Abstract
Breast cancer chemotherapy, although very potent against tumour tissue, results in significant cardiovascular toxicity. The focus of research in this area has been predominantly towards cardiotoxicity. There is limited evidence detailing the impact of such treatment on the vasculature despite its central importance within the cardiovascular system and resultant detrimental effects of damage and dysfunction. This review highlights the impact of chemotherapy for breast cancer on the vascular endothelium. We consider the most likely mechanisms of endothelial toxicity to be through direct damage and dysfunction of the endothelium. There are sharp consequences of these detrimental effects as they can lead to cardiovascular disease. However, there is potential for exercise to alleviate some of the vascular toxicity of chemotherapy, and the evidence for this is provided. The potential role of exercise in protecting against vascular toxicity is explained, highlighting the recent in-human and animal model exercise interventions. Lastly, the mediating mechanisms of exercise protection of endothelial health is discussed, focusing on the importance of exercise for endothelial health, function, repair, inflammation and hyperlipidaemia, angiogenesis, and vascular remodelling. These are all important counteracting measures against chemotherapy-induced toxicity and are discussed in detail.
Keywords: chemotherapy, cardiovascular toxicity, vasculo-oncology, endothelium, exercise, cardio-oncology
Introduction
Breast cancer is the most common form of cancer, with over two million women diagnosed globally in 2018 (1). The breast cancer 10-year survival rate increased over the past 40 years from 40 to 78% due to early detection and effective treatments (1). There are different types of breast cancer, which vary according to site, and as with all cancer types, there are different stages of breast cancer, according to progression, which influences the chosen treatment regimens (2). For early-stage breast cancer, lumpectomy followed by radiation therapy or simple mastectomy are the major treatment decisions. Additionally, adjuvant chemotherapy and 5–10 years of endocrine therapy are often recommended, with anti-herceptin2 agents, including trastuzumab, prescribed in addition for those with node-negative, hormone receptor-positive breast cancer (2). This review will focus specifically on the common chemotherapy regimens prescribed in early-stage breast cancer. This often consists of a combination of drugs including anthracyclines, antimetabolites, alkylating agents, and taxanes (3). These drugs work together in different ways to eradicate cancerous tumours. Anthracycline drugs, including doxorubicin and its analogue, epirubicin, inhibit topoisomerase, preventing mitosis and induces cellular damage via generation of oxidative stress by production of reactive oxygen species (ROS) (3). Antimetabolite drugs, such as 5-fluorouracil (5-FU), damage RNA, DNA and inhibit DNA synthesis (3). Alkylating agents, such as cyclophosphamide, alkylate nucleophilic bases in tumour DNA, forming crosslinks between and within DNA strands, creating breakages, and preventing DNA replication (3). Lastly, taxanes (e.g. paclitaxel and docetaxel) interfere with cell division through disruption of microtubule function (3). Together, these mechanisms cause activation of tumour cell apoptosis, making them effective in reducing neoplastic growth (3).
The systemic nature of intravenous chemotherapy means that the toxic effects of the drugs are not only carried out in the tumour cell but also in cells within the cardiovascular system (CVS). Cardiovascular toxicity is associated with clinically relevant detrimental effects, with cardiovascular disease (CVD) being the leading cause of mortality in breast cancer survivors, attributed to 15.9% of deaths, followed closely by breast cancer (15.1%) (4), highlighting that cardiovascular detriments may be more damaging than the cancer itself. Risk of CVD mortality is 1.9-fold higher in breast cancer survivors than in the general population (5). Research into cardiovascular toxicity of chemotherapy is essential to increase the likelihood of disease-free survival and to improve the quality of life in breast cancer survivors, and there is a growing body of evidence regarding the pathophysiology of the condition. However, the focus of research has been predominantly on the cardiac consequences of chemotherapy toxicity. The impact these drugs have on the vasculature have often been overlooked, despite its central and mediating role in the CVS. However, there is now emerging evidence for the importance of the vasculature in CVD development with chemotherapy exposure.
The vascular endothelium is the first point of contact with any intravenous chemotherapy treatment and is adversely affected by this exposure, presenting as endothelial dysfunction (3, 6) and increased cell death (7). The endothelium is responsible for the production of the potent vasodilator, nitric oxide (NO), which plays a key role in regulating normotension, and has an important role in maintaining cardiovascular health via anti-thrombotic and anti-platelet properties (8). Endothelial damage may in fact be one of the mediating mechanisms behind the initial cardiotoxicity which leads to chronic cardiovascular conditions and, ultimately, premature death in cancer sufferers (6). Hence, it is important to understand the role of the endothelium to provide a mechanistic basis for the initiation and progression of CVD with chemotherapy exposure. This will help to elucidate if there are potential interventions or treatments which can prevent, reverse, or attenuate the cardiotoxicity of cancer treatment.
Since cardiotoxicity is a poignant clinical issue for breast cancer survivors and their clinicians, there is a requirement for interventions which reduce toxicity. Exercise has been proposed as an effective low-cost, low-risk, and low-burden adjunct treatment for those undergoing chemotherapy. For example, exercise interventions during adjuvant breast cancer treatment have resulted in lower risk for cardiovascular events (9), greater blood pressure reduction (10), improved vascular function (11, 12), prevention of atherosclerosis (12), de-stiffening of arteries (13, 14, 15), and increased skeletal muscle angiogenesis (16). As with research into the toxicity itself, research into exercise interventions have also focused mainly on cardiac benefits, despite the importance of the vasculature for prevention of progression to CVD (6). Therefore, this review will focus on the vascular endothelial involvement in potential exercise protection against cardiovascular toxicity, highlighting current knowledge, potential pathways of protective mechanisms, and the requirement for further research in this field.
Cardiotoxicity of chemotherapy clinical cardiac manifestations
Acute toxicity of chemotherapy
Chemotherapy drugs have different associated cardiovascular toxicologies depending on the chosen regimen. Some of these effects can be observed immediately, whereas some cardiovascular detriments develop more slowly. Antimetabolites such as 5-FU have immediate effects, with 1–68% of patients developing cardiotoxicity during chemotherapy cycles, resulting in 2–13% of the affected patients dying during the treatment course (17). Cyclophosphamide also effects the CVS immediately during treatment with a 5% incidence of cardiac complications (18). Cyclophosphamide has also been associated with development of heart failure with reduced left ventricular ejection fraction (LVEF), which often manifests 1–10 days after the first cycle, with a prevalence of 7–28%, depending on the dose (17). Taxane exposure associates with 5% incidence of cardiac complications, occurring around 7 months post-treatment (19), including a 2–8% incidence of heart failure, with significantly reduced LVEF and 1.7% incidence of ischaemia (17)
Chronic toxicity of chemotherapy
In breast cancer patients, it is reported that the overall incidence of heart failure is 5% in patients treated with anthracyclines (20). The incidence of cardiotoxicity from anthracyclines varies depending on dosage and which drug is used, with doxorubicin eliciting incidence of toxicity of 3–26% and epirubicin eliciting a 1–3% incidence (3). Anthracycline-induced cardiotoxicity often manifests as significantly reduced LVEF (15). This cardiotoxicity occurs during treatment and may never recover fully, with only 11% of patients having full recovery of LVEF and 71% of patients having partial recovery (LVEF remaining <50%) 5-years post-treatment (20).
Protective effects of exercise on the cardiovascular system in cancer survivors
To prevent cardiotoxicity, clinicians consider reducing the treatment dose and using cardiac monitoring to detect the early phase of cardiotoxicity (17). However, dose attenuation may interfere with efficacy of treatment. Therefore, there is scope for other adjunct therapies to attenuate cardiotoxicity. So far, pharmaceutical interventions have been used, including neurohormonal blockers and antihypertensives (17), but these increase treatment costs and have their own side effects. There is hope for exercise as an adjunct treatment to attenuate cardiovascular toxicity as it is both effective, cost-efficient, and has almost no detrimental effects on cancer patients (9, 10, 11, 12, 16).
The American Heart Association has proposed development of a model to detect those at risk of cardiotoxicity with chemotherapy and to implement a comprehensive cardiac rehabilitation programme within cancer care to tackle the ever-growing problem (21). This proposal has strong justification as there is evidence emerging for the protective effects of exercise on the CVS in cancer survivors (9, 10, 11, 12, 16). In cancer patients who had completed treatment, Framingham risk scores post-exercise intervention were 2% in the exercise group and 13% in the usual care group, associating with an 11% decrease in the predicted 10-year risk of developing CVD (9). Additionally, more leisure-time physical activity was associated with reduced CVD risk in an 8-year follow-up study of breast cancer women treated with chemotherapy (22). Moreover, aerobic fitness has an inverse association with chemotherapy-induced cardiac fibrosis incidence (23), and higher baseline physical activity is associated with an attenuation in the usual decline in LVEF (24). Aerobic fitness can be enhanced with exercise training and therefore adds to the evidence that exercise could potentially reduce cardiotoxicity of chemotherapy. However, it is important to note that these studies present associations only and highlight a need for randomized controlled trials of exercise.
As with the studies investigating the cardiotoxicity of chemotherapy, exercise and physical activity studies have focused mainly on cardiac outcomes. However, beneficial effects of exercise during chemotherapy have also been observed for vascular health and function (9, 10, 11, 12). The importance of the vasculature’s role in treatment toxicity and the potential for exercise to attenuate this, as well as mechanistic links, will be reviewed in subsequent sections.
Vascular dysfunction as a predictor for cardiotoxicity
Vascular endothelial damage and dysfunction may have a role in early detection and development of chemotherapy toxicity. Flow-mediated dilation (FMD) is a non-invasive measurement of NO-mediated endothelium-dependent dilation, assessing the vasodilatory response to a sudden return of blood flow after a period of venous occlusion (8). An increase in blood flow increases shear stress across the endothelium, stimulating release of NO (potent vasodilator), inducing vasodilation (8). The relative change in artery diameter is predictive of the endothelium’s capacity to release NO (8). A 2.7% decrease in FMD associates with a 37% increased likelihood for LVEF reduction at 3 months post-chemotherapy in breast cancer patients (25). Furthermore, arterial stiffness has recently been described as a key measure for CVD risk profiling in patients treated with similar anti-cancer drugs (26). Pulse wave velocity (PWV) is a non-invasive bioassay for arterial stiffness and is an important predictor of cardiovascular disease and mortality in breast cancer patients (27). PWV is determined by the velocity at which the blood pressure pulse propagates through the circulatory system by measuring the time for a pulse wave to travel from the carotid to the femoral arteries, relative to the distance travelled. A 27% increase in PWV after the third cycle of chemotherapy independently predicts LVEF decrease after chemotherapy completion (28). Vascular remodelling also predicts cardiotoxicity, with a 4% increase in arterial stiffness associated with a 7% reduction in LVEF (29). This indicates that changes within the vasculature occur alongside changes in cardiac function and hence could be used as screening tools to predict cardiotoxicity risk at an early stage. This is important as toxicity may be reversed at an early stage but irreversible when LVEF is affected (15). Early detection and intervention are superior methods of care over managing late-stage CVD and heart failure. However, measurements of vascular health, including FMD, PWV, and arterial stiffness, are not incorporated into routine clinical care, despite the potential to detect early cardiotoxicity to allow effective intervention.
Vascular damage/dysfunction with chemotherapy and potential exercise protection
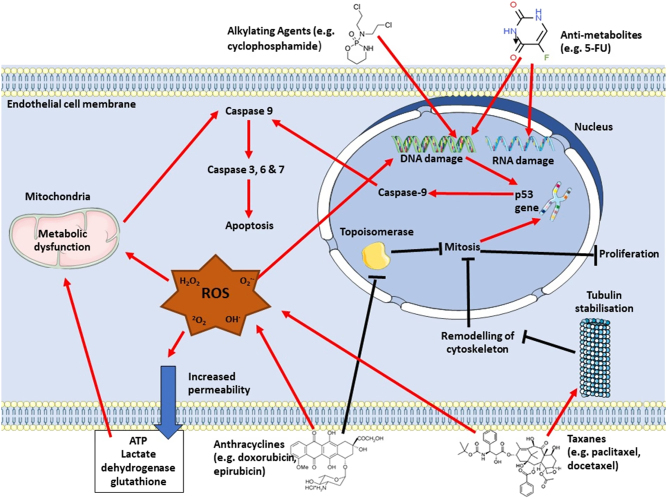
There is substantial cellular harm within the vasculature (Fig. 1) which may contribute to the detrimental impact of chemotherapy on the whole CVS. The endothelium is the first organ in contact with intravenous chemotherapy and is damaged by this exposure (3, 6, 7, 30). Chemotherapy-induced endothelial damage may play a significant role in aiding the development of cardiotoxicity, and there is evidence implicating vascular injury and dysfunction as an initiating step for CVD onset and progression (6, 31). Therefore, this gives an opportunity to assess vascular health as a detector of early cardiotoxicity, allowing for early intervention or treatment attenuation. As with cardiac detriments, exercise may alleviate vascular toxicity of chemotherapy. Exercise has well-documented benefits, including lower risk for overall cardiovascular events (9), reduced blood pressure (10), improved endothelial function (11, 12), prevention of atherosclerotic development (12), de-stiffening of arteries (13, 14, 15), and increased angiogenesis (16). The potential vascular-mediated mechanisms for exercise protection will be outlined with respect to attenuating chemotherapy-induced cardiovascular toxicity.
Figure 1.
Mechanisms of chemotherapy-induced apoptosis via activation of caspase cascade. ROS, reactive oxygen species.
The detrimental effects of chemotherapy on the endothelium manifest as vascular dysfunction and may contribute to myocardial ischaemia which is particularly prevalent in those treated with 5-FU with an incidence >20% (32). The most common symptom of this cardiotoxicity is angina due to myocardial ischaemia, as shown by ST-segment changes (32). This ischemia often occurs without obstruction of the coronary arteries but with increased vasoconstriction, indicative of vasospasm due to vascular endothelial dysfunction – and likely detrimental impacts on the vascular smooth muscle are also implicated – reducing oxygen supply to cardiac tissue (31, 32). Myocardial ischaemia also occurs with cyclophosphamide (33) and taxane (3) exposure. Although less common (<5%) than observed with 5-FU, coronary vasospasm is also the proposed mechanism of cardiotoxicity for cyclophosphamide (33). Due to this proposed underlying mechanism, cyclophosphamide, taxane, and 5-FU toxicity research should focus on vascular pathophysiology to elucidate potential strategies to attenuate myocardial ischaemia to increase the chances of disease-free survival.
The role of endothelial damage in toxicity and the potential for attenuation by exercise
Hypertension is a side effect of chemotherapy treatment, particularly well-documented in cyclophosphamide (3, 31), with this drug attributing to 43% of severe pulmonary hypertension in animal models (3). Cyclophosphamide-induced hypertension associates with acute endothelial damage, consequently disrupting the signalling pathway essential for control of NO-mediated vasodilation and blood pressure regulation (31). Endothelial damage may also lead to the enlarged myocardium which is seen with patients treated with cyclophosphamide chemotherapy (34). Direct endothelial damage from exposure to cyclophosphamide results in leakage of plasma proteins and erythrocytes, causing wall thickening due to interstitial oedema and haemorrhage which may reduce left ventricular diastolic function and presents as cardiomyopathy (34). Damage to the endothelium in the coronary vasculature results in leakage of toxic metabolites which directly damage cardiomyocytes (33). This may create an access route for other chemotherapy drugs into the heart, contributing to cardiotoxicity. The mechanism behind anthracycline- and docetaxel-mediated endothelial injury has been linked to increased intracellular levels of oxidative stress, leading to the activation of apoptotic signalling pathways (3, 29). This occurs within the endothelium, leading to further vascular damage and dysfunction. Oxidative stress also causes increased permeability of the endothelium by exerting a detrimental effect on endothelial junction proteins (35), with anthracyclines increasing permeability of arterial endothelial cells by ~10-fold, resulting in large decreases in ATP and antioxidants from the endothelium (36), further inducing metabolic dysfunction, oxidative stress, and apoptosis.
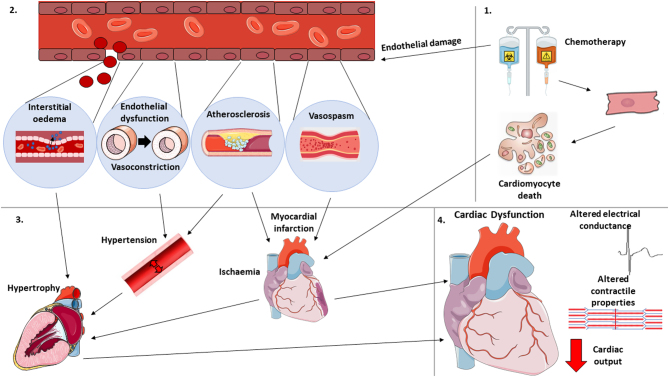
Overall, chemotherapy treatment increases the susceptibility to vascular damage and dysfunction, with cellular apoptosis within the CVS outlined as the main mechanism for cardiotoxicity (31). Mechanisms of action include damaging RNA and DNA, inhibition of topoisomerase, increasing oxidative stress, and binding and stabilizing tubulin (3). These mechanisms ultimately lead to inhibition of cell division and initiation of cellular apoptosis (3). Since these actions are non-specific, they induce apoptosis throughout the whole CVS. The potential mechanisms by which chemotherapy induces cellular apoptosis are outlined in Fig. 1. Vascular manifestations of this damage has a consequential ripple effect on each other and on overall cardiovascular health, and there is now evidence suggesting that vascular injury and dysfunction is an initiating step in CVD development (31). For example, coronary artery disease usually occurs due to an accumulation of atherosclerotic plaques, mediated by vascular damage and inflammation (37). Narrowing of the vessels results in hypertension, increasing cardiac stress due to pressure overload (37). Restricted coronary arteries lead to reduction of blood supply to the myocardium, and depending on the severity of blood flow reduction may result in myocardial infarction (MI). MI may occur with chemotherapy exposure (33) due to the increased risk of atherosclerosis, myocardial ischaemia, and hypertension, increasing the risk of the embolism breaking away from the localised site (33). The sudden deprivation of blood supply with an MI causes apoptosis and necrosis of the myocardium and alteration of cardiac electrical stimulation. If the patient survives an acute MI, the myocardium attempts to recover via growth of cardiomyocytes. This results in pathological remodelling and hypertrophy, accompanied by fibrous tissue development (38), creating mechanical stiffness and cardiac dysfunction, leading to heart failure and an endless cycle of cardiovascular detriments. The mechanisms by which chemotherapy-induced cardiomyocyte and vascular damage may lead to heart failure are illustrated in Fig. 2.
Figure 2.
Potential mechanisms by which chemotherapy induces cardiac dysfunction via endothelial and cardiomyocyte damage.
Exercise training has been found to reduce apoptosis in cardiomyocytes exposed to doxorubicin (39), most likely due to enhanced levels of antioxidants and reduced levels of ROS (40). Therefore, a potential mechanism for exercise protection against endothelial toxicity is through attenuation of oxidative stress and apoptosis. Reduced oxidative stress and subsequent reductions in apoptosis may protect against CVD risk, as oxidative stress and endothelial apoptosis are contributing factors to chemotherapy toxicity (2, 28, 30).
The role of endothelial function (eNOS) in toxicity and the potential for attenuation with exercise
One of the most critical effects of chemotherapy is likely to be the detrimental effects of these drugs on the key vasodilator and anti-thrombotic enzyme, endothelial nitric oxide synthase (eNOS). Chemotherapy drugs have been found to reduce eNOS bioavailability and/or activation within the endothelium (3, 6, 30, 41), likely attributed to oxidative stress (41). Overall, detriments in the endothelial vasodilatory pathway are likely responsible for the subsequent vasospasm and vasoconstriction, as seen with chemotherapy (6, 30). As NO is anti-thrombotic, impairment in its production is also likely to be involved in the increased incidence of venous thromboembolism in patients treated with chemotherapy (42). Interestingly, cyclophosphamide has been associated with increased microcirculatory relaxation and eNOS content, but this is likely to be a compensatory mechanism for the high levels of endothelial injury induced by cyclophosphamide exposure (7). Disruption of NO regulation is also seen within anthracycline chemotherapy, as assessed by FMD (12). There is a 7% reduction in FMD from pre- to post-anthracycline treatment (12) due to anthracycline-induced endothelial damage (3). This indicates that there is a reduction in NO bioavailability which increases the risk for CVD (25). Reduced endothelial-dependent vasodilation is clinically significant as FMD inversely associates with LVEF reduction in breast cancer patients treated with chemotherapy (25) and may be a mediating mechanism behind the 5% incidence of heart failure observed with anthracycline treatment (20).
An exercise intervention during chemotherapy increased FMD by 4% in breast cancer patients, suggesting enhanced endothelial function and eNOS production (12). This is promising for reducing risk of cardiovascular events as eNOS has anti-atherogenic properties, reducing the risk of atherosclerosis development. These results are echoed in non-chemotherapy studies of exercise training, and FMD improvements have been found to be mediated by shear stress (43). With 12 weeks of thrice weekly cycling during treatment (60–100% VO2Peak, 30–45 min), a non-significant increase in FMD was observed from pre-to post-doxorubicin–cyclophosphamide chemotherapy in the exercise group, with no change in usual care controls (11). More promising results were found with an 8-week thrice weekly high-intensity interval training (HIIT) cycling intervention alongside anthracycline chemotherapy, whereby post-intervention, brachial FMD increased by 4% with exercise compared to the 7% reduction in FMD in the usual care group (12). This is a clinically meaningful finding, as a 2.7% increase in FMD associates with a 37% decreased likelihood for LVEF reduction at 3 months post-chemotherapy in breast cancer patients.
Although there are now some, although few, in-human studies of exercise training before and during chemotherapy for breast cancer, some preliminary evidence from murine models should be highlighted. Eight, but not 4 weeks of exercise training (5 days/week, 30 min running 20–25 m/min) prior to exposure to chemotherapy was associated with enhanced endothelium-dependent vasodilation in rats (44), suggesting that exercise-induced protective effects on the vasculature are dose-dependent. Exercise training (6 weeks, 5 days/week, 30 min running at 50–60% maximum velocity) during doxorubicin treatment was associated with reduction in mortality, alongside an improved endothelium-independent but not endothelium-dependent vasodilation, in rats with doxorubicin-induced cardiac dysfunction (45). This suggests that exercise restores vascular function, but the mechanism may not be via increased NO bioavailability but rather via improved vascular structure and/or vascular smooth muscle properties. The discrepancies in mechanistic findings between Matsuura et al. (2010) and Hayward et al. (2004) highlight a requirement for further investigation.
The role of vasoconstrictors in toxicity and the potential for exercise modulation
Another mediating mechanism behind exercise-induced protection of vascular toxicity is likely to be the downregulation of vasoconstrictive factors, such as the potent vasoconstrictor, endothelin-1. Chemotherapy treatment increases the activity of endothelin-1 leading to a phenotype in favour of vasoconstriction and hypertension and is likely a postulating mechanism of 5-FU-induced vasospasm (3). In addition, endothelin-1 inhibits eNOS activity and is a likely mediator of the downregulation of eNOS observed in 5-FU (6). Vasoconstriction is also upregulated with exposure to anthracyclines (6), but endothelin-1 expression has not been investigated with anthracycline exposure. Nevertheless, the increase in vasoconstriction leads to hypertension which poses a risk to cardiovascular health as an increased pressure in the vessels increases the likelihood of plaque rupture and vascular events, alongside an increased pre-load and strain on the myocardium, worsening any cardiac dysfunction, consequently leading to heart failure if left untreated (Fig. 1). In contrast to other chemotherapy drugs, taxanes have been found to reduce endothelin-1 expression in breast cancer patients, which may improve overall survival (46). On the contrary, exercise studies in non-cancer older women have shown that exercise is associated with reduction in vasoconstrictive factors occurring alongside improved blood pressure profiles (47). An exercise intervention in healthy older women decreased plasma endothelin-1 concentration from 2.9 ± 0.2 to 2.2 ± 0.2 pg/mL, occurring alongside a reduction in systolic (127 ± 4 to 112 ± 3 mmHg) and diastolic (79 ± 2 to 65 ± 2 mmHg) blood pressure (47). Overall, exercise appears to promote a pro-vasodilatory phenotype which subsequently reduces hypertension risk and improves overall cardiovascular health.
Exercise has the potential to attenuate chemotherapy-induced arterial stiffness
Arterial stiffness is another key component of vascular dysfunction with anti-cancer therapies (26). Chemotherapy treatment results in remodelling of the resistance vessels whereby the structure of the vessels changed towards a larger lumen, suggesting significant endothelial injury, as this is a compensatory mechanism to decrease blood pressure (7). However, remodelling leads to stiffened blood vessels with reduced compliance related to increased oxidative stress with taxane treatment (29) and decreased NO-dependent vasodilation with doxorubicin exposure (48). Arterial stiffness with chemotherapy also occurs due to structural remodelling initiated by elastin degradation and advanced glycation end products formation, mediated by TNFA-dependent vascular inflammation (49). Changes in vascular compliance associate with reductions in cardiac function due to a consequently hypertensive state increasing cardiac afterload and cardiac stress, inducing significant cardiac detriments, with LVEF reducing by 7% from pre- to post-treatment (29). This indicates that reductions in cardiac function occur alongside changes in vascular structure.
Interestingly, exercise has the potential to reduce arterial stiffness, with those participating in lifelong exercise have lower arterial stiffness relative to age-matched sedentary counterparts (14, 15). Exercise during and after chemotherapy is associated with reduced circulating inflammatory cytokines IL6, IL10, IL1B, and TNFA (50), which has the potential to attenuate TNFA-induced arterial stiffness (49). In vivo animal models have provided mechanistic links for this exercise effect, with improved PWV in mice after voluntary wheel running attributed to reduced oxidative stress, mechanical stiffness, and accumulation of collagen-I and advanced glycation end products (13). This may well be the case with exercise during chemotherapy but as yet, no study has undertaken this investigation. Nevertheless, this may be a contributory effect for reduction of cardiotoxicity with exercise training during chemotherapy, as this reduces the likelihood of hypertension and subsequent organ damage, reducing treatment toxicity (51).
Exercise reduces thrombosis risk
The incidence of venous thromboembolism in patients treated with FEC (5-Fluorouracil, epirubicin, cyclophosphamide) was 17%, which is higher than any other chemotherapy regime (1.5–10%) (42). The reason for this particularly high incidence is not well understood, and current suggestions for the effects may be due to participant characteristics and high treatment dosage (42). It must be considered that the increased incidence of hypertension with cyclophosphamide will be a contributing factor to thromboembolism as increased pressure can cause plaque rupture. Cyclophosphamide and 5-FU alone also associate with thrombosis (3), leading to MI (26) and stroke (31). The mechanisms of action associated with these drugs pose a high risk to the health and integrity of the vasculature due to endothelial cell damage (3), increasing susceptibility to pro-thrombotic phenotypes. The issue of thrombotic risk needs to be addressed to improve quality of life and survival in those undergoing chemotherapy regimens.
Exercise may help to reverse the dysfunctional shift towards a pro-thrombotic endothelial cell morphology induced by chemotherapy. An 8-week exercise intervention during the course of chemotherapy prevented the usual increase in carotid intima-media thickness with chemotherapy treatment, suggesting a protective effect of exercise against atherosclerotic risk (12). Basally, the endothelium has low adhesion molecule expression which is upregulated by stimuli such as inflammation and disturbed blood flow. Endothelial activation presents as increased adhesion molecule expression which is important for thrombotic risk (52). E-selectin, vascular cell adhesion molecule 1 (VCAM1), and intracellular adhesion molecule 1 (ICAM1) are involved in the rolling and adhesion of leukocytes to the activated endothelium, initiating the development of atherosclerosis (52). The expression of ICAM1 is of particular importance as this associates with overall and disease-free survival (52). ICAM1 is upregulated with taxane exposure (52) and anthracycline exposure (53) and is the likely mechanism of action for vascular toxicity of cyclophosphamide (3). As eNOS downregulates endothelial adhesion molecule expression, the increased expression of adhesion molecules is likely a consequence of the reduced expression of eNOS which occurs with chemotherapy exposure (54), increasing the likelihood of atherosclerosis development. However, there is not yet enough evidence for this effect in chemotherapy drugs and, therefore requires further research for confirmation. Exercise, through increasing eNOS expression and activation, may reduce adhesion molecule expression which is an important mediator of vascular risk. There has been no study to-date which has investigated this in cancer patients but is likely to be a mediating mechanism as exercise in non-cancer clinical populations has been found to reduce circulating plasma levels of endothelial-derived adhesion molecule expression, specifically E-selectin and ICAM1 (55). One of the potential reasons for the downregulation of adhesion molecules on endothelial cells is thought to be shear stress. Exercise results in an increase in laminar blood flow across the vascular endothelium, and in vitro studies have shown that increasing shear stress results in reduced expression of VCAM1 and E-selectin, but interestingly an increase in ICAM1 (56). This suggests another mechanism responsible for exercise-induced downregulation of ICAM1 and requires further investigation.
Another potential mechanism for exercise protection against thrombotic risk is the ability to regulate inflammation and cholesterol flux (50). With chemotherapy-induced vascular toxicity, the anti-inflammatory properties of the endothelium are diminished (31), and chemotherapy has also been found to increase triglycerides, total cholesterol, and low-density lipoprotein, which are associated with elevated cardiovascular disease risk (57). This, together with the downregulation of eNOS (3, 6, 30) and upregulation of adhesion molecules (52, 53), is likely to lead to the onset and/or progression of atherosclerosis with the accumulation of inflammatory and lipid molecules within the vasculature. Exercise during and after chemotherapy is associated with a reduction in inflammatory cytokines IL6, IL10, IL1B, and TNFA (50) and improved lipid profiles with increased high-density lipoprotein and reduced total cholesterol (9). This may be a contributory effect for reduction of cardiotoxicity with exercise training during chemotherapy, as this reduces the likelihood of atherosclerotic development, posing a counteraction against anti-neoplastic treatment toxicity.
Exercise promotes skeletal muscle angiogenesis
As well as macrovascular health, muscle angiogenesis also improves with exercise during chemotherapy treatment (16). Skeletal muscle biopsies show that capillary density was reduced by 10% with chemotherapy (16). This reduced skeletal muscle perfusion may contribute to muscle atrophy and dysfunction commonly observed in cancer patients undergoing chemotherapy. However, 16 weeks of twice weekly HIIT cycling plus 20 min continuous cycling or plus resistance exercise training alongside chemotherapy increased capillary density by 30%, attenuating this vascular toxicity (16). With cyclophosphamide and docetaxel exposure, circulating levels of the pro-angiogenic signalling factor, vascular endothelial growth factor (VEGF) are reduced which is directly associated with hypertension and stroke risk (3, 31). VEGF is required for efficient angiogenesis as it signals the formation of new blood vessels in hypoxic regions and facilitates repair of damaged vessels – a key process to protect against vascular disease. Disruption of the VEGF pathway with docetaxel has been associated with the reduction in eNOS activation (3). Therefore, VEGF is not only important for angiogenesis but is also essential for NO production, subsequent vasodilation, and anti-thrombotic properties which are protective to the vasculature. Targeting VEGF has shown to be a therapeutic strategy for preventing doxorubicin-mediated endothelial dysfunction (58), potentially through modulation of doxorubicin-induced vascular mitochondrial ROS upregulation (41). Even though exercise-induced increases in circulating VEGF could possibly prevent chemotherapy-induced cardiotoxicity, there has been no observable effect of exercise during chemotherapy on levels of circulating VEGF (11). However, this has only been investigated in one study and requires further investigation for confirmation. Despite the minimal evidence against VEGF upregulation with exercise during chemotherapy, angiogenesis has been shown to increase by 30% in skeletal muscle, compared to a 10% decrease in non-exercise controls, helping to combat chemotherapy-induced muscular atrophy and dysfunction (16). Therefore, skeletal muscle angiogenesis may well improve with exercise training during chemotherapy. Whether this is mediated by exercise-induced alterations in VEGF should be explored further. It may be a concern that the pro-angiogenic effect of exercise may reduce treatment efficacy as some treatments work through reduction of tumour angiogenesis, but in the same study, exercise was also associated with decreased tumour blood flow (16). It can be argued that reducing tumour blood flow is preferable as this starves the tumour of its oxygen and nutrient supply, thereby killing the tumour. However, in some cases, such as in mice, exercise during chemotherapy increased tumour microvessel density, vessel maturity and perfusion, and reduced intratumoral hypoxia, associating with significantly reduced tumour growth and increased tumour apoptosis (59). Therefore, improving angiogenesis within tumours may actually increase efficacy of chemotherapy as this is improving the supply route for the drugs to reach the tumour (60). Importantly, exercise induces a normalization of tumour vasculature due to shear stress-induced angiogenesis, as compared with angiogenesis as a hallmark of cancer which produces ‘leaky’ and disorganised pathological vessels which reduce treatment efficacy and increase the chance of metastasis (60). Therefore, exercise may protect from vascular toxicity by increasing capillary number, which in turn can be beneficial for efficacy of the chemotherapy treatment itself.
Exercise promotes vascular regenerative capacity
To expand our understanding of exercise-induced endothelial protection, evidence for the implications of chemotherapy on endothelial repair must also be outlined. Endothelial repair is an important function of the endothelium to protect against dysfunction and plaque formation as endothelial damage without repair is often the initiating step for progression to pathological vascular states (5, 30). A wound healing assay showed impaired ability of endothelial cells to migrate across the site of injury when exposed to 5-FU and epirubicin (6); epirubicin and 5-FU have also been found to decrease migration, as shown by Boyden’s chamber assay, likely due to cell cycle arrest and downregulation of NO which is involved in the migration signalling pathway (6). Endothelial progenitor cells (EPCs) are implicated in reparative processes to maintain the integrity of the endothelial layer (61). There is limited evidence for the effect of chemotherapy on EPCs and therefore is a potential area for future research. However, docetaxel has been shown to reduce levels of circulating EPCs, likely due to increased apoptosis and inflammatory mediators (62). This is associated with CVD and mortality, highlighting the problem of anti-neoplastic drugs on the CVS (62). This, together with the previously mentioned disruption of the VEGF signalling pathway with docetaxel (3), leads to an endothelium with reduced capacity for repair of vascular damage, increasing the risk for development of CVD. Positively, exercise in breast cancer patients has been found to increase circulating EPCs and angiogenic factors (11). More evidence for the potential benefits of effects on EPCs comes from non-cancer studies, with acute execise found to mobilize EPCs and regular exercise found to increase resting EPC numbers in both healthy and heart failure populations, likely contributing to exercise-induced improvements in endothelial function (61). Hence, exercise may protect from vascular toxicity by increasing repair, possibly through upregulation of EPCs and pro-angiogenic factors, and fortunately, improved vascular networks do not appear to affect treatment efficacy.
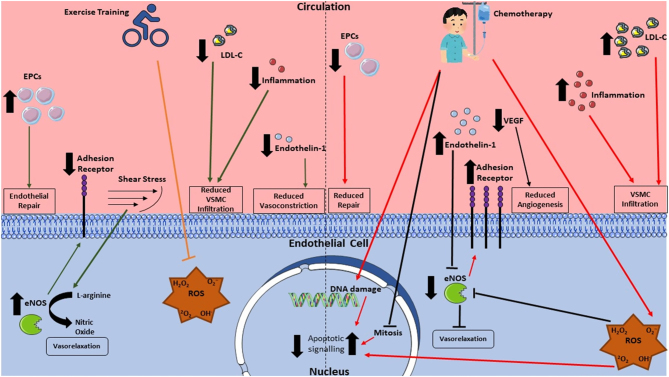
A summary of exercise interventions in humans and animal models can be found in Table 1. Additionally, overall mechanisms by which exercise may attenuate vascular damage and dysfunction from chemotherapy are outlined in Fig. 3.
Table 1.
Summary of exercise interventions in rodents and humans investigating vascular function and health during and after chemotherapy exposure.
| Participants | Exercise intervention | Chemotherapy treatment | Vascular outcomes | Overall findings | |
|---|---|---|---|---|---|
| Rodent studies | |||||
| Matsuura et al. (34) | Sprague–Dawley rats: n = 40 per group |
Intervention group: Treadmill 5 days/week for 4 or 8 weeks, 30 min/day, 20–25 m/min, 15% gradient Sedentary group: No exercise |
Ex vivo exposure to 5-fluorouracil (7 × 10−5 to 7 × 10−3 M) | Endothelium-dependent vasodilation (aortic ring) | No diff intervention vs sedentary group at 4 weeks ↑ EDV intervention group only at 8 weeks |
| eNOS protein content | No diff intervention vs sedentary group at 4 weeks ↑ eNOS content intervention group only at 8 weeks |
||||
| Betof et al. (35) | Sprague–Dawley rats: n = 10 per group |
Intervention group: Treadmill 5 days/week, for 6 weeks, 30 min/day, 50–60% max velocity Sedentary group: No exercise |
Intraperitoneal doxorubicin injection (1 mg/kg/day, 10 days) | Vasodilation response to acetylcholine | Impaired in both groups No diff intervention vs sedentary group |
| eNOS content and activity | No diff intervention vs sedentary group | ||||
| Scott et al. (36) | BALB/c female mice: n = 11 per group |
Intervention group: voluntary wheel running for 18 days Sedentary group: No wheel |
Intraperitoneal cyclophosphamide injection (100 mg/kg on days 7, 9, and 11) | Microvessel density | ↑ Microvessel density intervention vs sedentary group |
| Human studies | |||||
| Jones et al. (11) | Stage IIB–IIIC breast cancer, age 51± 6 years, BMI 29 ± 5 kg/m2, n = 10 per group |
Intervention:12 weeks, three sessions/week cycling at 60–100% for 30–45 min Control: usual care only |
Four cycles of neoadjuvant doxorubicin (60 mg/m2) plus cyclophosphamide (600 mg/m2) every 3 weeks | baFMD | No diff pre- to post-chemotherapy treatment No diff intervention vs sedentary group |
| Lee et al. (12) | Stage I–III breast cancer, age 49 ± 8 years, BMI 33 ± 8 kg m2, n = 15 per group |
Intervention: 8 weeks, three sessions/week 20-min cycling HIIT (7×1-min at 90% PPO) Control: usual care only |
(neo)Adjuvant doxorubicin and cyclophosphamide administered every 2 weeks for four cycles (dosage details not available) | baFMD cIMT pre- to post- treatment |
↓ baFMD pre- to post-chemotherapy treatment sedentary group ↑ baFMD pre- to post-chemotherapy treatment intervention group ↑ cIMT pre- to post-chemotherapy treatment sedentary group No change cIMT pre- to post-chemotherapy treatment intervention group |
| Mijwel et al. (13) | Stage I–IIIa breast cancer, age 51 ± 13 years, BMI 25 kg/m2 (n = 23) |
Resistance training: 12 weeks, two sessions/week 2–3 sets of 8–12 reps 70–80% 1-RM, 3×3-min of HIIT cycling (n = 6) Aerobic training: 12 weeks, two sessions/week, 20 min cycling/running RPE 13–15, 3×3-min of HIIT cycling (n = 7) Control: usual care only (n = 10) |
Anthracyclines, taxanes, or a combination of the two (dosage details not provided) | Capillaries per fibre pre to post-treatment: | ↓ Capillaries pre- to post-chemotherapy treatment sedentary group ↑ Capillaries pre- to post-chemotherapy treatment aerobic intervention group ↑ Capillaries pre- to post-chemotherapy treatment resistance intervention group |
baFMD, brachial flow-mediated dilation; cIMT, carotid intima media thickness; EDV, endothelium-dependent vasodilation; PPO, peak power output; RPE, rating of perceived exertion.
Figure 3.
Mechanisms of chemotherapy toxicity on the endothelium and counteractive effects of exercise. eNOS, endothelial nitric oxide synthase; EPCs, endothelial progenitor cells; VEGF, vascular endothelial growth factor; VSMC, vascular smooth muscle cell.
Exercise mimetics
Interestingly, exercise mimetics have been proposed as therapeutics to prevent CVD in those ‘at risk’ from the disease. The concept of exercise mimetics is that a polypill containing several compounds which lower blood lipids reduce blood pressure, are anti-thrombotic, blunt autonomic responses, and lower blood glucose concentration, may protect against CVD, in a similar way to exercise (63). In-human studies have shown that the short-term use of a polypill (amlodipine 2.5 mg, losartan 25 mg, hydrochlorothiazide 12.5 mg, and simvastatin 40 mg) can reduce CVD risk by ~80% (63). This may be an alternative therapeutic strategy for preventing/treating vascular dysfunction with chemotherapy drugs, given the barriers to exercise in cancer populations (64). This said, exercise interventions have been successfully implemented in cancer patients undergoing chemotherapy (8, 9, 10, 11, 12) and hence may be a more wide-reaching and low-cost therapeutic strategy.
Research gaps – a call for action
There are several research gaps which should be outlined with regards to both cardiovascular toxicity and potential exercise protection. Until recently, research has focused on cardiotoxicity of chemotherapy but now research is considering vascular consequences of cancer treatment. Despite initial evidence, there is still a paucity of research into the vasculature, with only a small number of in-human studies which focus on vascular endothelial health upon chemotherapy exposure. More research is required to confirm and expand on current knowledge regarding the endothelium’s role in initiation and development of chemotherapy-induced CVD and the mechanisms involved. Despite the proposed mechanisms in this review having a high likelihood for involvement in cardiotoxicity of chemotherapy, they have not yet been fully elucidated in cancer care. Therefore, further investigations to confirm the underlying mechanisms of vascular toxicity are needed. In addition to this, most evidence for cardiotoxicity comes from studies investigating doxorubicin, and there are very few studies investigating chemotherapy for breast cancer, despite also causing cardiotoxicity and their use being common in patient care (3).
More research gaps are evident when scrutinizing the literature that has investigated exercise protection against toxicity. There are only a small number of studies investigating vascular outcomes with exercise interventions during chemotherapy, and there are even fewer studies proposing underlying mechanisms for this. Furthermore, most exercise studies have investigated anthracyclines despite the multiplicity of chemotherapy drugs utilized in breast cancer care. A full review of the mechanisms by which exercise may improve vascular function in the specific setting of anthracycline chemotherapy is discussed in depth elsewhere (65). Despite gaps in the literature, these studies show promise for the potential inclusion of exercise therapy during cancer treatment. Future randomized controlled trials of exercise in those undergoing chemotherapy should assess vascular health and function outcomes including blood pressure, FMD, circulating endothelial cells, EPCs and PWV to fully determine the vascular benefits of exercise in this population. As yet, the majority of evidence for exercise protective mechanisms comes from non-chemotherapy-treated populations, and hence, assumptions regarding interdisciplinary consistency have been drawn. Despite the likelihood that these studies are still applicable to cancer treatment, there is a strong requirement for conformational studies regarding the mechanisms of exercise protection against vascular damage and dysfunction with chemotherapy.
Conclusion
Chemotherapy exposure is associated with cardiovascular toxicity, which is linked to CVD development and mortality, and is the number one cause of death in breast cancer patients (4). This is highly likely to be due to, in part, the toxic effects of chemotherapy drugs on the vasculature. The underlying pathology involves vascular dysfunction which results in ischaemia, hypertension, and thrombosis which can lead to cardiovascular events including arrythmias, heart failure, and MI. This is likely due to increased activation and apoptosis of vascular cells inducing a significant shift in the endothelial health from an anti-thrombotic, anti-coagulative, vasodilatory phenotype to a phenotype which promotes vasoconstriction, atherosclerosis, and thrombosis. Focusing on attenuation of vascular endothelial damage could provide a much-needed alleviation of cardiotoxicity. Exercise shows promise as an adjunct therapy to reduce vascular toxicity, by improving or maintaining endothelial function, reducing inflammation and hyperlipidaemia, as well as promoting endothelial repair. The emerging evidence outlined provides promise for exercise as a potential therapeutic, but there are still several research gaps. Future research should include studies elucidating potential mechanisms behind endothelial protection of exercise in this patient population, and large exercise trials in breast cancer patients are required to ensure exercise effects are applicable and feasible for breast cancer survivors.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This work was funded by the Peter KK Lee Fund at Edinburgh Napier University.
Author contribution statement
The manuscript was written by M M, M R, and G F J. All authors approved the final version of the manuscript.
References
- 1.Worldwide Cancer Data. Worldwide cancer data | World Cancer Research Fund International, 2018. (available at: https://www.wcrf.org/dietandcancer/worldwide-cancer-data/). Accessed on 27 August 2021. [Google Scholar]
- 2.Henry NL, Shah PD, Haider I, Freer PE, Jagsi R, Sabe MS. Cancer of the breast. In Abeloff’s Clinical Oncology, pp. 1560.e12–1603.e12. Elsevier. ( 10.1016/B978-0-323-47674-4.00088-8) [DOI] [Google Scholar]
- 3.Campia U.Vascular effects of cancer treatments. Vascular Medicine 202025226–234. ( 10.1177/1358863X20914978) [DOI] [PubMed] [Google Scholar]
- 4.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Research 201113 R64. ( 10.1186/bcr2901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gernaat SAM, Ho PJ, Rijnberg N, Emaus MJ, Baak LM, Hartman M, Grobbee DE, Verkooijen HM. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Research and Treatment 2017164537–555. ( 10.1007/s10549-017-4282-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gajalakshmi P, Priya MK, Pradeep T, Behera J, Muthumani K, Madhuwanti S, Saran U, Chatterjee S. Breast cancer drugs dampen vascular functions by interfering with nitric oxide signaling in endothelium. Toxicology and Applied Pharmacology 2013269121–131. ( 10.1016/j.taap.2013.03.011) [DOI] [PubMed] [Google Scholar]
- 7.Al-Hashmi S, Boels PJM, Zadjali F, Sadeghi B, Sällström J, Hultenby K, Hassan Z, Arner A, Hassan M. Busulphan-cyclophosphamide cause endothelial injury, remodeling of resistance arteries and enhanced expression of endothelial nitric oxide synthase. PLoS ONE 20127 e30897. ( 10.1371/journal.pone.0030897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 20071151285–1295. ( 10.1161/CIRCULATIONAHA.106.652859) [DOI] [PubMed] [Google Scholar]
- 9.Lee K, Tripathy D, Demark-Wahnefried W, Courneya KS, Sami N, Bernstein L, Spicer D, Buchanan TA, Mortimer JE, Dieli-Conwright CM. Effect of aerobic and resistance exercise intervention on cardiovascular disease risk in women with early-stage breast cancer: a randomized clinical trial. JAMA Oncology 20195710–714. ( 10.1001/jamaoncol.2019.0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sturgeon KM, Ky B, Libonati JR, Schmitz KH. The effects of exercise on cardiovascular outcomes before, during, and after treatment for breast cancer. Breast Cancer Research and Treatment 2014143219–226. ( 10.1007/s10549-013-2808-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones LW, Fels DR, West M, Allen JD, Broadwater G, Barry WT, Wilke LG, Masko E, Douglas PS, Dash RCet al. Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Prevention Research 20136925–937. ( 10.1158/1940-6207.CAPR-12-0416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee K, Kang I, Mack WJ, Mortimer J, Sattler F, Salem G, Lu J, Dieli-Conwright CM. Effects of high-intensity interval training on vascular endothelial function and vascular wall thickness in breast cancer patients receiving anthracycline-based chemotherapy: a randomized pilot study. Breast Cancer Research and Treatment 2019177477–485. ( 10.1007/s10549-019-05332-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gioscia-Ryan RA, Clayton ZS, Fleenor BS, Eng JS, Johnson LC, Rossman MJ, Zigler MC, Evans TD, Seals DR. Late-life voluntary wheel running reverses age-related aortic stiffness in mice: a translational model for studying mechanisms of exercise-mediated arterial de-stiffening. GeroScience 202143423–432. ( 10.1007/s11357-020-00212-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 20001021270–1275. ( 10.1161/01.cir.102.11.1270) [DOI] [PubMed] [Google Scholar]
- 15.Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arteriosclerosis, Thrombosis, and Vascular Biology 199818127–132. ( 10.1161/01.atv.18.1.127) [DOI] [PubMed] [Google Scholar]
- 16.Mijwel S, Cardinale DA, Norrbom J, Chapman M, Ivarsson N, Wengström Y, Sundberg CJ, Rundqvist H. Exercise training during chemotherapy preserves skeletal muscle fiber area, capillarization, and mitochondrial content in patients with breast cancer. FASEB Journal 2018325495–5505. ( 10.1096/fj.201700968R) [DOI] [PubMed] [Google Scholar]
- 17.Yeh ETH, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. Journal of the American College of Cardiology 2009532231–2247. ( 10.1016/j.jacc.2009.02.050) [DOI] [PubMed] [Google Scholar]
- 18.Nieto Y, Cagnoni PJ, Bearman SI, Shpall EJ, Matthes S, Jones RB. Cardiac toxicity following high-dose cyclophosphamide, cisplatin, and BCNU (STAMP-1) for breast cancer. Biology of Blood and Marrow Transplantation 20006198–203. ( 10.1016/s1083-8791(0070043-7) [DOI] [PubMed] [Google Scholar]
- 19.Sparano JA, Makhson AN, Semiglazov VF, Tjulandin SA, Balashova OI, Bondarenko IN, Bogdanova NV, Manikhas GM, Oliynychenko GP, Chatikhine VAet al. Pegylated liposomal doxorubicin plus docetaxel significantly improves time to progression without additive cardiotoxicity compared with docetaxel monotherapy in patients with advanced breast cancer previously treated with neoadjuvant-adjuvant anthracycline therapy: results from a randomized phase III study. Journal of Clinical Oncology 2009274522–4529. ( 10.1200/JCO.2008.20.5013) [DOI] [PubMed] [Google Scholar]
- 20.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano Get al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 20151311981–1988. ( 10.1161/CIRCULATIONAHA.114.013777) [DOI] [PubMed] [Google Scholar]
- 21.Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, La Gerche A, Ligibel JA, Lopez G, Madan Ket al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation 2019139e997–e1012. ( 10.1161/CIR.0000000000000679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones LW, Habel LA, Weltzien E, Castillo A, Gupta D, Kroenke CH, Kwan ML, Quesenberry CP, Scott J, Sternfeld Bet al. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. Journal of Clinical Oncology 2016342743–2749. ( 10.1200/JCO.2015.65.6603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkham AA, Paterson DI, Haykowsky MJ, Beaudry RI, Mackey JR, Pituskin E, Grenier JG, Thompson RB. Aerobic fitness is related to myocardial fibrosis post-anthracycline therapy. Medicine and Science in Sports and Exercise 202153267–274. ( 10.1249/MSS.0000000000002469) [DOI] [PubMed] [Google Scholar]
- 24.Upshaw JN, Hubbard RA, Hu J, Brown JC, Smith AM, Demissei B, Schmitz KH, Ky B. Physical activity during and after breast cancer therapy and associations of baseline physical activity with changes in cardiac function by echocardiography. Cancer Medicine 202096122–6131. ( 10.1002/cam4.3277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anastasiou M, Oikonomou E, Zagouri F, Siasos G, Antonopoulos AS, Psaltopoulou T, Bamias A, Dimopoulos MA, Tousoulis D. Flow-mediated dilation of brachial artery as a screening tool for anthracycline-induced cardiotoxicity. Journal of the American College of Cardiology 201770 3072. ( 10.1016/j.jacc.2017.09.1140) [DOI] [PubMed] [Google Scholar]
- 26.Parr SK, Liang J, Schadler KL, Gilchrist SC, Steele CC, Ade CJ. Anticancer therapy-related increases in arterial stiffness: a systematic review and meta-analysis. Journal of the American Heart Association 20209e015598. ( 10.1161/JAHA.119.015598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parr SK, Steele CC, Hammond ST, Turpin VRG, Ade CJ. Arterial stiffness is associated with cardiovascular and cancer mortality in cancer patients: insight from NHANESIII. International Journal of Cardiology: Hypertension 20219100085. ( 10.1016/j.ijchy.2021.100085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mihalcea D, Florescu M, Bruja R, Patrascu N, Vladareanu A-M, Vinereanu D. 3D echocardiography, arterial stiffness, and biomarkers in early diagnosis and prediction of CHOP-induced cardiotoxicity in non-Hodgkin’s lymphoma. Scientific Reports 2020101–11. ( 10.1038/s41598-020-75043-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Florescu M, Mihalcea D, Enescu OA, Radu E, Chirca A, Acasandrei AM, Magda LS, Rimbas RC, Cirstoiu C, Vinereanu D. Taxanes-induced cardiotoxicity is related to increased arterial stiffness and oxidative stress. European Heart Journal 201334P3006–P3006. ( 10.1093/eurheartj/eht309.P3006) [DOI] [Google Scholar]
- 30.Altieri P, Murialdo R, Barisione C, Lazzarini E, Garibaldi S, Fabbi P, Reggeri C, Borile S, Carbone F, Armirotti Aet al. Themed section: new insights into cardiotoxicity caused by chemotherapeutic agents effects of ranolazine in a model of doxorubicin-induced left ventricle diastolic dysfunction linked articles. British Journal of Pharmacology 2017174 3713–3726. ( 10.1111/bph.13725) [DOI] [Google Scholar]
- 31.Cameron AC, Touyz RM, Lang NN. Vascular complications of cancer chemotherapy. Canadian Journal of Cardiology 201632852–862. ( 10.1016/j.cjca.2015.12.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polk A, Vaage-Nilsen M, Vistisen K, Nielsen DL. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treatment Reviews 201339974–984. ( 10.1016/j.ctrv.2013.03.005) [DOI] [PubMed] [Google Scholar]
- 33.Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents. Incidence, treatment and prevention. Drug Safety 200022263–302. ( 10.2165/00002018-200022040-00002) [DOI] [PubMed] [Google Scholar]
- 34.Dhesi S, Chu MP, Blevins G, Paterson I, Larratt L, Oudit GY, Kim DH. Cyclophosphamide-induced cardiomyopathy: a case report, review, and recommendations for management. Journal of Investigative Medicine High Impact Case Reports 201312324709613480346. ( 10.1177/2324709613480346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkinson EL, Sidaway JE, Cross MJ. Cardiotoxic drugs herceptin and doxorubicin inhibit cardiac microvascular endothelial cell barrier formation resulting in increased drug permeability. Biology Open 201651362–1370. ( 10.1242/bio.020362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf MB, Baynes JW. The anti-cancer drug, doxorubicin, causes oxidant stress-induced endothelial dysfunction. Biochimica et Biophysica Acta 20061760267–271. ( 10.1016/j.bbagen.2005.10.012) [DOI] [PubMed] [Google Scholar]
- 37.Hansson GK.Inflammation, atherosclerosis, and coronary artery disease. New England Journal of Medicine 20053521685–1695. ( 10.1056/NEJMra043430) [DOI] [PubMed] [Google Scholar]
- 38.Brower GL, Gardner JD, Forman MF, Murray DB, Voloshenyuk T, Levick SP, Janicki JS. The relationship between myocardial extracellular matrix remodeling and ventricular function. European Journal of Cardio-Thoracic Surgery 200630604–610. ( 10.1016/j.ejcts.2006.07.006) [DOI] [PubMed] [Google Scholar]
- 39.Werner C, Hanhoun M, Widmann T, Kazakov A, Semenov A, Pöss J, Bauersachs J, Thum T, Pfreundschuh M, Müller Pet al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. Journal of the American College of Cardiology 200852470–482. ( 10.1016/j.jacc.2008.04.034) [DOI] [PubMed] [Google Scholar]
- 40.Scott JM, Jones LW, Hornsby WE, Koelwyn GJ, Khouri MG, Joy AA, Douglas PS, Lakoski SG. Cancer therapy-induced autonomic dysfunction in early breast cancer: implications for aerobic exercise training. International Journal of Cardiology 2014171e50–e51. ( 10.1016/j.ijcard.2013.11.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clayton ZS, Brunt VE, Hutton DA, VanDongen NS, D’Alessandro A, Reisz JA, Ziemba BP, Seals DR. Doxorubicin-induced oxidative stress and endothelial dysfunction in conduit arteries is prevented by mitochondrial-specific antioxidant treatment. JACC: Cardio-Oncology 20202475–488. ( 10.1016/j.jaccao.2020.06.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoy J, Neeman T, Stuart-Harris R, Davis A. Risk of venous thromboembolism in patients receiving adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide for early breast cancer. Asia-Pacific Journal of Clinical Oncology 20095129–136. ( 10.1111/j.1743-7563.2009.01205.x) [DOI] [Google Scholar]
- 43.Tinken TM, Thijssen DHJ, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 201055312–318. ( 10.1161/HYPERTENSIONAHA.109.146282) [DOI] [PubMed] [Google Scholar]
- 44.Hayward R, Ruangthai R, Schneider CM, Hyslop RM, Strange R, Westerlind KC. Training enhances vascular relaxation after chemotherapy-induced vasoconstriction. Medicine and Science in Sports and Exercise 200436428–434. ( 10.1249/01.mss.0000117130.91142.38) [DOI] [PubMed] [Google Scholar]
- 45.Matsuura C, Brunini TMC, Carvalho LCMM, Resende AC, Carvalho JJ, De Castro JPW, Mendes-Ribeiro AC. Exercise training in doxorubicin-induced heart failure: effects on the L-arginine-NO pathway and vascular reactivity. Journal of the American Society of Hypertension 201047–13. ( 10.1016/j.jash.2009.10.005) [DOI] [PubMed] [Google Scholar]
- 46.Alacacioglu A, Kebapcilar L, Sari I, Gokgoz Z, Tarhan O, Somali I, Yuksel A, Bozkaya G, Sop G. Taxane-based adjuvant chemotherapy reduces endothelin-1 and symmetric dimethylarginine levels in patients with breast cancer. Journal of the Balkan Union of Oncology 201015572–576. [PubMed] [Google Scholar]
- 47.Maeda S, Tanabe T, Miyauchi T, Otsuki T, Sugawara J, Iemitsu M, Kuno S, Ajisaka R, Yamaguchi I, Matsuda M. Aerobic exercise training reduces plasma endothelin-1 concentration in older women. Journal of Applied Physiology 200395336–341. ( 10.1152/japplphysiol.01016.2002) [DOI] [PubMed] [Google Scholar]
- 48.Bosman M, Favere K, Neutel CHG, Jacobs G, De Meyer GRY, Martinet W, Van Craenenbroeck EM, Guns PDF. Doxorubicin induces arterial stiffness: a comprehensive in vivo and ex vivo evaluation of vascular toxicity in mice. Toxicology Letters 202134623–33. ( 10.1016/j.toxlet.2021.04.015) [DOI] [PubMed] [Google Scholar]
- 49.Clayton ZS, Brunt VE, Hutton DA, Casso AG, Ziemba BP, Melov S, Campisi J, Seals DR. Tumor necrosis factor alpha-mediated inflammation and remodeling of the extracellular matrix underlies aortic stiffening induced by the common chemotherapeutic agent doxorubicin. Hypertension 2021771581–1590. ( 10.1161/HYPERTENSIONAHA.120.16759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LaVoy ECP, Fagundes CP, Dantzer R. Exercise, inflammation, and fatigue in cancer survivors. Exercise Immunology Review 20162282–93. [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell GF, Moyé LA, Braunwald E, Rouleau JL, Bernstein V, Geltman EM, Flaker GC, Pfeffer MA. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular Enlargement. Circulation 1997964254–4260. ( 10.1161/01.cir.96.12.4254) [DOI] [PubMed] [Google Scholar]
- 52.Dowlati A, Gray R, Sandler AB, Schiller JH, Johnson DH. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab – an Eastern Cooperative Oncology Group Study. Clinical Cancer Research 2008141407–1412. ( 10.1158/1078-0432.CCR-07-1154) [DOI] [PubMed] [Google Scholar]
- 53.Mills PJ, Parker B, Jones V, Adler KA, Perez CJ, Johnson S, Cohen-Zion M, Marler M, Sadler GR, Dimsdale JEet al. The effects of standard anthracycline-based chemotherapy on soluble ICAM-1 and vascular endothelial growth factor levels in breast cancer. Clinical Cancer Research 2004104998–5003. ( 10.1158/1078-0432.CCR-0734-04) [DOI] [PubMed] [Google Scholar]
- 54.Gao F, Lucke-Wold BP, Li X, Logsdon AF, Xu LC, Xu S, LaPenna KB, Wang H, Talukder MAH, Siedlecki CAet al. Reduction of endothelial nitric oxide increases the adhesiveness of constitutive endothelial membrane ICAM-1 through Src-mediated phosphorylation. Frontiers in Physiology 20178 1124. ( 10.3389/fphys.2017.01124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saetre T, Enoksen E, Lyberg T, Stranden E, Jørgensen JJ, Sundhagen JO, Hisdal J. Supervised exercise training reduces plasma levels of the endothelial inflammatory markers E-selectin and ICAM-I in patients with peripheral arterial disease. Angiology 201162301–305. ( 10.1177/0003319710385338) [DOI] [PubMed] [Google Scholar]
- 56.Chiu JJ, Lee PL, Chen CN, Lee CI, Chang SF, Chen LJ, Lien SC, Ko YC, Usami S, Chien S. Shear stress increases ICAM-1 and decreases VCAM-1 and E-selectin expressions induced by tumor necrosis factor-α in endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology 20042473–79. ( 10.1161/01.ATV.0000106321.63667.24) [DOI] [PubMed] [Google Scholar]
- 57.He T, Wang C, Tan Q, Wang Z, Li J, Chen T, Cui K, Wu Y, Sun J, Zheng Det al. Adjuvant chemotherapy-associated lipid changes in breast cancer patients: a real-word retrospective analysis. Medicine 202099 e21498. ( 10.1097/MD.0000000000021498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Räsänen M, Degerman J, Nissinen TA, Miinalainen I, Kerkelä R, Siltanen A, Backman JT, Mervaala E, Hulmi JJ, Kivelä Ret al. VEGF-B gene therapy inhibits doxorubicin-induced cardiotoxicity by endothelial protection. PNAS 201611313144–13149. ( 10.1073/pnas.1616168113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, Palmer G, Jones LW, Dewhirst MW. Modulation of murine breast tumor vascularity, hypoxia, and chemotherapeutic response by exercise. Journal of the National Cancer Institute 2015107djv040. ( 10.1093/jnci/djv040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schadler KL, Thomas NJ, Galie PA, Bhang DH, Roby KC, Addai P, Till JE, Sturgeon K, Zaslavsky A, Chen CSet al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget 2016765429–65440. ( 10.18632/oncotarget.11748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ross MD, Malone E, Florida-James G. Vascular ageing and exercise: focus on cellular reparative processes. Oxidative Medicine and Cellular Longevity 201620163583956. ( 10.1155/2016/3583956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muta M, Yanagawa T, Sai Y, Saji S, Suzuki E, Aruga T, Kuroi K, Matsumoto G, Toi M, Nakashima E. Effect of low-dose paclitaxel and docetaxel on endothelial progenitor cells. Oncology 200977182–191. ( 10.1159/000236016) [DOI] [PubMed] [Google Scholar]
- 63.Wald DS, Morris JK, Wald NJ. Randomized polypill crossover trial in people aged 50 and over. PLoS ONE 20127 e41297. ( 10.1371/journal.pone.0041297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rogers LQ, Courneya KS, Shah P, Dunnington G, Hopkins-Price P. Exercise stage of change, barriers, expectations, values and preferences among breast cancer patients during treatment: a pilot study. European Journal of Cancer Care 20071655–66. ( 10.1111/j.1365-2354.2006.00705.x) [DOI] [PubMed] [Google Scholar]
- 65.Clayton ZS, Hutton DA, Mahoney SA, Seals DR. Anthracycline chemotherapy-mediated vascular dysfunction as a model of accelerated vascular aging. Aging and Cancer 2021245–69. ( 10.1002/aac2.12033) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a