Abstract
Background:
HIV-1 incidence calculation currently includes recency classification by HIV-1 incidence assay and unsuppressed viral load (VL≥1000 copies/mL) in a recent infection testing algorithm (RITA). However, persons with recent classification not virally suppressed and taking antiretroviral medication (ARV) may be misclassified.
Setting:
We used data from 13 African household surveys to describe the impact of an ARV-adjusted RITA on HIV-1 incidence estimates.
Methods:
HIV-seropositive samples were tested for recency using the HIV-1 Limiting Antigen (LAg)-Avidity enzyme immunoassay, HIV-1 viral load, ARVs used in each country, and ARV drug resistance. LAg-recent result was defined as normalized optical density values ≤1.5. We compared HIV-1 incidence estimates using two RITA; RITA1: LAg-recent + VL ≥1000 copies/mL and RITA2: RITA1 + undetectable ARV. We explored RITA2 with self-reported ARV use and with clinical history.
Results:
Overall, 357 adult HIV-positive participants were classified as having recent infection with RITA1. RITA2 reclassified 55 (15.4%) persons with detectable ARV as having long-term infection. Those with detectable ARV were significantly more likely to be aware of their HIV-positive status (84% vs. 10%) and had higher levels of drug resistance (74% vs. 26%) than those without detectable ARV. RITA2 incidence was lower than RITA1 incidence (range, 0%–30% decrease), resulting in decreased estimated new infections from 390,000 to 341,000 across the 13 countries. Incidence estimates were similar using detectable or self-reported ARV (R2>0.995).
Conclusions:
Including ARV in RITA2 improved the accuracy of HIV-1 incidence estimates by removing participants with likely long-term HIV infection.
Keywords: HIV, incidence, RITA, ARV, viral load, surveys
INTRODUCTION
In 2014, the Joint United Nations Programme on HIV/AIDS (UNAIDS) set global targets to control the HIV epidemic: 90% of people living with HIV know their status; of these, 90% receive antiretroviral medication (ARV); and of these, 90% are virally suppressed. Achievement of these targets by 2020 could decrease HIV incidence and mortality rates by 90% by 2030.1 HIV-focused population-based surveys have been measuring progress toward the 90–90-90 targets in multiple sub-Saharan African countries since 2014. The primary objectives of these surveys are to estimate HIV prevalence, HIV-1 incidence, and viral load (VL) suppression as indicators of treatment and prevention program impact.2 For example, results from cross-sectional population-based surveys in Eswatini showed that adult HIV VL suppression doubled from 2011 to 2016, decreasing HIV incidence by 44%.3
The World Health Organization (WHO) recommends including biomarker testing for HIV incidence in population-based surveys for countries with HIV prevalence >5% and HIV incidence >0.3%.4 HIV incidence estimation in cross-sectional household surveys relies on laboratory-based assays to test for recent infection. Incidence assay performance to identify recent infections varies by assay and by population characteristics, such as treatment status, CD4 cell count, and HIV subtype.5,6 To reduce misclassification of recent infection, WHO recommends that incidence calculation includes both HIV recency assay and and VL test results in the recent infection test algorithm (RITA).7 The WHO RITA was validated comparing HIV-1 Limiting Antigen (LAg)-Avidity enzyme immunoassay (EIA) plus unsuppressed VL in a cross-sectional survey with incidence estimates from a prospective cohort in Eswatini.8 In 2015, WHO updated these recommendations to suggest that testing for ARV in the RITA could reduce misclassification, although additional research was needed before the guidance was changed.9,10 Results from two national population-based households surveys conducted in South Africa and Kenya in 2012 showed that including participant ARV exposure in the RITA resulted in HIV incidence estimates that were more closely aligned with UNAIDS modeled incidence.11 However, robust data were needed to rationalize adding ARV testing to the RITA.
We used results from the Population-based HIV Impact Assessment (PHIA) surveys in 13 African countries to examine the importance and impact of including ARV exposure in the RITA to improve HIV-1 incidence estimates.
METHODS
PHIA survey methods
PHIA data from Cameroon, Côte d’Ivoire, Eswatini, Ethiopia, Kenya, Lesotho, Malawi, Namibia, Rwanda, Tanzania, Uganda, Zambia, and Zimbabwe were used in our analysis. The PHIA survey methods were previously described (Patel/Duong, manuscript in current JAIDS supplement, Radin/Sachathep, manuscript in current JAIDS supplement). The analysis was limited to adult participants aged 15–59 years. Eligible consenting adult participants were asked the month, year, and result of their most recent HIV test. Those who reported an HIV-positive result were asked the month and year of ARV initiation. Participants received home-based HIV testing and counseling using two or three rapid tests according to the national HIV diagnostic rapid test algorithm for each country. HIV-positive field test results were confirmed using Geenius HIV 1/2 Supplemental Assay (BioRad, Marnes-la-Coquette, France) in a satellite laboratory.
HIV viral load and recency testing
Specific methods for VL testing varied by country (Sleeman, manuscript in preparation). Plasma VL testing was conducted using the Roche COBAS AmpliPrep/COBAS TaqMan (CAP/CTM) HIV-1 Test, version 2.0 (Roche Diagnostics, Pleasanton, CA USA) on a Roche CAP/CTM with either a 48 or a 96 analyzer or the Abbott RealTime HIV-1 assay (Abbott Molecular, Des Plaines, IL USA) on the Abbott fully automated m2000 platform (m2000sp and m20000rt). Dried blood spot (DBS) VL testing was conducted using the Roche HIV-1 Test, version 2.0, free virus elution protocol for DBS on a Roche CAP/CTM, or the Abbott RealTime HIV-1 VL optimized one spot assay on the Abbott m2000 platform for DBS VL testing, or the NucliSENSTM EasyQ HIV-1 v2.0 assay on the NucliSENSTM EasyMAG/EasyQ platform (bioMérieux, Marcy-l’Étoile, France).
HIV recency testing for confirmed HIV-seropositive participants with plasma samples was conducted using the HIV-1 LAg-Avidity EIA (Sedia Biosciences Corporation, Portland, OR USA) and with DBS using the Maxim HIV-1 LAg DBS EIA (Maxim Biomedical, Bethesda, MD USA) in a central reference laboratory by laboratorians trained by the U.S. Centers for Disease Control and Prevention (CDC).12 Staff from the Division of Clinical Pharmacology of the Department of Medicine at the University of Cape Town used qualitative high-performance liquid chromatography and tandem mass spectrometry assay to detect first-line and second-line ARV in DBS samples from each country.13 All LAg-recent samples were tested using a CDC-developed multiplex allele-specific drug resistance assay for mutations in the HIV protease and reverse transcriptase genes that confer ARV drug resistance.14
HIV recency incidence calculation
Incidence calculations used the formula recommended by the WHO Incidence Working Group and Consortium for Evaluation and Performance of Incidence Assays with the following parameters: mean duration of recent infection (MDRI) was 130 days (95% confidence interval [CI]: 118–142 days) in all countries except Uganda (MDRI, 153 days (95% CI: 127–178 days); proportion false recent (PFR) was 0.00; and time cutoff (T) was 1 year.5 A weighted MDRI of 153 days was used in Uganda to account for reported differences of MDRI between subtype A and D15 and distribution of subtype A (80%) and subtype D (20%) in this survey (data not shown). A LAg-recent result was defined as normalized optical density (ODn) values ≤1.5 (plasma) or ≤1.0 (DBS). The difference in ODn cut point by specimen is due to differences in calibrator specimens that define respective cutoffs of plasma and DBS kits while keeping consistent MDRI. Unsuppressed VL was defined as ≥1000 RNA copies/mL.7,16 Kassanjee17 derived an estimator for instantaneous HIV incidence as
where R is the number of recent cases, is the proportion of false recent cases, Q is the number of HIV-positive people tested, is the MDRI, and T is a cutoff time for the assay set at 365 days. N’ is the adjusted number of HIV-negative individuals in the sample. N and P are the numbers of HIV-negative and HIV-positive individuals in the sample, respectively. If all HIV-positive individuals were tested for recency, N’ = N.
We set the PFR cases , which simplified the equation for instantaneous incidence to
The number of recent cases as a proportion of individuals at risk was scaled by 365/130 (~2.81) to calculate the instantaneous HIV incidence rate. The annual incidence rate was calculated using
Using estimated values for the number of people in the sample, the numbers of HIV-positive and HIV-negative individuals, and the number of recent cases, we derived an estimate for the annual incidence rate, which was then multiplied by the number of people at risk from the country-specific census data to estimate the number of new HIV cases per year. HIV incidence estimates were calculated using SAS software (SAS Institute Inc., Cary, NC USA). The SAS incidence macro is available in the PHIA Data Use Manual.18
We calculated HIV incidence estimates using two RITA algorithms: LAg-recent result + VL≥1000 copies/mL (current WHO algorithm; RITA1) and LAg-recent + VL ≥1000 copies/mL + undetectable ARVs (RITA2). We compared the characteristics and laboratory results for participants who were classified as RITA2-recent with those who were RITA1-recent with detectable ARVs (i.e., reclassified as long-term infections under RITA2) to identify factors that may be associated with misclassification of long-term infection. We used RITA1 and RITA2 to compare incidence estimates and calculate annual new HIV infections. Additionally, we compared the PHIA incidence estimates using both RITAs with the UNAIDS SPECTRUM model estimates.19 We also considered self-reported ARV use in place of ARV detection to estimate incidence and assess the comparability of the two approaches. Since September 2015, WHO has recommended immediate treatment for individuals with a new HIV diagnosis.20 As a result of this expanding “Test-and-Start” strategy, some recently infected individuals may have received a diagnosis and initiated ARV treatment within the last 12 months and may not have VL suppression. These recent infections may be misclassified as long-term because of the presence of ARV. Therefore, we conducted an additional analysis using self-reported clinical history to identify potential cases with the most recent positive HIV test and self-reported ARV initiation <12 months before the survey interview date.
The PHIA surveys were approved by Institutional Review Boards at CDC, Columbia University, Westat, and in the respective countries. HIV rapid tests and VL test results were returned to participants and to the participants’ chosen facility, respectively.21 The LAg Avidity EIA and ARV detection results, which are not approved for clinical use and did not impact participant care and treatment decisions, were not returned to participants. The genotyping and drug resistance results were returned to the participants’ providers if mutations conferring HIV drug-resistance were identified.
RESULTS
Participant characteristics
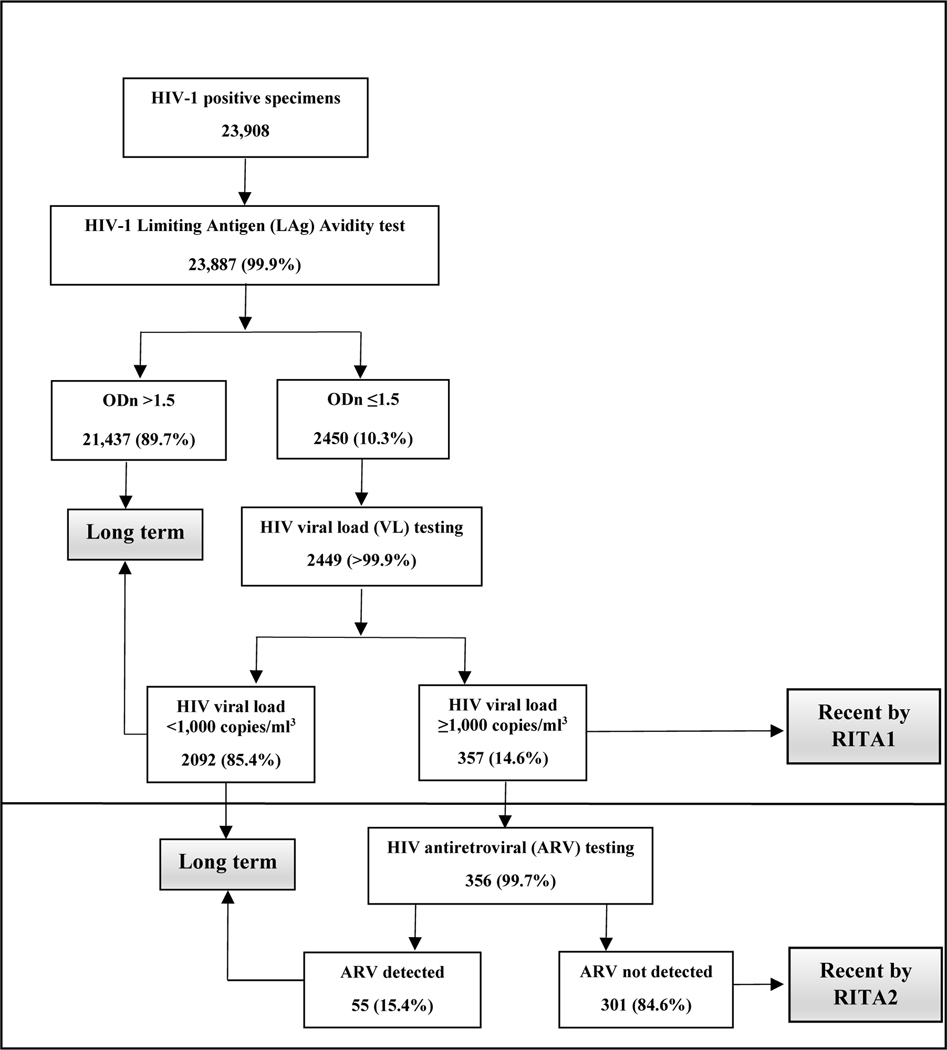
Of the 23,908 participants with confirmed HIV-positive status, LAg-Avidity EIA results were available for 23,887 (99.9%) participants. Of these, 2,450 (10.3%) were LAg-recent, and 2,449 of these had a VL test result. Among those with a VL result, 357 (14.6%) had a VL ≥1000 RNA copies/mL and were therefore classified as RITA1-recent cases. Of the 356/357 participants with ARV test results, 301 (84.6%) had no detectable ARVs and were classified as RITA2-recent, and 55 (15.4%) participants with detectable ARVs and VL ≥1000 copies/mL were reclassified as having long-term HIV infection (Figure 1).
Figure 1.
HIV-1 recent infection testing algorithm test outcomes for HIV-1 Limiting Antigen (LAg) Avidity test, HIV viral load test, and antiretroviral test results, Population-based HIV Impact Assessment, 2015–2019
There were no statistical differences when comparing the 301 participants who were RITA2-recent with the 55 RITA1-recent with detectable ARVs by sex (p=0.2) or age category (p=0.10). However, female RITA1-recent participants with detectable ARVs were significantly older than female RITA2-recent participants without detectable ARVs (p=0.006; Table 1). Participants who were RITA1-recent with detectable ARVs were significantly more likely to report awareness of their HIV-positive status (84% vs. 10%, p<0.001) and to have evidence of HIV ARV drug resistance (74% vs. 26%, p<0.001) compared to RITA2-recent participants. RITA2-recent participants had significantly higher median HIV-1 VL than RITA1-recent participants with detectable ARVs (51,179 vs. 13,905 RNA copies/mL; p<0.001). RITA1-recent participants with detectable ARVs were more likely than RITA2-recent participants to be from countries with ARV coverage ≥70% (52.7% vs. 35.5%, p<0.001).
Table 1.
Characteristics of participants with recent HIV infection identified by laboratory-based antiretroviral detection in the Population-based HIV Impact Assessments, 2015–2019
| Characteristics | ARV not detected | ARV detected | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | P-value* | |
| Male | 91 | (30.2) | 12 | (21.8) | |
| 15–29 years | 35 | (38.5) | 7 | (58.3) | 0.19 |
| 30–59 years | 56 | (61.5) | 5 | (41.7) | |
| Female | 210 | (69.8) | 43 | (78.2) | |
| 15–29 years | 122 | (58.1) | 15 | (34.9) | <0.01 |
| 30–59 years | 88 | (41.9) | 28 | (65.1) | |
| Self-reported HIV-positive status awareness | |||||
| Yes | 31 | (10.3) | 46 | (83.6) | <0.001 |
| No | 270 | (89.7) | 9 | (16.4) | |
| Self-reported ARV status | |||||
| ≥24 months or more | 6 | (2.0) | 21 | (38.2) | <0.01 |
| 12–23 months | 0 | (0.0) | 6 | (10.9) | |
| 5–11 months | 1 | (0.3) | 4 | (7.3) | |
| <5 months | 0 | (0.0) | 11 | (20.0) | |
| ARV use, no date reported | 2 | (0.7) | 3 | (5.5) | |
| No ARV use | 292 | (97.0) | 10 | (18.2) | |
| Median HIV-1 viral load | 51,179 | 13,905 | <0.001 | ||
| Resistance mutation† | |||||
| Any resistance | 71 | (25.8) | 31 | (73.8) | <0.01 |
| Nucleoside reverse transcriptase inhibitor | 9 | (3.3) | 25 | (59.5) | <0.01 |
| Non-nucleoside reverse transcriptase inhibitor | 63 | (22.9) | 28 | (66.7) | <0.01 |
| Protease inhibitor | 7 | (2.5) | 3 | (7.1) | <0.01 |
| National antiretroviral treatment coverage¶ | |||||
| ≥70% | 107 | (35.5) | 29 | (52.7) | 0.02 |
| <70% | 194 | (64.5) | 26 | (47.3) | |
|
| |||||
| Total participants | 301 | 55 | |||
Abbreviations: ARV=antiretroviral
Mantel-Haenszel chi-squared test; Median comparison using Wilcoxon rank-sum test
Genotyping successfully conducted for 317 samples [275 (91.4%) with no ARV detected and 42 (75.4%) with ARV detected].
≥70% ARV countries: Eswatini, Ethiopia, Kenya, Lesotho, Namibia, Rwanda; <70% ARV countries=Cameroon, Côte d’Ivoire, Malawi, Tanzania, Uganda, Zambia, Zimbabwe
HIV-1 incidence comparisons by RITA1 and RITA2 (ARV testing)
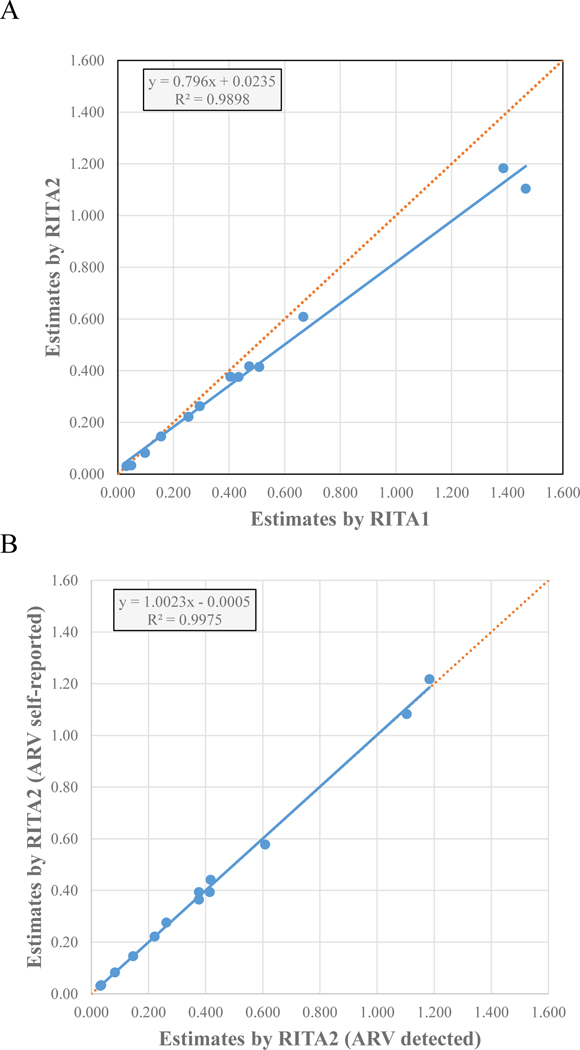
The percent decline in incidence from RITA1 to RITA2 ranged from 0% (Côte d’Ivoire) to 30% (Ethiopia; Table 2). Women had a higher percent change from RITA1 to RITA2-based incidence than men in most countries except Malawi, Namibia, Rwanda, and Zambia (Supplemental Table 1). Participants aged 30–59 years had a higher percent change from RITA1 to RITA2 than participants aged 15–29 years in most countries except Ethiopia, Namibia, Rwanda, Zambia, and Zimbabwe (Supplemental Table 1). There was not a linear relationship between the overall proportion of persons living with HIV receiving ARV and the percent change in incidence from RITA1 to RITA2 (R2=0.25). There was a strong correlation between RITA1 to RITA2-based incidence (R2=0.9898) with an approximate 20% decline for ARV-adjusted incidence using RITA2 (Figure 2A, slope=0.79). Overall, the estimated number of new infections decreased 12.5% from 390,000 to 341,000 across the 13 countries (Table 2).
Table 2.
Recent HIV cases, HIV incidence, HIV prevalence, antiretroviral treatment coverage, estimated annual new cases among persons aged 15–59 years, and percent change in incidence by country by recent infection testing algorithm in the Population-based HIV Impact Assessments, 2015–2019
| LAg/VL (RITA1) | LAg/VL/ARV (RITA2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Country | HIV Prevalence | ARV coverage | Recent cases | Incidence (95% CI) | Annual new cases | Recent cases | Incidence (95%CI) | Annual new cases | Percent change |
| Côte d’Ivoire | 2.7 | 46.0 | 5 | 0.03 (0.00–0.08) | 4,055 | 5 | 0.03 (0.00 – 0.08) | 4,055 | 0.0% |
| Cameroon | 3.7 | 51.2 | 22 | 0.25 (0.12–0.38) | 32,036 | 20 | 0.22 (0.10 – 0.34) | 27,973 | −12.7% |
| Tanzania | 5.0 | 57.1 | 37 | 0.30 (0.18–0.41) | 80,953 | 33 | 0.26 (0.16 – 0.37) | 72,121 | −10.9% |
| Zambia | 12.0 | 62.2 | 45 | 0.67 (0.45–0.88) | 46,982 | 41 | 0.61 (0.41 – 0.81) | 42,855 | −8.8% |
| Uganda | 6.2 | 65.1 | 51 | 0.51 (0.33–0.69) | 89,067 | 42 | 0.42 (0.26 – 0.57) | 72,615 | −18.5% |
| Zimbabwe | 14.1 | 67.6 | 32 | 0.47 (0.29–0.66) | 32,067 | 28 | 0.42 (0.24 – 0.59) | 28,333 | −11.6% |
| Malawi | 10.5 | 69.9 | 29 | 0.41 (0.23–0.58) | 30,080 | 25 | 0.38 (0.20 – 0.55) | 27,974 | −7.0% |
| Lesotho | 25.6 | 74.4 | 48 | 1.47 (0.95–1.99) | 13,066 | 35 | 1.10 (0.68 – 1.52) | 9,835 | −24.7% |
| Kenya | 4.9 | 75.6 | 13 | 0.16 (0.07–0.24) | 38,351 | 11 | 0.15 (0.06 – 0.23) | 35,921 | −6.3% |
| Eswatini | 27.9 | 76.6 | 33 | 1.39 (0.88–1.89) | 6,695 | 27 | 1.18 (0.71 – 1.65) | 5,716 | −14.6% |
| Ethiopia | 3.0 | 76.7 | 6 | 0.05 (0.00–0.10) | 5,827 | 4 | 0.03 (0.00 – 0.08) | 4,062 | −30.3% |
| Rwanda | 3.0 | 81.2 | 10 | 0.10 (0.03–0.16) | 6,438 | 8 | 0.08 (0.02 – 0.14) | 5,419 | −15.8% |
| Namibia | 12.6 | 82.4 | 26 | 0.43 (0.24–0.63) | 5,157 | 22 | 0.38 (0.19 – 0.57) | 4,468 | −13.4% |
|
| |||||||||
| Total | 357 | 390,774 | 301 | 341,347 | |||||
Abbreviations: LAg, HIV-1 Limiting Antigen Avidity test; VL, HIV viral load test; RITA, recent infection testing algorithm; ARV, antiretroviral medication
Incidence estimates were calculated using the formula recommended by the World Health Organization Incidence Working Group and Consortium for Evaluation and Performance of Incidence Assays, with time cutoff (T)=1.0 year, residual proportion false recent (PFR)=0.00, and mean duration of recent infection (MDRI) of 130 days (Eswatini, Lesotho, Malawi, Namibia, Tanzania, Zambia, and Zimbabwe) and 153 days (Uganda). Survey weights are utilized for all estimates.
RITA1= LAg normalized optical density values ≤1.5 + VL ≥1000 RNA copies/mL
RITA2= LAg normalized optical density values ≤1.5 + VL ≥1000 RNA copies/mL + no ARV detected
Figure 2.
Correlation of HIV-1 incidence estimates by RITA adjusting for exposure to ARV. A) Comparison of incidence estimates by RITA1 (LAg ODn ≤1.5 + VL ≥1000 RNA copies/mL) and RITA2 (LAg ODn ≤1.5 + VL ≥1000 RNA copies/mL + no ARV detected) B) Comparison of incidence estimates by RITA2 using ARV detection and RITA2 using self-reported ARV. Solid lines represent linear correlation trendlines with equation parameters and R2 shown for both plots. Dotted lines represent ideal fit with R2=1.0 and slope of 1.0 for comparison purposes.
Abbreviations: LAg, HIV-1 Limiting Antigen Avidity test; VL, HIV viral load test; RITA, recent infection testing algorithm; proportion false recent, PFR; normalized optical density values, ODn; ribonucleic acid, RNA; ARV, antiretroviral medication
When compared to the UNAIDS Spectrum modeled HIV incidence estimates, PHIA incidence estimates using RITA1 from Côte d’Ivoire, Eswatini, Lesotho, Malawi, Namibia, and Tanzania were lower on average by 24% and were higher in Cameroon, Rwanda, Uganda, and Zambia on average by 51% (Table 3). PHIA incidence in Kenya using RITA1 were the same as Spectrum incidence estimates. Using RITA2, HIV incidence estimates in PHIA surveys in Côte d’Ivoire, Eswatini, Kenya, Lesotho, Malawi, Namibia, and Tanzania were lower on average by 34% and were higher in Cameroon, Rwanda, Uganda, and Zambia on average by 29%. Overall, Spectrum modeled estimates were highly correlated with RITA1 (R2=0.956) and RITA2 (R2=0.962).
Table 3.
HIV prevalence and incidence by country among persons aged 15–49 years from the Joint United Nations Programme on HIV and AIDS (UNAIDS) model estimates and the Population-based HIV Impact Assessments, 2015–2019
| Country | PHIA survey data collection (month/year) | UNAIDS incidence estimate* (uncertainty bounds) | PHIA incidence estimate (RITA1) (95% CI) | PHIA incidence estimate (RITA2) (95% CI) | Percent change RITA1 | Percent change RITA2 |
|---|---|---|---|---|---|---|
| Rwanda | 10/18–3/19 | 0.05 (0.04–0.07) | 0.10 (0.03–0.16) | 0.08 (0.02–0.14) | 100 | 60 |
| Côte d’Ivoire | 8/17–3/18 | 0.12 (0.06–0.22) | 0.03 (0.00–0.08) | 0.03 (0.00–0.08) | −75 | −75 |
| Kenya | 6/18–2/19 | 0.16 (0.09–0.29) | 0.16 (0.07–0.26) | 0.15 (0.06–0.24) | 0 | −6 |
| Cameroon | 6/17–2/18 | 0.18 (0.15–0.21) | 0.26 (0.12–0.40) | 0.24 (0.11–0.38) | 44 | 33 |
| Tanzania | 10/16–8/17 | 0.28 (0.25–0.32) | 0.27 (0.16–0.38) | 0.24 (0.14–0.35) | −4 | −14 |
| Uganda | 8/16–3/17 | 0.33 (0.27–0.41) | 0.48 (0.31–0.65) | 0.39 (0.24–0.54) | 45 | 18 |
| Malawi | 11/15–8/16 | 0.51 (0.46–0.57) | 0.36 (0.19–0.53) | 0.33 (0.17–0.49) | −29 | −35 |
| Namibia | 6/17–12/17 | 0.54 (0.45–0.62) | 0.47 (0.25–0.68) | 0.40 (0.19–0.61) | −13 | −26 |
| Zimbabwe | 10/15–8/16 | 0.59 (0.41–0.75) | 0.50 (0.30–0.69) | 0.44 (0.25–0.62) | −15 | −25 |
| Zambia | 3/16–8/16 | 0.61 (0.43–0.89) | 0.70 (0.47–0.93) | 0.64 (0.42–0.86) | 15 | 5 |
| Lesotho | 11/16–5/17 | 1.66 (1.47–1.85) | 1.55 (0.99–2.11) | 1.19 (0.73–1.65) | −7 | −28 |
| Eswatini | 8/16–3/17 | 1.92 (1.77–2.11) | 1.48 (0.93–2.03) | 1.28 (0.77–1.79) | −23 | −33 |
UNAIDSEstimates2019: Country estimates were from the year that the PHIA survey was conducted. The Ethiopia PHIA survey included urban areas only. Urban HIV incidence is not available from the UNAIDS website.
Abbreviations: CI, confidence interval; LAg, HIV-1 Limiting Antigen Avidity test; VL, HIV viral load test; RITA, recent infection testing algorithm; proportion false recent, PFR; normalized optical density values, ODn; ribonucleic acid, RNA; ARV, antiretroviral medication
Incidence estimates were calculated using the formula recommended by the World Health Organization Incidence Working Group and Consortium for Evaluation and Performance of Incidence Assays, with time cutoff (T)=1.0 year, residual PFR=0.00 and mean duration of recent infection (MDRI) of 130 days (Eswatini, Lesotho, Malawi, Namibia, Tanzania, Zambia, and Zimbabwe) and 153 days (Uganda). Survey weights are utilized for all estimates.
RITA1= LAg ODn ≤1.5 + VL ≥1000 RNA copies/mL
RITA2= LAg ODn ≤1.5 + VL ≥1000 RNA copies/mL + no ARV detected
HIV-1 incidence estimates viaself-reported ARV use
Of the 357 RITA1-recent participants, 54 (15.1%) reported ARV use, and 49 participants provided the date of ARV initiation (Table 1). HIV incidence using RITA-2 with self-reported ARV use was highly correlated with RITA-2 using detectable ARVs (Figure 2B). Among the 49 participants who were RITA1-recent and reported ARV use, 11 (22.4%) reported initiating ARV <5 months before the interview, and an additional five (10.2%) reported initiating ARV in the 12 months before the interview (Table 1). Of these 16 participants who reported ARV use, 13 reported an HIV diagnosis in the past 12 months, two reported receiving an HIV diagnosis >12 months before the survey, and one did not recall the date of the last HIV-positive result. HIV incidence using RITA2 with self-reported ARV use, including the 13 participants with self-reported HIV diagnosis and ARV initiation <12 months before the survey interview date (clinical history), was highly correlated with HIV incidence using RITA2 with self-reported ARV use (R2=0.995). The percent change in incidence from RITA2 with detectable ARVs to RITA2 with self-reported ARVs or RITA2 with self-reported ARV initiation <12 months was <10% in all countries except in Cameroon, where change was 15% (data not shown).
DISCUSSION
This report shows the rationale to include ARV detection in RITA2 so that those with detectable ARVs are reclassified as long-term HIV infection. In late 2017, PHIA surveys began using the RITA2 algorithm for incidence calculation based on preliminary results from four PHIA surveys. Our analysis of 13 PHIA countries shows that participants who were RITA1-recent but had detectable ARVs were significantly more likely to report knowing their HIV status and to be receiving ARV for ≥1 year, suggesting that these were long-term infections that were misclassified as recent infections. Further, the disproportionately high level of drug resistance (74%) in this group suggests that unsuppressed VL could be attributable to treatment failure. Although VL was >1000 copies/mL in both groups, median VL was significantly lower in participants with detectable ARV than in those with no detectable ARV. We also found that female RITA1-recent cases with detectable ARVs who had received ARVs for several years were also significantly older than those without detectable ARV and that the likelihood of drug resistance increases over time for participants receiving ARV. Taken together, these results show that participants with detectable ARV have likely been misclassified as having recent infection using the current WHO-recommended RITA (RITA1), and these findings support the recommendation to include ARV results in RITA2 to identify recent HIV infections.10
RITA2 incidence estimates overall were 20% lower than RITA1 incidence estimates but varied by country during the time period of these surveys. The resulting estimated number of new infections decreased by approximately 51,000 cases across the 13 countries. The RITA2 HIV incidence estimates from each PHIA country generally approximated the UNAIDS HIV incidence estimates from the Spectrum models. The PHIA estimates were generally higher for countries with low incidence (Rwanda and Côte d’Ivoire) and lower for countries with high incidence (Lesotho and Eswatini) compared to the UNAIDS Spectrum estimates. The UNAIDS incidence estimates were calculated using various methods and data, and some assumptions and inputs were based in part on the PHIA data.22
All HIV-positive samples were tested for recency using the LAg Avidity-EIA, VL levels, and detection of ARVs to estimate HIV-1 incidence in the PHIA surveys. PFR was assumed to be zero in the PHIA incidence calculations because VL corrected most misclassified cases. Using a specific PFR value would not be justified due to anticipated variations of residual PFR, if any, among populations and subgroups over time.23 Notably, using non-zero PFR has resulted in negative incidence in some sub-populations when incidence is already low.8 Moreover, using a non-zero PFR decreases recent infections arbitrarily but does not improve positive predictive value of recent infection detection by removing misclassified individuals. Therefore, using a non-zero value for PFR cannot be justified across populations. Instead, we identified factors that likely contributed to misclassification. Using ARV detection in the RITA2 as an objective measurable biomarker helps account for variation in misclassification by populations and brings the PFR closer to zero.
There are challenges to including ARV detection in the RITA. The cost of testing is approximately US$50–$60 per specimen. At the time of the surveys, there was only one reference laboratory in Africa that could accurately perform ARV testing. Specimens were submitted and tested in order of survey completion, resulting in delays of several months in ARV-adjusted incidence estimates. This occurred because we sought to conduct ARV testing of all HIV-positive specimens to determine progress toward the UNAIDS 90-90-90 targets. However, using a serial algorithm with ARV testing as the last step of RITA2 (Figure 1) would require testing only a few specimens. This should substantially decrease the cost and shorten the turnaround time.
To overcome challenges of ARV detection, we explored using self-reported ARV exposure instead of ARV detection in the RITA2. HIV incidence estimates using ARV detection were highly correlated with those using self-reported ARV exposure (Figure 2B). However, participants who are included or excluded differ depending on how ARV status is assessed. Nine (3%) of 301 RITA2-recent participants reported receiving HIV treatment although ARVs were not detected, and nine (16%) of 55 RITA1-recent participants with detectable ARVs reported being unaware of their HIV-positive status. As a result, there are small differences in age-specific and sex-specific HIV incidence depending on the method of ARV adjustment (detected or self-reported ARV exposure).
Both RITA1 and RITA2 may misclassify some recent infections as long-term infections because of the presence of ARVs due to expanding Test-and-Start programs. To correct for these misclassified long-term infections due to detectable ARVs, reclassification as recent infection can be based on self-reported HIV testing and clinical history. Among the participants who reported their last HIV-positive result in the 12 months before the survey and who were receiving treatment, most reported starting ARVs in the 4 months before the survey. As global Test-and-Start efforts expand, HIV treatment initiation in an earlier phase of infection will result in increased false-recent classification with LAg Avidity EIA (unpublished data). We found that HIV incidence estimates using RITA2 with or without accounting for testing and clinical history were highly correlated with estimates using PHIA survey data. However, using RITA2, which incorporates accurate testing and clinical history of survey participants with a new HIV diagnosis who are receiving treatment, may be warranted in the future to improve recency classification as the overall coverage and duration of ARV treatment continues to expand.24
Our analysis has several limitations. Although collectively these 13 PHIA surveys represent many recent infections, the number of recent infections in some countries is relatively small (≤10). Therefore, the calculated HIV incidence was inherently imprecise, particularly when stratified by country, age, and sex categories. Additionally, all national HIV incidence estimates, except for Zambia, Lesotho, and Eswatini, were ≤0.5 per 100 person-years. As a result, the percent change comparisons between RITA1 and RITA2 and to UNAIDS Spectrum models for low HIV incidence countries should be interpreted with caution. However, in the absence of a gold standard for measuring incidence in the study populations, the assessment of the best method is based on our finding that removing the ARV-positive persons from the RITA will decrease misclassification.
This report is the most comprehensive evaluation to date that addresses the WHO suggestion of providing additional data before recommending a modification to the current RITA.10 The expansion of national ARV program coverage and longer duration of ARV use among PLHIV will likely result in more drug resistance and misclassification by the RITA1. Our results show that HIV-positive PHIA participants living in countries with >70% ARV program coverage were more likely to have detectable ARVs and were classified as having a long-term infection by RITA2. In this context, accurate incidence estimation through surveys and routine surveillance activities may require inclusion of both clinical test results and reliable patient histories.
Supplementary Material
Acknowledgments
Conflicts of Interest and Sources of Funding: As an inventor of LAg-Avidity EIA, BP receives royalties from sale of test kits sold by the manufacturer per U.S. government policy. There are no other conflicts of interests. This research has been supported by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of cooperative agreements #U2GGH001271 and #U2GGH001226. The findings and conclusions of this document are those of the authors and do not necessarily represent the official position of the funding agencies.
REFERENCES
- 1.90–90-90 An ambitious treatment target to help end the AIDS epidemic. 2014. 2020, at https://files.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2014/90-90-90_en.pdf.)
- 2.Justman JE, Mugurungi O, El-Sadr WM. HIV Population Surveys - Bringing Precision to the Global Response. The New England journal of medicine 2018;378:1859–61. [DOI] [PubMed] [Google Scholar]
- 3.Nkambule RN-BH, Mnisi Z, Ao TT, Ginindza C, Duong YT, Patel H, Saito S, Philip NM, Brown K, Draghi C, Voetsch AC, Mabuza K, Zwane A, Sahabo R, Okello V, Dobbs T, Parekh B, Ryan C, Justman J. Substantial progress in confronting the HIV epidemic in Swaziland: first evidence of national impact. 9th IAS Conference on HIV Science 2017; Paris, France. p. 564. [Google Scholar]
- 4.Monitoring HIV Impact Using Population-based Surveys. 2015. at https://www.unaids.org/sites/default/files/media_asset/JC2763_PopulationBasedSurveys_en.pdf.)
- 5.Duong YT, Kassanjee R, Welte A, et al. Recalibration of the limiting antigen avidity EIA to determine mean duration of recent infection in divergent HIV-1 subtypes. PloS one 2015;10:e0114947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kassanjee R, Pilcher CD, Keating SM, et al. Independent assessment of candidate HIV incidence assays on specimens in the CEPHIA repository. Aids 2014;28:2439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.When and how to use assays for recent infection to estimate HIV incidence at a population level. 2011. at https://www.who.int/hiv/pub/surveillance/sti_surveillance/en/.)
- 8.Duong YT, Dobbs T, Mavengere Y, et al. Field Validation of Limiting-Antigen Avidity Enzyme Immunoassay to Estimate HIV-1 Incidence in Cross-Sectional Survey in Swaziland. AIDS research and human retroviruses 2019;35:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Technical Update on HIV Incidence Assays for Surveillance and Monitoring Purposes. 2015. at https://www.unaids.org/sites/default/files/media_asset/HIVincidenceassayssurveillancemonitoring_en.pdf.)
- 10.Meeting Report 3–4 March 2018. 2018. at https://www.who.int/diagnostics_laboratory/links/180622_boston_meeting_report.pdf?ua=1.)
- 11.Kim AA, Rehle T. Short Communication: Assessing Estimates of HIV Incidence with a Recent Infection Testing Algorithm That Includes Viral Load Testing and Exposure to Antiretroviral Therapy. AIDS research and human retroviruses 2018;34:863–6. [DOI] [PubMed] [Google Scholar]
- 12.Duong YT, Qiu M, De AK, et al. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies. PloS one 2012;7:e33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koal T, Burhenne H, Romling R, Svoboda M, Resch K, Kaever V. Quantification of antiretroviral drugs in dried blood spot samples by means of liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2005;19:2995–3001. [DOI] [PubMed] [Google Scholar]
- 14.Zhang G, Cai F, de Rivera IL, et al. Simultaneous Detection of Major Drug Resistance Mutations of HIV-1 Subtype B Viruses from Dried Blood Spot Specimens by Multiplex Allele-Specific Assay. Journal of clinical microbiology 2016;54:220–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laeyendecker O, Gray RH, Grabowski MK, et al. Validation of the Limiting Antigen Avidity Assay to Estimate Level and Trends in HIV Incidence in an A/D Epidemic in Rakai, Uganda. AIDS research and human retroviruses 2019;35:364–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welte A, McWalter TA, Barnighausen T. A Simplified Formula for Inferring HIV Incidence from Cross-Sectional Surveys Using a Test for Recent Infection. AIDS research and human retroviruses 2009;25:125–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kassanjee R, McWalter TA, Barnighausen T, Welte A. A new general biomarker-based incidence estimator. Epidemiology 2012;23:721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.PHIA Data Use Manual: Reference Guide for Using Data from the Population-based HIV Impact Assessments. 2019. at https://dms-filesystem.s3.amazonaws.com/uploads/44adf91f274078963217183c84c4ac66/01_PHIA%20Data%20Use%20Manual.pdf.) [Google Scholar]
- 19.Eaton JW, Brown T, Puckett R, et al. The Estimation and Projection Package Age-Sex Model and the r-hybrid model: new tools for estimating HIV incidence trends in sub-Saharan Africa. Aids 2019;33 Suppl 3:S235-S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva2015. [PubMed] [Google Scholar]
- 21.Saito S, Duong YT, Metz M, et al. Returning HIV-1 viral load results to participant-selected health facilities in national Population-based HIV Impact Assessment (PHIA) household surveys in three sub-Saharan African Countries, 2015 to 2016. Journal of the International AIDS Society 2017;20 Suppl 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stover J, Brown T, Puckett R, Peerapatanapokin W. Updates to the Spectrum/Estimations and Projections Package model for estimating trends and current values for key HIV indicators. Aids 2017;31 Suppl 1:S5–S11. [DOI] [PubMed] [Google Scholar]
- 23.Hallett TB, Ghys P, Barnighausen T, Yan P, Garnett GP. Errors in ‘BED’-derived estimates of HIV incidence will vary by place, time and age. PloS one 2009;4:e5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mermin J, Musinguzi J, Opio A, et al. Risk factors for recent HIV infection in Uganda. JAMA : the journal of the American Medical Association 2008;300:540–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.