Abstract
OBJECTIVE:
The purpose of the study was to assess the effect of neutrophil-to-lymphocyte ratio (NLR) on recurrence and survival in patients with Esophageal Squamous Cell Carcinoma (ESCC) undergoing surgery.
METHODS:
This was a retrospective analysis of the 80 resectable ESCC patients who underwent surgery at Yuzuncu Yil University Faculty of Medicine between 2008 and 2018. Receiver operator characteristics curve of NLR was plotted for disease-free survival (DFS). The area under the curve of NLR was 0.692 (p=0.008) with 65.2% sensitivity and 2.8 with 69.5% specificity. Patients were divided into two groups based on the NLR as follows: NLR <2.8 and NLR ≥2.8.
RESULTS:
Among 80 ESCC patients, 54 (65.5%) were female. The median age was 55 years (range, 26–77). The NLR was <2.8 in 47 (58.7%) patients. Median DFS was 55 months in patients with NLR ≥2.8, whereas it was not reached in those with NLR <2.8 (p=0.008), with corresponding overall survival (OS) durations of 71 months and not reached (p=0.027). Eastern Cooperative Oncology Group performance score 2, presence of obstruction at diagnosis, lower 1/3 esophageal localization, neoadjuvant treatment, and NLR ≥2.8 were found to be the factors related to survival.
CONCLUSION:
The present study demonstrated that high pre-treatment NLR was associated with worse DFS and OS in patients with resectable esophageal cancer. We believe that pre-treatment NLR may help guide predicting treatment outcomes in non-metastatic resectable ESCC patients.
Keywords: Esophageal cancer, neutrophil-to-lymphocyte ratio, squamous cell carcinoma
Esophageal cancer (EC) ranks the 8th among all cancers and is the 6th cause of cancer-related deaths worldwide. Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) account for more than 95% of all EC cases. As well as the decline in the incidence of ESCC, the incidence of EAC has increased dramatically over the past few years; however, ESCC still dominates the ES landscape worldwide [1, 2].
At the time of diagnosis, 50–80% of EC patients have locally advanced or metastatic disease. Surgical resection for non-metastatic EC is the basis of curative treatment. Current advances in perioperative treatments, staging methods, and surgical management have improved mortality and morbidity associated with EC. However, despite new advances in diagnostic and therapeutic strategies, patients still have poor prognosis [3, 4].
Patients’ performance status and other clinical features such as weight loss, tumor stage, tumor localization, tumor grade, extent of surgical resection, pre-operative chemoradiotherapy (CRT), and response to pre-operative CRT as well as molecular markers including vascular endothelial growth factor, epidermal growth factor, and p53 have been shown to have prognostic value in EC patients [5–7].
Systemic inflammatory response (SIR) is associated with tumor development, apoptosis inhibition, and angiogenesis. In addition, SIR has been shown to be an important factor, leading to tumor progression and metastasis [8]. The neutrophil-to-lymphocyte ratio (NLR), which is an inflammatory- and immunological-based score, can be easily obtained by dividing the total neutrophil count (TNC) by the total lymphocyte count (TLC). Its prognostic value has previously been investigated in a variety of cancers such as lung, stomach, pancreas, hepatocellular, colorectal, and ovarian cancer [9–11]. The aim of this study was to investigate the relation of pre-treatment NLR with recurrence and survival in operated ESCC patients.
MATERIALS AND METHODS
Study Population
After analyzing the medical records of 430 EC patients who were followed up and treated at the Department of Medical Oncology, Yuzuncu Yil University Faculty of Medicine from 2008 to 2018, a total of 80 patients were included in the final analysis, excluding the patients with the following criteria; age <18 years, benign or malignant hematologic disease, chronic or acute infection, history of immunosuppressive drug use, non-squamous histology (adenocarcinoma, undifferentiated, and small cell carcinoma), unoperated patients, multiple primary tumors, and cases with incomplete data (Fig. 1).
FIGURE 1.
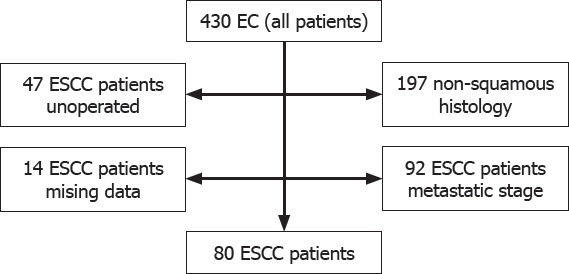
Patient selection for study; a flow diagram.
Data Collection
The demographic features including age, gender, comorbid disease (essential hypertension [HT], diabetes mellitus [DM], chronic obstructive pulmonary disease [COPD], chronic ischemic heart disease [CIHD], and initial symptoms, e.g., obstruction, dysphagia, abdominal pain, and weight loss), Eastern Cooperative Oncology Group performance score (ECOG PS), tumor localization (middle 1/3 vs. lower 1/3), tumor grade, disease stage, neoadjuvant chemoradiotherapy (NACRT), adjuvant treatment, recurrence and site of recurrence, final status (dead or alive) of patients, and initial laboratory data including carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), hemoglobin (Hb), TLC, total monocyte count (TMC), TNC, and total platelet count (TPC), were obtained from hospital medical records. In all patients, we utilized the initial laboratory data which were obtained after the first diagnosis. NLR was calculated by dividing the TNC by TLC. The platelet-to-lymphocyte ratio (PLR) was found by dividing the TPC by TLC. Patients were grouped according to ECOG PS as 0–1 and 2. Tumor localization was classified as middle 1/3 and lower 1/3. Tumor grade was categorized into two groups as good (1) + moderate (2) and poorly differentiated (3). Receiver operator characteristics curve of NLR was plotted for disease-free survival (DFS). The area under the curve of NLR was found to be 0.692 (95% confidence interval [CI] =0.569–0.812, p=0.008) with 65.2% sensitivity and 2.8 with 69.5% specificity (Fig. 2). Patients were categorized into two groups according to NLR as NLR <2.8 and NLR ≥2.8.
FIGURE 2.
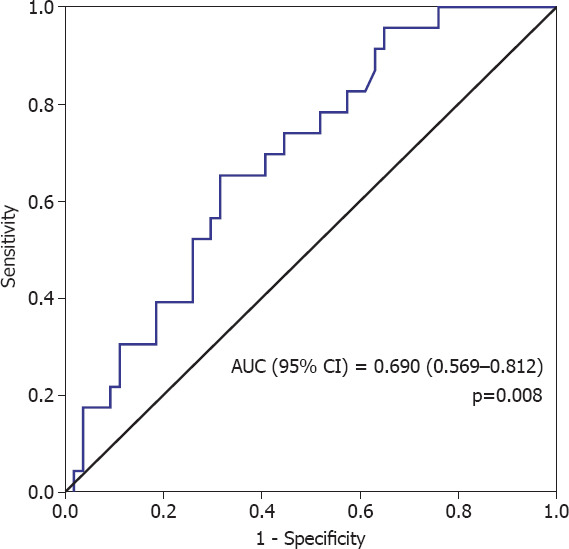
Receiver operator characteristics curve of neutrophil-to-lymphocyte ratio for disease-free survival.
Highlight key points
Esophageal cancer (EC) is the 6th cause of cancer-related deaths worldwide.
At the time of diagnosis, 50–80% of EC patients have locally advanced or metastatic disease.
The neutrophil-to-lymphocyte ratio (NLR), which is an inflammatory- and immunological-based score can be easily calculated by total neutrophil count and the total lymphocyte count.
Systemic inflammatory response has been shown to be an important factor, leading to tumor progression and metastasis.
High pre-operative NLR was associated with worse survival in patients with ESCC.
Statistical Analysis
Statistical Package for the Social Sciences 22.0 for Windows software (Armonk NY, IBM Corp. 2013) was used for all statistical analysis. Statistical alpha significance level was accepted as p<0.05. Student’s t-test was conducted if the numerical variable met the normal distribution condition in two independent groups, whereas Mann–Whitney U-test was performed when the normal distribution condition was not met. Comparison of the rates in the groups was performed using Chi-square analysis. Survival analysis was carried out using Kaplan–Meier method. The determinative factors were examined with Cox regression analysis. Forward stepwise model was used for the factors with p<0.150 values determined in univariate analysis. DFS was defined as the time period from the date of diagnosis until the date of recurrence and overall survival (OS) were defined as the time interval from the date of diagnosis until the date of death or last follow-up.
RESULTS
Among 80 patients, 26 (32.5%) were male and 54 (65.5%) were female, with a median age of 55 years (range, 26–77). There was HT in 12 (15.0%) patients, DM in 3 (3.8%) patients, CIHD in 2 (2.5%) patients, and COPD in 4 (5.0%) patients. Twenty-one (26.3%) patients were active smokers. Presenting symptoms with a decreasing frequency were as follows; dysphagia (93.8), weight loss (23.8%), and obstruction (13.8%). At the time of diagnosis, ECOG PS was 2 in 21 (26.3%) patients. The primary tumor was localized in the lower third of the esophagus in 22 (27.5%) patients. Fourteen (17.5%) patients had Grade 3 tumor. NACRT was administered to 32 (40%) patients. Twenty (25%) patients had pathological Stage III disease. At 37-month median follow-up time, recurrence developed in 23 (28.8%) patients and 26 (32.5%) patients died (Table 1).
TABLE 1.
Clinical and demographic data
| Characteristics | All patients (n=80) % | NLR <2.8 (n=47) % | NLR ≥2.8 (n=33) % | p |
|---|---|---|---|---|
| Gender | 0.894 | |||
| Male | 32.5 | 31.9 | 33.3 | |
| Female | 67.5 | 68.1 | 66.7 | |
| Age (Year) | ||||
| Median (Min–max) | 55 (26–77) | 58 (32–77) | 52 (26–74) | 0.028 |
| HT | ||||
| Yes | 15.0 | 17.0 | 12.1 | 0.752 |
| DM | ||||
| Yes | 3.8 | 6.4 | 0.0 | 0.264 |
| CIHD | ||||
| Yes | 2.5 | 2.2 | 3.0 | 0.664 |
| COPD | ||||
| Yes | 5.0 | 8.5 | 0.0 | 0.139 |
| Smoking | ||||
| Yes | 26.3 | 27.7 | 24.2 | 0.732 |
| Dysphagia | ||||
| Yes | 93.8 | 93.6 | 93.9 | 0.953 |
| Abdominal pain | ||||
| Yes | 12.5 | 8.5 | 18.2 | 0.304 |
| Weight loss | ||||
| Yes | 23.8 | 29.8 | 15.2 | 0.130 |
| Obstruction | ||||
| Yes | 13.8 | 12.8 | 15.2 | 0.754 |
| ECOG-PS | 0.391 | |||
| 0–1 | 73.8 | 70.2 | 78.8 | |
| 2 | 26.3 | 29.8 | 21.2 | |
| Localization | 0.585 | |||
| Middle 2/3 | 72.5 | 70.2 | 75.8 | |
| Lower 1/3 | 27.5 | 29.8 | 24.2 | |
| Grade | 0.137 | |||
| 1+2 | 82.5 | 76.6 | 90.9 | |
| 3 | 17.5 | 23.4 | 9.1 | |
| Neoadjuvant | ||||
| Yes | 40.0 | 40.4 | 39.4 | 0.926 |
| Stage | 0.149 | |||
| I+II | 75.0 | 80.9 | 66.7 | |
| III | 25.0 | 19.1 | 33.3 | |
| Adjuvant treatment | 0.012 | |||
| Yes | 13.2 | 4.7 | 24.2 | |
| Recurrence | ||||
| Yes | 28.8 | 17.0 | 45.5 | 0.006 |
| Site of recurrence | ||||
| Locoregional | 30.4 | 0.0 | 46.7 | 0.040 |
| Lung | 8.7 | 0.0 | 13.3 | |
| Liver | 26.1 | 50.0 | 13.3 | |
| Distant LN | 34.8 | 50.0 | 26.7 | |
| Final status | 0.011 | |||
| Dead | 32.5 | 21.3 | 48.5 | |
| Alive | 67.5 | 78.7 | 51.5 |
CIHD: Chronic ischemic heart disease; DM: Diabetes mellitus; ECOG-PS: Eastern Cooperative Oncology Group Performance Score; HT: Hypertension; LN: Lymph node.
The NLR in 47 (58.7%) patients was <2.8, while it was ≥2.8 in 33 patients. There was no remarkable difference between NLR groups (<2.8 vs. ≥2.8) in regard to CEA, CA19-9, TMC, and TPC, whereas there was statistically significant difference between the groups in terms of TNC, TLC, Hb, and PLR (Table 2).
TABLE 2.
Laboratory data
| Total Mean±SD | NLR <2.8 Mean±SD | NLR ≥2.8 Mean±SD | p | |
|---|---|---|---|---|
| CEA | 2.23±1.64 | 2.36±1.53 | 2.04±1.80 | 0.460 |
| CA19-9 | 9.66±8.71 | 10.25±9.13 | 8.77±8.16 | 0.524 |
| TNC | 4784.67±2175.59 | 3789.78±1193.67 | 6260.96±2466.53 | <0.001 |
| TLC | 1872.20±692.93 | 2147.60±641.78 | 1463.54±556.50 | <0.001 |
| TMC | 526.36±20.36 | 507.82±165.83 | 553.87±243.18 | 0.326 |
| Hb | 13.31±1.95 | 14.02±1.39 | 12.25±2.19 | <0.001 |
| TPC | 260.914.28±99.140.95 | 245.408.69±58.145.36 | 283.922.58±137.966.63 | 0.096 |
| NLR | 3.08±2.46 | 1.85±0.63 | 4.89±3.01 | <0.001 |
| PLR | 158.62±93.81 | 121.59±38.94 | 213.57±121.65 | <0.001 |
SD: Standard deviation; CA19-9: Carbohydrate antigen 19–9; CEA: Carcinoembryonic antigen; Hb: Hemoglobin; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; TLC: Total lymphocyte count; TMC: Total monocyte count; TNC: Total neutrophil count; TPC: Total platelet count; SD: Standard deviation.
Patients with NLR ≥2.8 had mDFS of 55 months (95% CI, 11.2–90.8) versus “not reached” in those with NLR <2.8 (Log rank p=0.008) (Fig. 3), with corresponding mOS of 71 months (95% CI, 12.1–136.0) and “not reached” (Fig. 4) (Log rank p=0.027).
FIGURE 3.
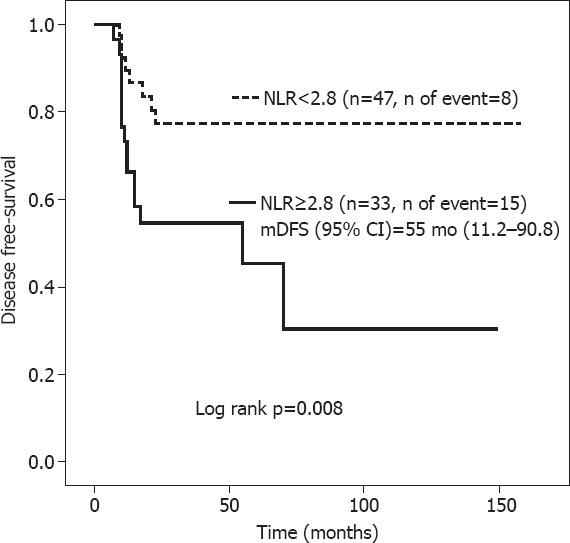
Disease-free survival according to neutrophil-to-lymphocyte ratio.
FIGURE 4.
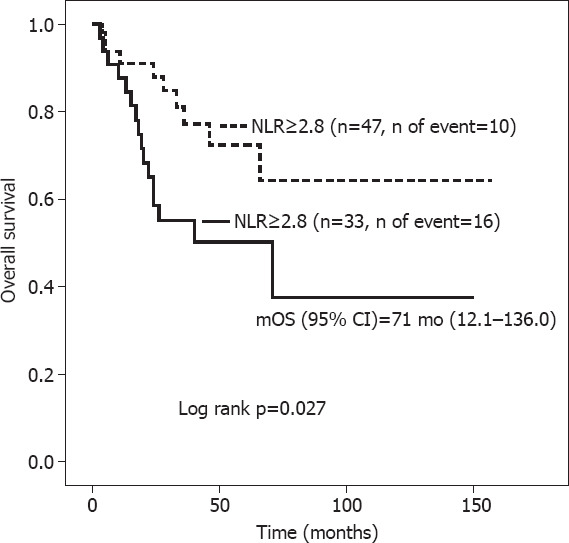
OS according to neutrophil-to-lymphocyte ratio.
In univariate analysis, lower 1/3 localization (hazard ratio [HR], 0.52, 95% CI, 0.45–0.92), NACRT (HR, 0.50, 95% CI, 0.18–0.95), Stage III disease (HR, 2.12, 95% CI, 1.91–4.91), and NLR ≥2.98 (HR, 2.98, 95% CI, 1.26–7.05) were detected as the factors associated with DFS. However, multivariate analysis showed that ECOG PS (HR, 2.99, 95% CI, 1.16–7.71), presence of obstruction at diagnosis (HR, 3.25, 95% CI, 1.13–9.32), lower 1/3 localization (HR, 0.62, 95% CI, 0.42–0.85), NACRT (HR, 0.58, 95% CI, 1.25–5.96), and NLR ≥2.8 (HR, 5.44, 95% CI, 2.03–4.54) were found to be the factors related to DFS (Table 3).
TABLE 3.
Univariate and multivariate analysis for DFS
| Characteristics | Univariate analysis for DFS | Multivariate analysis for DFS | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95.0% CI for HR | p | HR | 95.0% CI for HR | p | |
| Age | ||||||
| Year | 0.988 | 0.955–1.021 | 0.459 | |||
| Gender | ||||||
| Female vs. male | 0.703 | 0.304–1.626 | 0.410 | |||
| ECOG PS | ||||||
| 0+1 vs. 2 | 1.810 | 0.764–4.282 | 0.177 | 2.991 | 1.160–7.713 | 0.023 |
| HT | ||||||
| Yes vs. no | 2.255 | 0.885–5.739 | 0.088 | |||
| DM | ||||||
| Yes vs. no | 0.945 | 0.568–4.321 | 0.440 | |||
| CIHD | ||||||
| Yes vs. no | 3.506 | 0.815–15.080 | 0.092 | |||
| COPD | ||||||
| Yes vs. no | 0.760 | 0.102–5.602 | 0.789 | |||
| Smoking | ||||||
| Yes vs. no | 0.989 | 0.389–2.514 | 0.982 | |||
| Dysphagia | ||||||
| Yes vs. no | 1.961 | 0.902–4.649 | 0.466 | |||
| Abdominal pain | ||||||
| Yes vs. no | 1.727 | 0.584–5.104 | 0.323 | |||
| Weight loss | ||||||
| Yes vs. no | 1.434 | 0.584–3.522 | 0.431 | |||
| Obstruction | ||||||
| Yes vs. no | 2.391 | 0.877–6.517 | 0.088 | 3.252 | 1.134–9.323 | 0.028 |
| Localization | ||||||
| Middle 2/3 vs. lower 1/3 | 0.526 | 0.452–0.927 | 0.045 | 0.623 | 0.427–0.859 | 0.042 |
| Grade | ||||||
| 3 vs. 1+2 | 1.617 | 0.542–4.819 | 0.388 | |||
| Neoadjuvant | ||||||
| Yes vs. no | 0.506 | 0.187–0.951 | 0.017 | 0.585 | 0.207–0.952 | 0.011 |
| Stage | ||||||
| III vs. I+II | 2.121 | 1.916–4.910 | 0.019 | 2.541 | 1.258–5.968 | 0.001 |
| CEA | 0.875 | 0.573–1.334 | 0.534 | |||
| CA19-9 | 0.962 | 0.898–1.301 | 0.272 | |||
| TNC | 1.000 | 0.999–1.002 | 0.155 | |||
| TLC | 1.000 | 0.999–1.000 | 0.516 | |||
| TMC | 0.999 | 0.997 | 0.481 | |||
| Hb | 0.892 | 0.726–1.094 | 0.273 | |||
| TPC | 1.000 | 0.999–1.001 | 0.836 | |||
| NLR | ||||||
| ≥2.8 vs. <2.8 | 2.983 | 1.260–7.055 | 0.013 | 5.445 | 2.081–4.547 | 0.001 |
| PLR | 1.002 | 0.997–1.005 | 0.383 | |||
DFS: Disease-free survival; HR: Hazard ratio; CI: Confidence interval; ECOG PS: Eastern Cooperative Oncology Group Performance Score; HT: Hypertension; DM: Diabetes mellitus; CIHD: Chronic ischemic heart disease; COPD: Chronic obstructive pulmonary disease; CEA: Carcinoembryonic antigen; CA19-9: Carbohydrate antigen 19-9; TNC: Total neutrophil count; TLC: Total lymphocyte count; TMC: Total monocyte count; Hb: Hemoglobin; TPC: Total platelet count; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; vs.: Versus.
DISCUSSION
EC is a very aggressive disease with poor prognosis. Besides TNM staging to determine patients at high risk for recurrence, the identification of new markers augments the chances of early diagnosis and intervention, providing more favorable treatment outcomes. NLR can be easily calculated through a simple hemogram analysis, which is easy, affordable, and cost-effective blood test. The present study investigated the relation of NLR with recurrence and survival in non-metastatic ESCC patients treated with curative surgery and concluded that both DFS and OS were significantly lower in patients with NLR ≥2.8, with NLR ≥2.8 increasing the risk of recurrence by 5.4 times.
Systemic inflammation can contribute to tumor development through some pathways such as genomic instability, genetic mutations, and epigenetic modification. Inflammation activates tissue repair responses that induce the proliferation of premalignant cells and ensure their survival. In addition, inflammation leads to immunosuppression, resulting in formation of microenvironments through which malignant cells can survive. Systemic inflammation also promotes metastatic spread by stimulating angiogenesis [8]. Systemic inflammatory markers such as NLR, which can reflect the inflammatory status of patients, have been shown to predict mortality and recurrence in various cancers in the previous studies [9–13]. In a study of 295 curatively operated EC patients, 75 of whom were ESCC, the cutoff value for NLR was found to be 5 and patients with high pre-operative NLR were shown to have worse OS and DFS than those with low pre-operative NLR [12]. Duan et al. [14] found that patients with high pre-operative NLR had significantly worse tumor-specific survival and recurrence-free survival rates than those with low pre-operative NLR in ESCC patients who underwent curative surgery, indicating that NLR had the most significant predictive effect, particularly for patients with Stage IIIA disease. Similarly, Xu et al. [15] reported the cutoff value for NLR to be 2.99 in their study with ESCC patients and concluded that pre-operative NLR was significantly associated with long-term prognosis, especially in patients with lymph node metastasis and Stage II–III disease. However, Rashid et al. [16] reported that NLR (cutoff; 3.5) was not associated with DFS in their study including 294 EC patients, only 50 of whom were ESCC.
In our study, cutoff value of NLR for DFS was detected 2.8, with 65.2% sensitivity and 69.5% specificity. We found no significant difference between NLR groups in terms of disease stage and NACRT rates. Besides, the locoregional relapse rate was significantly higher in the patients with NLR ≥2.8. Similar to the findings observed in other studies, we showed that high pre-operative NLR negatively affected both DFS and OS [12, 14, 15].
In the neoplastic process, neutrophilia can be observed due to the granulocyte colony-stimulating factor produced by malignant cells. Moreover, myeloid growth factors are produced as a part of paraneoplastic syndrome, which can contribute to neutrophilia. Interleukin-6 and tumor necrosis factor-a secreted by tumor cells can also cause neutrophilia and therefore inflammation. In addition, a significant decrease in CD-4 helper lymphocytes and increase in CD-8 suppressor lymphocytes represent a depression in natural cellular immunity. For many types of cancer, lymphocytopenia indicates a general immunosuppression condition, which seems to affect survival. Depression in T-cell function can weaken the tumor-specific response [17–21]. These mechanisms above may explain, albeit partially, how NLR affects oncological results.
The strengths of our study were as follows; *follow-up period was relatively longer, *unlike other studies, only ESCC patients were included, *NLR groups were homogeneous [16, 12], and *comorbidities and presenting symptoms were also analyzed. However, our study was designed as a retrospective and single-center study. In addition, our study was conducted in an area where ESCC is endemic; we, therefore, do not know how these conditions affect our results.
Conclusion
This study revealed that high pre-operative NLR was associated with worse DFS and OS in patients with ESCC. The determination of NLR is pretty simple, cost effective, and easily available in daily oncological practice. Based on the findings mentioned above, we believe that pre-treatment NLR can provide important information to predict treatment outcomes in non-metastatic operable ESCC patients.
Footnotes
Ethics Committee Approval: The Yuzuncu Yil University Clinical Research Ethics Committee granted approval for this study (date: 22.05.2020, number: 2020/03-26).
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Authorship Contributions: Concept – AS, OA, SS, CK; Design – AS, MA, SS; Supervision – OA, MNA, CK, AbS; Fundings – SS, MNA, CK; Materials – AS, OA, SS, AbS; Data collection and/or processing – AS,MA, OA; Analysis and/or interpretation – AS, SS, AbS; Literature review – MNA, AbS; Writing – AS, SS, CK; Critical review – AbS, MA.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence:are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19:1468–70. doi: 10.1158/1055-9965.EPI-10-0012. [DOI] [PubMed] [Google Scholar]
- 3.Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Australasian Gastro-Intestinal Trials Group. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma:an updated meta-analysis. Lancet Oncol. 2011;12:681–92. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 4.Sakin A, Urun YY, Sahin S, Atci MM, Arici S, Geredeli C, et al. Factors affecting survival in esophageal squamous cell carcinoma:Single-center experience. North Clin Istanb. 2020;7:267–74. doi: 10.14744/nci.2019.31384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice TW, Rusch VW, Ishwaran H, Blackstone EH Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction:data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763–73. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Cai E, Huang J, Yu P, Li K. Prognostic value of vascular endothelial growth factor expression in patients with esophageal cancer:a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:1126–34. doi: 10.1158/1055-9965.EPI-12-0020. [DOI] [PubMed] [Google Scholar]
- 7.Wang XL, Zhang CM, Shi LY, Yu HP, Xu SQ. Significance of p53 gene mutation and P53 protein expression abnormality on the prognosis of esophageal cancer:a meta-analysis study. [Article in Chinese] Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:769–74. [PubMed] [Google Scholar]
- 8.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakin A, Sahin S, Yasar N, Demir C, Arici S, Geredeli C, et al. The relation between hemogram parameters and survival in extensive-stage small cell lung cancer. Oncol Res Treat. 2019;42:506–15. doi: 10.1159/000501595. [DOI] [PubMed] [Google Scholar]
- 10.Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors:a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:1204–12. doi: 10.1158/1055-9965.EPI-14-0146. [DOI] [PubMed] [Google Scholar]
- 11.Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416–21. doi: 10.1038/bjc.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, et al. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18:3362–9. doi: 10.1245/s10434-011-1754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura K, Nagasaka T, Nishida T, Haruma T, Ogawa C, Kusumoto T, et al. Neutrophil to lymphocyte ratio in the pre-treatment phase of final-line chemotherapy predicts the outcome of patients with recurrent ovarian cancer. Oncol Lett. 2016;11:3975–81. doi: 10.3892/ol.2016.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan H, Zhang X, Wang FX, Cai MY, Ma GW, Yang H, et al. Prognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21:5591–7. doi: 10.3748/wjg.v21.i18.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu GW, Wu HR, Xiong R, Li CW, Liu CQ, Xu MQ, et al. Value of the preoperative neutrophil-to-lymphocyte ratio as a prognostic factor for long-term survival in postoperative esophageal squamous cell carcinoma patients. Thorac Cancer. 2018;9:1707–15. doi: 10.1111/1759-7714.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rashid F, Waraich N, Bhatti I, Saha S, Khan RN, Ahmed J, et al. A pre-operative elevated neutrophil:lymphocyte ratio does not predict survival from oesophageal cancer resection. World J Surg Oncol. 2010;8:1. doi: 10.1186/1477-7819-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lord BI, Bronchud MH, Owens S, Chang J, Howell A, Souza L, et al. The kinetics of human granulopoiesis following treatment with granulocyte colony-stimulating factor in vivo. Proc Natl Acad Sci U S A. 1989;86:9499–503. doi: 10.1073/pnas.86.23.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulich TR, del Castillo J, Guo K, Souza L. The hematologic effects of chronic administration of the monokines tumor necrosis factor, interleukin-1, and granulocyte-colony stimulating factor on bone marrow and circulation. Am J Pathol. 1989;134:149–59. [PMC free article] [PubMed] [Google Scholar]
- 19.Ulich TR, del Castillo J, Guo KZ. In vivo hematologic effects of recombinant interleukin-6 on hematopoiesis and circulating numbers of RBCs and WBCs. Blood. 1989;73:108–10. [PubMed] [Google Scholar]
- 20.Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154:1805–13. doi: 10.1016/S0002-9440(10)65436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182:318–24. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]


