Abstract
Susceptibility and resilience to stress depend on the timing of the exposure with respect to development, when across the lifespan effects are measured, and the behavioral or biological phenotype under consideration. This translational review examines preclinical stress models providing clues to causal mechanisms and their relationship to more the complex phenomenon of “stress-related” psychiatric and cognitive disorders in humans. We examine how genetic sex and epigenetic regulation of hormones contribute to the proximal and distal effects of stress at different epochs of life. Stress during the pre-natal period and early post-natal life put males at risk of developing diseases involving socialization, attention and cognition, such as autism spectrum disorders, and attention deficit disorder, respectively. While females show resilience to some of the proximal effects of pre-natal/early post-natal stress there is evidence that risk associated with developmental insults is unmasked in females following periods of hormonal activation and flux including puberty, pregnancy and perimenopause. Likewise, stress exposures during puberty have stronger proximal effects on females including an increased risk of developing mood- and stress- related illnesses such as depression, anxiety and, post-traumatic stress disorder. Hormonal changes during menopause and andropause impact the processes of memory and emotion in both sexes, though females are preferentially at risk for dementia and childhood adversity impacts estradiol effects on neural function. We propose that studies to determine mechanisms for stress risk and resilience across the lifespan must consider the nature and timing of stress exposures as well as the sex of the organism under investigation.
Keywords: mood disorders, stress, sex differences, epigenetics, estrogens, CRF
How do we define resilience to stress? In preclinical models, resilience is ascribed to animals that experience a stressor, yet demonstrate biological or behavioral phenotypes similar to unstressed controls. In clinical research, the ability to experience significant stress(es) without subsequent psychopathology is considered a sign of resilience. However, studies of immune, hypothalamic pituitary adrenal (HPA) axis, and brain function suggest that such exposures have a physiologic impact even in asymptomatic individuals (1-3). Such alterations create risk for adverse health conditions later in life. That many individuals suffer psychopathology in the setting of acute stress, but fully recover, highlights the complexity of risk and resilience research in humans.
Biological and behavioral adaptations in response to stress along with nurturing environments mitigate the adverse effects of significant stress in rodent, primate and human subjects research (4-8). Preclinical studies indicate that milder, repeated stress or being housed in a nurturing environment leads to epigenetic and neurohormonal profiles associated with less behavioral dysregulation later in life, even when exposed to additional stressors. Support from family and the wider social environment at the time of trauma exposure and throughout life is critical to promote resilience in humans (8).
Studies that use stress to induce behavioral endophenotypes, focus mostly on proximal effects. However, most psychiatric disorders have prodromes that can appear years before the individual reaches symptom threshold for psychiatric illness (9, 10). The events that contribute to the occurrence of the disorder may also occur years prior to the emergence of frank symptoms (11). Finally, the majority of basic research examining the mechanisms of resilience has done so only in male animals (12) when the majority of humans experiencing stress-related disorders are female (13, 14).
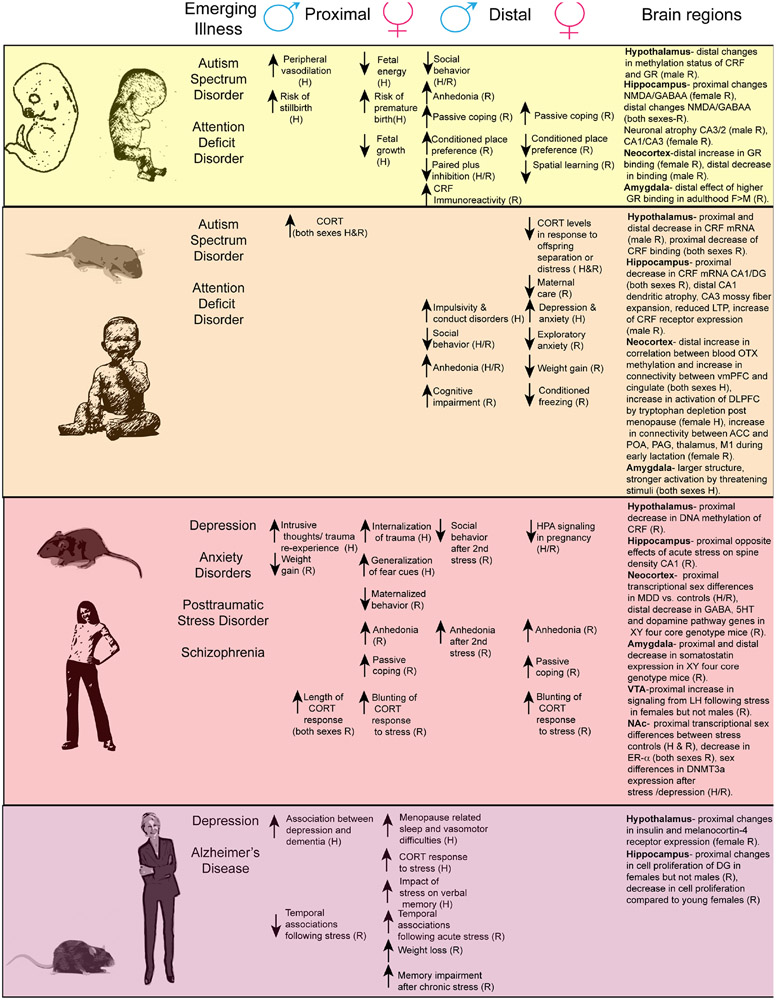
We examine the concept of resilience as both a proximal and distal response to stress. We are defining proximal as responses measured within the same developmental epoch as the stress exposure and distal as responses that occur in a subsequent developmental time point. We discuss work with similar measurable endpoints from basic and clinical research examining sex differences in vulnerability and resilience of response to stress across the lifespan (Figure 1). We propose that resilience is an active and dynamic process that is shaped, in part by genetic sex, gonadal steroids and epigenetic regulation of stress physiology and changes across epochs.
Figure 1. The relationship between stress effects on behavior across the lifespan and psychiatric disease.
For each epoch of the lifespan the associated emerging diseases are listed next to the proximal and distal effects of stress exposure at that time point and known changes in brain sub-regions. Abbreviations: N-methyl-D aspartate (NMDA), Gamma–Aminobutyric acid (GABA), ventral medial prefrontal cortex (vmPFC), dorsal lateral prefrontal cortex (DLPFC), anterior cingulated cortex (ACC), Preoptic area (POA), periaquaductal grey (PAG), Motor cortex 1 (M1), 5HT-serotonin, Lateral habenula (LH), dentate gyrus (DG).
Prenatal stress shapes an individual’s response to the environment
The prenatal experience shapes an individual’s brain, body and behavior for their lifetime and potentially even affects the response of subsequent generations through trans-generational mechanisms. Human offspring exposed to extreme gestational stress such as starvation during the Dutch Hunger Winter had increased risk of psychiatric disorders including affective disorders (15), addiction (16), and schizophrenia (17). Effects of famine were also associated with increased risk of schizophrenia in a separate Chinese population (18). In humans, it is difficult to separate the out the effects of maternal psychological distress due to famine from calorie and nutrient restriction which can have multiple physiologic effects on the offspring, including activation of the HPA axis. Most data have been collected from famine situations, but even maternal immune activation, which induces sickness behavior, results in temporary decreased food intake (19, 20). The flexibility of fetal female energy consumption in response to stress or inflammation may confer proximal protection. Female, but not male, growth is restricted by in utero exposure to maternal uncontrolled asthma (21), a chronic medical condition associated with maternal hypertension, poor oxygenation, heightened immune activation and risk for pre-eclampsia [32]. It has been proposed that the ability of the female fetus to respond to an adverse maternal environment is protective against fetal stillbirth, which is higher in males exposed to maternal asthma and other medical adversities such as pre-eclampsia (22-24). Male risk bias is thought to be secondary to male adaptations in placental blood flow allowing the male fetus continue to grow in utero, but be born with greater peripheral vasodilation, a condition associated with worse neonatal outcome [32]. However, the timing of the stress exposure during gestation also contributes to sex differences on its impact. Acute early gestational stress in humans caused by experiencing an earthquake (2-3 months after conception) increased the rate of preterm birth to a greater degree in female offspring (25).
Maternal immune activation in humans’ mirrors the physiologic effects found during extreme stress and have been linked to increased risk of schizophrenia and autism spectrum disorders (26-29). Similarly, rodent models of maternal immune activation or stress demonstrate lasting effects predominantly on male offspring’s behavior (30-33) although effects are also reported in females (Table 1) (34-37). There is evidence of greater distal effects of prenatal stress in females on subsequent stress related behavior and physiology responses later in life (38). Later gestational stress or cytokine exposure (PND 11- parturition) leads to greater behavioral changes in females than males (Table 1) (39-41). Translating findings from rodents to humans is complicated for obvious reasons, but is also made more complex by the asynchrony between developmental epochs between species. Particularly, the effects of gestational stress are difficult to translate from human to rodent as rodent parturition occurs at roughly the equivalent of the end of the human second trimester (42).
Table 1.
Sex differences in the effects of stress on HPA axis regulation and behavior in male and female preclinical studies across the lifespan.
| Epoch of Stress exposure |
Males | Females |
|---|---|---|
| Pre-conception | Distal- Increased anxiety/depression associated behaviors. Increased response to subsequent stress M > F (46, 48, 49). | Distal- Increased anxiety/depression associated behaviors. Increased response to subsequent stress M > F (46, 48, 49). |
| Pre-natal stress |
Distal- Increased CORT in response to restraint (38). Decreased sociability and decreased paired plus inhibition (30-33). PND1-7 Distal -Feminized patterns of spatial navigation in Barnes maze/increased immobility in FST (35). Passive coping/ anhedonia (34, 35). Decreased spatial learning (37) PND 11- Birth Distal -Increased sensitivity to reward/ increased conditioned place preference for chocolate/cocaine (40). Alterations in circadian rhythm and REM sleep includes increased paradoxical/ fragmented sleep and decreased slow wave sleep (145) |
Distal- Increased CORT, ACTH and corticosterone binding globulin in response to restraint (38). PND 11- Birth Distal- Passive coping (FST) M < F Decreased sensitivity to reward/ decreased conditioned place preference for chocolate/cocaine (40). |
| Birth- peri-puberty |
Proximal- Increased CORT Decreased growth (62-65). Distal –Anhedonia. Decreased play/decreased social behavior (66-68). Decreased social interaction (146). Cognitive impairments(63, 69). Increased susceptibility to adult social defeat (76). |
Proximal- Increased CORT Decreased growth (62-65). Distal -Decreased weight (16) Decreased exploratory anxiety like behavior/ decreased contextual fear conditioning (147). Decreased maternal care (148). Decreased social interaction (146). Blunted HPA activity in response to acute stress pregnancy and pup separation/distress (78, 79). |
| Puberty |
Proximal- Increased period for CORT to return to baseline following acute stress (99, 100). Altered weight gain (102, 103). Distal- Increased submissive response to conditioned defeat (Syrian hamsters)(115). |
Proximal- Increased period for CORT to return to baseline following acute stress during puberty (99, 100). Blunted CORT response to restraint stress following exposure to chronic stressors (both proximal and distal)(101). Decreased maternalization of virgin females to pups(79) Anhedonia passive coping (101). Distal-Increased submissive response to conditioned defeat (Syrian hamsters) (115). |
| Adult |
Proximal- 21 day variable stress Decreased grooming following 6 day variable stress (splash test), increased latency to eat (NSF), increased immobility (FST) (111, 112). Increased ability to learn trace eyeblink conditioning(135) |
Proximal- 6 day variable stress- Decreased grooming (splash test), increased latency to eat (NSF), increased immobility (FST) decreased sucrose preference (111). 21 day variable stress Decreased grooming (splash test), increased latency to eat (NSF), increased immobility ( FST) decreased sucrose preference (111, 112). Conditioned defeat Decreased ability to learn trace eyeblink conditioning (135) |
| Senescence | Proximal-No effect of stress on trace eyeblink conditioning (136). |
Proximal- No effect of stress on trace eyeblink conditioning (136). Decreased spatial learning (radial arm maze) (65). |
Abbreviations: CORT- corticosterone; ACTH- adrenocorticotropic hormone; FST- forced swim test; NSF-novelty suppressed feeding
Potential Mechanisms for Intergenerational Transmission of Stress: Consideration of In Utero Adaptation by Sex
Initially, female development was characterized as a passive default. However, pre-clinical research suggests that female brain development is an active process regulated by DNA methylation (43), an epigenetic process through which genetic transcription is silenced (44). Female fetuses have higher placental levels of DNA methyltransferase 1 (DNMT 1) involved in maintenance methylation and respond to maternal variable stress respond with a further increases supporting the likelihood of continued recreation of methylation patterns (35). Additionally, prenatal stress only impacts de novo methylation patterns in the brains of the male offspring resulting in changes of methylation status of the corticotropin releasing factor (CRF) promoter and glucocorticoid receptor (GR) promoter in the hypothalamus (35) that contribute to lifelong changes in stress hormone signaling.
The epigenetic impact of prenatal stress on the offspring is not limited to stressors that affect the mother. Nor do stressors have to occur during gestation. Rodent studies in which either females or males were stressed during the peripubertal window, prior to conception, implicate germ cell epigenetic transmission of the stress phenotype from one generation to the next (45). Stress effects on gene expression through DNA methylation, histone modifications and noncoding RNAs impact cell-specific gene expression that can lead to alterations in normal cellular functions in the parent as well as offspring. Preclinical studies in males found that stress and/or exposure to drugs of abuse produce lasting epigenetic changes in sperm (46, 47) that impact the offspring behavior (Table 1) (46-49).
In humans, maternal exposure to famine prior to concieving was associated with worse mental health and quality of life for adult offspring, though sex of the offspring was not considered (50). Studies of children and grandchildren of Holocaust survivors have demonstrated clear adverse effects on the mental health of the F1 generation, but the adverse impact of the Holocaust on grandchildren’s mental health appears less pronounced (51-53). While the adverse effects of childhood and preconception trauma exposures on parental mental health and parenting practices mediate, in part, the effects of parental trauma on offspring mental health, recent data implicate epigenetic mechanisms. Most notably a study on the offspring of Holocaust survivors identified altered methylation status of the gene encoding FK506 binding protein, a regulator of glucocorticoid receptor sensitivity, in blood (54). These same offspring self reported depression and anxiety symptoms. Furthermore, female pre-pubertal exposure to the Holocaust effected transmission of risk phenotype to the offspring. Reduction in activity of the enzyme 11ß-hydroxysteroid dehydrogenase type 2 (11ß-HSD) which inactivates cortisol has been noted in Holocaust survivors, though an opposite effect was noted for their offspring. Emphasizing the importance of timing of adversity, the impact of having a mother who suffered in the Holocaust was more pronounced with respect to offspring 11ß-HSD activity if the mother was pre-pubertal at the time and her subsequent offspring was male (55).
Stress across the lifespan: Proximal and Distal Ramifications
Early life stress:
Studies of children isolated in orphanages due to previous polices of the Romanian government have provided insight into the lasting damage of neglect and early life stress even when basic needs such as food and shelter are met. Caregiver deprivation is associated with an accentuation of the female bias towards anxiety and depressive disorders and the male bias towards impulsivity and conduct disorders (56, 57). Neuroimaging studies show abnormalities in amygdala development and subsequent response to stimuli, influencing connections to brain regions critical to the evaluation and appropriate response to rewarding and aversive stimuli, in adults (58). Whether imaging outcomes vary by sex is not known as most studies do not examine data separately for males and females who have suffered severe caregiver deprivation.
In humans, another form of early life stress- high maternal allostatic load, measured by maternal psychological distress and parenting stress was associated with increased risk for socio-emotional problems in a prospective, longitudinal study of 1 year-old offspring (59). Offspring hair cortisol levels showed a complex relationship with maternal factors, with offspring levels positively correlated with parent stress, but negatively correlated with maternal depression scores (59). In humans, significant adversity before the age of 18 is associated with a host of adverse psychiatric and medical health outcomes regardless of sex (60, 61).
In rodents, early life stress that disrupts maternal care (62-65) has proximal effects (Table 1) including increased HPA activity in a traditionally refractory period. Distally in females there is reduced functional connectivity within cortical areas to brain regions implicated in maternal care, pain modulation and emotion along with sex differences in behavior (Table 1)(63, 66-69). Males but not females have cognitive deficits that become more pronounced in males as they age (70). Decreased ability to perform cognitive tasks in these males were associated with changes in excitability in subregions in the hippocampus that were unmasked only at an advanced age (70). CRF was also increased and the later cognitive effects were blocked by administration of a CRF antagonist immediately after the period of early life stress exposure (71).
In humans, studies of older adults reflect these preclinical findings. Significant childhood adversity is associated with worse cognitive aging and more rapid declines in processing speed, particularly when there are current depressive symptoms (72). However, the sex differences reported in the preclinical literature may not be relevant to human aging. Childhood adversity accentuates the effects of later-life care-giving stress on inflammation and telomere length, a marker of cellular aging (73) and older females are more likely to be caregivers than males. Women are also at roughly twice the risk of Alzheimer Disease than males (74). Severity of childhood trauma was associated with greater risk for a dementia diagnosis and Alzheimer Disease though sex differences were not investigated (75).
Pre-Peripubescent stress:
The timing of early life stress with respect to offspring development may also contribute to lasting effects. Preclinical work in male mice demonstrated that late postnatal stress (PND 10-20) produced distal effects on adult responses to social stress (76). The authors discovered that a transcription factor orthrodenticle homeobox 2 (OTX 2) contributed by mediating hedonic programming in the ventral tegmental area. A subsequent study in children (8-15) that have experienced childhood maltreatment found that the methylation status of peripheral OTX2 predicted depression and was associated with increased functional connectivity between key brain structures associated with mood disorders (77).
Early life stress alters the relationship between HPA axis activation and pregnancy or postpartum stress (Table 1) (78, 79). Similarly, psychologically healthy women who self-reported exposure to 2 or more types of adversities during childhood-adolescence (age 0-18) using the Adverse Childhood Experiences (ACE) Questionnaire also showed a blunted HPA response to separation from their 6 month-old infants that was mirrored by the offspring who underwent a restraint and noise stressor (78). Sample size was too small to examine offspring sex differences and it is unclear whether the blunted maternal and infant cortisol response to stress is a sign of risk or resilience. That individuals who experience significant childhood adversity are more likely to experience significant psychosocial stress later in life (80), a degree of HPA-axis blunting may be preferable.
Women who report experiencing two or more types of childhood adversities are also more likely to experience a first onset of major depressive disorder during the perimenopause transition compared to those who reported no childhood adversities. Experience of 1 type of adversity during the pre-pubertal window was associated with resilience to depression even if the individual went on to experience additional childhood adversities during the post-pubertal period (81). Some women are resilient to depression despite exposures to significant early life stress until they experience perimenopause suggesting an important interaction between gonadal steroids, early life stress and brain changes during aging. Non-human primate studies of social subordinant stress (82) and recent neuroimaging studies in postmenopausal females (83) suggest that early life stress is associated with enduring alterations estradiol driven changes on serotonergic functioning and may underlie a risk for depression or cognitive decline during periods of hypogonadism in females (84). Whether this same relationship holds true for older males is not known, but it is important to consider that adult males do not typically undergo a dramatic change in gonadal steroids with aging. Those males who experience natural or iatrogenically-induced hypogonadism are also at greater risk for cognitive declines and depression, though the impact of childhood adversity on their risk for depression during hypogonadism is not known (85, 86).
Stress during puberty and early adulthood:
The adolescent period is a risk factor in the occurrence of many psychiatric disorders in both sexes (87). By age 14 half of the people who will experience mental illness have had their first occurrence and this figure rises to 75% by age 24 (88). In girls, early onset of menstruation (prior to 11.5) increases circulating levels of estrogens during adolescence and increases the risk of depression (89). While the developmental changes of adolescence are usually associated with activational effects of gonadal hormones bringing on secondary sexual characteristics, there is growing evidence from the basic and clinical literature recognizing that, genetic sex (90, 91), organizational effects of gonadal hormones (92-94) and epigenetic mechanisms (95-97) contribute to the development of brain, body and behavior during this critical period.
Elegant studies have delineated the contribution of the genetic sex compliment on gene expression in the frontal cortex of the four core genotype model following chronic stress in early adulthood (90) . This mouse model allows for the dissociation of gonadal sex from genetic sex through manipulation of location of the SRY gene. Having an XY chromosome regardless of gonadal sex reduced gene expression for pathway members of GABA, serotonin and dopamine signaling in the frontal cortex (90). XY mice in the absence of testosterone expressed increased anxiety associated behavior compared to XX mice. Testosterone administration was anxiolytic indicating that the higher levels found in males promote resilience by compensating for an underlying vulnerability. Additional studies found a similar pattern in the relationship of somatostatin expression to anxiety associated behavior in chronically stressed males in the basal lateral amygdala (91). Future work should examine distal effects of stress within this model along with earlier life stress exposure.
Pre-clinical research indicated that there are proximal neuroendocrine changes in stress reactivity and sex differences in behavior (Table 1) as an individual moves from the pre-pubertal state to early adulthood (98-103). The effects of stress during puberty are long lasting and have implications for the human development of PTSD and other stress based mood disorders (104). During adolescence girls are 3 times more likely to develop PTSD than boys (105). Sex or gender differences in cognitive styles contribute to resilience for PTSD and other mood disorders. Females who experienced either early life or adolescent abuse are more likely to use internalizing coping strategies predictive for increased risk for PTSD (106). Traumatized girls entering puberty (age 8-13) are more likely to engage in self-blame and avoidance whereas traumatized boys tend to report intrusive or re-experiencing symptoms (107). When non-traumatized children were exposed to fear conditioning in an experimental setting, girls but not boys showed generalized fear and lack of ability to discriminate a safety signal.
Gonadal steroid fluctuations across the menstrual cycle are also thought to contribute directly to risk and symptoms of PTSD (108). Among women with PTSD, phobic anxiety is increased during the follicular phase when estradiol levels are low. A finding not observed in traumatized women without PTSD and non-trauma controls (109). In healthy adult women, low estradiol levels are also associated with stronger intrusive memories in an experimental paradigm (110). Together these data support that timing of traumatic exposures with respect to menstrual cycle phase, and thus natural gonadal steroid levels may contribute to risk or resilience for subsequent PTSD. Moreover, behavioral treatments for PTSD may be more successful if menstrual cycle phases and use of steroid contraceptives are considered.
Gonadal hormones also contribute directly to the effects of stress on behavior in animal. Female but not male mice respond to 6 days of variable stress whereas both sexes respond to 21 days of stress (Table 1) (111, 112). These sex differences involve increased signaling between the lateral habenula and the ventral tegmental area that only occurs in females (113). Ovariectomy blocks the behavioral responses to 6 days of stress (114), it has not known whether gonadectomy also blocks the effects of longer periods of variable stress in females, nor whether male resilience is dependent on gonadal hormones. The existing literature suggests that resilience for other stress models may be regulated via gonadal hormones (115). In males, submissive behavior following conditioned defeat was dependent upon testosterone. Castrated males express more submissive behavior following fewer attacks. Testosterone or dihydrotestosterone replacement reduced submissive behavior in castrated males (116). Studies of social stress of young adult mice also indicate a neuromodulatory role for hormones, as estrogen receptor alpha (ER-α) expression was functionally linked to resilience. Male mice that were susceptible to social defeat stress had decreased ER-α expression in the nucleus accumbens (NAc) and increasing expression of ER-α promoted resilience (117). Nuclear ER-α expression was also decreased in NAc of male and female mice that underwent variable stress and over-expression promoted resilience in both sexes (117). Different transcriptional targets were regulated in males and females following over-expression of ER-α indicating that different downstream molecular mechanisms regulate resilience in males and females. Molecular sex differences in response to stress have been replicated in other species including Syrian hamsters (118) and post-mortem tissue taken from humans with a diagnosis of major depressive disorder (112).
DNMT3a is a de novo methyltransferase that regulates sex differences in the adult transcriptome (111). Masculinizing female transcriptional signatures by reducing levels of DNMT3a blocked the effects of variable stress in females. Gene ontogeny identified the CRF pathway shifting most towards a male transcriptional pattern. Viral mediated overexpression of DNMT3a made males and females responsive to sub-threshold variable stress and micro defeat (111, 119). Post-mortem NAc tissue from men and women with major depressive disorder had increased DNMT3a expression and a history of antidepressant treatment at time of death partially attenuated the increase (111). In male mice that were behaviorally susceptible to social defeat stress infusion of a DNA methylation inhibitor, reversed social avoidance behavior similar to the effects of 28 days of systemic treatment with fluoxetine (119).
Some of the individual differences in CRF signaling in response to adult stress are due to epigenetic regulation of the CRF promoter. Stress susceptible male mice have higher levels of CRF expression in the hypothalamus than resilient animals due to decreased methylation of the CRF promoter (120). Antidepressant treatment blocks CRF promoter methylation and social anhedonia, whereas infusion into the hypothalamus of short interfering RNA (siRNA) sequences targeted to CRF promoted resilience. There is growing evidence of sex differences in CRF activation in response to stress in adulthood (121). Stress induced activation of CRF negatively impacts attention and cognitive function in adult males but not females. Males are more sensitive to cholinergic CRF activation whereas females respond with a noradrenergic mediated hyper arousal and vigilant state (122). These effects likely arise from region specific sex differences in the CRF1 receptor internalization (123, 124).
Stress during senescence.
Menopause and andropause are another period of changing hormones during which there are increased risks for emergence of psychiatric and cognitive issues. About 20% of women experience a debilitating menopause characterized by depression, cognitive changes, sleep difficulties and moderate to severe vasomotor symptoms (125). Perimenopausal women are at increased risk of affective and cognitive complaints though the risk for new onset and recurrence of major depression declines in the years following the final menstrual period (126). Premature menopause, defined as final menstrual period before the age of 40, is associated with even greater risk for affective and cognitive disturbances. That males typically experience later onset and a more protracted decline in testosterone production may contribute to their resilience to adverse cognitive and mood changes with aging. When andropause occurs prematurely, men too experience adverse effects on health and quality of life (127).
Menopause leads to changes in the HPA axis (128) that may enhance the adverse effects of stress. Postmenopausal women not using estradiol therapy (ET) tend to have greater cortisol response to acute stress than age matched males and younger adults (129) and increasing levels of urinary cortisol over time have been associated with worse baseline memory recall and increased memory decline over a two year period (130). Impact of ET on HPA axis response to stress in postmenopausal women is inconsistent and may be influenced by timing of administration onset with respect to the final menstrual period, dose of ET and duration of use. Short-term estradiol administration in women who have been hypogonadal for many years appears to negatively sensitize cognitive and behavioral response to stress while the opposite occurs in premenopausal women (131, 132). Longer term use of ET in postmenopausal women appears to blunt cortisol response to stress and reduce the negative effects of stress on working memory (133). Among individuals ages 54 to 72, only females showed a negative impact of a stressor on verbal memory. Women in the age group are likely to be postmenopausal and again cortisol levels were associated with worse performance in females only (134).
While preclinical studies on the effects of stress on cognition in aging mice are very limited, they imply that acute and chronic stress have different impacts on cognitive function aged animals compared to young (Table 1)(135, 136) and these effects are in part mediated by sex specific NMDA receptor dependent changes in CA1 spine density (137). Acute stress also decreased cell proliferation in the hippocampus of female but not male aged mice in the absence of effects on spatial learning and could be ameliorated by group housing (138). Additional studies demonstrate drastically reduced levels of neurogenesis in the hippocampus of females as they age and behavioral effects (Table 1)(65). These aged female mice had different regulation of insulin and melanocortin-4 receptors in the hypothalamus compared to young stressed females. Based on the clinical literature there is a genuine unmet need to examine the mechanisms through which hormones interact with stress in both sexes to promote cognitive and emotional resilience an individual ages.
It was originally proposed that depression during the lifespan was a risk factor for Alzheimer’s disease (AD) as a earlier meta-analysis showed a significant correlation between the duration of time between depression and AD diagnoses and risk for AD (139). However, recent longitudinal studies found that people with earlier onset of depression are not at greater risk of AD, even if they experienced episodes later in life (139, 140). Instead, our current understanding is that late onset depression is a prodrome of dementia. Those with a first episode of depression after the age of 50 are at 46% greater risk of all cause dementia and significant depressive symptoms at age 65 or older was associated with a 71% greater risk of dementia. Retrospective analysis of data over 28 years showed that differences in depression symptoms was apparent 11 years prior to the diagnosis of dementia with an accelerated increase in depressive symptoms occurring in the decade prior to dementia diagnosis (140). Similarly, increasing but not steady or declining depressive symptoms over a 10-year period was associated with increased risk for all cause dementia and AD (141). Like depression, AD is more common in women than in men (142, 143). Most studies examining the relationship between depression symptoms and risk for dementia control for sex in their analyses, and do not report findings for males and female separately. The Baltimore Longitudinal Study of Aging, the one study to do so, reported that the relationship between depression symptoms and all dementia including AD was significant for males, but not females (144).
Conclusions
In summary, males are at greater risk of adverse proximal and some distal behavioral effects of gestational and early life stress, due to a lack of compensatory mechanisms and alterations in epigenetic regulation and organizational effects of hormones. The greatest distal impact of stress in males at all lifespan epochs seems to be on cognitive ability, particularly spatial learning and may contribute symptoms associated with autism spectrum disorder, attention deficit disorder and the relationship between depression and AD later in life. While females demonstrate compensatory mechanisms that protect them from the effects of early life stress on cognition, the impact is still present when emotion related behavior is measured later in life and are unmasked during periods of dynamic hormonal changes including puberty, pregnancy and menopause. In general periods of fluctuating hormones appear to be a greater risk factor for both the proximal and distal effects of stress in females.
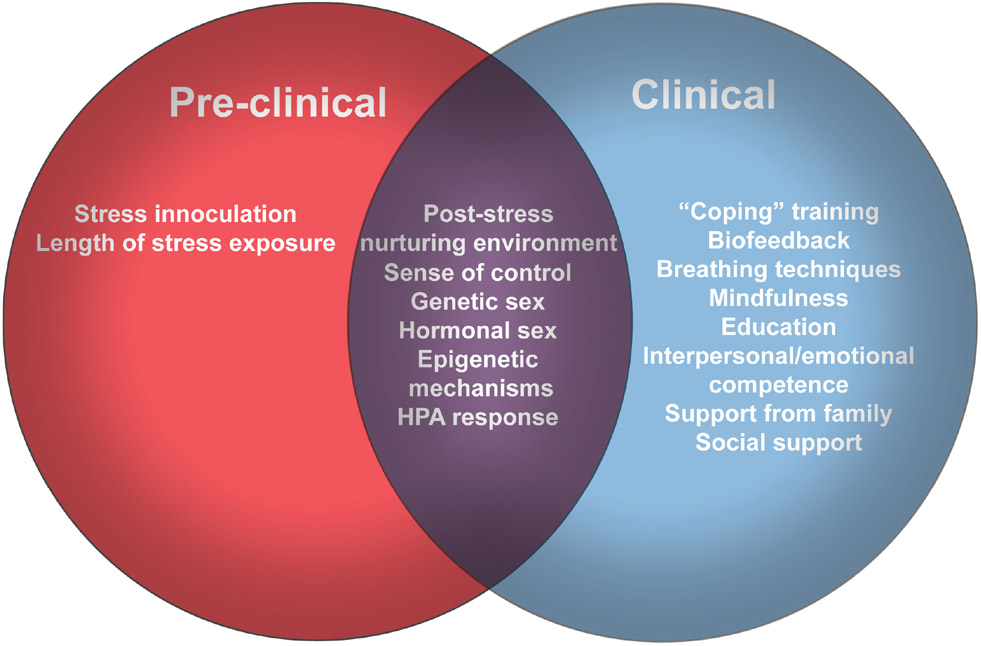
The impact of sex on risk and resilience to stress is complex, varying according to characteristics of the stressor such as type, timing and duration as well as development with its associated changes in brain structure and function as well as central and peripheral levels of gonadal steroids. Similarly, the negative effects of stress can be observed immediately in both animal and human models or endure for years without apparent health effects many years later. In fact, effects of childhood adversity on mood and cognition may require specific hormonal and/or developmental states such as those that occur at menopause and with aging in order to be revealed. Epigenetic regulation of hormonal state combined with genetic sex differences are driving factors effecting stress susceptibility and resilience in animal models and are also implicated in complex human disease states. As pre-clinical and clinical research works towards personalized treatments we will need to start considering that mechanisms contributing to disease state may differ by sex and by age. As researchers we need to start examining corresponding endpoints in clinical and preclinical studies. Only then will we be able to understand how mechanisms of resilience (Figure 2) protect individuals from specific symptoms across diseases.
Figure 2. Methods and mechanisms of promoting resilience.
The Venn diagrams display methods of promoting resilience in animal models (red), human subjects (blue) and methods/mechanisms that translate across species (purple).
Acknowledgements
This work was supported by NCI R01CA201295 and NIDA R01DA037289 to CNE. and a young investigator NARSAD grant from the brain and behavior research foundation to GEH.
Footnotes
Disclosures
Dr. Epperson serves as a consultant for Sage Therapeutics and on the advisory board for Asarina Pharma. Dr. Epperson or her spouse have personal investments in Merck & Co., Inc., Abbott Laboratories, Abbvie Inc, Johnson and Johnson, Bristol Myers Squibb. Dr. Hodes reports no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Menard C, Pfau ML, Hodes GE, Russo SJ (2017): Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology 42:62–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEwen BS (2002): Sex, stress and the hippocampus: Allostasis, allostatic load and the aging process. Neurobiol Aging 23:921–939. [DOI] [PubMed] [Google Scholar]
- 3.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ (2012): Neurobiology of resilience. Nat Neurosci 15:1475–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brockhurst J, Cheleuitte-Nieves C, Buckmaster CL, Schatzberg AF, Lyons DM (2015): Stress inoculation modeled in mice. Transl Psychiatry 5:e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hourani L, Tueller S, Kizakevich P, Lewis G, Strange L, Weimer B, et al. (2016): Toward preventing post-traumatic stress disorder: Development and testing of a pilot predeployment stress inoculation training program. Mil Med 181:1151–1160. [DOI] [PubMed] [Google Scholar]
- 6.Morrison KE, Narasimhan S, Fein E, Bale TL (2016): Peripubertal stress with social support promotes resilience in the face of aging. Endocrinology 157:2002–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillman L, Adams J, Kovac R, Kilcullen A, House A, Doyle C (2015): Strategies to promote coping and resilience in oncology and palliative care nurses caring for adult patients with malignancy: A comprehensive systematic review. JBI Database System Rev Implement Rep 13:131–204. [DOI] [PubMed] [Google Scholar]
- 8.Domhardt M, Munzer A, Fegert JM, Goldbeck L (2015): Resilience in survivors of child sexual abuse: A systematic review of the literature. Trauma Violence Abuse 16:476–493. [DOI] [PubMed] [Google Scholar]
- 9.Jackson A, Cavanagh J, Scott J (2003): A systematic review of manic and depressive prodromes. J Affect Disord 74:209–217. [DOI] [PubMed] [Google Scholar]
- 10.Savill M, D’Ambrosio J, Cannon TD, Loewy RL (2018): Psychosis risk screening in different populations using the Prodromal Questionnaire: A systematic review. Early Interv Psychiatry 12:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Secinti E, Thompson EJ, Richards M, Gaysina D (2017): Research review: Childhood chronic physical illness and adult emotional health—a systematic review and meta-analysis. J Child Psychol Psychiatry 58:753–769. [DOI] [PubMed] [Google Scholar]
- 12.Zucker I, Beery AK (2010): Males still dominate animal studies. Nature 465:690. [DOI] [PubMed] [Google Scholar]
- 13.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB (1993): Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord 29:8–96. [DOI] [PubMed] [Google Scholar]
- 14.Kessler RC (2003): Epidemiology of women and depression. J Affect Disord 74:5–13. [DOI] [PubMed] [Google Scholar]
- 15.Brown AS, Susser ES, Lin SP, Neugebauer R, Gorman JM (1995): Increased risk of affective disorders in males after second trimester prenatal exposure to the Dutch hunger winter of 1944-45. Br J Psychiatry 166:601–606. [DOI] [PubMed] [Google Scholar]
- 16.Franzek EJ, Sprangers N, Janssens AC, Van Duijn CM, Van De Wetering BJ (2008): Prenatal exposure to the 1944-45 Dutch ‘hunger winter’ and addiction later in life. Addiction 103:433–438. [DOI] [PubMed] [Google Scholar]
- 17.Hoek HW, Brown AS, Susser E (1998): The Dutch famine and schizophrenia spectrum disorders. Soc Psychiatry Psychiatr Epidemiol 33:373–379. [DOI] [PubMed] [Google Scholar]
- 18.St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, et al. (2005): Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. JAMA 294:557–562. [DOI] [PubMed] [Google Scholar]
- 19.Dantzer R (2004): Cytokine-induced sickness behaviour: A neuroimmune response to activation of innate immunity. Eur J Pharmacol 500:399–411. [DOI] [PubMed] [Google Scholar]
- 20.Hart BL (1988): Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12:123–137. [DOI] [PubMed] [Google Scholar]
- 21.Clifton VL, Murphy VE (2004): Maternal asthma as a model for examining fetal sex-specific effects on maternal physiology and placental mechanisms that regulate human fetal growth. Placenta 25(Suppl A):S45–S52. [DOI] [PubMed] [Google Scholar]
- 22.Clifton VL (2010): Review: Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta 31(Suppl):S33–S39. [DOI] [PubMed] [Google Scholar]
- 23.Murphy VE, Gibson PG, Giles WB, Zakar T, Smith R, Bisits AM, et al. (2003): Maternal asthma is associated with reduced female fetal growth. Am J Respir Crit Care Med 168:1317–1323. [DOI] [PubMed] [Google Scholar]
- 24.Murphy VE, Smith R, Giles WB, Clifton VL (2006): Endocrine regulation of human fetal growth: The role of the mother, placenta, and fetus. Endocr Rev 27:141–169. [DOI] [PubMed] [Google Scholar]
- 25.Torche F, Kleinhaus K (2012): Prenatal stress, gestational age and secondary sex ratio: The sex-specific effects of exposure to a natural disaster in early pregnancy. Hum Reprod 27:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown AS (2012): Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol 72:1272–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, et al. (2008): Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry 65:146–152. [DOI] [PubMed] [Google Scholar]
- 28.Kinney DK, Miller AM, Crowley DJ, Huang E, Gerber E (2008): Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord 38:481–488. [DOI] [PubMed] [Google Scholar]
- 29.Ronald A, Pennell CE, Whitehouse AJ (2010): Prenatal maternal stress associated with ADHD and autistic traits in early childhood. Front Psychol 1:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jouda J, Wohr M, Del Rey A (2019): Immunity and ultrasonic vocalization in rodents. Ann N Y Acad Sci 1437:68–82. [DOI] [PubMed] [Google Scholar]
- 31.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH (2012): Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun 26:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi L, Fatemi SH, Sidwell RW, Patterson PH (2003): Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci 23:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holloway T, Moreno JL, Umali A, Rayannavar V, Hodes GE, Russo SJ, et al. (2013): Prenatal stress induces schizophrenia-like alterations of serotonin 2A and metabotropic glutamate 2 receptors in the adult offspring: Role of maternal immune system. J Neurosci 33:1088–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goel N, Bale TL (2009): Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J Neuroendocrinol 21:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mueller BR, Bale TL (2008): Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci 28:9055–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller BR, Bale TL (2007): Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav 91:55–65. [DOI] [PubMed] [Google Scholar]
- 37.Weinstock M (2008): The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev 32:1073–1086. [DOI] [PubMed] [Google Scholar]
- 38.McCormick CM, Smythe JW, Sharma S, Meaney MJ (1995): Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res 84:55–61. [DOI] [PubMed] [Google Scholar]
- 39.Alonso SJ, Arevalo R, Afonso D, Rodriguez M (1991): Effects of maternal stress during pregnancy on forced swimming test behavior of the offspring. Physiol Behav 50:511–517. [DOI] [PubMed] [Google Scholar]
- 40.Reynaert ML, Marrocco J, Mairesse J, Lionetto L, Simmaco M, Deruyter L, et al. (2016): Hedonic sensitivity to natural rewards is affected by prenatal stress in a sex-dependent manner. Addict Biol 21:1072–1085. [DOI] [PubMed] [Google Scholar]
- 41.Samuelsson AM, Jennische E, Hansson HA, Holmang A (2006): Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol 290:R1345–R1356. [DOI] [PubMed] [Google Scholar]
- 42.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ (2013): Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106–107:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, et al. (2015): Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci 18:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bogdanovic O, Veenstra GJ (2009): DNA methylation and methyl-CpG binding proteins: Developmental requirements and function. Chromosoma 118:549–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan JC, Nugent BM, Bale TL (2018): Parental advisory: Maternal and paternal stress can impact offspring neurodevelopment. Biol Psychiatry 83:886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodgers AB, Morgan CP, Leu NA, Bale TL (2015): Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci U S A 112:13699–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC (2013): Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci 16:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White SL, Vassoler FM, Schmidt HD, Pierce RC, Wimmer ME (2016): Enhanced anxiety in the male offspring of sires that self-administered cocaine. Addict Biol 21:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, et al. (2011): Paternal transmission of stress-induced pathologies. Biol Psychiatry 70:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein AD, Pierik FH, Verrips GH, Susser ES, Lumey LH (2009): Maternal exposure to the Dutch famine before conception and during pregnancy: Quality of life and depressive symptoms in adult offspring. Epidemiology 20:909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sagi-Schwartz A, van IMH, Bakermans-Kranenburg MJ (2008): Does intergenerational transmission of trauma skip a generation? No meta-analytic evidence for tertiary traumatization with third generation of Holocaust survivors. Attach Hum Dev 10:105–121. [DOI] [PubMed] [Google Scholar]
- 52.Kassai SC, Motta RW (2006): An investigation of potential Holocaust-related secondary traumatization in the third generation. Int J Emerg Ment Health 8:35–47. [PubMed] [Google Scholar]
- 53.Zerach G, Solomon Z (2016): Low levels of posttraumatic stress symptoms and psychiatric symptomatology among third-generation Holocaust survivors whose fathers were war veterans. J Psychiatr Res 73:25–33. [DOI] [PubMed] [Google Scholar]
- 54.Yehuda R, Daskalakis NP, Bierer LM, Bader HN, Klengel T, Holsboer F, et al. (2016): Holocaust exposure induced intergenerational effects on FKBP5 methylation. Biol Psychiatry 80:372–380. [DOI] [PubMed] [Google Scholar]
- 55.Bierer LM, Bader HN, Daskalakis NP, Lehrner AL, Makotkine I, Seckl JR, et al. (2014): Elevation of 11beta-hydroxysteroid dehydrogenase type 2 activity in Holocaust survivor offspring: Evidence for an intergenerational effect of maternal trauma exposure. Psychoneuroendocrinology 48:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ellis BH, Fisher PA, Zaharie S (2004): Predictors of disruptive behavior, developmental delays, anxiety, and affective symptomatology among institutionally reared romanian children. J Am Acad Child Adolesc Psychiatry 43:1283–1292. [DOI] [PubMed] [Google Scholar]
- 57.Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, et al. (2009): Institutional rearing and psychiatric disorders in Romanian preschool children. Am J Psychiatry 166:777–785. [DOI] [PubMed] [Google Scholar]
- 58.Fareri DS, Tottenham N (2016): Effects of early life stress on amygdala and striatal development. Dev Cogn Neurosci 19:233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palmer FB, Anand KJ, Graff JC, Murphy LE, Qu Y, Volgyi E, et al. (2013): Early adversity, socioemotional development, and stress in urban 1-year-old children. J Pediatr 163:1733–1739.e1731. [DOI] [PubMed] [Google Scholar]
- 60.Van Niel C, Pachter LM, Wade R Jr, Felitti VJ, Stein MT (2014): Adverse events in children: predictors of adult physical and mental conditions. J Dev Behav Pediatr 35:549–551. [DOI] [PubMed] [Google Scholar]
- 61.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. (1998): Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14:245–258. [DOI] [PubMed] [Google Scholar]
- 62.Nephew BC, Bridges RS (2011): Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress 14:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rice CJ, Sandman CA, Lenjavi MR, Baram TZ (2008): A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 149:4892–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bolton JL, Molet J, Ivy A, Baram TZ (2017): New insights into early-life stress and behavioral outcomes. Curr Opin Behav Sci 14:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lotan A, Lifschytz T, Wolf G, Keller S, Ben-Ari H, Tatarsky P, et al. (2018): Differential effects of chronic stress in young-adult and old female mice: Cognitive-behavioral manifestations and neurobiological correlates. Mol Psychiatry 23:1432–1445. [DOI] [PubMed] [Google Scholar]
- 66.Bolton JL, Molet J, Regev L, Chen Y, Rismanchi N, Haddad E, et al. (2018): Anhedonia following early-life adversity involves aberrant interaction of reward and anxiety circuits and is reversed by partial silencing of amygdala corticotropin-releasing hormone gene. Biol Psychiatry 83:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ, et al. (2016): Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl Psychiatry 6:e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bolton JL, Ruiz CM, Rismanchi N, Sanchez GA, Castillo E, Huang J, et al. (2018): Early-life adversity facilitates acquisition of cocaine self-administration and induces persistent anhedonia. Neurobiol Stress 8:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanatsou S, Karst H, Kortesidou D, van den Akker RA, den Blaauwen J, Harris AP, et al. (2017): Overexpression of mineralocorticoid receptors in the mouse forebrain partly alleviates the effects of chronic early life stress on spatial memory, neurogenesis and synaptic function in the dentate gyrus. Front Cell Neurosci 11:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, et al. (2005): Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci 25:9328–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ivy AS, Rex CS, Chen Y, Dube C, Maras PM, Grigoriadis DE, et al. (2010): Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci 30:13005–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Korten NC, Penninx BW, Pot AM, Deeg DJ, Comijs HC (2014): Adverse childhood and recent negative life events: Contrasting associations with cognitive decline in older persons. J Geriatr Psychiatry Neurol 27:128–138. [DOI] [PubMed] [Google Scholar]
- 73.Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R (2011): Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med 73:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Podcasy JL, Epperson CN (2016): Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci 18:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Radford K, Delbaere K, Draper B, Mack HA, Daylight G, Cumming R, et al. (2017): Childhood stress and adversity is associated with late-life dementia in Aboriginal Australians. Am J Geriatr Psychiatry 25:1097–1106. [DOI] [PubMed] [Google Scholar]
- 76.Pena CJ, Kronman HG, Walker DM, Cates HM, Bagot RC, Purushothaman I, et al. (2017): Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 356:1185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaufman J, Wymbs NF, Montalvo-Ortiz JL, Orr C, Albaugh MD, Althoff R, et al. (2018): Methylation in OTX2 and related genes, maltreatment, and depression in children. Neuropsychopharmacology 43:2204–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morrison KE, Epperson CN, Sammel MD, Ewing G, Podcasy JS, Hantsoo L, et al. (2017): Preadolescent adversity programs a disrupted maternal stress reactivity in humans and mice. Biol Psychiatry 81:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shors TJ, Tobomicronn K, DiFeo G, Durham DM, Chang HY (2016): Sexual Conspecific Aggressive Response (SCAR): A model of sexual trauma that disrupts maternal learning and plasticity in the female brain. Sci Rep 6:18960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manyema M, Norris SA, Richter LM (2018): Stress begets stress: The association of adverse childhood experiences with psychological distress in the presence of adult life stress. BMC Public Health 18:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Epperson CN, Sammel MD, Bale TL, Kim DR, Conlin S, Scalice S, et al. (2017): Adverse childhood experiences and risk for first-episode major depression during the menopause transition. J Clin Psychiatry 78:e298–e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA (1991): Social behavior and gender in biomedical investigations using monkeys: studies in atherogenesis. Lab Anim Sci 41:334–343. [PubMed] [Google Scholar]
- 83.Shanmugan S, Loughead J, Cao W, Sammel MD, Satterthwaite TD, Ruparel K, et al. (2017): Impact of tryptophan depletion on executive system function during menopause is moderated by childhood adversity. Neuropsychopharmacology 42:2398–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shanmugan S, Epperson CN (2014): Estrogen and the prefrontal cortex: Towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp 35:847–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khosravi S, Ardebili HE, Larijani B, Nedjat S, Nikbakht Nasrabadi A, Ardebili ME, et al. (2015): Are andropause symptoms related to depression? Aging Clin Exp Res 27:813–820. [DOI] [PubMed] [Google Scholar]
- 86.Sato Y, Tanda H, Kato S, Onishi S, Nakajima H, Nanbu A, et al. (2007): Prevalence of major depressive disorder in self-referred patients in a late onset hypogonadism clinic. Int J Impot Res 19:407–410. [DOI] [PubMed] [Google Scholar]
- 87.Breslau J, Gilman SE, Stein BD, Ruder T, Gmelin T, Miller E (2017): Sex differences in recent first-onset depression in an epidemiological sample of adolescents. Transl Psychiatry 7:e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005): Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602. [DOI] [PubMed] [Google Scholar]
- 89.Joinson C, Heron J, Lewis G, Croudace T, Araya R (2011): Timing of menarche and depressive symptoms in adolescent girls from a UK cohort. Br J Psychiatry 198:17–23. [DOI] [PubMed] [Google Scholar]
- 90.Seney ML, Ekong KI, Ding Y, Tseng GC, Sibille E (2013): Sex chromosome complement regulates expression of mood-related genes. Biol Sex Differ 4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Puralewski R, Vasilakis G, Seney ML (2016): Sex-related factors influence expression of mood-related genes in the basolateral amygdala differentially depending on age and stress exposure. Biol Sex Differ 7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schulz KM, Sisk CL (2016): The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neurosci Biobehav Rev 70:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Lorme KC, Sisk CL (2016): The organizational effects of pubertal testosterone on sexual proficiency in adult male Syrian hamsters. Physiol Behav 165:273–277. [DOI] [PubMed] [Google Scholar]
- 94.Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL (2004): Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav 45:242–249. [DOI] [PubMed] [Google Scholar]
- 95.Bessa DS, Maschietto M, Aylwin CF, Canton APM, Brito VN, Macedo DB, et al. (2018): Methylome profiling of healthy and central precocious puberty girls. Clin Epigenetics 10:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lomniczi A, Wright H, Castellano JM, Matagne V, Toro CA, Ramaswamy S, et al. (2015): Epigenetic regulation of puberty via Zinc finger protein-mediated transcriptional repression. Nat Commun 6:10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lomniczi A, Loche A, Castellano JM, Ronnekleiv OK, Bosch M, Kaidar G, et al. (2013): Epigenetic control of female puberty. Nat Neurosci 16:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Foilb AR, Lui P, Romeo RD (2011): The transformation of hormonal stress responses throughout puberty and adolescence. J Endocrinol 210:391–398. [DOI] [PubMed] [Google Scholar]
- 99.Romeo RD, Karatsoreos IN, McEwen BS (2006): Pubertal maturation and time of day differentially affect behavioral and neuroendocrine responses following an acute stressor. Horm Behav 50:463–468. [DOI] [PubMed] [Google Scholar]
- 100.Romeo RD, Lee SJ, McEwen BS (2004): Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology 80:387–393. [DOI] [PubMed] [Google Scholar]
- 101.Bourke CH, Neigh GN (2011): Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav 60:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kaplowitz ET, Savenkova M, Karatsoreos IN, Romeo RD (2016): Somatic and neuroendocrine changes in response to chronic corticosterone exposure during adolescence in male and female rats. J Neuroendocrinol 28:12336. [DOI] [PubMed] [Google Scholar]
- 103.Weintraub A, Singaravelu J, Bhatnagar S (2010): Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res 1343:83–92. [DOI] [PubMed] [Google Scholar]
- 104.Westfall NC, Nemeroff CB (2015): The preeminence of early life trauma as a risk factor for worsened long-term health outcomes in women. Curr Psychiatry Rep 17:90. [DOI] [PubMed] [Google Scholar]
- 105.McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, et al. (2013): Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. J Am Acad Child Adolesc Psychiatry 52:815–830.e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, et al. (2013): Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci U S A 110:19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gamwell K, Nylocks M, Cross D, Bradley B, Norrholm SD, Jovanovic T (2015): Fear conditioned responses and PTSD symptoms in children: Sex differences in fear-related symptoms. Dev Psychobiol 57:799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garcia NM, Walker RS, Zoellner LA (2018): Estrogen, progesterone, and the menstrual cycle: A systematic review of fear learning, intrusive memories, and PTSD. Clin Psychol Rev 66:80–96. [DOI] [PubMed] [Google Scholar]
- 109.Pineles SL, Nillni YI, King MW, Patton SC, Bauer MR, Mostoufi SM, et al. (2016): Extinction retention and the menstrual cycle: Different associations for women with posttraumatic stress disorder. J Abnorm Psychol 125:349–355. [DOI] [PubMed] [Google Scholar]
- 110.Wegerer M, Kerschbaum H, Blechert J, Wilhelm FH (2014): Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiol Learn Mem 116:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, et al. (2015): Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci 35:16362–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. (2017): Sex-specific transcriptional signatures in human depression. Nat Med 23:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang S, Zhang H, Ku SM, Juarez B, Morel C, Tzavaras N, et al. (2018): Sex differences in the neuroadaptations of reward-related circuits in response to subchronic variable stress. Neuroscience 376:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.LaPlant Q, Chakravarty S, Vialou V, Mukherjee S, Koo JW, Kalahasti G, et al. (2009): Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol Psychiatry 65:874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rosenhauer AM, McCann KE, Norvelle A, Huhman KL (2017): An acute social defeat stressor in early puberty increases susceptibility to social defeat in adulthood. Horm Behav 93:31–38. [DOI] [PubMed] [Google Scholar]
- 116.Solomon MB, Karom MC, Norvelle A, Markham CA, Erwin WD, Huhman KL (2009): Gonadal hormones modulate the display of conditioned defeat in male Syrian hamsters. Horm Behav 56:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lorsch ZS, Loh YE, Purushothaman I, Walker DM, Parise EM, Salery M, et al. (2018): Estrogen receptor alpha drives pro-resilient transcription in mouse models of depression. Nat Commun 9:1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McCann KE, Sinkiewicz DM, Rosenhauer AM, Beach LQ, Huhman KL (2019): Transcriptomic analysis reveals sex-dependent expression patterns in the basolateral amygdala of dominant and subordinate animals after acute social conflict. Mol Neurobiol 56:3768–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.LaPlant Q, Vialou V, Covington HE 3rd, Dumitriu D, Feng J, Warren BL, et al. (2010): Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci 13:1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A (2010): Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci 13:1351–1353. [DOI] [PubMed] [Google Scholar]
- 121.Bangasser DA, Eck SR, Ordones Sanchez E (2019): Sex differences in stress reactivity in arousal and attention systems. Neuropsychopharmacology 44:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bangasser DA, Eck SR, Telenson AM, Salvatore M (2018): Sex differences in stress regulation of arousal and cognition. Physiol Behav 187:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, et al. (2010): Sex differences in corticotropin-releasing factor receptor signaling and trafficking: Potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15:877, 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McAlinn HR, Reich B, Contoreggi NH, Kamakura RP, Dyer AG, McEwen BS, et al. (2018): Sex differences in the subcellular distribution of corticotropin-releasing factor receptor 1 in the rat hippocampus following chronic immobilization stress. Neuroscience 383:98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hu LY, Shen CC, Hung JH, Chen PM, Wen CH, Chiang YY, et al. (2016): Risk of psychiatric disorders following symptomatic menopausal transition: A nationwide population-based retrospective cohort study. Medicine (Baltimore) 95:e2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Freeman EW, Sammel MD, Boorman DW, Zhang R (2014): Longitudinal pattern of depressive symptoms around natural menopause. JAMA Psychiatry 71:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saad F, Gooren LJ (2014): Late onset hypogonadism of men is not equivalent to the menopause. Maturitas 79:52–57. [DOI] [PubMed] [Google Scholar]
- 128.Woods NF, Carr MC, Tao EY, Taylor HJ, Mitchell ES (2006): Increased urinary cortisol levels during the menopausal transition. Menopause 13:212–221. [DOI] [PubMed] [Google Scholar]
- 129.Bale TL, Epperson CN (2015): Sex differences and stress across the lifespan. Nat Neurosci 18:1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW (1997): Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. J Clin Endocrinol Metab 82:2458–2465. [DOI] [PubMed] [Google Scholar]
- 131.Albert K, Pruessner J, Newhouse P (2015): Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology 59:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Newhouse PA, Dumas J, Wilkins H, Coderre E, Sites CK, Naylor M, et al. (2010): Estrogen treatment impairs cognitive performance after psychosocial stress and monoamine depletion in postmenopausal women. Menopause 17:860–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Herrera AY, Hodis HN, Mack WJ, Mather M (2017): Estradiol therapy after menopause mitigates effects of stress on cortisol and working memory. J Clin Endocrinol Metab 102:4457–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Almela M, Hidalgo V, Villada C, Espin L, Gomez-Amor J, Salvador A (2011): The impact of cortisol reactivity to acute stress on memory: Sex differences in middle-aged people. Stress 14:117–127. [DOI] [PubMed] [Google Scholar]
- 135.Hodes GE, Shors TJ (2005): Distinctive stress effects on learning during puberty. Horm Behav 48:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hodes GE, Shors TJ (2007): Learning during middle age: A resistance to stress? Neurobiol Aging 28:1783–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shors TJ, Falduto J, Leuner B (2004): The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur J Neurosci 19:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tzeng WY, Wu HH, Wang CY, Chen JC, Yu L, Cherng CG (2016): Sex differences in stress and group housing effects on the number of newly proliferated cells and neuroblasts in middle-aged dentate gyrus. Front Behav Neurosci 10:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D (2006): Depression and risk for Alzheimer disease: Systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 63:530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Singh-Manoux A, Dugravot A, Fournier A, Abell J, Ebmeier K, Kivimaki M, et al. (2017): Trajectories of depressive symptoms before diagnosis of dementia: A 28-year follow-up study. JAMA Psychiatry 74:712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mirza SS, Wolters FJ, Swanson SA, Koudstaal PJ, Hofman A, Tiemeier H, et al. (2016): 10-year trajectories of depressive symptoms and risk of dementia: A population-based study. Lancet Psychiatry 3:628–635. [DOI] [PubMed] [Google Scholar]
- 142.Mielke MM, Vemuri P, Rocca WA (2014): Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clin Epidemiol 6:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, et al. (1997): Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology 49:1498–1504. [DOI] [PubMed] [Google Scholar]
- 144.Dal Forno G, Palermo MT, Donohue JE, Karagiozis H, Zonderman AB, Kawas CH (2005): Depressive symptoms, sex, and risk for Alzheimer’s disease. Ann Neurol 57:381–387. [DOI] [PubMed] [Google Scholar]
- 145.Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O (2003): Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev 27:119–127. [DOI] [PubMed] [Google Scholar]
- 146.Babb JA, Carini LM, Spears SL, Nephew BC (2014): Transgenerational effects of social stress on social behavior, corticosterone, oxytocin, and prolactin in rats. Horm Behav 65:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kanatsou S, Ter Horst JP, Harris AP, Seckl JR, Krugers HJ, Joels M (2015): Effects of mineralocorticoid receptor overexpression on anxiety and memory after early life stress in female mice. Front Behav Neurosci 9:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nephew BC, Febo M, Huang W, Colon-Perez LM, Payne L, Poirier GL, et al. (2018): Early life social stress and resting state functional connectivity in postpartum rat anterior cingulate circuits. J Affect Disord 229:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]