suture on the anteroposterior commissure (Figure 3).
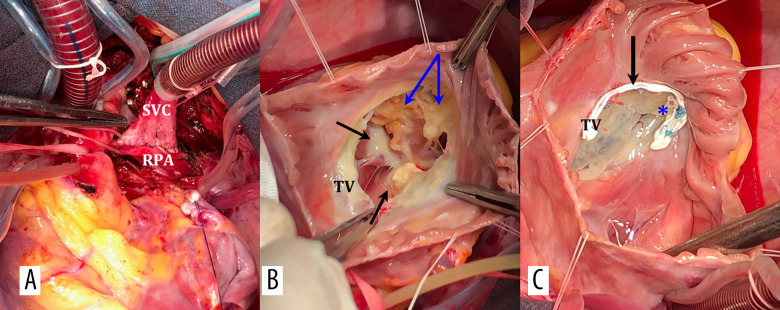
Figure 3.
Intraoperative photographs. (A) Bidirectional cavopulmonary anastomosis (B) Visualization of TV: dilatation of fibrous anulus, thin leaflets, black arrows indicate fibrosis and calcification of papillary muscles, blue – calcification of the ventricle wall. (C) Annuloplasty of TV on a soft PTFE half-ring, valvuloplasty RV, U-shaped Prolene suture along the anteroposterior commissure. SVC – superior vena cava; RPA – right pulmonary artery; TV – tricuspid valve; FA – fibrosus annulus; RV – right ventricle.
Just before the discharge, the patient noted no dyspnea at exercise. Echocardiography showed LV EDD 4.3cm, LV EF 60%, RV EDV 80 ml, ESV 58 ml, RV EF 27%, and TAPSE 11 mm, and RV myocardial diastolic function was E/A 2.4, E/e 13.8, and Vs 6.2 cm/s. TV leaflets were movable, with total cooptation and satisfactory amplitude, with minimal regurgitation. The diameter of the TV fibrous annulus was 30 mm (Figure 1). Estimated RV pressure was 36 mmHg.
Discussion
Acute lymphoblastic leukemia is a malignant disease of the hematopoietic system and is the most common cancer in children and adolescents (accounting for 33% of all pediatric cancers). Treatment of this disease includes administration of high doses of cell growth blocker drugs (cytostatics), with a highly likely cardiotoxic effect at the same time. Their pronged usage can cause heart muscle damage that ultimately develops into diffuse myocardial fibrosis and symptoms of heart failure [9–11].
Restrictive cardiomyopathy treatment aims to reduce heart failure symptoms. Routine therapy includes diuretic therapy, ACE inhibitors or angiotensin II receptor antagonists, beta-blockers, digitalis, and anticoagulants to prevent thrombosis [12–14]. There are different approaches to the indications for surgical treatment and the timing of its implementation. The effectiveness of the surgery and prognosis depend on an earlier intervention; therefore, one should not expect a significant deterioration in the condition and severe myocardial damage [15].
Contrast MRI tomography is the main method in the diagnosis of restrictive CMP, since echocardiography fails to reveal the degree and nature of myocardial damage and the volumetric characteristics of the RV.
Regarding the suggestion of Mocumbi et al (2013) to verify the diagnosis of endomyocardial fibrosis [16,17], our patient met 2 major diagnostic criteria (obliteration of the apex of the right ventricle and endomyocardial thickenings of more than 2 mm) and 2 minor diagnostic criteria (increased right atrium and restrictive flow through the TV).
Surgery is considered if a patient is resistant to conservative therapy, has episodes of thromboembolic complications, and is NYHA class III–IV [18–20]. The effectiveness of the surgery and prognosis depend on an earlier intervention; therefore, one should not expect a significant deterioration in the condition and severe myocardial damage [21,22].
Dubost was the first to perform surgical treatment, in 1971 [23]. The surgical techniques included resection of endomyocardial ventricular fibrosis and replacement or repair of atrioventricular valves if necessary. Surgical treatment of endomyocardial fibrosis should be considered as a palliative procedure, but surgery may be the only hope for survival in cases of severe heart failure [24].
The most important report on surgical treatment of endomyocardial fibrosis was presented by Moraes et al, in 1999. Endomyocardectomy was performed on 83 patients in NYHA class III–IV, aged 4–59 years (average age 31 years). Hospital mortality was 18%. In the group of 51 surviving patients with AV valve replacement, long-term mortality was 23.5%, and it was 17.6% in the group of 17 patients with AV valve repair. Overall survival at follow-up 17 years after surgery was 55±7.8%. Less than half of the survivors were in NYHA class I–II, and relapse of fibrosis was noted in 5.8%. The authors concluded that surgical treatment of endomyocardial fibrosis should be considered as a palliative procedure, but surgery may be the only hope for survival in patients with severe heart failure [24]. There is an opposite point of view regarding active surgical treatment tactics. Sultan et al concluded that conservative therapy should be preferred because surgical treatment does not affect the cause and progressive nature of the disease [25]. Unsatisfactory results in the described series may be associated with the initial preoperative severity of patients [26].
The other methods of surgical treatment reported in patients with CMP are intracardiac assist device implantation and heart transplantation, with 87%, 81%, and 74% survival at 1, 3, and 5 years, respectively [27]. The use of these methods is limited by their inaccessibility and existence of specific complications [28,29].
There are few case reports in which it is proposed to use a bidirectional cavopulmonary anastomosis as a method of treating acquired right ventricular dysfunction of non-congenital etiology; in 2 cases these were patients with endomyocardial fibrosis, and the 2 other cases were patients with arrhythmogenic right ventricular dysplasia [30–33] (Table 1).
Table 1.
Surgical treatment of acquired severe right ventricular dysfunction of non-congenital ethiology by BDCA.
| Author | Diagnosis | Age/sex | RA/RV | Function of RV | TV regurgitation | Pressure, mm Hg | Surgery | Result |
|---|---|---|---|---|---|---|---|---|
| Gorgulu S. et al., 200 5 | ARVD | 16/F | Dilated/dilated | Severe RV systolic dysfunction | Severe | PA - 23/5 (10) | BDCA + TV annuloplasty by DeVega | At discharge the cyanosis disappeared, the O2 saturation increased from 87 to 97% |
| Vaidyanathan S. et al., 2017 | ARVD | 26/F | Dilated/dilated | Severe RV systolic dysfunction | Normal | PA - 20/6 (11) | Glenn shunt | At discharge saturation improved from 80 to 89%.Patient is asymptomaticat 18-month follow-up: no ventricular arrhythmia |
| Anbarasu M. et al., 2004 | RV EMF | 27/M | Enlargement/obliteration of RV apex and a discrete shelf at the infundibulum causing RVOT obstruction | RV function was normal | Moderate | PA - 20/14 (15) | Endocardial shelf removed, outflow, widened with a patch of pericardium+TV annuloplasty by DeVega+BDCA | At discharge no systemic desaturation, improvement in RV size, mild tricuspid regurgitation |
| Mishra A. et al., 2002 | RV EMF | 25/M | Gross enlargement/severe RV endomyo-cardial fibrosis with severe obliteration of the RV cavity and apex | N/A | Severe | RA - 25 RV end-diastolic - 24 | BDCA | The postoperative recovery was stormy. After 8 years of follow-up, he was in NYHA FC II and back at work. Echocardiography showed an EF of 71%, no progression of disease |
ARVD – arrhythmogenic right ventricular dysplasia; RV EMF – right ventricular endomyocardial fibrosis; F – female; M – male; RV – right ventricle; TV – tricuspid valve; RA – right atrium; EF – ejection fraction; PA – pulmonary artery; BDCA – bidirectional cavopulmonary anastomosis; N/A – not applicable.
Conclusions
The treatment of lymphoblastic leukemia includes administration of high-dose cytostatics, with a highly likely cardiotoxic effect at the same time. In the long-term, it can lead to restrictive cardiomyopathy. It should be noted that the oneand-a-half ventricular correction, commonly used in patients with Ebstein’s anomaly and RV dysfunction or in patients with congenital heart defects associated with RV hypoplasia, was proposed as a method of choice for this type of RV dysfunction (a significant decrease and the absence of the trabecular part of the RV cavity, considered as a kind of “acquired” RV hypoplasia).
References
- 1.Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: A position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–76. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 2.Elliott P, Charron PH, Gimeno Blanes JR, et al. The EORP Cardiomyopathy Registry Pilot Investigators, European Cardiomyopathy Pilot Registry: EURObservational Research Programme of the European Society of Cardiology. Eur Heart J. 2016;37(2):164–73. doi: 10.1093/eurheartj/ehv497. [DOI] [PubMed] [Google Scholar]
- 3.Amosova EN. [Cardiomyopathy.] Kniga plus. 1999:421. [in Russian] [Google Scholar]
- 4.Camm J, Thomas F, Lusher, Serruys PW. The ESC textbook of cardiovascular medicine. Oxford University Press; 2011. [Google Scholar]
- 5.Mogensen J, Arbustini E. Restrictive cardiomyopathy. Curr Opin Cardiol. 2009;24:214–20. doi: 10.1097/hco.0b013e32832a1d2e. [DOI] [PubMed] [Google Scholar]
- 6.Zhao WJ, Wei SN, Zeng XJ, et al. Gene expression profiling identifies the novel role of immunoproteasome in doxorubicin-induced cardiotoxicity. Toxicology. 2015;333:76–88. doi: 10.1016/j.tox.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Hess OM, Turina M, Senning A, et al. Pre- and postoperative findings in patients with endomyocardial fibrosis. Heart. 1978;40(4):406–15. doi: 10.1136/hrt.40.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madi D, Achappa B, Pai N, Kamath P. Right ventricular endomyocardial fibrosis – a case report. AMJ. 2013;6:88–90. doi: 10.4066/AMJ.2013.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhury UK, Airan B, Sharma R, et al. One and a half ventricle repair with pulsatile bidirectional Glenn: Results and guidelines for patient selection. Ann Thorac Surg. 2001;71:1995–2002. doi: 10.1016/s0003-4975(01)02517-6. [DOI] [PubMed] [Google Scholar]
- 10.Podzolkov VP, Samsonov VB, Makhachev OA, et al. [Comparative study of the results of one and a half ventricle repair and univentricle correction of hypoplastic right ventricle syndrome.] Detskiebolezni serdtsa i sosudov. 2008;3:31–36. [in Russian] [Google Scholar]
- 11.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–84. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 12.Snegovoy AV, Vitsenya MV, Kopp MV, Larionova VB. Prakticheskiye rekomendatsii po korrektsii kardiovaskulyarnoy toksichnosti. indutsirovannoy khimioterapiyey i targetnymi preparatami. Zlokachestvennyye opukholi. 2016;4:418–27. [in Russian] [Google Scholar]
- 13.Schweiger M, Dave H, Lemme F, Cavigelli-Brunner A, et al. Acute chemo-therapy-induced cardiomyopathy treated with intracorporeal left ventricular assist device in an 8-year-old child. ASAIO J. 2013;59(5):520–22. doi: 10.1097/MAT.0b013e3182a0d242. [DOI] [PubMed] [Google Scholar]
- 14.Hyun YK, Cho YH, Lee B, Park HB. Unusual presentation chronic pulmonary embolism due to calcified right ventricular mass. J Cardiovasc Ultrasound. 2011;19(2):91–94. doi: 10.4250/jcu.2011.19.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Mendonça JT, Carvalho MR, da Costa RK, et al. [Endomyocardial fibrosis: Surgical treatment.] Arq Bras Cardiol. 1989;52(1):13–17. [in Portuguese] [PubMed] [Google Scholar]
- 16.Mocumbi AO, Yacoub MH, Yokohama H, Ferreira MB. Right ventricular endomyocardial fibrosis. Cardiovasc Pathol. 2009;18(1):64–65. doi: 10.1016/j.carpath.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Zotova LA, Petrov VS, Vuleh VM, Trunina TP. [Case restrictive cardiomyopathy in real clinical practice.] Nauka molodykh (Eruditio juvenium) 2018;1:74–86. [in Russian] [Google Scholar]
- 18.Qureshi N, Amin F, Chatterjee D, et al. MR imaging of endomyocardial fibrosis (EMF) Int J Cardiol. 2011;149:e36–37. doi: 10.1016/j.ijcard.2009.03.091. [DOI] [PubMed] [Google Scholar]
- 19.Bunte M, Liao K, Manivel JC, et al. Forme fruste of isolated right ventricular endomyocardial fibrosis: A case report. J Med Case Rep. 2014;8:94. doi: 10.1186/1752-1947-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira B, Matsika-Claquin MD, Hausse-Mocumbi AO, et al. Geographic origin of endomyocardial fibrosis treated at the central hospital of Maputo (Mozambique) between 1987 and 1999. Bull Soc Pathol Exot. 2002;95:276–79. [PubMed] [Google Scholar]
- 21.Barretto AC, Pileggi F. [Endomyocardial fibrosis: 100 cases, 10 years’ experience.] Arq Bras Cardiol. 1988;51:117–120. [in Portuguese] [PubMed] [Google Scholar]
- 22.Mady C, Barretto AC, Oliveira SA, et al. Evolution of the endocardial fibrotic process in endomyocardial fibrosis. Am J Cardiol. 1991;68:402–3. doi: 10.1016/0002-9149(91)90841-8. [DOI] [PubMed] [Google Scholar]
- 23.Dubost C. [Endocardial resection: Surgical treatment of constrictive fibrous endocarditis.] C R Acad Hebd Seances Acad Sci D. 1975;281(12):855–57. [PubMed] [Google Scholar]
- 24.Moraes F, Lapa C, Hazin S, et al. Surgery for endomyocardial fibrosis revisited. Eur J Cardiothorac Surg. 1999;15:309–12. doi: 10.1016/s1010-7940(99)00027-5. ; discussion 312–13. [DOI] [PubMed] [Google Scholar]
- 25.Sultan FAT, Ahmed SW. Cardiac magnetic resonance evaluation of cardiac masses in patients with suspicion of cardiac masses on echo or computed tomography. J Clin Imaging Sci. 2020;10:57. doi: 10.25259/JCIS_137_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salemi VMC, Rochitte CE, Shiozaki AA, et al. Late gadolinium enhancement magnetic resonance imaging in the diagnosis and prognosis of endomyocardial fibrosis patients. Circ Cardiovasc Imaging. 2011;4:304–11. doi: 10.1161/CIRCIMAGING.110.950675. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira GH, Dupont M, Naftel D, et al. Increased need for right ventricular support in patients with chemotherapy-induced cardiomyopathy undergoing mechanical circulatory support: Outcomes from the INTERMACS Registry (Interagency Registry for Mechanically Assisted Circulatory Support) J Am Coll Cardiol. 2014;63(3):240–48. doi: 10.1016/j.jacc.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira GH, Hardaway BW, Kucheryavaya AY, et al. Characteristics and survival of patients with chemotherapy-induced cardiomyopathy undergoing heart transplantation. J Heart Lung Transplant. 2012;31(8):805–10. doi: 10.1016/j.healun.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Asai T, Miyashita F, Nota H, Vigers PN. Tricuspid valve relocation with endomyocardial fibrosis removal for Löeffler’s endocarditis. Eur J Cardiothorac Surg. 2019;56(3):622–24. doi: 10.1093/ejcts/ezy487. [DOI] [PubMed] [Google Scholar]
- 30.Mishra A, Krishna Manohar SR, Sankar Kumar R, Valiathan MS. Bidirectional Glenn shunt for right ventricular endomyocardial fibrosis. Asian Cardiovasc Thorac Ann. 2002;10(4):351–53. doi: 10.1177/021849230201000419. [DOI] [PubMed] [Google Scholar]
- 31.Anbarasu M, Krishna Manohar SR, et al. One-and-a-half ventricle repair for right ventricular endomyocardial fibrosis. Asian Cardiovasc Thorac Ann. 2004;12(4):363–65. doi: 10.1177/021849230401200418. [DOI] [PubMed] [Google Scholar]
- 32.Gorgulu S, Nurkalem Z, Celebi A, et al. Unusual presentation of a patient with arrhythmogenic right ventricular dysplasia treated with a Glenn shunt. Int J Cardiol. 2006;113(3):410–13. doi: 10.1016/j.ijcard.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 33.Vaidyanathan S, Kothandam S, Kumar R, et al. Cavopulmonary anastomosis in a patient with arrhythmogenic right ventricular cardiomyopathy with severe right ventricular dysfunction. World J Pediatr Congenit Heart Surg. 2017;11(4):60–62. doi: 10.1177/2150135117707459. [DOI] [PubMed] [Google Scholar]