Key Points
Question
Is parathyroidectomy associated with a lower risk of fracture compared with nonoperative management among older adults with primary hyperparathyroidism (PHPT)?
Findings
This population-based longitudinal cohort study of 210 206 Medicare beneficiaries with PHPT from 2006 to 2017 found that treatment with parathyroidectomy was associated with a lower adjusted risk of any clinical fracture and hip fracture compared with nonoperative management.
Meaning
The findings of this longitudinal cohort study indicate that parathyroidectomy is associated with lower fracture risk among older adults with PHPT and should be considered for this age group.
Abstract
Importance
Primary hyperparathyroidism (PHPT) contributes to the development and progression of osteoporosis in older adults. The effectiveness of parathyroidectomy for reducing fracture risk in older adults is unknown.
Objective
To compare the incidence of clinical fracture among older adults with PHPT treated with parathyroidectomy vs nonoperative management.
Design, Setting, and Participants
This was a population-based, longitudinal cohort study of all Medicare beneficiaries with PHPT from 2006 to 2017. Multivariable, inverse probability weighted Cox proportional hazards and Fine-Gray competing risk regression models were constructed to determine the association of parathyroidectomy vs nonoperative management with incident fracture. Data analysis was conducted from February 17, 2021, to September 14, 2021.
Main Outcomes and Measures
The primary outcome was clinical fracture at any anatomic site not associated with major trauma during the follow-up period.
Results
Among the 210 206 Medicare beneficiaries with PHPT (mean [SD] age, 75 [6.8] years; 165 637 [78.8%] women; 183 433 [87.3%] White individuals), 63 136 (30.0%) underwent parathyroidectomy within 1 year of diagnosis, and 147 070 (70.0%) were managed nonoperatively. During a mean (SD) follow-up period of 58.5 (35.5) months, the unadjusted incidence of fracture was 10.2% in patients treated with parathyroidectomy. During a mean (SD) follow-up of 52.5 (33.8) months, the unadjusted incidence of fracture was 13.7% in patients observed nonoperatively. On multivariable analysis, parathyroidectomy was associated with lower adjusted rates of any fracture (hazard ratio [HR], 0.78; 95% CI, 0.76-0.80]) and hip fracture (HR, 0.76; 95% CI, 0.72-0.79). At 2, 5, and 10 years, parathyroidectomy was associated with adjusted absolute fracture risk reduction of 1.2% (95% CI, 1.0–1.4), 2.8% (95% CI, 2.5–3.1), and 5.1% (95% CI, 4.6–5.5), respectively, compared with nonoperative management. On subgroup analysis, there were no significant differences in the association of parathyroidectomy with fracture risk by age group, sex, frailty, history of osteoporosis, or meeting operative guidelines. Fine-Gray competing risk regression confirmed parathyroidectomy was associated with a lower probability of any fracture and hip fracture when accounting for the competing risk of death (HR, 0.84; 95% CI, 0.82-0.85; and HR, 0.83; 95% CI, 0.80-0.85, respectively).
Conclusions and Relevance
This longitudinal cohort study found that parathyroidectomy was associated with a lower risk of any fracture and hip fracture among older adults with PHPT, suggesting a clinically meaningful benefit of operative management in this population.
This population-based, longitudinal cohort study of all Medicare beneficiaries with primary hyperparathyroidism compares the incidence of clinical fracture among older patients treated with parathyroidectomy vs nonoperative management.
Introduction
Primary hyperparathyroidism (PHPT) is a common endocrine disorder among older adults that is associated with the development and progression of osteoporosis1,2 in addition to kidney stones,3 declining kidney function,4 and impaired quality of life.5 The condition primarily affects patients 65 years and older in the US, and its prevalence has been increasing during the past 3 decades.6 Parathyroidectomy is the only treatment for PHPT and is recommended for all patients with symptomatic disease and for asymptomatic patients who meet consensus operative criteria.7 Guidelines for operative management have expanded during the past 20 years, coinciding with advancements in imaging for parathyroid localization and minimally invasive operative techniques. However, the vast majority of older adults with PHPT are observed nonoperatively, and rates of parathyroidectomy among this population have been declining over time.8,9 Low utilization of parathyroidectomy is likely related to the absence of high-quality comparative effectiveness studies of parathyroidectomy among older adults with PHPT, especially among those in their 70s and 80s.
Primary hyperparathyroidism is associated with an increased risk of fractures.10 Randomized clinical trials have shown that parathyroidectomy results in improvements in bone mineral density, while nonoperative management is associated with declining bone mass over time.1,2,11 There are limited data to indicate whether this translates into fracture risk reduction for older adults specifically. A large observational study of patients with PHPT11 showed that parathyroidectomy was associated with a reduced risk of fractures compared with observation or treatment with bisphosphonates; however, the operatively managed cohort was much younger than the nonoperatively managed cohort, and the analysis did not account for relevant competing risks. Therefore, the findings cannot be extrapolated to make treatment decisions for older adults, who are the predominant population diagnosed with PHPT.
The aim of this study was to determine if parathyroidectomy is independently associated with a reduced risk of clinical fracture in older adults with PHPT using methods that control for treatment selection bias and account for the competing risk of death. We also analyzed short- and long-term absolute fracture risk reduction and heterogeneity of treatment effects among subgroups of interest, including those individuals with and without osteoporosis. We hypothesized that the benefits of parathyroidectomy associated with fracture risk reduction would also be evident among older adults and that the time horizon to benefit from parathyroidectomy could be established to inform treatment decisions among this population.
Methods
We performed a population-based longitudinal cohort study of all Medicare beneficiaries diagnosed with PHPT using 100% Medicare fee-for-service claims from January 1, 2006, to December 31, 2017, including outpatient claims, carrier claims, Medicare Provider Analysis and Review files, and Master Beneficiary Summary File files. Claims were accessed through the Virtual Research Data Center housed on a secure server managed by the Centers for Medicare & Medicaid Services (CMS).
The institutional review board at Stanford University approved this study and waived the need for informed consent because this was research involving minimal risk to participants that could not be carried out practicably without the waiver. This study was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement guidelines for observational research.12
Study Population
Details on the cohort creation are available in a previously published article.9 In brief, patients 65 years and older with incident diagnosis of PHPT were identified by the International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9) or Tenth Revision (ICD-10) diagnosis codes for PHPT (252.01, E21.0) appearing on any of their Medicare insurance claims during the study period (2006-2017). Patients were excluded based on codes indicating possible secondary or tertiary hyperparathyroidism or prior parathyroidectomy; missing demographic information (age, sex, race or ethnicity); or having a mailing zip code outside of the US or unavailable in US Census or Area Deprivation Index (ADI) data. Included patients were required to have at least 12 months of continuous enrollment in Medicare Parts A and B before and after PHPT diagnosis to allow ascertainment of preexisting medical comorbidities or PHPT and to identify patients treated with parathyroidectomy within 1 year of diagnosis. Beneficiaries enrolled in Medicare Part C (Medicare Advantage Plans) were excluded from the analyses to ensure complete evaluation of claims.
Treatment for PHPT
Patients were assigned to the parathyroidectomy treatment group if they were treated with parathyroidectomy within 1 year of diagnosis. All other patients were included in the nonoperative management group, including those who underwent delayed parathyroidectomy; the goal was to mimic an intention-to-treat analysis. We identified patients who underwent parathyroidectomy by using the related ICD-9 codes (06.81, 06.89, 06.99), ICD-10 codes (0GBLxxx, 0GBMxxx, 0GBNxxx, 0GBPxxx, 0GBQxxx, 0GBRxxx), and Current Procedural Terminology codes (60500, 60502, 60505) on claims from all health care settings. The treatment date for the parathyroidectomy group was the date of surgery. For the nonoperatively managed group, a random treatment date was generated for each beneficiary. These treatment dates had the same overall distribution as those of the parathyroidectomy group to ensure that follow-up times would be of similar duration.
Outcomes
The primary outcome of interest was incident clinical fracture at any site. The secondary outcome of interest was incident clinical hip fracture. Fractures were identified using a modified claims-based algorithm13 that has been shown to have high validity in Medicare claims when compared with gold standard medical record review (positive predictive value >85% for all fracture sites). Fractures associated with major accidents (eg, motor vehicle accidents, strike by falling object, accidents caused by firearms) were excluded based on ICD-9 and ICD-10 external cause-of-injury codes (E-codes). Fractures associated with falls were identified using E-codes and were included because patients with PHPT-associated osteopenia or osteoporosis may be at higher risk of fractures associated with falls. We assessed the harms of parathyroidectomy by analyzing the occurrence of recurrent laryngeal nerve injury based on relevant ICD-9 and ICD-10 diagnosis codes or tracheostomy procedure codes within 6 months of surgery.
Covariates
Demographic characteristics (age, sex, race and ethnicity) and information on place of residence (ADI, urban/rural area) were assessed at the time of PHPT diagnosis (cohort entry). Mailing address zip code was used to estimate each patient’s socioeconomic status based on area deprivation index (ADI) score, a validated measure of neighborhood disadvantage in the US.14,15 Beneficiary mailing zip code was also used to classify urban vs rural inhabitance based on US Census Bureau data, as previously described.9,16 Comorbidities and frailty were assessed during a 1-year period before treatment. We used the Charlson-Deyo Comorbidity Index to assess patient comorbidity.17,18 A validated claims-based frailty index using the deficit accumulation model was used to classify patients as robust, prefrail, mildly frail, or moderately-to-severely frail.19 A history of stage 3 chronic kidney disease (CKD) or kidney stones, both of which are associated with PHPT and are indications for parathyroidectomy based on multidisciplinary guidelines,7 was identified based on ICD-9 and ICD-10 diagnosis codes from all available claims prior to treatment. Patients were identified as having received endocrinologist specialty care 6 months before or after treatment based on at least 1 outpatient or carrier claim with a CMS provider specialty code indicating subspecialty training in endocrinology and a diagnosis code for PHPT or related sequelae (eg, hypercalcemia, kidney stones, osteoporosis).
Risk factors for fractures included as covariates were a history of osteoporosis, a prior fracture, tobacco use, alcohol use disorder, and obesity. History of osteoporosis was identified based on the CMS Chronic Conditions Data Warehouse “osteoporosis ever” indicator variable, which is based on ICD-9 and ICD-10 codes for osteoporosis, evaluated from all available claims before the treatment date.20 Beneficiaries with prior fractures were identified by applying the fracture algorithm described13 to all claims before the treatment date. Tobacco use, alcohol use disorders, and obesity were identified using CMS Chronic Conditions Data Warehouse indicator variables with these names in the year of treatment, which have reference periods of 2 years of claims.20 We conducted a subgroup analysis of patients with continuous enrollment in Medicare Part D (prescription drug coverage) for 1 year before treatment to account for pharmacologic therapies that affect fracture risk and the use of cinacalcet, a calcimimetic that lowers serum calcium but has not been associated with improvement in bone mineral density in patients with PHPT.21 Beneficiaries were classified as receiving long-term steroid treatment according to Medicare Part D claims for oral glucocorticoids (prednisone, prednisolone, methylprednisolone, hydrocortisone, dexamethasone, or budesonide) if they had filled prescriptions for more than a 180-day supply in the year before treatment. Patients receiving pharmacologic therapy for osteoporosis were identified using National Drug Code and the Healthcare Common Procedure Coding System codes from claims for bisphosphonates (alendronate, risedronate, ibandronate, zoledronate, pamidronate), denosumab, parathyroid hormone and parathyroid hormone-related protein analogs (teriparatide, abaloparatide), and selective estrogen receptor modulators (raloxifene) at any time before treatment.
Statistical Analysis
The analytic approach used in this study sought to identify whether parathyroidectomy is independently associated with the rate of incident clinical fractures after adjusting for patient factors that could be confounders or contribute to treatment selection bias and accounting for the competing risk of death. Univariate comparisons were performed using χ2 and Student t tests.
To reduce treatment selection bias, we performed propensity score weighting and estimated the average treatment effect using the weighted population. For the propensity score model, we selected a priori demographic and clinical characteristics that likely influence receipt of parathyroidectomy and development of the primary outcome (ie, incident clinical fracture) including: age; sex; race or ethnicity; Charlson-Deyo Comorbidity Index; frailty; ADI; urban or rural residence; history of stage 3 CKD, kidney stones, or osteoporosis at any point before PHPT diagnosis; endocrinologist care; and additional fracture risk factors (ie, prior fracture, tobacco use disorder, alcohol use disorder, obesity). Standard mean differences (SMDs) were used to assess covariate balance between patients who underwent parathyroidectomy and patients who were observed nonoperatively after inverse probability weighting22; SMDs greater than 10% were considered meaningful differences. We then calculated inverse probability weighted (IPW) cause-specific Cox proportional hazard time-to-event regression models to estimate the association between parathyroidectomy and the primary and secondary outcomes (ie, incident fracture and incident hip fracture). To assess if parathyroidectomy is associated with a decreased risk of fracture when accounting for the competing risk of death, IPW Fine-Gray competing risk regression models were calculated as well.23,24 We doubly adjusted for all demographic and clinical factors that were included in the propensity score model.25 Patients were censored at loss of Medicare coverage for both treatment groups, and death was accounted for as a competing risk in the Fine-Gray analysis. We included interaction terms to test for effect modification of treatment by a priori specified subgroups (sex, age group, frailty, history of osteoporosis, and meeting operative guidelines for parathyroidectomy based on a history of stage 3 CKD, kidney stones, or osteoporosis at diagnosis) and generated hazard ratios (HRs) for parathyroidectomy vs nonoperative management within each specified subgroup. As a sensitivity analysis to assess the likelihood of residual confounding, we calculated E-values for the primary and secondary outcomes, as previously described.26,27 Statistical significance was assessed at α = .05 and all analyses were 2-tailed. Statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc) from February 17, 2021, to September 14, 2021.
Results
We identified 210 206 Medicare beneficiaries diagnosed with PHPT from January 1, 2006, to December 31, 2017 (eFigure in the Supplement). The mean (SD) age of the study cohort was 75 (6.8) years; 165 637 (78.8%) participants were women; 453 (0.2%) were American Native; 1953 (0.9%), Asian; 19 797 (9.4%), Black; 2280 (1.1%), Hispanic; and 83 433 (87.3%), White (Table 1). Of the cohort, 63 136 (30.0%) patients underwent parathyroidectomy and 147 070 (70.0%) were observed nonoperatively within 1 year. Patients treated with parathyroidectomy were younger compared with the nonoperative group (mean age, 73.5 vs 76.0 years; P < .001), and more likely to be White, nonfrail, and have a lower comorbidity burden (Table 1). Patients in the operatively managed group were less likely to have a history of osteoporosis and prior fracture than patients in the nonoperatively managed group (41.6% vs 44.1% and 7.4% vs 9.9%, respectively; P < .001). A total of 12 089 patients underwent delayed parathyroidectomy during follow-up, which occurred an average of 35 months after PHPT diagnosis. There was no clinically significant difference in the mean age of patients who underwent delayed (73.9 years) vs early (73.5 years) treatment with parathyroidectomy. Baseline characteristics were well-balanced between treatment groups after propensity score IPW.
Table 1. Baseline Characteristics of Medicare Beneficiaries With Primary Hyperparathyroidism (2006-2017).
| Characteristic | Unadjusted, No. (%) | SMD | IPW-adjusted, %a | SMD | ||||
|---|---|---|---|---|---|---|---|---|
| Overall cohort | Parathyroidectomy | Nonoperative | Overall cohort | Parathyroidectomy | Nonoperative | |||
| Total No. | 210 206 | 63 136 | 147 070 | NA | 210 206 | 63 136 | 147 070 | NA |
| Sex | ||||||||
| Male | 44 569 (21.2) | 13 684 (21.7) | 30 885 (21.0) | −0.016 | 21.1 | 20.9 | 21.1 | 0.007 |
| Female | 165 637 (78.8) | 49 452 (78.3) | 116 185 (79.0) | 0.016 | 78.9 | 79.1 | 78.9 | −0.007 |
| Age group, y | ||||||||
| 66-75 | 117 960 (56.1) | 42 024 (66.6) | 75 936 (51.6) | −0.307 | 56.0 | 55.8 | 56.1 | 0.005 |
| 76-85 | 73 491 (35.0) | 19 010 (30.1) | 54 481 (37.0) | 0.147 | 35.1 | 35.2 | 35.0 | −0.005 |
| ≥86 | 18 755 (8.9) | 2102 (3.3) | 16 653 (11.3) | 0.310 | 8.9 | 8.9 | 8.9 | 0.000 |
| Race or ethnicity | ||||||||
| American Native | 453 (0.2) | 150 (0.2) | 303 (0.2) | −0.007 | 0.2 | 0.2 | 0.2 | −0.001 |
| Asian | 1953 (0.9) | 390 (0.6) | 1563 (1.1) | 0.049 | 0.9 | 1.0 | 0.9 | −0.005 |
| Black | 19 797 (9.4) | 4741 (7.5) | 15 056 (10.2) | 0.096 | 9.5 | 9.6 | 9.4 | −0.007 |
| Hispanic | 2280 (1.1) | 480 (0.8) | 1800 (1.2) | 0.047 | 1.1 | 1.1 | 1.1 | 0.000 |
| White | 183 433 (87.3) | 56 888 (90.1) | 126 545 (86.0) | −0.126 | 87.2 | 87.0 | 87.2 | 0.007 |
| Unknown | 2290 (1.1) | 487 (0.8) | 1803 (1.2) | 0.046 | 1.1 | 1.1 | 1.1 | 0.001 |
| ADI group | ||||||||
| Most advantaged | 52 899 (25.2) | 12 717 (20.1) | 40 182 (27.3) | 0.169 | 25.1 | 25.0 | 25.1 | 0.002 |
| Slightly advantaged | 68 471 (32.6) | 20 784 (32.9) | 47 687 (32.4) | −0.011 | 32.5 | 32.4 | 32.5 | 0.004 |
| Slightly disadvantaged | 65 887 (31.3) | 22 093 (35.0) | 43 794 (29.8) | −0.112 | 31.4 | 31.5 | 31.4 | −0.003 |
| Most disadvantaged | 22 949 (10.9) | 7542 (11.9) | 15 407 (10.5) | −0.047 | 11.0 | 11.1 | 10.9 | −0.005 |
| Geography | ||||||||
| Urban | 178 718 (85.0) | 51 689 (81.9) | 127 029 (86.4) | 0.123 | 84.9 | 84.9 | 85.0 | 0.003 |
| Rural | 31 488 (15.0) | 11 447 (18.1) | 20 041 (13.6) | −0.123 | 15.1 | 15.1 | 15.0 | −0.003 |
| Frailty | ||||||||
| Robust | 72 794 (34.6) | 24 450 (38.7) | 48 344 (32.9) | −0.122 | 34.7 | 34.8 | 34.7 | −0.004 |
| Prefrail | 109 087 (51.9) | 32 866 (52.1) | 76 221 (51.8) | −0.005 | 51.8 | 51.8 | 51.9 | 0.002 |
| Mildly frail | 19 462 (9.3) | 4041 (6.4) | 15 421 (10.5) | 0.147 | 9.3 | 9.3 | 9.3 | 0.000 |
| Moderately-to-severely frail | 8863 (4.2) | 1779 (2.8) | 7084 (4.8) | 0.104 | 4.2 | 4.1 | 4.2 | 0.005 |
| Charlson-Deyo Comorbidity Index score | ||||||||
| 0 | 47 672 (22.7) | 16 201 (25.7) | 31 471 (21.4) | −0.101 | 22.7 | 22.7 | 22.7 | −0.001 |
| 1 | 46 003 (21.9) | 14 515 (23.0) | 31 488 (21.4) | −0.038 | 21.9 | 22.0 | 21.9 | −0.002 |
| ≥2 | 116 531 (55.4) | 32 420 (51.3) | 84 111 (57.2) | 0.117 | 55.4 | 55.3 | 55.4 | 0.002 |
| Indications for parathyroidectomy | ||||||||
| History of osteoporosis | 91 122 (43.4) | 26 247 (41.6) | 64 875 (44.1) | 0.051 | 43.3 | 43.3 | 43.3 | 0.000 |
| History of kidney stones | 30 470 (14.5) | 11 197 (17.7) | 19 273 (13.1) | −0.128 | 14.4 | 14.3 | 14.4 | 0.003 |
| History of stage 3 CKD | 20 435 (9.7) | 4300 (6.8) | 16 135 (11.0) | 0.147 | 9.7 | 9.6 | 9.7 | 0.004 |
| Endocrinologist care ≤6 mo of diagnosis | 98 205 (46.7) | 35 546 (56.3) | 62 659 (42.6) | −0.277 | 46.8 | 47.0 | 46.7 | −0.005 |
| Fracture risk factors | ||||||||
| Prior fracture | 19 222 (9.1) | 4689 (7.4) | 14 533 (9.9) | 0.087 | 9.2 | 9.2 | 9.2 | −0.001 |
| Tobacco use | 9317 (4.4) | 3282 (5.2) | 6035 (4.1) | −0.052 | 4.4 | 4.4 | 4.4 | 0.001 |
| Alcohol abuse disorder | 1715 (0.8) | 457 (0.7) | 1258 (0.9) | 0.015 | 0.8 | 0.8 | 0.8 | −0.002 |
| Obesity | 23 916 (11.4) | 7541 (11.9) | 16 375 (11.1) | −0.025 | 11.3 | 11.3 | 11.4 | 0.003 |
Abbreviations: ADI, area deprivation index; CKD, chronic kidney disease; IPW, inverse probability weighted; SMD, standardized mean difference.
The propensity score used for IPW was based on all characteristics listed.
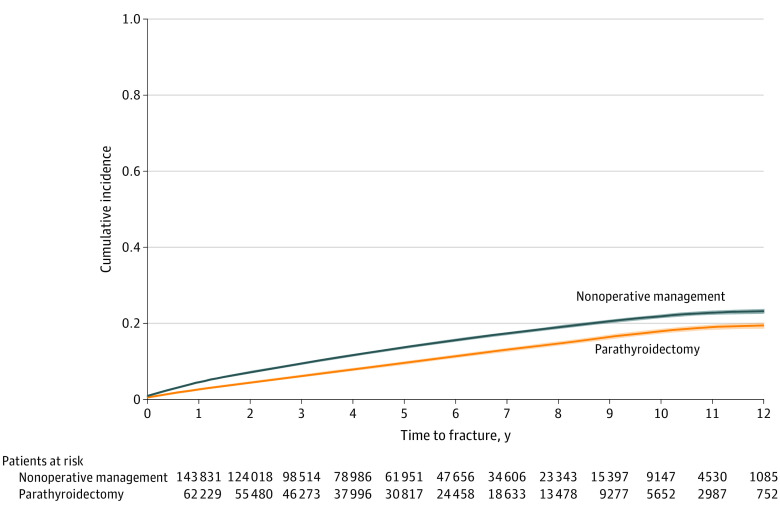
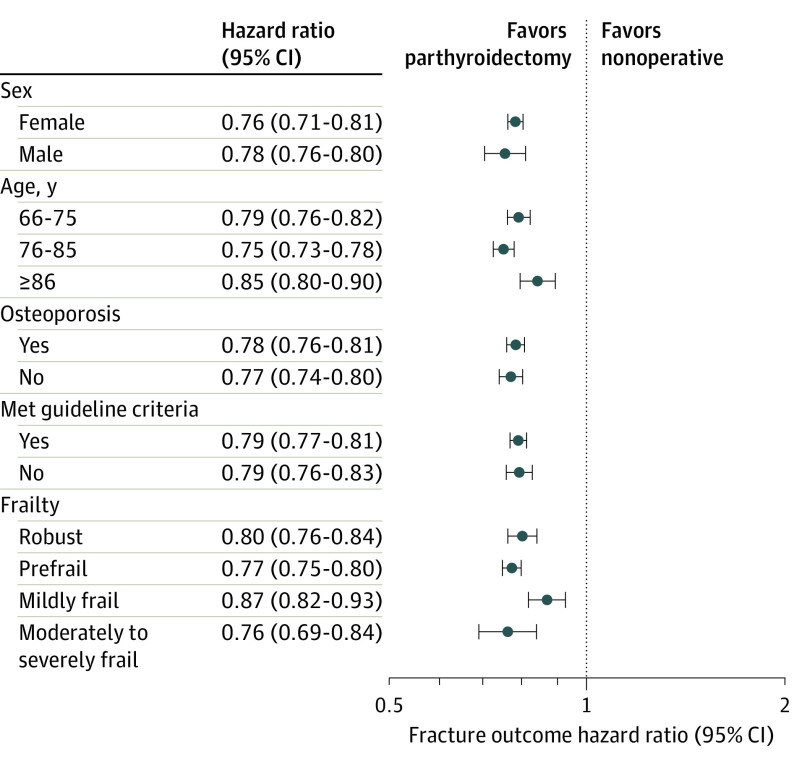
During a mean (SD) follow-up period of 58.5 (35.5) months, 6447 (10.2%) and 1831 (2.9%) patients treated with parathyroidectomy developed any fracture and hip fracture, respectively. With a mean (SD) follow-up period of 52.5 (33.8) months, 20 076 (13.7%) and 6190 (4.2%) patients treated nonoperatively developed any fracture and hip fracture, respectively. Recurrent laryngeal nerve injury was observed in 622 (0.99%) of 63 136 patients treated with parathyroidectomy. On multivariable analysis, parathyroidectomy was associated with lower adjusted rate of any fracture (HR, 0.78; 95% CI, 0.76-0.80) and hip fracture (HR, 0.76; 95% CI, 0.72-0.79) compared with nonoperative management (Table 2). At 1, 2, 5, and 10 years, parathyroidectomy was associated with statistically significant adjusted absolute fracture risk reduction compared with nonoperative management (Table 3). Figure 1 shows the cumulative incidence curve demonstrating the unadjusted incidence of any fracture among the parathyroidectomy and nonoperatively managed treatment groups. Fine-Gray competing risk regression confirmed parathyroidectomy was associated with a lower risk of any fracture (HR, 0.84; 95% CI, 0.82-0.85) and hip fracture (HR, 0.83; 95% CI, 0.80-0.85) when accounting for the competing risk of death (eTable 1 in the Supplement). On stratified subgroup analyses, there were no significant differences in the association of parathyroidectomy with fracture risk by sex, age group, frailty, history of osteoporosis, or meeting guideline criteria for parathyroidectomy (Figure 2). The E-value analyses demonstrated that an unmeasured confounder would need to have a minimum association (HR) of 1.88 (95% CI, 1.81) for any fracture and 1.96 (95% CI, 1.85) for hip fracture with both the treatment and the outcome, conditional on the measured covariates, to negate the observed treatment effect.
Table 2. Unadjusted Incidence Rate and Adjusted Rate of Any Fracture and Hip Fracture Based on Inverse-Probability Weighted Cox Proportional Hazards Regression Among Medicare Beneficiaries With Primary Hyperparathyroidisma.
| Characteristic | Any fracture | Hip fracture | |||
|---|---|---|---|---|---|
| Unadjusted incidence rate (per 100 person-y) | Cox proportional hazards regression, IPW adjusted HR (95% CI) |
Unadjusted incidence rate (per 100 person-y) |
Cox proportional hazards regression, IPW adjusted HR (95% CI) |
||
| Treatment | |||||
| Nonoperative management | 3.19 | 1 [Reference] | 0.90 | 1 [Reference] | |
| Parathyroidectomy | 2.15 | 0.78 (0.76-0.80) | 0.57 | 0.76 (0.72-0.79) | |
| Sex | |||||
| Male | 1.66 | 1 [Reference] | 0.52 | 1 [Reference] | |
| Female | 3.17 | 1.41 (1.36-1.47) | 0.86 | 1.28 (1.19-1.37) | |
| Age group, y | |||||
| 66-75 | 1.80 | 1 [Reference] | 0.40 | 1 [Reference] | |
| 76-85 | 3.93 | 1.69 (1.64-1.74) | 1.16 | 2.37 (2.25-2.50) | |
| ≥86 | 6.76 | 2.41 (2.32-2.51) | 2.28 | 4.04 (3.77-4.34) | |
| Race or ethnicity | |||||
| American Native | 2.69 | 0.98 (0.75-1.27) | 0.52 | 0.78 (0.46-1.33) | |
| Asian | 2.12 | 0.70 (0.61-0.80) | 0.55 | 0.73 (0.56-0.93) | |
| Black | 1.21 | 0.44 (0.42-0.47) | 0.36 | 0.42 (0.37-0.47) | |
| Hispanic | 3.01 | 0.90 (0.81-1.01) | 0.87 | 0.79 (0.63-0.98) | |
| White | 3.05 | 1 [Reference] | 0.84 | 1 [Reference] | |
| Unknown | 1.72 | 0.62 (0.53-0.72) | 0.43 | 0.53 (0.39-0.73) | |
| ADI group | |||||
| Most advantaged | 2.91 | 1 [Reference] | 0.77 | 1 [Reference] | |
| Slightly advantaged | 2.86 | 1.03 (1.00-1.07) | 0.79 | 1.09 (1.03-1.16) | |
| Slightly disadvantaged | 2.85 | 1.04 (1.00-1.07) | 0.81 | 1.14 (1.07-1.21) | |
| Most disadvantaged | 2.68 | 1.82 (1.03-1.13) | 0.81 | 1.28 (1.17-1.39) | |
| Geography | |||||
| Urban | 2.86 | 1 [Reference] | 0.79 | 1 [Reference] | |
| Rural | 2.80 | 1.03 (1.00-1.07) | 0.80 | 1.04 (0.98-1.11) | |
| Frailty | |||||
| Robust | 1.74 | 1 [Reference] | 0.49 | 1 [Reference] | |
| Prefrail | 3.12 | 1.40 (1.36-1.45) | 0.85 | 1.29 (1.22-1.37) | |
| Mildly frail | 5.68 | 1.98 (1.89-2.07) | 1.56 | 1.89 (1.74-2.05) | |
| Moderately-to-severely frail | 5.55 | 1.80 (1.70-1.91) | 1.53 | 1.80 (1.62-2.01) | |
| Charlson-Deyo Comorbidity Index score | |||||
| 0 | 2.16 | 1 [Reference] | 0.62 | 1 [Reference] | |
| 1 | 2.67 | 1.10 (1.06-1.14) | 0.75 | 1.07 (0.99-1.15) | |
| ≥2 | 3.25 | 1.16 (1.12-1.20) | 0.89 | 1.11 (1.04-1.18) | |
| Operative indications for parathyroidectomy | |||||
| History of osteoporosis | 4.31 | 1.66 (1.62-1.71) | 1.11 | 1.40 (1.33-1.47) | |
| History of kidney stones | 2.65 | 0.96 (0.92-0.99) | 0.69 | 0.92 (0.86-0.99) | |
| History of stage 3 CKD | 3.03 | 0.93 (0.89-0.98) | 0.91 | 1.08 (1.00-1.17) | |
| Endocrinologist care ≤6 mo of diagnosis | 2.81 | 0.97 (0.95-0.99) | 0.74 | 0.92 (0.88-0.97) | |
| Fracture risk factors | |||||
| Prior fracture | 9.01 | 2.34 (2.26-2.41) | 1.81 | 1.58 (1.48-1.68) | |
| Tobacco use | 3.65 | 1.38 (1.31-1.47) | 1.15 | 1.76 (1.59-1.94) | |
| Alcohol use disorder | 5.12 | 1.54 (1.37-1.74) | 1.34 | 1.55 (1.24-1.94) | |
| Obesity | 2.30 | 0.95 (0.90-0.99) | 0.48 | 0.76 (0.69-0.84) | |
Abbreviations: ADI, area deprivation index; CKD, chronic kidney disease; HR, hazard ratio; IPW, inverse probability weighted.
The propensity score used for the IPW was based on all characteristics listed.
Table 3. Adjusted Absolute Risk Reduction of Any Fracture and Hip Fracture Associated With Parathyroidectomy at 1, 2, 5, and 10 Years After Treatmenta.
| Fracture type | Time point, y | Adjusted ARR, % (95% CI) |
|---|---|---|
| Any fracture | ||
| 1 | 0.67 (0.52-0.82) | |
| 2 | 1.2 (1.0-1.4) | |
| 5 | 2.8 (2.5-3.1) | |
| 10 | 5.1 (4.6-5.5) | |
| Hip fracture | ||
| 1 | 0.18 (0.11-0.26) | |
| 2 | 0.36 (0.26-0.46) | |
| 5 | 0.98 (0.81-1.2) | |
| 10 | 2.3 (2.0-2.6) | |
Abbreviations: ADI, area deprivation index; ARR, absolute risk reduction; PHPT, primary hyperparathyroidism.
Inverse probability weighted and adjusted for sex, age group, race and ethnicity, ADI, urban or rural inhabitance, frailty, Charlson-Deyo Comorbidity Index, operative indications for parathyroidectomy (history of osteoporosis, kidney stones, or stage 3 chronic kidney disease), endocrinologist care within 6 months of PHPT diagnosis, prior fracture, tobacco use, alcohol use disorder, and obesity.
Figure 1. Unadjusted Cumulative Incidence of Any Fracture Among Older Adults With PHPT Treated With Parathyroidectomy vs Nonoperative Management, Accounting for the Competing Risk of Death.
PHPT denotes primary hyperparathyroidism.
Figure 2. Adjusted HRs for the Association of Parathyroidectomy vs Nonoperative Management With Any Clinical Fracture According to Sex, Age Group, Osteoporosis History, Meeting Consensus Guideline Criteria for Parathyroidectomy, and Frailty.
The HRs are adjusted for all other characteristics in addition to race and ethnicity, ADI, urban or rural inhabitance, Charlson-Deyo Comorbidity Index, endocrinologist care within 6 months of PHPT diagnosis, prior fracture, tobacco use, alcohol use disorder, and obesity.
ADI denotes area deprivation index; HR, hazard ratio; PHPT, primary hyperparathyroidism; and ADI, area deprivation index.
We performed a secondary analysis of patients with pretreatment Medicare Part D (prescription drug coverage). A total of 110 552 (52.6%) patients had continuous enrollment in Medicare Part D for 1 year before their treatment date and were included in the subgroup analysis accounting for pharmacologic therapy. Among the 54 853 patients with PHPT and a history of osteoporosis, 26 673 (50.5%) received pharmacologic therapy for osteoporosis before treatment, suggesting more severe disease (eTable 2 in the Supplement). In addition, 1408 (1.3%) patients received long-term steroids, and 2757 patients (2.5%) received cinacalcet. On multivariable analysis, parathyroidectomy remained associated with a lower adjusted rate of any fracture (HR, 0.77; 95% CI, 0.74-0.80) and hip fracture (HR, 0.70; 95% CI, 0.68-0.71) compared with nonoperative management after also adjusting for a history of pharmacologic treatment for osteoporosis, long-term steroid use, and cinacalcet use (eTable 3 in the Supplement).
Discussion
In this long-term follow-up of a nationally representative population of older adults with PHPT, we found that treatment with parathyroidectomy was associated with a lower rate of any fracture and hip fracture compared with nonoperative management, with statistically significant absolute fracture risk reduction evident as early as 1 year following treatment. Fracture risk reduction was similar when accounting for the severity of osteoporosis based on a history of treatment with bisphosphonates or other pharmacologic therapy and the competing risk of death. Parathyroidectomy was associated with robust fracture risk reduction in patients of all age groups and frailty categories. In addition, there were no significant differences in the association of parathyroidectomy with fracture risk among women of postmenopausal age and older men, those individuals with and without osteoporosis, or those who met and did not meet operative guidelines, suggesting the skeletal benefits of parathyroidectomy are not limited to specific populations at higher risk of fractures. Overall, the study results show a clinically meaningful association of parathyroidectomy with a lower risk of overall and hip fractures among older adults with PHPT and suggest operative management should be strongly considered in this population to prevent fracture-related morbidity and disability.
Prior research8,9,28,29 has shown that fewer than one-third of older adults with PHPT in the US are initially treated with parathyroidectomy, that life expectancy or meeting operative guideline criteria have little bearing on the rate of operative management, and that delayed parathyroidectomy is rare, which indicates that initial treatment decisions are often final. The findings of this study suggest that the preferential nonoperative management of PHPT in older adults may lead to excess skeletal morbidity that could be prevented with parathyroidectomy. These findings are consistent with randomized clinical trials1,2 demonstrating that parathyroidectomy improves bone mineral density compared with nonoperative management. In addition, the overall findings of the present study and the magnitude of absolute fracture risk reduction associated with parathyroidectomy are consistent with those reported by Yeh and colleagues,11 who assessed fracture outcomes among a cohort of 6272 patients with biochemical diagnosis of PHPT treated with parathyroidectomy, nonoperative management, and bisphosphonates. Given that the mean age of our operative cohort was significantly older than that of the prior study (73.5 years vs median 56 years),11 our results suggest that the benefits of parathyroidectomy related to fracture risk reduction extend to older adults with PHPT.
Fractures are associated with significant morbidity and mortality among older adults. The 1-year mortality rate following hip fracture among this population ranges from 10% to 66% depending on age, comorbid conditions, and functional status.30 In addition, hip fractures contribute to long-term disability and poor quality of life—reducing healthy life expectancy by an average of 2.7%31—and account for more than $5 billion in health care costs per year.32 Parathyroidectomy is generally a low-risk procedure that is often performed in the outpatient setting and has an overall complication rate of 1.1%.33 Although that risk is increased among frail patients and those of very advanced age,33 when weighed against the excess risk of fractures our findings suggest that parathyroidectomy should be strongly considered in older adults with substantial life expectancy who are at low or moderate risk of surgical complications. Among patients with limited life expectancy or high operative risk, the risks of surgery can now be weighed against the benefits using our estimates of short- and long-term absolute fracture risk reduction that are specific to older adults.
Consensus guidelines for the operative management of PHPT recommend parathyroidectomy for patients younger than 50 years and older adults with a T-score of 2.5 or higher (indicating osteoporosis) or asymptomatic vertebral fractures detected by radiography or computed tomography.7 These indications are based largely on expert opinion and randomized clinical trials with surrogate end points (eg, bone mineral density).34 The present study findings that parathyroidectomy was associated with a lower risk of fracture among patients with and without osteoporosis and patients who did and did not meet criteria for operative treatment suggest that these guidelines7 may require new consideration. A more appropriate strategy among older adults with PHPT would be to recommend parathyroidectomy for individuals with a greater than 3-year life expectancy, unless the patient’s frailty suggests that the risks of parathyroidectomy outweigh its benefits.
Strengths and Limitations
The strengths of the analysis include the identification of a large, nationally representative cohort of older adults with PHPT and the use of a validated claims-based algorithm to detect incident clinical fractures not associated with major trauma. The limitations of this study are related to the use of administrative claims data for analysis. Medicare data lack granular clinical information with which to determine the severity of PHPT or associated metabolic complications, and biochemical or bone mineral density data to more closely assess baseline fracture risk, identify patients who met all operative guidelines, or account for selection bias. The absence of these data and the observational nature of our analysis may lead to residual confounding of the association between parathyroidectomy and fracture risk reduction. The E-value analysis suggests a moderate to strong confounder would need to be present to negate the observed treatment effect. Lastly, we chose to include patients who underwent delayed parathyroidectomy in the nonoperatively managed group to mimic an intention-to-treat analysis and reduce treatment selection bias. Larger benefits of parathyroidectomy would have been observed in an as-treated analysis.
Conclusions
Parathyroidectomy was associated with a lower risk of any fracture and hip fracture among older adults with PHPT, suggesting a clinically meaningful benefit of operative management in this population. These benefits were observed in patients of all ages and frailty categories and patients with and without osteoporosis or guideline indications for operative management. Based on these findings, low utilization of parathyroidectomy in older adults requires further evaluation to guide focused interventions that target parathyroidectomy to older adults likely to benefit.
eFigure 1. Cohort Enrollment Criteria
eTable 1. Adjusted hazard of any fracture and hip fracture based on inverse-probability weighted Fine-Gray competing risk regression accounting for the competing risk of death among Medicare beneficiaries with PHPT.
eTable 2. Baseline characteristics of subgroup of older adults diagnosed with PHPT from 2006 – 2017 who have continuous enrollment in Medicare Part D (prescription) coverage for 12 months prior to diagnosis.
eTable 3. Unadjusted incidence rate and adjusted hazard of any fracture and hip fracture based on inverse-probability weighted Cox proportional hazard regression among subgroup of Medicare beneficiaries with PHPT who have continuous enrollment in Medicare Part D (prescription) coverage for 12 months prior to diagnosis.
References
- 1.Ambrogini E, Cetani F, Cianferotti L, et al. Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: a prospective, randomized clinical trial. J Clin Endocrinol Metab. 2007;92(8):3114-3121. doi: 10.1210/jc.2007-0219 [DOI] [PubMed] [Google Scholar]
- 2.Lundstam K, Heck A, Godang K, et al. ; SIPH Study Group . Effect of surgery versus observation: skeletal 5-year outcomes in a randomized trial of patients with primary HPT (the SIPH study). J Bone Miner Res. 2017;32(9):1907-1914. doi: 10.1002/jbmr.3177 [DOI] [PubMed] [Google Scholar]
- 3.Mollerup CL, Vestergaard P, Frøkjaer VG, Mosekilde L, Christiansen P, Blichert-Toft M. Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective follow up study. BMJ. 2002;325(7368):807. doi: 10.1136/bmj.325.7368.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assadipour Y, Zhou H, Kuo EJ, Haigh PI, Adams AL, Yeh MW. End-organ effects of primary hyperparathyroidism: A population-based study. Surgery. 2019;165(1):99-104. doi: 10.1016/j.surg.2018.04.088 [DOI] [PubMed] [Google Scholar]
- 5.Coker LH, Rorie K, Cantley L, et al. Primary hyperparathyroidism, cognition, and health-related quality of life. Ann Surg. 2005;242(5):642-650. doi: 10.1097/01.sla.0000186337.83407.ec [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeh MW, Ituarte PHG, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98(3):1122-1129. doi: 10.1210/jc.2012-4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the fourth international workshop. J Clin Endocrinol Metab. 2014;99(10):3561-3569. doi: 10.1210/jc.2014-1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seib CD, Meng T, Suh I, et al. Undertreatment of primary hyperparathyroidism in a privately insured US population: decreasing utilization of parathyroidectomy despite expanding surgical guidelines. Surgery. 2021;169(1):87-93. doi: 10.1016/j.surg.2020.04.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seib CD, Suh I, Meng T, et al. Patient factors associated with parathyroidectomy in older adults with primary hyperparathyroidism. JAMA Surg. 2021;156(4):334-342. doi: 10.1001/jamasurg.2020.6175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ejlsmark-Svensson H, Rolighed L, Harsløf T, Rejnmark L. Risk of fractures in primary hyperparathyroidism: a systematic review and meta-analysis. Osteoporos Int. 2021;32(6):1053-1060. doi: 10.1007/s00198-021-05822-9 [DOI] [PubMed] [Google Scholar]
- 11.Yeh MW, Zhou H, Adams AL, et al. The relationship of parathyroidectomy and bisphosphonates with fracture risk in primary hyperparathyroidism: an observational study. Ann Intern Med. 2016;164(11):715-723. doi: 10.7326/M15-1232 [DOI] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806-808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright NC, Daigle SG, Melton ME, Delzell ES, Balasubramanian A, Curtis JR. The design and validation of a new algorithm to identify incident fractures in administrative claims data. J Bone Miner Res. 2019;34(10):1798-1807. doi: 10.1002/jbmr.3807 [DOI] [PubMed] [Google Scholar]
- 14.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible—the neighborhood atlas. N Engl J Med. 2018;378(26):2456-2458. doi: 10.1056/NEJMp1802313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu J, Kind AJH, Nerenz D. Area deprivation index predicts readmission risk at an urban teaching hospital. Am J Med Qual. 2018;33(5):493-501. doi: 10.1177/1062860617753063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Census Bureau . Census Bureau Decennial Survey. 2010. Accessed April 2, 2020. https://data.census.gov/cedsci/table?g=0100000US.860000&tid=DECENNIALSF12010.H2&hidePreview=true
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 18.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 19.Kim DH, Glynn RJ, Avorn J, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2019;74(8):1271-1276. doi: 10.1093/gerona/gly197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Medicare & Medicaid Services . Chronic Conditions Data Warehouse. Accessed March 10, 2021. https://www2.ccwdata.org/web/guest/condition-categories
- 21.Peacock M, Bolognese MA, Borofsky M, et al. Cinacalcet treatment of primary hyperparathyroidism: biochemical and bone densitometric outcomes in a five-year study. J Clin Endocrinol Metab. 2009;94(12):4860-4867. doi: 10.1210/jc.2009-1472 [DOI] [PubMed] [Google Scholar]
- 22.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He P, Eriksson F, Scheike TH, Zhang MJ. A proportional hazards regression model for the sub-distribution with covariates adjusted censoring weight for competing risks data. Scand Stat Theory Appl. 2016;43(1):103-122. doi: 10.1111/sjos.12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36(27):4391-4400. doi: 10.1002/sim.7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bang H, Robins JM. Doubly robust estimation in missing data and causal inference models. Biometrics. 2005;61(4):962-973. doi: 10.1111/j.1541-0420.2005.00377.x [DOI] [PubMed] [Google Scholar]
- 26.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Website and R package for computing E-values. Epidemiology. 2018;29(5):e45-e47. doi: 10.1097/EDE.0000000000000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 28.Alore EA, Suliburk JW, Ramsey DJ, et al. Diagnosis and management of primary hyperparathyroidism across the Veterans Affairs Health Care System. JAMA Intern Med. 2019;179(9):1220-1227. doi: 10.1001/jamainternmed.2019.1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeh MW, Wiseman JE, Ituarte PHG, et al. Surgery for primary hyperparathyroidism: are the consensus guidelines being followed? Ann Surg. 2012;255(6):1179-1183. doi: 10.1097/SLA.0b013e31824dad7d [DOI] [PubMed] [Google Scholar]
- 30.Cenzer IS, Tang V, Boscardin WJ, et al. One-year mortality after hip fracture: development and validation of a prognostic index. J Am Geriatr Soc. 2016;64(9):1863-1868. doi: 10.1111/jgs.14237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papadimitriou N, Tsilidis KK, Orfanos P, et al. Burden of hip fracture using disability-adjusted life-years: a pooled analysis of prospective cohorts in the CHANCES consortium. Lancet Public Health. 2017;2(5):e239-e246. doi: 10.1016/S2468-2667(17)30046-4 [DOI] [PubMed] [Google Scholar]
- 32.Adeyemi A, Delhougne G. Incidence and economic burden of intertrochanteric fracture: a Medicare claims database analysis. JB JS Open Access. 2019;4(1):e0045. doi: 10.2106/JBJS.OA.18.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seib CD, Chomsky-Higgins K, Gosnell JE, et al. Patient frailty should be used to individualize treatment decisions in primary hyperparathyroidism. World J Surg. 2018;42(10):3215-3222. doi: 10.1007/s00268-018-4629-3 [DOI] [PubMed] [Google Scholar]
- 34.Wentworth K, Shoback D. Applying the Guidelines for Primary Hyperparathyroidism: the path not taken. JAMA Intern Med. 2019;179(9):1227-1229. doi: 10.1001/jamainternmed.2019.1738 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Cohort Enrollment Criteria
eTable 1. Adjusted hazard of any fracture and hip fracture based on inverse-probability weighted Fine-Gray competing risk regression accounting for the competing risk of death among Medicare beneficiaries with PHPT.
eTable 2. Baseline characteristics of subgroup of older adults diagnosed with PHPT from 2006 – 2017 who have continuous enrollment in Medicare Part D (prescription) coverage for 12 months prior to diagnosis.
eTable 3. Unadjusted incidence rate and adjusted hazard of any fracture and hip fracture based on inverse-probability weighted Cox proportional hazard regression among subgroup of Medicare beneficiaries with PHPT who have continuous enrollment in Medicare Part D (prescription) coverage for 12 months prior to diagnosis.