Abstract
Risk of metastatic disease in the cluster 2-related pheochromocytoma/paraganglioma (PPGL) is low. In MEN2 patients, identification of origin of metastases from pheochromocytoma (PCC) or medullary thyroid carcinoma (MTC) is challenging as both are of neuroendocrine origin. We aim to describe our experience and perform a systematic review to assess prevalence, demographics, biochemistry, diagnostic evaluation, management, and predictors of cluster 2-related metastatic PPGL. Retrospective analysis of 3 cases from our cohort and 43 cases from world literature was done. For calculation of prevalence, all reported patients (n = 3063) of cluster 2 were included. We found that the risk of metastasis in cluster 2-related PPGL was 2.6% (2% in RET, 5% in NF1, 4.8% in TMEM127 and 16.7% in MAX variation). In metastatic PCC in MEN2, median age was 39 years, bilateral tumors were present in 71% and median tumor size was 9.7 cm (range 4–19) with 43.5% mortality. All patients had a primary tumor size ≥4 cm. Origin of primary tumor was diagnosed by histopathology of metastatic lesion in 11 (57.9%), 131I-MIBG scan in 6 (31.6%), and selective venous sampling and CT in 1 (5.3%) patient each. In subgroup of neurofibromatosis 1 (NF1), median age was 46 years (range 14–59) with median tumor size 6 cm and 57% mortality. To conclude, the risk of metastatic disease in cluster 2-related PPGL is low, being especially high in tumors with size ≥4 cm and associated with high mortality. One-third patients of NF1 with metastatic PPGL had presented in second decade of life. Long-term studies are needed to formulate management recommendations.
Keywords: metastatic pheochromocytoma, MEN2A, MEN2B, NF1, cluster 2
Introduction
Pheochromocytomas (PCC) and paragangliomas (PGL), together known as pheochromocytoma/paraganglioma (PPGL), are rare neuroendocrine tumors originating from the chromaffin tissue in adrenal glands and sympathetic/parasympathetic ganglia, respectively, with an approximate incidence of 0.8/100,000 population per year (1). The Endocrine Society guidelines recommend genetic testing in all PPGL patients as the prevalence of germline mutations is almost 40% (1, 2). PPGL are categorized into three molecular clusters based on genetics. Cluster 1 (pseudohypoxia pathway)-related tumors secrete norepinephrine and mainly include germline mutations of succinate dehydrogenase subunits and assembly factor (SDHA, SDHB, SDHC, SDHD, andSDHAF2), and von Hippel–Lindau tumor suppressor (VHL) genes. Cluster 2 (Kinase-signaling pathway)-related tumors are epinephrine-producing and include germline mutations in the rearranged-during-transfection (RET) proto-oncogene, neurofibromin 1 (NF1) tumor suppressor, transmembrane protein 127 (TMEM127), Myc associated factor X (MAX), and somatic mutation in HRAS. The epinephrine-producing cluster 2-related PPGLs are more differentiated and have lesser malignant potential than cluster 1-related tumors. There are no recognized germline mutations with cluster 3 (Wnt signaling pathway)-related PPGL (3).
Risk of metastatic disease in the cluster 2-related PPGL is low (1, 4, 5). In multiple endocrine neoplasia 2 (MEN2) patients, identification of origin of metastases from PCC or medullary thyroid carcinoma (MTC) is challenging as both are of neuroendocrine origin. We aim to describe metastatic cluster 2-related PPGLs managed at our center with emphasis on this diagnostic challenge in MEN2 syndrome. We further aim to perform a systematic review to calculate the prevalence of metastases and attempt to describe distinct demographic, biochemical features, diagnostic evaluation, management, and predictors of malignancy in cluster 2-related metastatic PPGL.
Materials and methods
This retrospective study was conducted at Seth G.S. Medical College and KEM Hospital after approval from Institutional Ethical Committee (EC/OA-72/2021). The records of all patients diagnosed with PPGL between January 2001 and April 2021 were screened and eligible patients with cluster 2-related metastatic PPGL were included in the study. The diagnosis of PPGL was based on histopathology and/or combination of suggestive biochemistry (elevated, fractionated plasma-free metanephrines) and imaging. Neurofibromatosis 1 (NF1) was diagnosed on clinical grounds, whereas multiple endocrine neoplasia type 2 was diagnosed either by genetic confirmation or syndromic diagnosis due to presence or history of MTC, cutaneous lichen amyloidosis (CLA), primary hyperparathyroidism (PHPT), and/or mucosal neuromas in the patients and/or first-degree relatives. Genetic analysis for TMEM127 and MAX was not performed in our study. As per World Health Organization (WHO), metastatic PPGL was defined as the presence of a metastatic lesion(s) at the nonchromaffin site (6). Demographic characteristics (age at presentation, gender, and family history), clinical findings (hypertension), biochemical profile, contrast-enhanced computed tomography (CECT) findings of neck, abdomen, and pelvis, functional imaging viz, 68Ga-DOTATATE PET/CT, 18flourodeoxyglucose (18FDG)-PET/CT, 131metaiodobenzylguanidine (131I-MIBG) scintigraphy, histopathology of primary and/or metastatic lesions, treatment details, and outcome were recorded. Plasma fractionated free metanephrines, CECT, 68Ga-DOTATATE PET-CT, 18FDG PET-CT, 131I-MIBG, and RET mutation analysis were done as described previously (7, 8). The plasma free metanephrine (PFMN) and plasma-free normetanephrine (PFNMN) were measured using an enzyme immunoassay with upper limit for PFMN and PFNMN being 90 pg/mL and 180 pg/mL, respectively (9).
Systematic review of literature
A systematic review of the literature was performed as per Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines. The PubMed database was searched in August 2021 using the keywords ‘Metastatic pheochromocytoma AND MEN2A’, ‘Metastatic pheochromocytoma AND MEN2B’, ‘Metastatic pheochromocytoma AND NF1’, ‘Metastatic pheochromocytoma AND MAX’, ‘Metastatic pheochromocytoma AND TMEM127’, ‘Malignant pheochromocytoma AND MEN 2A’, ‘Malignant pheochromocytoma AND MEN2B’, ‘Malignant pheochromocytoma AND NF1’, ‘Malignant pheochromocytoma AND TMEM127’, and ‘Malignant pheochromocytoma AND MAX’ to find reports regarding cluster 2-related PPGL. A total of 1803 publications were screened. Cross-references of selected publications and review articles were searched to find additional articles. Only cases with available individual patient details were taken for the analysis. After exclusions for various reasons (as detailed in Fig. 1), 34 articles (43 patients) were included, and per-patient, details were recorded. Data were tabulated to include demographic, clinical, biochemical, radiological, genetic, management, and outcome details. The biochemical values were recorded as multiples of the upper normal reference range for uniform interpretation of results. In addition, the prevalence of metastatic PPGL was calculated from the reported studies on cluster 2 PCC. For deriving predictors of malignancy in MEN2, the collated data from individual per patient details were compared with the large cohorts of benign PCC and benign cases from our center.
Figure 1.
Preferred Reporting Items for Systematic Review and Meta-Analyses flowchart for literature search of cluster 2-related metastatic pheochromocytoma/paraganglioma.
Statistical analysis
Statistical analysis was performed using SPSS, version 25.0 (IBM). Categorical variables were expressed as actual numbers and percentages and the significance of difference between two groups was calculated using Fisher’s exact t-test. Continuous variables with normal distribution were expressed as mean ± s.d. and unpaired t-test was used for comparison whereas those with skewed distribution were expressed as median (Interquartile range) and Mann–Whitney U test was used for comparison. Two-sided P -value <0.05 was considered statistically significant.
Results
Of the 450 cases of PPGL registered at our institute, 28 (6.2%) cases had cluster 2-related phenotypes (19 MEN2A, 4 MEN2B, and 5 NF1). Among these, three (10.7%) cases (2 MEN2A, 1 NF1) had metastatic PPGL. On systematic review of world literature including our patients (n = 3063), the overall prevalence of cluster 2-related metastatic PPGL was 2.6% (2% in RET, 5% in NF1, 4.8% in TMEM127, and 16.7% in MAX variation) (Table 1 and Supplementary Table 1, see section on supplementary materials given at the end of this article). The detailed analysis of our patients and 43 cases in literature with cluster 2-related metastatic PPGL for whom adequate per-patient data were available, is described below.
Table 1.
Prevalence of cluster 2-related metastatic pheochromocytoma/paraganglioma.
| Gene | World literature | Our center | Overall |
|---|---|---|---|
| RET | 50/2608 (1.9%) | 2/23 (8.7%) | 52/2631 (2%) |
| NF1 | 11/235 (4.7%) | 1/5 (20%) | 12/240 (5%) |
| TMEM127 | 6/126 (4.8%) | – | 6/126 (4.8%) |
| MAX | 11/66 (16.7%) | – | 11/66 (16.7%) |
| Overall cluster 2 | 78/3035 (2.6%) | 3/28 (10.7%) | 81/3063 (2.6%) |
Metastatic PPGL in MEN2 syndrome
Case A
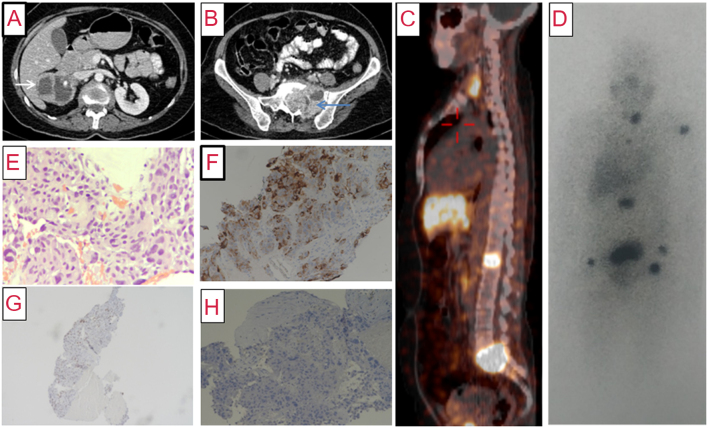
A 54-year-old-female was referred for evaluation of incidentally detected right suprarenal mass. There was no history of paroxysmal symptoms. She had a family history of MTC in her mother and younger sister. Physical examination was unremarkable. Investigations revealed elevated PFNMN (3586 pg/mL) and PFMN (1300 pg/mL), raised serum calcitonin level (1253 pg/mL; normal range: <6.3 pg/mL) and normal calcium profile. CECT showed a cystic right adrenal mass of size 6.1 × 5.5 × 5.5 cm with intense peripheral contrast enhancement, left adnexal mass (8.0 × 6.1 × 4.5 cm cystic lesion), lytic lesions in L1, L5-S1 with soft tissue component in sacral ala (Fig. 2), and hypodense nodules in both thyroid lobes (left: 20 × 18 × 28 mm, right: 10 × 8 mm) without any extrathyroidal extension or lymphadenopathy. 68Ga-DOTATATE PET-CT showed somatostatin receptor (SSTR) avid lesions in both lobes of the thyroid, right adrenal gland, left humerus, L1 vertebra, sacral mass at L5-S1 vertebrae, and non-SSTR avid left adnexal mass (Fig. 2). Genetic analysis showed a germline missense pathogenic variant (c.1852T>C, p.Cys618Arg) in RET proto-oncogene. The skeletal metastases were thought to be arising from MTC as malignant PCC in MEN2A is rare.
Figure 2.
(A) Contrast-enhanced computed tomography (axial section) of abdomen showing a predominantly cystic mass lesion (6.1 × 5.5 × 5.5 cm) in the right suprarenal region (white arrow) with peripherally enhancing solid component. (B) Caudal section in the same scan showing a large lytic lesion with soft tissue (7.3 × 5.9 × 6.8 cm) in the sacral body and the left ala (blue arrow), with similar enhancement characteristics. (C) 68Ga-DOTATATE PET-CT showing somatostatin receptor avid lesions in thyroid, L1 vertebral body, and sacrum. (D) 131I-MIBG scan (anterior view) showing areas of increased radiotracer uptake in the thyroid bed, left humerus, L1 vertebra, sacrum, and both pelvic bones (black). (E) Photomicrograph of biopsy of sacral mass showing metastatic pheochromocytoma with nuclear pleomorphism and moderate to abundant amphophilic cytoplasm with perivascular arrangement of tumor cells (×200, hematoxylin and eosin). (F) Tumor cells showing positive staining for chromogranin immunohistochemistry (IHC), suggesting neuroendocrine tumor (×100, 3,3'-diaminobenzidine (DAB)). (G) Tumor cells showing nuclear reactivity for GATA 3 IHC, favoring pheochromocytoma (×40, DAB). (H) Tumor cells showing negative staining for calcitonin IHC, ruling out medullary thyroid carcinoma (×100, DAB).
The patient underwent open right adrenalectomy along with left adnexal mass excision after α-blockade. On histopathology, the right adrenal mass was reported as PCC and left adnexal mass as benign mucinous cystadenoma. Two months after surgery, she had persistently elevated metanephrines (PFNMN: 3975 pg/mL, PFMN: 740 pg/mL); hence the skeletal metastases were suspected to arise from PCC. 131I-MIBG scan revealed uptake in the thyroid bed, left humerus, L1 vertebra, and sacral mass (Fig. 2). She underwent angioembolization along with CT-guided biopsy of the sacral mass to identify the primary malignancy. Histopathological report (HPR) confirmed the origin of metastasis from PCC (Fig. 2) with immunohistochemistry (IHC) positive for synapatophysin, chromogranin A, and GATA-3 and negative for calcitonin. The patient was planned for 131I-MIBG therapy for metastases and total thyroidectomy for MTC.
Case B
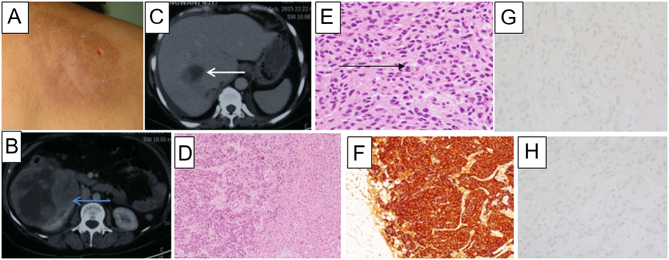
A 43-year-old female was referred for management of incidentally detected bilateral adrenal mass. She had a history of paroxysms and was found to be hypertensive for 5 years. She had hyperpigmented skin lesion over the interscapular area (Fig. 3). Biochemistry revealed elevated PFNMN (4289 pg/mL), PFMN (1300 pg/mL), serum calcitonin (597 pg/mL), and parathyroid hormone (PTH)-dependent hypercalcemia (calcium: 11.6 mg/dL, phosphorus: 2.8 mg/dL, alkaline phosphatase: 43 U/L, and PTH: 346.7 pg/mL). CECT showed a 1.7 × 1.6 cm hypodense nodule in right thyroid lobe, and bilateral adrenal masses (right: 8.3 × 7.2 cm, left: 4. 7 × 3 cm). 18FDG-PET/CT scan showed hypermetabolic lesions in thyroid and both adrenal glands. Skin biopsy from interscapular lesion showed CLA. Genetic analysis showed a germline missense pathogenic variant (c.1901G>A, p.Cys634Tyr) in RET proto-oncogene.
Figure 3.
(A) Hyperpigmented plaque in the interscapular region, suggestive of cutaneous lichen amyloidosis. (B) Contrast-enhanced computed tomography (CECT) (axial section) of abdomen showing a cystic mass lesion (16 × 12 × 12 cm) with peripheral enhancement in the right suprarenal region (blue arrow). (C) CECT (axial section) of abdomen showing a hypodense lesion with peripherally enhancing solid component in segment VIII of the liver (white arrow). (D) Low-power photomicrograph of liver biopsy showing liver parenchyma (right side) being infiltrated by a cellular tumor (left side) (×40, hematoxylin & eosin (H and E)). (E) High-power microphotograph to show sheets of tumor cells with eccentrically placed nuclei, and abundant granular eosinophilic cytoplasm are seen, consistent with pheochromocytoma. Brisk and atypical mitoses (black arrow) are seen (×400, H and E). (F) Tumor cells showing positive staining for chromogranin immunohistochemistry (IHC), suggesting neuroendocrine tumor (×100, DAB). Tumor cells with negative IHC for carcinoembryonic antigen (G) (×200, DAB) and calcitonin (H) (×400, DAB) ruling out medullary thyroid carcinoma.
She underwent laparoscopic bilateral adrenalectomy after α-blockade. Histopathology revealed bilateral PCC. Three months later, after documentation of normal metanephrines (PFNMN: 156 pg/mL, PFMN: 48 pg/mL), she underwent total thyroidectomy along with excision of three parathyroid glands. Histopathology showed MTC with parathyroid hyperplasia.
Two years later, she was presented with abdominal pain and vomiting. Biochemistry revealed elevated PFNMN level (3310 pg/mL) and normal calcitonin level (15 pg/mL). CECT showed 16 × 12 × 12 cm right adrenal mass with a hypodense lesion in right lobe of the liver, both of which were 18FDG-avid. Liver biopsy revealed metastatic neuroendocrine tumor with IHC positive for synaptophysin and chromogranin A but negative for calcitonin and carcinoembryonic antigen (CEA), thereby confirming the origin of metastasis from PCC (Fig. 3). As the disease was inoperable, chemotherapy was started, but the patient succumbed within 1 month.
Literature review
We found 29 cases of MEN2 with metastatic PCC on literature search. The details of these cases, including our two cases, are summarized in Table 2 (10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33).
Table 2.
Review of patients with metastatic pheochromocytoma/paraganglioma in MEN2 syndrome.
| Case | Age (year)/gender (M/F) | Syndromic features | Genetics (RET Codon) | Pheochromocytoma/paraganglioma | Metastasis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MTC (Y/N) | TTx (Y/N)/CTE (Y/N) | PHPT (Y/N) | HTN (Y/N) | Biochemistry (×UNL) | Primary site | Size (cm) | Metastatic site | Chronousity (SC/MC) | Adrenal surgery (Y/N)/CE (Y/N) | Modality for localization | HPR (Y/N) | Treatment | Follow-up (month) | Outcome at last FU death (Y/N) | |||
| 1. PFMN, 2. PFNMN, 3. UNMN, 4. UMN, 5. UE, 6. UNE | 1. MIBG, 2. FDG, 3. DOTA, 4. CT/MRI, 5. Intra-OP, 6. Autopsy, 7. SVS, 8. DOPA | ||||||||||||||||
| 1. (10) | 18/F | Y | Y | Y | – | B/L | 5 | – | – | – | – | – | – | 384 | N | ||
| 2. (10) | 28/F | Y | Y | Y | – | B/L | 12 | – | – | – | – | – | – | 384 | N | ||
| 3. (10) | 20/F | Y | Y | N | 4: 27.9 | B/L | 13 | – | – | – | – | – | – | – | Y | ||
| 4. (10) | 23/F | Y | Y | Y | – | B/L | 12 | – | – | – | – | – | – | – | Y | ||
| 5. (11) | 53/F | – | – | B/L | 12 | Liver | – | – | – | – | – | – | – | ||||
| 6. (11) | 38/F | – | – | B/L | – | Liver | – | – | – | – | – | – | – | ||||
| 7. (12) | 49/M | Y | N/– | – | – | B/L | 19 | Lung, heart | SC | N/– | 6: Lung, heart | Y | – | 0 | Y | ||
| 8. (13) | 20/F | Y | Y/– | Y | B/L | 11 | Liver, spleen, pancreas |
SC | N/– | 1: Liver, 4: Liver 5: Liver, spleen, pancreas |
– | – | – | – | |||
| 9. (14) | 44/F | Y | Y/N | – | – | B/L | – | Lung | MC | Y/Y | 1: Lung, 5: Lung | – | Surgery | – | N | ||
| 10. (15) | 26/M | Y | Y | 5: 5, 6: 63.4 | Left | – | – | – | – | – | – | – | – | N | |||
| 11. (15) | 40/F | Y | Y | – | B/L | – | – | – | – | – | – | – | – | Y | |||
| 12. (16) | 23/M | Y | N/Y | Y | 5: 7.8, 6: 4.5 | Left | – | Lung, liver | SC | Y/Y | 1: Lung, liver | MIBG therapy | 84 | N | |||
| 13. (17) | 35/M | Y | N | 3: 1.63 | B/L | 6 | Lymph node | – | – | – | Y | – | – | – | |||
| 14. (18) | 39/F | Y | N/Y | Y | 3: 29.8, 4: 8.3 5: 1.2, 6: 1.5 |
B/L | – | Lung, livera, bone |
MC | Y/Y | 1: Lung, liver, 4: Liver, 7: Liver |
Y | MIBG therapy, Chemotherapy |
48 | Y | ||
| 15. (19) | 28/M | Y | Y/Y | Y | 5: 21, 6: 4.0 | B/L | 12 | Bone | MC | Y/Y | 1: Bone, 4: Bone | – | MIBG therapy | 12 | N | ||
| 16. (20) | 53/M | Y | Y/ | Y | 634 | – | B/L | 9.4 | Lung, liver | SC | Y/– | – | Y | Angioembolisation | 2 | Y | |
| 17. (21) | 31/F | Y | Y/ | Y | – | Left | – | Brain | MC | Y/ | 4: Brain | Y | Surgery | 48 | N | ||
| 18. (22) | 47/M | Y | N/Y | 634 | Y | – | B/L | 7 | Bone, liver | SC | N/Y | 1: Bone, 4: Bone | Y | MIBG therapy, RT | 24 | N | |
| 19. (23) | 65/M | Y | N/Y | 5: 5.25, 6: 1.2 | B/L | 8 | Liver | MC | Y/Y | – | Y | – | – | Y | |||
| 20. (24) | 34/F | Y | N/Y | Y | 634 | Y | 4: >1 | B/L | – | Pancreas | SC | N/Y | 5: Pancreas | Y | Surgery | 6 | N |
| 21. (25) | 21/F | Y | N/Y | N | 634 | Y | 3: 104, 4:99.6 | Right | 12 | Heart | SC | N/Y | 4: Heart | Y | Surgery | 5 | N |
| 22. (26) | 41/M | N | N/– | – | – | – | B/L | 4 | Skin, lung | SC | N/– | – | Y | – | – | Y | |
| 23. (27) | 65/M | 804 | – | – | U/L | 9.5 | – | – |
– | – | – | – | – | – | |||
| 24 (28) | 47/F | 634 | – | – | Right | 10 | Bone | MC | Y/– | 1: Bone, 8: Bone | – | – | – | – | |||
| 25. (29) | 39/F | 791 | – | – | U/L | 10 | Lymph node | MC | – | – | – | –– | – | – | |||
| 26. (30) | 55/F | N | N/N | Y | 531 | Y | 3: 2.6, 4:0.9 | B/L | – | Liver, lung | MC | Y/– | 1: Liver, lung | – | MIBG therapy | 96 | N |
| 27. (31) | 48/F | Y | N/Y | N | 634 | Y | 3: 7.6, 4:18.3 | B/L | 4.1 | Liver | MC | Y/Y | 2: Liver, 3: Liver, 4: Liver | Y | Chemotherapy | 4 | N |
| 28. (32) | 25/F | 634 | – | – | B/L | 8 | – | – | – | – | – | – | – | – | |||
| 29. (33) | 19/M | Y | N/Y | N | 918 | – | 1: 12, 3:15.9 4: 14.8 |
Right | 11 | Bone, lung | SC | N/Y | 1: Bones, lung, 4: Bones, lung |
Y | Chemotherapy, sunitinib | 0 | Y |
| 30. Case A | 54/F | Y | N/Y | N | 618 | N | 1: 20 2: 18.3 |
Right | 6.1 | Bone | SC | Y/Y | 1: Bones, 3: Bones, 4: Bones | Y | MIBG therapy, angio-embolisation | 6 | N |
| 31. Case B | 45/F | Y | Y/N | Y | 634 | Y | 2: 16.9 | B/L | 8.3 | Liver | MC | Y/Y | 2: Liver, 5: Liver | Y | – | 0 | Y |
aDiagnosed by selective venous sampling of hepatic vein and inferior vena cava.
Adx, adrenal surgery; B/L, bilateral; CE, catecholamine excess; CTE, calcitonin excess; CVD, cyclophosphamide, vincristine, dacarbazine; DOTA, 68Ga-DOTATATE PET CT scan; F, female, FDG, 18fluorodeoxyglucose PET CT scan; FU, follow-up; HA, hepatic artery; HPR, histopathological report; HTN, hypertension; Intra-OP, intraoperatively; M, male; MC, metachoronous; MTC, medullary thyroid carcinoma; MIBG, 131metaiodobenzylguanidine; N, no; PFMN, plasma-free metanephrins; PFNMN, plasma-free normetanephrins; PHPT, primary hyperparathyroidism; RT, radiotherapy; SC, synchoronous; TTx, total thyroidectomy; U/L, unilateral; UNMN, 24 h urinary normetanephrines; UMN, 24 h urinary metanephrins; UE, 24 h urinary epinephrines; UNE, 24 h urinary norepinephrines; ×UNL, times the upper normal limit; Y, yes; –, data not available.
Out of a total of 31 cases (28 MEN2A, 3 MEN2B), 20 (64.5%) were females and 11 (35.5%) were males. The age ranged from 18 to 65 years (median: 39, interquartile range (IQR): 25–48 years). Majority (12/20, 60%) presented with symptoms of catecholamine excess, while eight (8/20, 40%) were detected incidentally. Family history was positive in nine cases (52.9%, n = 17). All cases were hypertensive, except for two. MTC was present in most (23/25) of the cases. The levels of catecholamines/metanephrines (either in plasma or urine) were elevated in all patients (n = 14). The adrenal involvement was bilateral in majority of the cases (22/31, 71%). The tumor size ranged from 4 to 19 cm (median: 9.7, IQR: 7–12). The genetic analysis was available for 12 cases of MEN2A; eight had mutation in codon 634, and one each in codon 618, 531, 709, and 804 of RET proto-oncogene. The most common site for metastasis was the liver (11/23, 47.8%), followed by lungs (8/23, 34.9%), bone (6/23, 26.1%), and pancreas, lymph node, and heart (2/23, 8.7%). Metastases to other sites, including spleen, brain, and skin were present in one case each. Metastasis was synchronous in 50% (10/20) of the cases. Among these, the identification of origin of metastasis was made prior to adrenal surgery in three cases on the basis of histopathology of metastatic lesion. In two cases, the metastasis was detected intraoperatively and later confirmed by histopathology and 131I-MIBG scan in one case each. In three cases, metastases were overlooked/misdiagnosed, and diagnosis was made after adrenal surgery by histopathology in two cases and 131I-MIBG scan in one case. The diagnosis was established on CECT in one case and on autopsy in another. In ten cases with metachronous metastasis, the modality of diagnosis was histopathology in four cases, 131I-MIBG scan in four cases, and selective venous sampling of hepatic vein in one case. Overall (n = 19), origin of primary was diagnosed by histopathology of metastatic lesion in 11 (57.9%) cases, 131I-MIBG scan in 6 (31.6%) cases, and selective venous sampling and CECT in 1 (5.3%) case each.
The management of metastasis was surgical resection in four (30.8%) cases, 131I-MIBG therapy in three (23.1%) cases, chemotherapy and angioembolization in one (7.7%) case each. The management was multimodal (131I-MIBG therapy with chemotherapy/radiotherapy/angioembolization or chemotherapy with tyrosine-kinase inhibitor) in four (30.8%) cases. The median follow-up duration was 9 months (IQR: 3–66), and ten (10/23, 43.5%) cases had died at the last follow-up.
On comparing the patient characteristics of metastatic PCC with reported cohorts of benign PCC in MEN2 to evaluate the predictors of malignancy, primary tumor size was significantly higher in metastatic cohort as compared to benign PCC cohorts (9.5 ± 3.5 vs 3.6 ± 2.2, P value <0.0001). There was no significant difference in age, gender, laterality, tumor, and RET mutation between metastatic and benign PCC (Table 3) (8, 34, 35). In addition, mortality was significantly high in metastatic cohort (43 vs 17.6%, P value = 0.009).
Table 3.
Comparison of metastatic pheochromocytoma/paraganglioma (PPGL) with benign PPGL in MEN 2 syndrome.
| Parameter | Current study (n = 31) | Thosani et al.a (n = 85) | Mucha et al.a (n = 85) | Diwaker et al.b (n = 21) | |||
|---|---|---|---|---|---|---|---|
| Data | Data | P value | Data | P value | Data | P value | |
| Age | |||||||
| Median (years) | 39 | 32 | – | – | – | 39 | 0.69 |
| Mean ± s.d. (years) | 37.8 ± 13.7 | – | – | 34.4 ± 11.6 | 0.18 | 39 ± 11.1 | 0.74 |
| Females (%) | 64.5 | 63.5 | 0.92 | 51.7 | 0.22 | 38.1 | 0.06 |
| Bilateral PCC tumor (%) | 71 | 72 | 0.9 | 55.3 | 0.12 | 72.2 | 0.92 |
| Tumor size | |||||||
| Median (cm) | 9.7 | 3.5 | – | – | – | 5.5 | 0.003 |
| Mean ± s.d. (cm) | 9.5 ± 3.5 | – | – | 3.6 ± 2.2 | <0.0001 | 6.2 ± 3 | 0.004 |
| RET 634 mutations (%) | 66.6 | 69 | 0.86 | 70.9 | 0.76 | 92.8 | 0.09 |
| Overall mortality (%) | 43.5 | 24 | 0.06 | 17.6 | 0.009 | – | – |
aStudies having only benign PPGL. bMetastatic PPGL were excluded and data was collected from benign cases only. – Data not available.
Metastatic PPGL in NF1
Case C
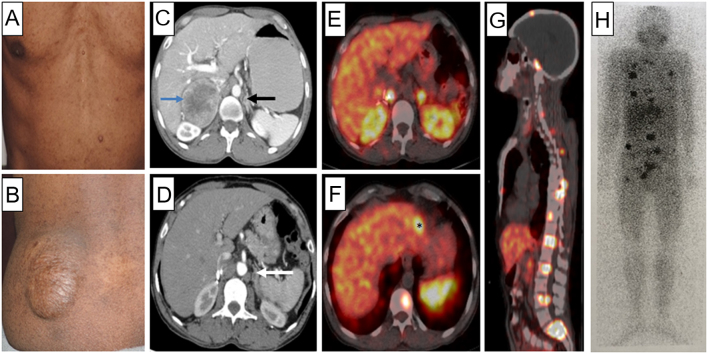
A 43-year-old male was presented with paroxysmal hypertension (6 months) and bilateral adrenal masses. He had multiple cafe-au-lait macules, neurofibromas, and a plexiform neurofibroma over the left buttock (Fig. 4). His daughter also had multiple cafe-au-lait macules and neurofibromas. Biochemistry revealed elevated PFNMN (3797 pg/mL) and PFMN (1057 pg/mL). CECT showed a 5.7 × 5.9 × 7.5 cm right adrenal mass and 0.7 × 0.9 × 0.8 cm left adrenal mass (Fig. 4). He was diagnosed with NF1 based on the clinical criteria. Genetic analysis revealed a previously reported monoallelic variant c.1393-9T>A, at a cryptic splice site in intron 12 of NF1 gene. It affects splicing and leads to premature truncation of NF1 protein. After α-adrenergic blockade, he underwent right laparoscopic adrenalectomy. Histopathology confirmed PCC. Plasma fractionated metanephrines (PFNMN: 115 pg/mL, PFMN: 23.9 pg/mL), 3 months post-surgery, were normal; he was normotensive off antihypertensive medications. Two years later, he was presented with similar paroxysmal episodes. Again, biochemistry confirmed elevated PFNMN (6422 pg/mL) and PFMN (532 pg/mL). In addition, CECT showed a left adrenal mass sized 0.9 × 0.9 × 0.8 cm and metastatic lesions in the liver, bone, and lungs, which were also avid on 68Ga-DOTATATE PET-CT and 131I-MIBG scan (Fig. 4). Given extensive metastasis, the patient is further planned for 131I-MIBG therapy.
Figure 4.
(A) Freckles and neurofibromas on trunk. (B) Plexiform neurofibroma on left buttock. (C) Contrast-enhanced computed tomography (CECT) of the abdomen (axial section) showing a heterogenously enhancing mass lesion (5.7 × 5.9 × 7.5 cm) in the right suprarenal region with central areas of necrosis (blue arrow). Another subcentimetric lesion with similar enhancement characteristics is seen in the body of left adrenal gland (black arrow). (D) Post-operative CECT; showing only the left adrenal lesion (white arrow). 68Ga DOTATATE PET-CT with somatostatin receptor avid lesions in right adrenal bed, left adrenal mass (E), segment 2 of liver(*) (F), and skull, dorsal, lumbar vertebrae, and sacrum (G), suggestive of metastatic disease. (H) 131I-MIBG scan (anterior view) showing areas of increased radiotracer uptake in the ribs, multiple vertebrae, and pelvic bones (black).
Literature review
We found nine cases of NF1 with metastatic PCC. The details of these cases, including one case from our center, are summarized in Table 4 (36, 37, 38, 39, 40, 41).
Table 4.
Review of cases with metastatic pheochromocytoma/paraganglioma in neurofibromatosis 1.
| Case | Age (year)/gender (M/F) | Syndromic features | Genetics (NF1) | Pheochromocytoma/paraganglioma | Metastasis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HTN (Yes/No) | Biochemistry (×UNL) | Primary site | Size (cm) | Metastatic site | Chronousity (SC/MC) | Adrenal surgery (Yes/No) | Modality for localization | HPR (Yes/No) | Treatment | Follow-up (months) | Outcome at last FU death (Yes/No) | ||||
| 1. PFNMN, 2. PFMN, 3. UNMN, 4. UMN | 1. MIBG | ||||||||||||||
| 1. (36) | 14/F | NF, CALMs | – | No | – | Right adrenal | – | Lung, bone, liver, lymph node | SC | No | – | Yes | Chemotherapy | 6 | Yes |
| 2. (37a) | 54/F | NF, CALMs | – | Yes | 1: 24.4 2: 4.64 |
Right adrenal | – | Lung, bone | MC | Yes | 1: Lung, bone | No | Chemotherapy | 3 | No |
| 3. (38) | 16/M | – | Yes | Yes | Left adrenal | 6.0 | Lymph node | SC | Yes | – | Yes | Surgery | – | No | |
| 4. (39) | 14/F | – | – | – | 1: 9.4 2: 17.2 3: 32.2 |
Left adrenal | 6.5 | Liver, bone | MC | Yes | – | – | Surgery, RFA, cryoablation | 228 | Yes |
| 5. (39) | 58/F | – | – | – | – | Bilateral adrenal, Paraaortic paraganglioma | 4.0 | Lymph node | MC | Yes | – | – | – | 168 | Yes |
| 6. (40) | 44/M | – | – | Yes | – | Right abdominal paraganglioma | 5 | Abdomen, chest, pelvis | MC | Yes | – | – | – | 60 | – |
| 7. (40) | 48/M | – | – | Yes | – | Left adrenal | – | Abdomen, liver, mediastinum | SC | Yes | – | – | – | – | – |
| 8. (41) | 52/M | – | – | No | 1: 7.1 2: 21 |
Left adrenal | 6 | Bone | SC | No | – | – | MIBG therapy | 60 | Yes |
| 9. (41) | 59/F | – | – | No | 4: 2.6 | Left adrenal | 3.3 | Lung | – | Yes | – | – | Chemotherapy | 60 | – |
| 10. (Case C) | 42/M | NF, CALMs | Yes | Yes | 1: 19.4 2: 16.3 |
Bilateral adrenal | 7.5 | Liver, lung, bone | MC | Yes | 1: Liver, lung, bone | No | MIBG therapy | 24 | No |
athis patient was included as it was an adrenaline producing tumor (characteristic of cluster 2) despite having SDHB mutation.
CVD, cyclophosphamide, vincristine, dacarbazine; CALMs, café au lait macules; F, female; FU, follow-up; HTN, hypertension; HPR, histopathological report; M, male; MC, metachronous; MIBG, 131metaiodobenzylguanidine; NF, neurofibroma; PFMN, plasma-free metanephrins; PFNMN, plasma-free normetanephrins; RFA, radiofrequency ablation; SC, synchronous; UNMN, 24 h urinary normetanephrines; UMN, 24 h urinary metanephrins; ×UNL, times the upper normal limit; –, data not available.
The age at presentation ranged from 14 to 59 years (median: 46, IQR: 16–54 years). There were five males. Most (n = 8) cases had adrenergic symptoms, while two were detected incidentally. Five cases were hypertensive (5/8, 62.5%). The plasma or urinary metanephrines were elevated in all patients. The most common primary site was unilateral PCC (n = 7), followed by abdominal PGL (n = 1), bilateral PCC (n = 1), and multifocal PPGL (bilateral PCC with an abdominal PGL). Tumor size ranged from 3.3 to 7.5 cm (median: 6, IQR: 4–6.5 cm). The commonest site for metastasis was the bones (5/10, 50%) followed by lungs (4/10, 40%), liver (4/10, 40%), and lymph nodes (3/10, 30%). The management of metastasis was chemotherapy in three (42.8%) cases, 131I-MIBG therapy in two (28.6%) cases, and surgical resection and multimodal management (surgical resection, radiofrequency ablation, and cryoablation) in one (7.7%) case each. The follow-up (n = 7) duration ranged from 0.5 to 19 years; four patients had died at the end of follow-up. Total cohort of metastatic NF1 PPGL comprised of only 10 patients; comparison with benign cohorts did not show consistent difference between parameters and predictors for malignancy could not be calculated (Supplementary Table 2).
Metastatic PPGL in patients with mutations in TMEM127 or MAX
Literature review
Patient details were available for four (age range: 45–51 years, males: 2, bilateral PCC: 2, unilateral PCC: 2) of the 11 reported cases of malignant PPGL with MAX mutation (Supplementary Table 3). All four had metastasis to lymph nodes; one patient had additional bony metastasis. Patient detail was available for one (59 years old female with bilateral PCC and bony metastasis) of the six reported cases of malignant PPGL with TMEM127 mutation (Supplementary Table 3).
Discussion
We present our experience and literature review with cluster 2-related metastatic PPGL and found that the risk of malignancy is 2.6% (2% in RET, 5% in NF1, 4.8% in TMEM127, and 16.7% in MAX variation). Reported prevalence in studies with malignant PPGL in MEN2 cohorts (n ≥ 50) is 0.35 to 4%; while in NF1, TMEM127, and MAX cohorts (n ≥ 10) is 4.9–12%, 5–10.3%, and 8.7–25%, respectively (39, 42, 43, 44, 45, 46, 47, 48). Previous studies with larger cohorts (n ≥ 50) have reported the prevalence varying from 4.3 to 5.4% in cluster 2 (4, 5). This contrasts with the high prevalence (41.9–43.8%) seen in cluster 1 tumors, especially in SDHB-related tumors (73.8–75.6%) (4, 5). This is proposedly due to immature chromaffin progenitors with arrested differentiation and immature phenotype in cluster 1 as compared to mature chromaffin tumors progenitors and differentiated tumors in cluster 2 (49).
The most common hereditary syndrome in cluster 2 is MEN2, which occurs due to gain of function mutations in RET (3). MEN2A is the most frequent subgroup representing 95% of the cases, and MEN2B seen in the rest 5%. PCC in MEN2 is usually bilateral, benign, and arises in the setting of hyperplasia. MTC is present in almost 95–100% cases of MEN2. In MEN2 patients, identification of origin of metastases from PCC or MTC poses challenges as exemplified in our cases. As PCC is usually benign, the metastases are believed to originate from MTC. Moreover, both MTC and PCC metastasis are of neuroendocrine origin, show similar uptake on functional imaging (131I-MIBG and 68Ga-DOTA scans), and immunostaining avidity for synaptophysin, chromogranin, and neuron-specific enolase. As also observed from the collated data, corroborative findings (elevated catecholamines and/or their metabolites after bilateral adrenalectomy, normal serum calcitonin, and CEA after total thyroidectomy, and negative IHC for calcitonin and CEA) can suggest PCC as the origin of metastasis. However, interpretation of catecholamine metabolites (especially PFMN) may be difficult post-adrenalectomy due to scarce data regarding normative values post-bilateral adrenalectomy or per se particularly if done using immunoassays. Weismann suggested that immunoassay measurements cannot be used to reliably determine presence or absence of disease when upper cut-offs used are >44 and >58 pg/mL for PFNMN and PFMN, respectively (50). Biopsy of an accessible metastatic lesion may be considered as a diagnostic aid after adequate α-blockade.
In this series of collated data of metastatic PCC in MEN2, the median age was 39 years, bilateral tumors were present in 71%, and median tumor size was 9.7 cm. In two large series of benign PCC in MEN2, the age of presentation was 32 and 34.4 years, bilateral tumors were seen in 72 and 55.2%, and median tumor size was 3.5 and 3.6 cm, respectively (34, 35). In our collated data, the common metastatic sites for MEN2 PCC are the liver (47.8%), lungs (34.9%), bone (26.1%), and lymph node (8.3%) which is in agreement with the data by Sue et al. where more liver metastases were associated with adrenal as primary site of tumor location (51). In contrast, common metastatic sites in patients with sporadic PPGL are bones (64%) followed by soft tissues (lungs (47%), lymph nodes (36%), and liver (32%)) (52). Metastases to the liver and lungs are known to be associated with increased mortality (52). This may correlate with higher (43.5%) mortality observed in the malignant MEN2 PCC patients. Surgery for patients with metastatic MEN2 PCC was curative with loco-regional lymphadenopathy and/or isolated resectable distant metastases, as observed in four cases (case 9, 16, 19, and 21 in Table 2). In progressive and/or unresectable tumors, the aim is palliative care and requires a multidisciplinary approach. The available treatment options are chemotherapy (CVD, most commonly used regime), 131I-MIBG therapy, 177Lu-DOTATATE therapy, tyrosine-kinase inhibitors, and immune checkpoint inhibitors (52). In our study, 67% patients of MEN2A with metastatic PCC had a mutation of codon 634 of exon 11 of RET proto-oncogene (high risk), which is also the most commonly encountered mutation in PCC in MEN2A. One of our cases (case A) was harboring a mutation of codon 618 of exon 10 (moderate risk). To the best of our knowledge, this is the first report of metastatic PCC with this mutation. There is a suggestion that the type of RET mutation may influence the penetrance of PCC; however, the implication on metastatic potential has not been yet described (35).
On comparing the patient characteristics (age, gender, laterality, tumor size, and RET mutation) of metastatic PCC with reported cohorts of benign PCC in MEN2, greater primary tumor size was found to be the potential predictor of malignant PPGL. In all metastatic cases, primary tumor size was ≥4 cm, and most (19/22, 86.4%) were ≥6 cm. This observation stresses the need for early tumor (size <4 cm) detection and timely surgical management. Cortical sparing adrenalectomy is suggested in the management of PCC in MEN2 to prevent lifelong adrenal insufficiency (42). Generalizing this approach to MEN2 patients with PCC of size > 4 cm needs reconsideration.
The prevalence of PPGL in NF1 ranges from 1.2 to 2%. Among these, the metastatic PPGL has been reported in 7% of the cases (53). We found similar prevalence (5%) of metastatic PPGL in NF1. In three large series of benign PCC in NF1, the median age range was from 39 to 42 years, median tumor size range was 3.8–4.5 cm, 76–80% were unilateral PCC and 2.6–6% had PGL. In our series of collated data of metastatic PPGL in NF1, the median age was 46 years, median tumor size was 6 cm, 80% were unilateral, and 20% had PGL. On comparing the patient characteristics (age, gender, laterality, tumor size, and presence of PGL) of metastatic PPGL with reported cohorts of benign PPGL in NF1, greater PCC size and higher proportion of PGL was observed with metastatic PPGL in NF1, although not consistent across various cohorts (Supplementary Table 2) (39, 40, 41). Recent studies have suggested lowering the age of screening to 14 years and extending screening to asymptomatic individuals as compared to conventional guidelines (39, 54). Metastatic PPGL in NF1 was present even among young (3/10, 30% in second decade), normotensive (3/8, 37.5%) and incidentally diagnosed cases (2/10, 20%) and was associated with a high mortality (57%); thus re-emphasizing the need for early screening for PPGL irrespective of the symptoms in NF1.
For PPGL with TMEM127 and MAX mutations, the prevalence rates of metastatic PPGL were 4.8 and 16.6%, respectively. Since detailed data are available for very few cases, characteristics of this cohort need to be studied in future.
This is the first systematic review studying prevalence, diagnosis, and predictors of metastatic cluster 2-related PPGL, with detailed description of three cases from our center and some novel observations. The major limitation of our study is its retrospective nature with its inherent drawbacks. Owing to resource constraints, genetic testing was performed for those of younger age group and associated syndromic features, resulting in lower prevalence (6.2%) of cluster 2-related PPGL as compared to 11.9% in a study by Pamporaki et al. (4). Further, none of the cases from our cohort as well as from reviewed cases underwent 18fluoro-l-dihydroxyphenylalanine PET-CT and 11C-hydroxy-ephedrine PET-CT scan which are more specific for PCC. Another limitation was use of immunoassay for measurement of plasma fractionated metanephrines, which can underestimate PFNMN and PFMN by 60 and 39%, respectively, as compared to liquid chromatography-tandem mass spectrometric measurement (50).
To conclude, the risk of metastatic disease in cluster 2-related PPGL is 2.6% in this review, the risk is especially high in tumors with size ≥4 cm and is associated with high mortality. Differentiating the origin of metastases between MTC and PPGL in MEN2 patients is challenging. Almost one-third patients of NF1 with metastatic PPGL presented as early as second decade of life. Long-term studies are needed to formulate management recommendations.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Acknowledgements
The authors thank Dr Vyankatesh Shivane and Dr Aparna Kamble for their assistance in conducting the research.
References
- 1.Nölting S, Ullrich M, Pietzsch J, Ziegler CG, Eisenhofer G, Grossman A, Pacak K. Current management of pheochromocytoma/paraganglioma: a guide for the practicing clinician in the era of precision medicine. Cancers 2019111, 505. ( 10.3390/cancers11101505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenders JWM, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SKG, Murad MH, Naruse M, Pacak K, Young WF. & Endocrine Society. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2014991915–1942. ( 10.1210/jc.2014-1498) [DOI] [PubMed] [Google Scholar]
- 3.Crona J, Taïeb D, Pacak K. New perspectives on pheochromocytoma and paraganglioma: toward a molecular classification. Endocrine Reviews 201738489–515. ( 10.1210/er.2017-00062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pamporaki C, Hamplova B, Peitzsch M, Prejbisz A, Beuschlein F, Timmers HJLM, Fassnacht M, Klink B, Lodish M, Stratakis CA.et al. Characteristics of pediatric vs adult pheochromocytomas and paragangliomas. Journal of Clinical Endocrinology and Metabolism 20171021122–1132. ( 10.1210/jc.2016-3829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bechmann N, Moskopp ML, Ullrich M, Calsina B, Wallace PW, Richter S, Friedemann M, Langton K, Fliedner SMJ, Timmers HJLM.et al. HIF2α supports pro-metastatic behavior in pheochromocytomas/paragangliomas. Endocrine-Related Cancer 202027625–640. ( 10.1530/ERC-20-0205) [DOI] [PubMed] [Google Scholar]
- 6.DeLellis RA. WHO Classification of Tumours: Pathology and genetics of tumours of endocrine organs. Lyon, France: IARC. [Google Scholar]
- 7.Jaiswal SK, Sarathi V, Memon SS, Goroshi M, Jadhav S, Prakash G, Dalvi A, Lila AR, Bandgar T, Shah NS. Sympathetic paraganglioma: a single-center experience from Western India. Endocrine Practice 201925211–219. ( 10.4158/EP-2018-0480) [DOI] [PubMed] [Google Scholar]
- 8.Diwaker C, Sarathi V, Jaiswal SK, Shah R, Deshmukh A, Thomas AE, Prakash G, Malhotra G, Patil V, Lila A.et al. Hereditary medullary thyroid carcinoma syndromes: experience from western India. Familial Cancer 202120241–251. ( 10.1007/s10689-020-00219-9) [DOI] [PubMed] [Google Scholar]
- 9.Sarathi V, Pandit R, Jagtap V, Lila AR, Bandgar TR, Menon PS, Varthakavi P, Raghavan VP, Shah NS. Performance of plasma fractionated free metanephrines by enzyme immunoassay in the diagnosis of pheochromocytoma and paraganglioma. Endocrine Practice 201117759–765. ( 10.4158/EP11058.OR) [DOI] [PubMed] [Google Scholar]
- 10.Carney JA, Sizemore GW, Sheps SG. Adrenal medullary disease in multiple endocrine neoplasia, type 2: pheochromocytoma and its precursors. American Journal of Clinical Pathology 197666279–290. ( 10.1093/ajcp/66.2.279) [DOI] [PubMed] [Google Scholar]
- 11.Wilson RA, Ibanez ML. A comparative study of 14 cases of familial and nonfamilial pheochromocytomas. Human Pathology 19789181–188. ( 10.1016/S0046-8177(7880109-9) [DOI] [PubMed] [Google Scholar]
- 12.Westfried M, Mandel D, Alderete MN, Groopman J, Minkowitz S. Sipple’s syndrome with a malignant pheochromocytoma presenting as a pericardial effusion. Cardiology 197863305–311. ( 10.1159/000169909) [DOI] [PubMed] [Google Scholar]
- 13.Sisson JC, Shapiro B, Beierwaltes WH. Scintigraphy with I-131 MIBG as an aid to the treatment of pheochromocytomas in patients with the multiple endocrine neoplasia type 2 syndromes. Henry Ford Hospital Medical Journal 198432254–261. [PubMed] [Google Scholar]
- 14.Spapen H, Gerlo E, Achten E, Bossuyt A, Somers G, Dupont A, Six R. Pre- and peroperative diagnosis of metastatic pheochromocytoma in multiple endocrine neoplasia type 2a. Journal of Endocrinological Investigation 198912729–731. ( 10.1007/BF03350044) [DOI] [PubMed] [Google Scholar]
- 15.Oishi S, Sasaki M, Yamauchi J, Umeda T, Sato T. Analysis of eight Sipple’s syndrome patients and review of eighty-two cases from the Japanese literature. Japanese Journal of Clinical Oncology 199020392–406. ( 10.1093/oxfordjournals.jjco.a039417) [DOI] [PubMed] [Google Scholar]
- 16.Namba H, Kondo H, Yamashita S, Kimura H, Yokoyama N, Tsuruta M, Sato A, Izumi M, Kinoshita H, Hakariya S. Multiple endocrine neoplasia type 2 with malignant pheochromocytoma – long term follow-up of a case by 131I-meta-iodobenzylguanidine scintigraphy. Annals of Nuclear Medicine 19926111–115. ( 10.1007/BF03164652) [DOI] [PubMed] [Google Scholar]
- 17.Bonnin F, Schlumberger M, Gardet P, Tenenbaum F, Lumbroso J, Leclere J, Comoy E, Megnigbeto A, Travagli JP, Parmentier C. Screening for adrenal medullary disease in patients with medullary thyroid carcinoma. Journal of Endocrinological Investigation 199417253–257. ( 10.1007/BF03348970) [DOI] [PubMed] [Google Scholar]
- 18.Sasaki M, Iwaoka T, Yamauchi J, Tokunaga H, Naomi S, Inoue J, Oishi S, Umeda T, Sato T. A case of Sipple’s syndrome with malignant pheochromocytoma treated with 131I-metaiodobenzyl guanidine and a combined chemotherapy with cyclophosphamide, vincristine and dacarbazine. Endocrine Journal 199441155–160. ( 10.1507/endocrj.41.155) [DOI] [PubMed] [Google Scholar]
- 19.Scopsi L, Castellani MR, Gullo M, Cusumano F, Camerini E, Pasini B, Orefice S. Malignant pheochromocytoma in multiple endocrine neoplasia type 2B syndrome. Case report and review of the literature. Tumori 199682480–484. ( 10.1177/030089169608200514) [DOI] [PubMed] [Google Scholar]
- 20.Hinze R, Machens A, Schneider U, Holzhausen HJ, Dralle H, Rath FW. Simultaneously occurring liver metastases of pheochromocytoma and medullary thyroid carcinoma – a diagnostic pitfall with clinical implications for patients with multiple endocrine neoplasia type 2a. Pathology, Research and Practice 2000196477–481. ( 10.1016/S0344-0338(0080049-7) [DOI] [PubMed] [Google Scholar]
- 21.Gentile S, Rainero I, Savi L, Rivoiro C, Pinessi L. Brain metastasis from pheochromocytoma in a patient with multiple endocrine neoplasia type 2A. Panminerva Medica 200143305–306. [PubMed] [Google Scholar]
- 22.Hamdan A, Hirsch D, Green P, Neumann A, Drozd T, Molad Y. Pheochromocytoma: unusual presentation of a rare disease. Israel Medical Association Journal 20024827–828. [PubMed] [Google Scholar]
- 23.Ishida E, Nakamura M, Shimada K, Matsuyoshi S, Tada K, Okajima E, Fujimoto K, Konishi N. Autopsy case of prostate cancer with multiple endocrine neoplasia 2A. Pathology International 200454918–923. ( 10.1111/j.1440-1827.2004.01773.x) [DOI] [PubMed] [Google Scholar]
- 24.Gullu S, Gursoy A, Erdogan MF, Dizbaysak S, Erdogan G, Kamel N. Multiple endocrine neoplasia type 2A/localized cutaneous lichen amyloidosis associated with malignant pheochromocytoma and ganglioneuroma. Journal of Endocrinological Investigation 200528734–737. ( 10.1007/BF03347557) [DOI] [PubMed] [Google Scholar]
- 25.Ku CF, Lo CY, Chan WF, Chiu SW, Fan ST, Lam KSL. Resection of phaeochromocytoma extending into the right atrium in a patient with multiple endocrine neoplasia type 2A. Hong Kong Medical Journal 20051159–62. [PubMed] [Google Scholar]
- 26.Duquia RP, Almeida de HL, Traesel M, Jannke HA. Cutaneous metastasis of pheochromocytoma in multiple endocrine neoplasia IIB. Journal of the American Academy of Dermatology 200655341–344. ( 10.1016/j.jaad.2005.11.1080) [DOI] [PubMed] [Google Scholar]
- 27.Crona J, Nordling M, Maharjan R, Granberg D, Stålberg P, Hellman P, Björklund P. Integrative genetic characterization and phenotype correlations in pheochromocytoma and paraganglioma tumours. PLoS ONE 20149 e86756. ( 10.1371/journal.pone.0086756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang BHH, Yu HW, Lo CY, Lee KE, Garcia-Barcelo MM, Woo YC, Lee PCH, Wong KP, Tam PKH, Lam KSL. Bilateral pheochromocytomas in MEN2A syndrome: a two-institution experience. World Journal of Surgery 2015392484–2491. ( 10.1007/s00268-015-3117-2) [DOI] [PubMed] [Google Scholar]
- 29.Kotecka-Blicharz A, Hasse-Lazar K, Jurecka-Lubieniecka B, Pawlaczek A, Oczko-Wojciechowska M, Bugajska B, Ledwon A, Król A, Michalik B, Jarząb B. Occurrence of phaeochromocytoma tumours in RET mutation carriers – a single-centre study. Endokrynologia Polska 20166754–58. ( 10.5603/EP.2016.0008) [DOI] [PubMed] [Google Scholar]
- 30.Martins AF, Martins JM, do Vale S, Dias T, Silveira C, da Silva IR, Carmo-Fonseca M. A rare missense variant in RET exon 8 in a Portuguese family with atypical multiple endocrine neoplasia type 2A. Hormones 201615435–440. ( 10.14310/horm.2002.1691) [DOI] [PubMed] [Google Scholar]
- 31.Pal R, Rastogi A, Kumar S, Bhansali A. Metastatic pheochromocytoma in MEN 2A: a rare association. BMJ Case Reports 20182018bcr2017222758. ( 10.1136/bcr-2017-222758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma X, Li M, Tong A, Wang F, Cui Y, Zhang X, Zhang Y, Chen S, Li Y. Genetic and clinical profiles of pheochromocytoma and paraganglioma: a single center study. Frontiers in Endocrinology 202011574662. ( 10.3389/fendo.2020.574662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jester G, Hassanein H, El-Far A. Late diagnosis of metastatic pheochromocytoma in multiple endocrine neoplasia 2B with rapid clinical decline. BMJ Case Reports 202114e240488. ( 10.1136/bcr-2020-240488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thosani S, Ayala-Ramirez M, Palmer L, Hu MI, Rich T, Gagel RF, Cote G, Waguespack SG, Habra MA, Jimenez C. The characterization of pheochromocytoma and its impact on overall survival in multiple endocrine neoplasia type 2. Journal of Clinical Endocrinology and Metabolism 201398E1813–E1819. ( 10.1210/jc.2013-1653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mucha L, Leidig-Bruckner G, Frank-Raue K, Bruckner T, Kroiss M, Raue F. & German Study Gro up for Rare Thyroid Cancer. Phaeochromocytoma in multiple endocrine neoplasia type 2: RET codon-specific penetrance and changes in management during the last four decades. Clinical Endocrinology 201787320–326. ( 10.1111/cen.13386) [DOI] [PubMed] [Google Scholar]
- 36.Nakagawara A, Ikeda K, Tsuneyoshi M, Daimaru Y, Enjoji M. Malignant pheochromocytoma with ganglioneuroblastomatous elements in a patient with von Recklinghausen’s disease. Cancer 1985552794–2798. () [DOI] [PubMed] [Google Scholar]
- 37.Otoukesh S, Cooper CJ, Lou W, Mojtahedzadeh M, Nasrazadani A, Wampler M, Nahleh Z. Combination chemotherapy regimen in a patient with metastatic malignant pheochromocytoma and neurofibromatosis type 1. American Journal of Case Reports 201415123–127. ( 10.12659/AJCR.890181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giovannoni I, Callea F, Boldrini R, Inserra A, Cozza R, Francalanci P. Malignant pheochromocytoma in a 16-year-old patient with neurofibromatosis type 1. Pediatric and Developmental Pathology 201417126–129. ( 10.2350/13-10-1397-CR.1) [DOI] [PubMed] [Google Scholar]
- 39.Gruber LM, Erickson D, Babovic-Vuksanovic D, Thompson GB, Young WF, Bancos I. Pheochromocytoma and paraganglioma in patients with neurofibromatosis type 1. Clinical Endocrinology 201786141–149. ( 10.1111/cen.13163) [DOI] [PubMed] [Google Scholar]
- 40.Petr EJ, Else T. Pheochromocytoma and paraganglioma in Neurofibromatosis type 1: frequent surgeries and cardiovascular crises indicate the need for screening. Clinical Diabetes and Endocrinology 20184 15. ( 10.1186/s40842-018-0065-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Sharefi A, Javaid U, Perros P, Ealing J, Truran P, Nag S, Kamaruddin S, Abouglila K, Cains F, Lewis L.et al. Clinical presentation and outcomes of phaeochromocytomas/paragangliomas in neurofibromatosis Type 1. European Endocrinology 20191595–100. ( 10.17925/EE.2019.15.2.95) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castinetti F, Qi XP, Walz MK, Maia AL, Sansó G, Peczkowska M, Hasse-Lazar K, Links TP, Dvorakova S, Toledo RA.et al. Outcomes of adrenal-sparing surgery or total adrenalectomy in phaeochromocytoma associated with multiple endocrine neoplasia type 2: an International Retrospective Population-Based Study. Lancet: Oncology 201415648–655. ( 10.1016/S1470-2045(1470154-8) [DOI] [PubMed] [Google Scholar]
- 43.Modigliani E, Vasen HM, Raue K, Dralle H, Frilling A, Gheri RG, Brandi ML, Limbert E, Niederle B, Forgas L. Pheochromocytoma in multiple endocrine neoplasia type 2: European study. The Euromen Study Group. Journal of Internal Medicine 1995238363–367. ( 10.1111/j.1365-2796.1995.tb01211.x) [DOI] [PubMed] [Google Scholar]
- 44.Bausch B, Borozdin W, Neumann HPH. & European-American Pheochromocytoma Study Group. Clinical and genetic characteristics of patients with neurofibromatosis type 1 and pheochromocytoma. New England Journal of Medicine 20063542729–2731. ( 10.1056/NEJMc066006) [DOI] [PubMed] [Google Scholar]
- 45.Yao L, Schiavi F, Cascon A, Qin Y, Inglada-Pérez L, King EE, Toledo RA, Ercolino T, Rapizzi E, Ricketts CJ.et al. Spectrum and prevalence of FP/TMEM127 gene mutations in pheochromocytomas and paragangliomas. JAMA 20103042611–2619. ( 10.1001/jama.2010.1830) [DOI] [PubMed] [Google Scholar]
- 46.Bausch B, Schiavi F, Ni Y, Welander J, Patocs A, Ngeow J, Wellner U, Malinoc A, Taschin E, Barbon G.et al. Clinical characterization of the pheochromocytoma and paraganglioma susceptibility genes SDHA, TMEM127, MAX, and SDHAF2 for gene-informed prevention. JAMA Oncology 201731204–1212. ( 10.1001/jamaoncol.2017.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Comino-Méndez I, Gracia-Aznárez FJ, Schiavi F, Landa I, Leandro-García LJ, Letón R, Honrado E, Ramos-Medina R, Caronia D, Pita G.et al. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nature Genetics 201143663–667. ( 10.1038/ng.861) [DOI] [PubMed] [Google Scholar]
- 48.Burnichon N, Cascón A, Schiavi F, Morales NP, Comino-Méndez I, Abermil N, Inglada-Pérez L, Cubas de AA, Amar L, Barontini M.et al. MAX mutations cause hereditary and sporadic pheochromocytoma and paraganglioma. Clinical Cancer Research 2012182828–2837. ( 10.1158/1078-0432.CCR-12-0160) [DOI] [PubMed] [Google Scholar]
- 49.Eisenhofer G, Klink B, Richter S, Lenders JW, Robledo M. Metabologenomics of phaeochromocytoma and paraganglioma: an integrated approach for personalised biochemical and genetic testing. Clinical Biochemist: Reviews 20173869–100. [PMC free article] [PubMed] [Google Scholar]
- 50.Weismann D, Peitzsch M, Raida A, Prejbisz A, Gosk M, Riester A, Willenberg HS, Klemm R, Manz G, Deutschbein T.et al. Measurements of plasma metanephrines by immunoassay vs liquid chromatography with tandem mass spectrometry for diagnosis of pheochromocytoma. European Journal of Endocrinology 2015172251–260. ( 10.1530/EJE-14-0730) [DOI] [PubMed] [Google Scholar]
- 51.Sue M, Martucci V, Frey F, Lenders JMW, Timmers HJ, Peczkowska M, Prejbisz A, Swantje B, Bornstein SR, Arlt W.et al. Lack of utility of SDHB mutation testing in adrenergic metastatic phaeochromocytoma. European Journal of Endocrinology 201517289–95. ( 10.1530/EJE-14-0756) [DOI] [PubMed] [Google Scholar]
- 52.Ilanchezhian M, Jha A, Pacak K, Del Rivero J. Emerging treatments for advanced/metastatic pheochromocytoma and paraganglioma. Current Treatment Options in Oncology 202021 85. ( 10.1007/s11864-020-00787-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neumann HP, Young WF, Krauss T, Bayley JP, Schiavi F, Opocher G, Boedeker CC, Tirosh A, Castinetti F, Ruf Jet al. 65 years OF THE Double HELIX: genetics informs precision practice in the diagnosis and management of pheochromocytoma. Endocrine-Related Cancer 201825T201–T219. ( 10.1530/ERC-18-0085) [DOI] [PubMed] [Google Scholar]
- 54.Stewart DR, Korf BR, Nathanson KL, Stevenson DA, Yohay K. Care of adults with neurofibromatosis type 1: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genetics in Medicine 201820671–682. ( 10.1038/gim.2018.28) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a