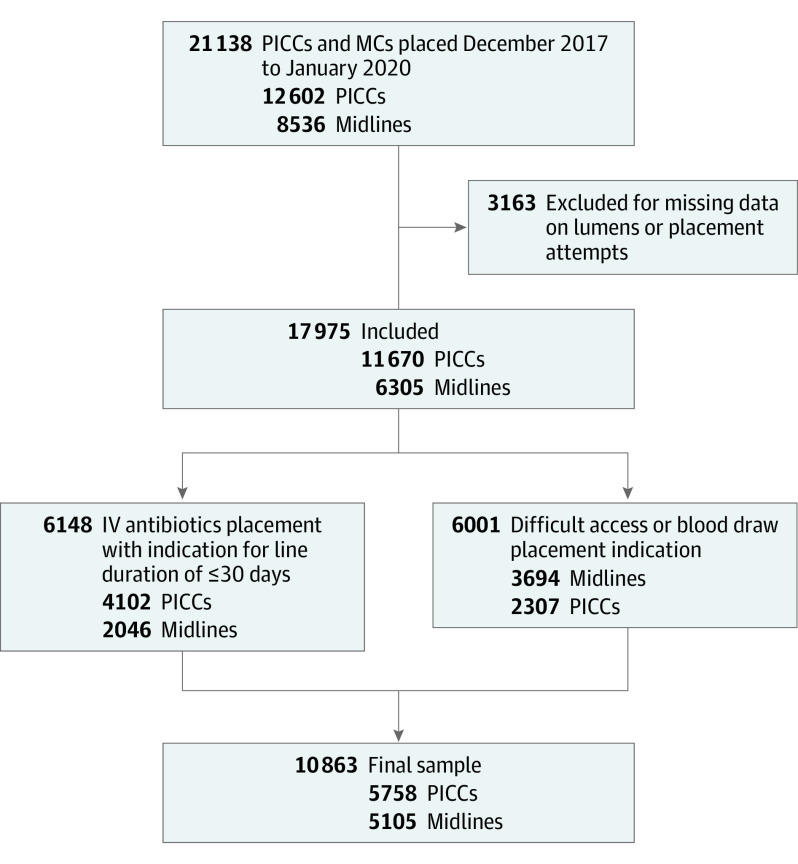
This cohort study compares outcomes of peripherally inserted central catheters vs midline catheters placed for patients with the indication of difficult vascular access or antibiotic therapy for 30 or fewer days.
Key Points
Question
In patients with difficult intravenous access or those who require short-term intravenous antibiotics, are midline catheters safer than peripherally inserted central catheters (PICCs)?
Findings
In this cohort study of 10 863 patients across multiple hospitals who received 5758 PICCs and 5105 midlines for difficult access or short-term antibiotic therapy, midlines were associated with fewer bloodstream infections and catheter occlusions but similar thrombosis events compared with PICCs.
Meaning
Judicious use of midlines over PICCs may improve patient safety; randomized clinical trials to compare these devices appear necessary.
Abstract
Importance
Peripherally inserted central catheters (PICCs) and midlines are frequently used for short-term venous access; whether one is safer than the other in this setting has not been adequately reported.
Objective
To compare outcomes between patients who had a PICC vs midline placed for the indication of difficult vascular access or antibiotic therapy for 30 or fewer days.
Design, Setting, and Participants
This cohort study analyzed data from a multihospital registry including patients admitted to a participating site from December 2017 through January 2020 who had a PICC or midline placement for the indications of difficult venous access or intravenous antibiotic therapy prescribed for 30 or fewer days. Data were analyzed from October 2020 to March 2021.
Exposures
PICC and midline placement.
Main Outcomes and Measures
Major complications, including a composite of symptomatic catheter-associated deep vein thrombosis (DVT), catheter-related bloodstream infection, and catheter occlusion. Logistic regression and Cox proportional hazards regression models (taking into account catheter dwell) were used to estimate risk for major complications, adjusting for patient and device characteristics and the clustered nature of the data. Sensitivity analyses limiting analyses to 10 days of device dwell were performed.
Results
Data on 10 863 patients, 5758 with PICCs and 5105 with midlines (median [IQR] age of device recipients, 64.8 [53.4-75.4] years; 5741 [52.8%] were female), were included. After adjusting for patient characteristics, comorbidities, catheter lumens, and dwell time in logit models, patients who received PICCs had a greater risk of developing a major complication compared with those who received midlines (odds ratio, 1.99; 95% CI, 1.61-2.47). Reduction in complications stemmed from lower rates of occlusion (2.1% vs 7.0%; P < .001) and bloodstream infection (0.4% vs 1.6%; P < .001) in midlines vs PICCs; no significant difference in the risk of DVT between PICCs and midlines was observed. In time-to-event models, similar outcomes for bloodstream infection and catheter occlusion were noted; however, the risk of DVT events was lower in patients who received PICCs vs midlines (hazard ratio, 0.53; 95% CI, 0.38-0.74). Results were robust to sensitivity analyses.
Conclusions and Relevance
In this cohort study among patients with placement of midline catheters vs PICCs for short-term indications, midlines were associated with a lower risk of bloodstream infection and occlusion compared with PICCs. Whether DVT risk is similar or greater with midlines compared with PICCs for short-term use is unclear. Randomized clinical trials comparing these devices for this indication are needed.
Introduction
Reliable venous access is essential in providing safe and effective care for hospitalized patients. In the US, an estimated 150 million peripheral and 5 million central venous catheters (CVCs) are inserted annually.1 Spurred by technological advances, various vascular access devices, including peripherally inserted central catheters (PICCs) and midlines, are available for use during and beyond hospitalization.2,3,4
Peripherally inserted central catheters are CVCs inserted into peripheral veins such that their tips end at the cavoatrial junction near the right atrium. While they can be conveniently placed at the bedside and provide extended venous access, like other central lines, they are associated with complications, including central line–associated bloodstream infection (CLABSI) and deep vein thrombosis (DVT).5 In contrast, midline catheters are peripheral vascular devices inserted in the veins of the upper extremity that terminate at or below the axillary vein, distal to the shoulder. Because they terminate outside the great vessels of the chest, some studies have shown that they are less likely to cause CLABSI or DVT.6,7 The emergence of appropriateness criteria to guide vascular access device use, greater awareness of PICC-related complications, and financial penalties associated with CLABSI have spurred the use of midlines in hospitalized patients.8,9 Numerous studies have reported a decrease in PICC use and complications by substituting midlines in hospitalized patients.1,2,3,10,11,12,13,14
As use of midlines has grown, concerns regarding premature failure and major complications from these devices have also emerged.15 For example, several observational studies have suggested that midline complications, including infection, thrombosis and failure, may rival or outnumber those from PICCs.16,17,18,19 However, almost all these studies are limited by retrospective design, single-center evaluations, and inclusion of heterogeneous patient populations. Thus, for short-term venous access, the optimal device that balances safety and meets clinical needs is unclear.
Given this gap, we used data from a statewide, multihospital quality improvement registry to compare outcomes of patients who received PICCs vs those who received midlines. We hypothesized that use of midline catheters placed specifically for the indications of difficult vascular access or antibiotic therapy for 30 or fewer days would be associated with fewer complications than use of PICCs.
Methods
Study Setting and Design
We used data from the Hospital Medicine Safety Consortium, a 48-hospital collaborative quality initiative supported by Blue Cross Blue Shield of Michigan and Blue Care Network. The design and setting of this consortium have been previously described.20,21 Hospitals belonging to the consortia collect data on a representative sample of hospitalized patients who are admitted to a general medicine ward or intensive care unit and receive a PICC or midline for any reason during clinical care. Patients who are (1) younger than 18 years; (2) pregnant; (3) admitted to nonmedical services (eg, general surgery); or (4) admitted under observation status are excluded. At each hospital, trained data abstractors collect detailed demographic (eg, age, sex, race, ethnicity) and clinical data using a defined protocol directly from patient records. Following PICC and midline placement, all patients are followed up until device removal, death, or 30 days (whichever occurs first). For this study, we included data on patients admitted to a participating site from December 2017 through January 2020. We restricted our sample to patients for whom the indication for PICC or midline use was documented as difficult venous access or short-term intravenous (IV) antibiotic therapy (defined as maximal duration of treatment ≤30 days). We focused on these indications because clinically, these are the 2 primary considerations when it comes to choosing between a PICC or midline in a hospitalized patient.8 To ensure rigor, data related to device indication were collected using 3 sources in the patient’s electronic health record: the device insertion note by the vascular access team or interventional radiologist, the indication for insertion in the medical order, and ordering physician documentation related to the device. Because the purpose of Hospital Medicine Safety Consortium is to improve the quality of care to hospitalized patients, it is deemed not regulated by the institutional review board of the University of Michigan (HUM-00179611). Patient consent was waived because deidentified data were collected.
Covariates and Definitions
Peripherally inserted central catheters were defined as vascular access devices that were inserted in the veins of the upper extremity that terminated in the superior vena cava or right atrium.9 Midlines were defined as vascular access devices inserted in the veins of the upper extremity that terminated in the brachial, basilic, or cephalic veins of the arm at or near the axillary line.3 Thus, short and long peripheral IV catheters placed in the forearm were excluded from this study. Patient demographic data, medical diagnoses, comorbidities, clinical findings, medications, laboratory values at the time of device placement, and duration of infusion therapy were abstracted directly from medical records. Device variables, including indication for insertion, number of insertion attempts, placement arm and vein of insertion, catheter gauge, and number of catheter lumens, were also abstracted from patient records. Catheter dwell time was recorded as days between insertion and removal and was reported as median dwell time with interquartile ranges.
Clinical Outcomes
Complications were defined as a composite of symptomatic upper extremity DVT or pulmonary embolism (PE), CLABSI (in the case of PICCs) or catheter-related bloodstream infection (CRBSI, in the case of midlines), and catheter occlusion. All venous thrombosis events were diagnosed in the setting of clinical suspicion (eg, patients reported arm pain or swelling) with imaging confirming DVT or PE. For patients with PICCs, we defined CLABSI in accordance with the Centers for Disease Control and Prevention/National Healthcare Safety Network criteria as present when a patient had a confirmed positive blood culture with a PICC in place for 48 hours or longer without another identified infection source, or if a PICC tip culture was positive in the setting of clinically suspected catheter infection, or if documentation of bacteremia, sepsis, or CLABSI due to the PICC was found in the medical record.22 Similarly, for midlines, we defined CRBSI as occurring when 1 or more of the following criteria was documented: (1) physician documentation reflecting bloodstream infection or line sepsis or line bacteremia in a patient with a midline; (2) the midline was removed specifically for suspected bloodstream infection; or (3) bloodstream infection was confirmed based on positive blood cultures with no other source of infection and a pathogen known to cause CRBSI either with or without a positive concordant catheter tip culture.23 Catheter occlusion was defined as present when documentation in the medical record indicated occlusion, or when tissue plasminogen activator was administered for problems with device aspiration or infusion.24
Statistical Analysis
Standardized mean differences (SMDs) were used to express differences across the midline and PICC populations. To compare complication rates by device type, we fit a logistic mixed-effect model with hospital-specific random intercepts. We used a published and validated conceptual model to clinically select variables for adjustment.5 Based on this schema, we adjusted for patient characteristics including age; sex; comorbidities; cancer history; recent or remote history of DVT, PE, CLABSI, or CRBSI; and previous CVC placement. For device factors, we adjusted for number of catheter lumens, device dwell time (days), and number of insertion attempts. We created separate models to examine risk of all major complications, then each major complication. Results were expressed as odds ratios (ORs) with 95% CIs. Given that the dwell time of PICCs and midlines may vary and be associated with the risk of complications, we also fit a Cox proportional hazards model for each complication to identify whether variation in catheter dwell time was associated with complication hazards. Results were expressed as hazard ratios (HRs) with 95% CIs. All models were adjusted to account for the clustered nature of the data. Finally, to further account for brief dwell influencing complication hazard, we performed sensitivity analyses and recalculated the baseline model, limiting complications to those occurring within 10 days of line placement. For patients with multiple device placements in the data set, only the first placement occurrence was retained for analysis. All statistical tests were 2-tailed; P < .05 was considered statistically significant. All analyses were performed in SAS, version 9.4 (SAS Institute).
Results
General Characteristics
Data on 5758 PICCs and 5105 midlines placed between December 2017 and January 2020 were available and included for analysis (Figure). The median (IQR) age of device recipients was 64.8 (53.4-75.4) years for both PICC and midline patients, and 5741 (52.8%) were female. Both PICC and midline recipients had similar comorbidity burden (median Charlson score = 3 for both cohorts). Single-lumen devices represented 63.2% of PICCs and 84.9% of midlines. In terms of indications for use, more patients with midlines had these inserted for the indication of difficult IV access or blood draws than PICCs (72.4% vs 40.1%, respectively; SMD = 0.688). Conversely, for the indication for short-term IV antibiotics, more patients had PICCs placed than midlines (72.4% vs 40.1%, respectively; SMD = −0.661). Differences by arm of insertion were observed, with the right arm used for placement in patients with PICCs more frequently than in patients with midlines (71.4% of PICCs and 54.2% of midlines, respectively; SMD = −0.360). The median (IQR) dwell time for PICCs was 14 (7-27) days vs 6 (3-12 ) days for midlines (SMD = −0.776). Patients with midline placement more frequently had a recent history of venous thromboembolism than patients with PICCs (6.7% vs 3.3%, SMD = 0.157). Conversely, with respect to prior bloodstream infection, the prevalence of CLABSI was lower than in patients with PICCs vs CRBSI in patients with midlines (1.0% vs 1.5%; SMD = −0.048) (Table 1).
Figure. Sample Selection.
IV indicates intravenous; MC, midline catheter; PICC, peripherally inserted central catheter.
Table 1. Patient, Device, and Hospital Characteristics (n = 10 863).
| Characteristic | No. (%) | Standardized mean difference | Total, No. (%) | |
|---|---|---|---|---|
| Midline (n = 5105) | PICC (n = 5758) | |||
| Patient characteristics | ||||
| Sex | ||||
| Female | 2969 (58.2) | 2772 (48.1) | 0.202 | 5741 (52.8) |
| Male | 2136 (41.8) | 2986 (51.9) | −0.202 | 5122 (47.2) |
| Race and ethnicitya | ||||
| Asian | 32 (0.6) | 27 (0.5) | 0.022 | 59 (0.5) |
| Black | 2001 (39.2) | 1065 (18.5) | 0.469 | 3066 (28.2) |
| White | 2877 (56.4) | 4373 (75.9) | −0.423 | 7250 (66.7) |
| Age group, ≥65 y | 2524 (49.4) | 2852 (49.5) | −0.002 | 5376 (49.5) |
| Age, median (IQR) | 64.8 (52.8-75.5) | 64.9 (53.8-75.4) | −0.019 | 64.8 (53.4-75.4) |
| Charlson, median (IQR) | 3 (2-5) | 3 (2-5) | 0.041 | 3 (2-5) |
| BMI, median (IQR) | 28.8 (23.9-35.8) | 29.2 (24.2-35.7) | −0.007 | 29.0 (24.1-35.8) |
| Admitted from home | 4499 (88.1) | 4978 (86.5) | 0.050 | 9477 (87.2) |
| Level of care | ||||
| ICU | 1145 (22.5) | 1557 (27.1) | −0.106 | 2702 (24.9) |
| Inpatient medical floor | 3874 (76.1) | 4165 (72.4) | 0.085 | 8039 (74.1) |
| Outpatient/emergency department | 74 (1.4) | 33 (0.6) | 0.08809 | 107 (0.1) |
| Medical history | ||||
| Kidney failure/hemodialysis | 4042 (79.2) | 4800 (83.4) | −0.107 | 8842 (81.4) |
| Hypertension | 3475 (68.1) | 3769 (65.5) | 0.055 | 7244 (66.7) |
| Cerebrovascular disease/CVA/TIA | 3301 (64.7) | 3737 (64.9) | −0.005 | 7038 (64.8) |
| Former/current smoker | 2875 (56.3) | 3344 (58.1) | −0.036 | 6219 (57.2) |
| Peripheral vascular disorders | 1996 (39.1) | 2251 (39.1) | 0.000 | 4247 (39.1) |
| Sepsis | 1950 (38.2) | 2222 (38.6) | −0.008 | 4172 (38.4) |
| Swollen legs | 1764 (34.6) | 2249 (39.1) | −0.094 | 4013 (36.9) |
| Lung disease | 1809 (35.4) | 1892 (32.9) | 0.054 | 3701 (34.1) |
| Hemiplegia/paraplegia | 1575 (30.9) | 1501 (26.1) | 0.106 | 3076 (28.3) |
| Peptic ulcer disease | 1508 (29.5) | 1403 (24.4) | 0.117 | 2911 (26.8) |
| Pneumonia | 1279 (25.1) | 1201 (20.9) | 0.100 | 2480 (22.8) |
| Cancer history | 1003 (19.6) | 1215 (21.1) | −0.036 | 2218 (20.4) |
| Diabetes-uncomplicated | 883 (17.3) | 938 (16.3) | 0.027 | 1821 (16.8) |
| Cellulitis | 605 (11.9) | 1195 (20.8) | −0.108 | 1800 (16.6) |
| Dementia | 625 (12.2) | 920 (16.0) | −0.243 | 1545 (14.2) |
| Osteomyelitis | 188 (3.7) | 904 (15.7) | −0.415 | 1092 (10.1) |
| Venous stasis | 298 (5.8) | 371 (6.4) | −0.025 | 669 (6.2) |
| Moderate/severe liver disease | 229 (4.5) | 345 (6.0) | −0.067 | 574 (5.3) |
| Coagulopathy | 171 (3.3) | 233 (4.0) | −0.037 | 404 (3.7) |
| Lymphoma | 224 (4.4) | 176 (3.1) | 0.070 | 400 (3.7) |
| Life-threatening illness | 167 (3.3) | 10 (0.2) | 0.240 | 177 (1.6) |
| Diabetes-complicated | 2969 (58.2) | 92 (1.6) | −0.049 | 145 (1.3) |
| IBD | 84 (1.6) | 54 (0.9) | 0.063 | 138 (1.3) |
| CLABSI history | 49 (1.0) | 86 (1.5) | −0.048 | 135 (1.2) |
| VTE history | ||||
| Any | 765 (15.0) | 760 (13.2) | 0.051 | 1525 (14.0) |
| <30 d | 341 (6.7) | 188 (3.3) | 0.157 | 529 (4.9) |
| eGFR, median (IQR), mL/min | 65 (45-94) | 63 (54-93) | −0.019 | 64 (50-93.74) |
| WBC count, median (IQR), /μL | 9200 (6700-12 930) | 9000 (6600-12 400) | −0.019 | 9100 (6630-12 630) |
| Hemoglobin, median (IQR), g/dL | 10.10 (8.50-11.80) | 10.20 (8.70-11.80) | 0.006 | 10.20 (8.60-11.80) |
| Platelets, median (IQR), ×103/μL | 230 (165-312) | 242 (170-330) | −0.075 | 236 (168-321) |
| INR, median (IQR) | 1.12 (1.03-1.30) | 1.17 (1.05-1.36) | −0.023 | 1.15 (1.04-1.33) |
| Device characteristics | ||||
| Presence of another CVC | 549 (10.8) | 608 (10.6) | 0.006 | 1157 (10.7) |
| PICC/CVC in prior 6 mo | 712 (13.9) | 1179 (20.5) | −0.174 | 1891 (17.4) |
| Placement attempts | ||||
| 1 | 4456 (87.3) | 5121 (88.9) | −0.051 | 9577 (88.2) |
| ≥2 | 649 (12.7) | 637 (11.1) | 0.051 | 1286 (11.8) |
| Inserted in right arm | 2761 (54.2) | 4107 (71.4) | −0.360 | 6868 (63.3) |
| Power injectable devices | 4388 (86.0) | 5411 (94.0) | −0.269 | 9799 (90.2) |
| Insertion vein | ||||
| Basilic | 2488 (48.7) | 3492 (60.6) | −0.241 | 5980 (55.0) |
| Brachial | 1893 (37.1) | 1873 (32.5) | 0.096 | 3766 (34.7) |
| Other/unknown | 724 (14.2) | 393 (6.8) | −0.241 | 1117 (10.3) |
| Inserted by | ||||
| Vascular access nurse | 4652 (98.1) | 4492 (83.5) | 0.520 | 9144 (90.3) |
| Interventional radiologist | 77 (1.6) | 877 (16.3) | −0.532 | 954 (9.4) |
| Other/unknown | 15 (0.3) | 12 (0.2) | 0.019 | 27 (0.3) |
| Placement indication | ||||
| Short-term antibiotics | 2046 (40.1) | 4102 (71.2) | −0.661 | 6148 (56.6) |
| Chemotherapy | 5 (0.1) | 76 (1.3) | −0.146 | 81 (0.7) |
| Difficult access/blood draws | 3694 (72.4) | 2307 (40.1) | 0.688 | 6001 (55.2) |
| Multiple fluids | 62 (1.2) | 239 (4.2) | −0.183 | 301 (2.8) |
| TPN | 2 (<0.1) | 218 (3.8) | −0.276 | 220 (2.0) |
| No. of device lumens | ||||
| Single | 4334 (84.9) | 3637 (63.2) | 0.512 | 7971 (73.4) |
| Double | 762 (14.9) | 1750 (30.4) | −0.376 | 2512 (23.1) |
| Triple/quadruple | 9 (0.2) | 371 (6.4) | −0.355 | 380 (3.5) |
| Gauge | ||||
| 4F | 4464 (87.4) | 3425 (59.5) | 0.668 | 7889 (72.6) |
| 5F | 638 (12.5) | 2202 (38.2) | −0.619 | 2840 (26.1) |
| ≥6F | 3 (0.1) | 131 (2.3) | −0.208 | 134 (1.2) |
| Device length, median (IQR), cm | 14 (10-16) | 42 (39-46) | −6.601 | 35 (14-43) |
| Device duration, median (IQR), d | 6 (3-12) | 14 (7-27) | −0.776 | 10 (5-20) |
| Device removal (for any complication) | 371 (7.3) | 300 (5.2) | 0.085 | 671 (6.2) |
| Hospital characteristics | ||||
| Bed size, median (IQR) | 342 (273-632) | 372 (250-573) | 0.109 | 348 (255-584) |
| Discharges, median (IQR) | 16 160 (11 072-30 375) | 19 097 (11 241-30 611) | −0.006 | 18 439 (11 241-30 375) |
| Area, rural | 6 (0.1) | 163 (2.8) | −0.226 | 169 (1.6) |
| Profit, for profit | 580 (11.4) | 304 (5.3) | 0.221 | 884 (8.1) |
| Academic, yes | 3589 (70.3) | 3615 (62.8) | 0.160 | 7204 (66.3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CLABSI, central line–associated bloodstream infection; CVA/TIA, cerebrovascular accident/transient ischemic attack; CVC, central venous catheter; eGFR, estimated glomerular filtration rate; IBD, inflammatory bowel disease; ICU, intensive care unit; INR, international normalized ratio; PICC, peripherally inserted central catheter; TPN, total parenteral nutrition; VTE, venous thromboembolism; WBC, white blood cell.
SI conversion factors: To convert hemoglobin to g/L, multiply by 10.0; platelets to ×109/L, multiply by 1.0; WBC count to ×109/L, multiply by 0.001.
Race and ethnicity were indicated as other or unknown for n = 488 (4.5%) of the sample. Data were not available or interpretable because of missing or unclear entries or other categories not listed.
Unadjusted Risk of Complications
Overall, 9.9% (n = 569) of patients with a PICC vs 3.9% (n = 200) of patients with a midline experienced a major complication (SMD = −0.237) (Table 2). The most prevalent complication was catheter occlusion, which occurred in 7.0% (405) of PICCs and 2.1% (105) of midlines, respectively (SMD = −0.240). With respect to bloodstream infection, a total of 93 PICCs (1.6%) met criteria for CLABSI. In comparison, only 19 midlines (0.4%) met criteria for CRBSI (SMD = −0.126). Deep vein thrombosis occurred in 86 patients with PICCs (1.5%) and 74 patients with midlines (1.4%; SMD = −0.003). The incidence of PE was low in both devices, occurring in 14 patients with PICCs and 8 (0.2%) patients with midlines (SMD = −0.018; Table 3). Catheter removal owing to a complication occurred in 7.3% of all midlines (371 of 5105) and 5.2% of PICCs (300 of 578) (Table 3).
Table 2. Overall Frequency of Device Complications Stratified by Indication for Use.
| Outcome | No. (%) | ||
|---|---|---|---|
| Total | Midline | PICC | |
| Short-term IV antibiotic therapy | |||
| No. | 6148 | 2046 | 4102 |
| Any major complication | 433 (7.0) | 71 (3.5) | 362 (8.8) |
| Primary BSI | 77 (1.3) | 5 (0.2) | 72 (1.8) |
| Catheter occlusion | 281 (4.6) | 45 (2.2) | 236 (5.8) |
| DVT | 81 (1.3) | 20 (1.0) | 61 (1.5) |
| Pulmonary embolism | 12 (0.2) | 3 (0.1) | 9 (0.2) |
| Difficult IV access | |||
| No. | 6001 | 3694 | 2307 |
| Any major complication | 426 (7.1) | 146 (4.0) | 280 (12.1) |
| Primary BSI | 42 (0.7) | 15 (0.4) | 27 (1.2) |
| Catheter occlusion | 291 (4.8) | 69 (1.9) | 222 (9.6) |
| DVT | 99 (1.6) | 60 (1.6) | 39 (1.7) |
| Pulmonary embolism | 13 (0.2) | 7 (0.2) | 6 (0.3) |
Abbreviations: BSI, bloodstream infection; DVT, deep vein thrombosis; IV, intravenous; PICC, peripherally inserted central catheter.
Table 3. Device Complications (per 1000 Catheter Days) Stratified by Device Indication.
| Outcome | No./d (rate per 1000 catheter days) | ||
|---|---|---|---|
| Total | Midline | PICC | |
| Short-term IV antibiotic therapy | |||
| No. | 6148 | 2046 | 4102 |
| Any major complication | 433/84 342 (5.1) | 71/19 077 (3.7) | 362/65 265 (5.6) |
| Primary BSI | 77/84 342 (0.9) | 5/19 077 (0.3) | 72/65 265 (1.1) |
| Catheter occlusion | 281/84 342 (3.3) | 45/19 077 (2.4) | 236/65 265 (3.6) |
| DVT | 81/84 342 (1.0) | 20/19 077 (1.1) | 61/65 265 (0.9) |
| Pulmonary embolism | 12/84 342 (0.1) | 3/19 077 (0.2) | 9/65 265 (0.1) |
| Difficult IV access | |||
| No. | 6001 | 3694 | 2307 |
| Any major complication | 426/67 487 (6.3) | 146/31 644 (4.6) | 280/35 843 (7.8) |
| Primary BSI | 42/67 487 (0.6) | 15/31 644 (0.5) | 27/35 843 (0.8) |
| Catheter occlusion | 291/67 487 (4.3) | 69/31 644 (2.2) | 222/35 843 (6.2) |
| DVT | 99/67 487 (1.5) | 60/31 644 (1.9) | 39/35 843 (1.1) |
| Pulmonary embolism | 13/67 487 (0.2) | 7/31 644 (0.2) | 6/35 843 (0.2) |
Abbreviations: BSI, bloodstream infection; DVT, deep vein thrombosis; IV, intravenous; PICC, peripherally inserted central catheter.
Adjusted Risk of Complications
After adjusting for patient characteristics including age; sex; Charlson score; history of cancer; history of CLABSI, DVT, or PE; number of insertion attempts; catheter dwell; number of catheter lumens; and prior CVC placement, patients who received a PICC were almost twice as likely to develop a major complication than patients who received a midline (OR, 1.99; 95% CI, 1.61-2.47). Results were similar across both indications for device insertion. For example, in patients for whom difficult IV access was the indication for device insertion, PICCs were more likely to result in a complication than midlines (OR, 2.04; 95% CI, 1.50-2.78). Similarly, for patients for whom IV antibiotics was the primary indication for use, PICCs were also more likely to result in a complication (OR, 2.12; 95% CI, 1.56-2.88).
With respect to individual complications, risk of both catheter occlusion and bloodstream infection was greater in patients who received PICCs vs midlines. Specifically, PICCs were associated with more than 2-fold odds of occlusion (OR, 2.24; 95% CI, 1.70-2.96) and greater than 4-fold risk of bloodstream infection (OR 4.44; 95% CI, 2.52-7.82) vs midline devices. No significant difference in symptomatic DVT or PE was observed in patients who received either PICCs or midlines (OR, 0.93; 95% CI, 0.63-1.37; and OR, 1.29; 95% CI, 0.46-3.61, for DVT and PE, respectively).
When outcomes were fit using a Cox proportional hazard model to take device dwell into account, higher hazard of major complications including bloodstream infection and catheter occlusion were observed in patients who received PICCs vs midlines consistent with logistic regression models. However, with respect to DVT, a lower hazard of DVT events for PICCs vs midlines (HR, 0.53; 95% CI, 0.38-0.74) was observed, possibly reflecting the higher number of events occurring over fewer total catheter days in midlines compared with PICCs (Table 4).
Table 4. Multivariate Analysis Showing Odds of Major Complications Stratified by Device Typea.
| Outcome | No. (%) | OR (95% CI) | P value | HR (95% CI) | P value | ||
|---|---|---|---|---|---|---|---|
| Total (n = 10 863) | Midline (n = 5105) | PICC (n = 5758) | |||||
| Any major complication | 769 (7.1) | 200 (3.9) | 569 (9.9) | 1.99 (1.61-2.47) | <.001 | 1.21 (1.02-1.44) | .03 |
| Primary BSI | 112 (1.0) | 19 (0.4) | 93 (1.6) | 4.44 (2.52-7.82) | <.001 | 1.76 (1.06-2.92) | .03 |
| Catheter occlusion | 510 (4.7) | 105 (2.1) | 405 (7.0) | 2.24 (1.70-2.96) | <.001 | 1.58 (1.26-1.97) | <.001 |
| DVT | 160 (1.5) | 74 (1.4) | 86 (1.5) | 0.93 (0.63-1.37) | .70 | 0.53 (0.38-0.74) | <.001 |
| PE | 22 (0.2) | 8 (0.2) | 14 (0.2) | 1.29 (0.46-3.61) | .62 | 0.92 (0.36-2.32) | .85 |
Abbreviations: BSI, bloodstream infection; CLABSI, central line–associated blood stream infection; CVC, central venous catheter; DVT, deep vein thrombosis; HR, hazard ratio; OR, odds ratio; PE, pulmonary embolism; PICC, peripherally inserted central catheter.
For logistic mixed-effect models, results were estimated using robust sandwich covariance matrix estimates to account for hospital-level correlation. Patient- and device-level adjustments include age, sex, catheter lumens, line duration, Charlson comorbidity score, previous CVC placements, and history of prior DVT, PE, CLABSI, or cancer. For Cox proportional hazards models, results were adjusted for hospital clustering by calculating SEs using robust sandwich estimates for each site. Patient- and device-level adjustments include age, sex, catheter lumens, line duration, Charlson comorbidity score, previous CVC placements, and history of prior DVT, PE, CLABSI, or cancer.
In sensitivity analyses limiting complications to those occurring in the first 10 days after device placement, greater odds for major complications (OR, 1.45; 95% CI, 1.12-1.88), bloodstream infection (OR, 2.83; 95% CI, 1.37-5.84), and catheter occlusion (OR, 1.66; 95% CI, 1.19-2.32) were observed for PICCs vs midlines consistent with the primary analysis. Findings for DVT did not reach statistical significance (OR, 0.84; 95% CI, 0.54-1.32) (eTable in the Supplement).
Discussion
The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) recommends midlines as the preferred vascular access in patients with difficult vascular access, for treatment that will likely exceed 6 days, and for patients requiring infusions including antibiotics for up to 14 days.9 However, variation in use of midlines across hospitals not explained by illness severity or hospital factors such as bed size or patient volumes is known to exist.6 Additionally, evidence to date comparing complications of PICCs and midlines has been inconclusive and at times contradictory.16,17,25 This multicenter study shows that PICCs appear to have twice the risk of major complications compared with midlines when used for patients who have difficult vascular access or require IV antibiotics for up to 30 days. Thus, our study supports recommendations from MAGIC and other vascular access guidelines that promote midlines as the optimal device for use for these indications. However, given variation in dwell times and the possibility of confounding by indication, head-to-head randomized clinical trials comparing midlines with PICCs for short-term indications are needed to confirm these findings.
We observed that midlines were associated with a significantly lower risk of CRBSI compared with PICCs, even after adjusting for important predictors, including number of lumens and line duration. Some authors have argued that lack of robust surveillance of midline-related bloodstream infections and longer duration of IV access are important confounding variables when it comes to this comparison.7 Because we specifically adjusted for line duration, used rigorous methods for adjudicating bloodstream infection for both devices, and conducted sensitivity analyses to take into account differences in line dwell, our findings help inform this debate. Of note, our results are in line with findings from many US hospitals that have launched midline programs as part of their CLABSI reduction efforts and have also reported positive outcomes with greater substitution of these devices for PICCs.11,14
Catheter-associated DVT is an important and potentially lethal complication of vascular access devices. The literature suggests that rates of midline DVT range from 1% to 4%.6,7 Some studies have found that midlines have a higher risk of developing catheter-associated thrombosis compared with PICCs.17 However, these studies often do not adjust for key confounders when making this comparison. After adjusting for patient, device, and hospital characteristics, our study showed no statistically significant difference between the overall risk of DVT or PE when comparing these 2 devices in logistic regression models. However, when examining time-to-event models, we observed that midlines appeared to be associated with greater daily hazard of DVT, potentially owing to a similar number of events occurring within a shorter catheter dwell time associated with these devices. This finding serves as a reminder to not dismiss the risk of thrombosis associated with midlines, especially in patients with hypercoagulability or preexisting risk factors for DVT (eg, cancer). In view of these findings and absence of randomized clinical trial data, a thoughtful, evidence-based deliberation of the pros and cons of device use before placement is needed in clinical decision-making. Limiting catheter use—both midline and PICC—or reducing days of dwell (especially in the case of midlines) may be vital to ensuring safety.
Prior studies have shown catheter occlusion as a common complication occurring in up to 12% of PICCs in contrast to 4% for midlines.7,26 Our study confirms these findings and shows that PICCs were twice as likely to become occluded vs midlines. While occlusion itself may not be life threatening, they have the potential for treatment delays, are associated with significant declotting costs, and could lead to premature device removal.24 Thus, when considering safety, it is important to not forget about this event, especially from a patient-centered perspective. Our findings suggest that midlines outperform PICCs when it comes to this outcome.
Limitations and Strengths
Our study has several limitations. First, as this is an observational study, unmeasured confounding may affect study conclusions. Second, although the variables included in models were clinically chosen and based on plausible pathways, our models do not imply causation and only show associations between exposure and outcomes. Third, we did not account for difference in manufacturers, coatings, or device-specific features that may be peculiar to various PICCs and midlines. Because these are inherently different devices and such factors are on the causal pathway, adjusting for these explanatory variables would not be appropriate. Finally, our findings should be viewed as preliminary in regard to the debate of midline vs PICCs. Only a well-designed randomized clinical trial comparing the 2 devices head-to-head can definitively evaluate the performance and safety of these devices.
Despite these limitations, our study has important strengths. To our knowledge, this is the largest multicenter study to compare PICC vs midline catheter complications for prespecified indications. Through use of robust statistical techniques, sensitivity analyses, inclusion of patients receiving care in large and small hospitals, manual medical record abstraction to capture and adjudicate findings, and long-term follow-up of device outcomes, our results are clinically relevant and internally valid and lend a high degree of generalizability to various settings. Finally, by validating guidelines recommending midlines as preferred vascular devices for select hospitalized patients, our study also has important implications for clinicians, hospitals, and policy makers alike. These data help affirm the validity of appropriateness criteria when making decisions regarding vascular access in hospitalized patients.
Conclusions
In this cohort study among hospitalized patients who required venous access for the indications of difficult access and IV antibiotics for up to 30 days, PICCs appeared to have twice the rate of major complications compared with midlines. Thoughtful selection between these 2 devices, balancing the risk of venous thrombosis, appears necessary in clinical care. Randomized clinical trials comparing these devices are needed to help inform patient safety.
eTable. Multivariate analysis showing odds of major complications by device type
References
- 1.Magill SS, Edwards JR, Bamberg W, et al. ; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team . Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198-1208. doi: 10.1056/NEJMoa1306801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galen B, Baron S, Young S, Hall A, Berger-Spivack L, Southern W. Reducing peripherally inserted central catheters and midline catheters by training nurses in ultrasound-guided peripheral intravenous catheter placement. BMJ Qual Saf. 2020;29(3):245-249. doi: 10.1136/bmjqs-2019-009923 [DOI] [PubMed] [Google Scholar]
- 3.Adams DZ, Little A, Vinsant C, Khandelwal S. The midline catheter: a clinical review. J Emerg Med. 2016;51(3):252-258. doi: 10.1016/j.jemermed.2016.05.029 [DOI] [PubMed] [Google Scholar]
- 4.Seo H, Altshuler D, Dubrovskaya Y, et al. The safety of midline catheters for intravenous therapy at a large academic medical center. Ann Pharmacother. 2020;54(3):232-238. doi: 10.1177/1060028019878794 [DOI] [PubMed] [Google Scholar]
- 5.Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. doi: 10.1016/j.amjmed.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 6.Chopra V, Kaatz S, Swaminathan L, et al. Variation in use and outcomes related to midline catheters: results from a multicentre pilot study. BMJ Qual Saf. 2019;28(9):714-720. doi: 10.1136/bmjqs-2018-008554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tripathi S, Kumar S, Kaushik S. The practice and complications of midline catheters: a systematic review. Crit Care Med. 2021;49(2):e140-e150. doi: 10.1097/CCM.0000000000004764 [DOI] [PubMed] [Google Scholar]
- 8.Moureau N, Chopra V. Indications for peripheral, midline and central catheters: summary of the MAGIC recommendations. Br J Nurs. 2016;25(8):S15-S24. doi: 10.12968/bjon.2016.25.8.S15 [DOI] [PubMed] [Google Scholar]
- 9.Chopra V, Flanders SA, Saint S, et al. ; Michigan Appropriateness Guide for Intravenouse Catheters (MAGIC) Panel . The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6)(suppl):S1-S40. doi: 10.7326/M15-0744 [DOI] [PubMed] [Google Scholar]
- 10.Lescinskas EH, Trautner BW, Saint S, et al. Use of and patient-reported complications related to midline catheters and peripherally inserted central catheters. Infect Control Hosp Epidemiol. 2020;41(5):608-610. doi: 10.1017/ice.2020.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swaminathan L, Flanders S, Rogers M, et al. Improving PICC use and outcomes in hospitalised patients: an interrupted time series study using MAGIC criteria. BMJ Qual Saf. 2018;27(4):271-278. doi: 10.1136/bmjqs-2017-007342 [DOI] [PubMed] [Google Scholar]
- 12.Nielsen EB, Antonsen L, Mensel C, et al. The efficacy of midline catheters-a prospective, randomized, active-controlled study. Int J Infect Dis. 2021;102:220-225. doi: 10.1016/j.ijid.2020.10.053 [DOI] [PubMed] [Google Scholar]
- 13.Kleinman Sween J, Lowrie A, Kirmse JM, Laughlin RK, Wodziak B, Sampathkumar P. A quality improvement project to decrease utilization of multilumen peripherally inserted central catheters. Infect Control Hosp Epidemiol. 2021;42(2):222-224. doi: 10.1017/ice.2020.411 [DOI] [PubMed] [Google Scholar]
- 14.DeVries M, Lee J, Hoffman L. Infection free midline catheter implementation at a community hospital (2 years). Am J Infect Control. 2019;47(9):1118-1121. doi: 10.1016/j.ajic.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 15.Bundgaard Madsen E, Sloth E, Skov Illum B, Juhl-Olsen P. The clinical performance of midline catheters—an observational study. Acta Anaesthesiol Scand. 2020;64(3):394-399. doi: 10.1111/aas.13516 [DOI] [PubMed] [Google Scholar]
- 16.Hogle NJ, Balzer KM, Ross BG, et al. A comparison of the incidence of midline catheter-associated bloodstream infections to that of central line-associated bloodstream infections in 5 acute care hospitals. Am J Infect Control. 2020;48(9):1108-1110. doi: 10.1016/j.ajic.2019.11.004 [DOI] [PubMed] [Google Scholar]
- 17.Bahl A, Karabon P, Chu D. Comparison of venous thrombosis complications in midlines versus peripherally inserted central catheters: are midlines the safer option? Clin Appl Thromb Hemost. 2019;25:1076029619839150. doi: 10.1177/1076029619839150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Underwood J, Marks M, Collins S, Logan S, Pollara G. Intravenous catheter-related adverse events exceed drug-related adverse events in outpatient parenteral antimicrobial therapy. J Antimicrob Chemother. 2019;74(3):787-790. doi: 10.1093/jac/dky474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campagna S, Gonella S, Zerla PA, et al. The risk of adverse events related to extended-dwell peripheral intravenous access. Infect Control Hosp Epidemiol. 2018;39(7):875-877. doi: 10.1017/ice.2018.79 [DOI] [PubMed] [Google Scholar]
- 20.Chopra V, O’Malley M, Horowitz J, et al. Improving peripherally inserted central catheter appropriateness and reducing device-related complications: a quasiexperimental study in 52 Michigan hospitals. BMJ Qual Saf. 2021;bmjqs-2021-013015. doi: 10.1136/bmjqs-2021-013015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paje D, Rogers MAM, Conlon A, Flanders SA, Bernstein SJ, Chopra V. Use of peripherally inserted central catheters in patients with advanced chronic kidney disease: a prospective cohort study. Ann Intern Med. 2019;171(1):10-18. doi: 10.7326/M18-2937 [DOI] [PubMed] [Google Scholar]
- 22.O’Grady NP, Alexander M, Burns LA, et al. ; Healthcare Infection Control Practices Advisory Committee (HICPAC) (Appendix 1) . Summary of recommendations: guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):1087-1099. doi: 10.1093/cid/cir138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoe DS, Anderson DJ, Berenholtz SM, et al. ; Society for Healthcare Epidemiology of America (SHEA) . A compendium of strategies to prevent healthcare-associated infections in acute care hospitals: 2014 updates. Infect Control Hosp Epidemiol. 2014;35(8):967-977. doi: 10.1086/677216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SN, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: the 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756. doi: 10.1016/j.jvir.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 25.Xu T, Kingsley L, DiNucci S, et al. Safety and utilization of peripherally inserted central catheters versus midline catheters at a large academic medical center. Am J Infect Control. 2016;44(12):1458-1461. doi: 10.1016/j.ajic.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 26.Chopra V, Smith S, Swaminathan L, et al. Variations in peripherally inserted central catheter use and outcomes in Michigan hospitals. JAMA Intern Med. 2016;176(4):548-551. doi: 10.1001/jamainternmed.2015.8402 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Multivariate analysis showing odds of major complications by device type