Abstract
Background:
Recurrent Clostridioides difficile infection (CDI) is a major public health threat. While clinical prediction tools exist, they do not incorporate the newest Infectious Diseases Society of America guidelines.
Methods:
Prospective longitudinal study of patients experiencing their first episode of uncomplicated CDI. Patients were followed from diagnosis through 8 weeks post completion of their anti-CDI therapy to assess recurrence. Stool was collected at diagnosis and weekly for 8-weeks following treatment. Recurrence was defined as diarrhea as well as a positive stool test by toxin EIA (EIA) for C. difficile. Fisher’s exact test for binary variables and student’s t-test for continuous variables were performed. Cox regression was performed to assess for predictors of CDI recurrence.
Results:
75 patients were enrolled between Aug 1, 2015 and Sept 1, 2018. Mean age 58.1 years +/− 15.5, 69.3% female, 74.7% were white, 11.3% had baseline irritable bowel syndrome, and 54.7% were actively using PPIs. Over the 8-week follow up period 22 patients developed a confirmed CDI recurrence. Univariate predictors of recurrence included treatment with metronidazole (40.9% vs 15.1%, p=0.03), initially diagnosis by EIA (77.3% vs 43.4%, p=0.007) and platelet count (206 +/− 72.1 vs 270.9 +/− 114.8, p=0.03). A cox regression model revealed primary diagnosis by EIA (HR 3.39, 95%CI 1.23, 9.31, p=0.018) and treatment with metronidazole (HR 3.27 95% CI 1.31–8.19, p=0.01) remain predictors for CDI recurrence.
Conclusion:
In a large prospective longitudinal cohort of uncomplicated CDI patients, treatment with metronidazole, and diagnosis via EIA were the most robust predictors of CDI recurrence.
Keywords: Clostridioides difficile, toxin, metronidazole, vancomycin, microbiome
Background:
Clostridioides difficile, a gram positive spore-forming bacterium that colonizes the human gut, has the potential to elaborate potent toxins that cause mucosal damage and pseudomembranous colitis.[1] After an initial infection, recurrent disease will occur in 20–30% of patients.[2] Recurrent Clostridioides difficile infection (CDI) is a major public health problem, and has become the most prevalent nosocomial infection in the US.
The capacity to identify patients at risk for recurrence would support timely interventions, including altering potential antibiotic exposures and other risks, with the hopes of preventing the significant morbidity and mortality that occurs with recurrent C. difficile infections. Clinical prediction tools for recurrence exist[3]; however many use retrospectively collected data as opposed to real time stool assessments for recurrent disease.
Additionally, in the newest 2018 Infectious Diseases Society of America guidelines[4] changes in diagnostic laboratory testing for C. difficle, and the treatment recommendations for uncomplicated infection have been updated. Specifically, the guidelines recommend the use of stool toxin as part of a multi-step algorithm. With regards to treatment, either vancomycin or fidaxomicin are now recommended over metronidazole as first line therapy.
We thus undertook studying a longitudinal cohort of patients with primary, uncomplicated CDI to assess potential intervenable predictors of recurrence. Improved clinical and diagnostic markers to predict patients at risk for recurrent infections will enable the development of better strategies to treat primary disease and prevent recurrences.
Methods:
We conducted a prospective longitudinal cohort study of patients experiencing their first episode of uncomplicated CDI. Patients were recruited from the inpatient service at Brigham and Women’s Hospital (BWH), identified by daily reports of patients with positive tests for C. difficile provided by the BWH Clinical Microbiology Laboratory, as well as two surrounding community hospitals. Patients with diarrhea and a positive C. difficile test by either glutamate dehydrogenase (GDH) and enzyme immunoassay (EIA) toxin or Polymerase Chain Reaction (depending on the testing methods used at the associated hospital lab), and who were being treated for CDI were eligible for inclusion. Primary CDI was defined as no episodes of CDI within the past 6 months. Patients with inflammatory bowel disease, inherited or acquired immunodeficiencies, severe or fulminant CDI or ongoing non-CDI antibiotic use that continued past the CDI antibiotic course were excluded. Patients were followed from the time of CDI diagnosis through 8 weeks post completion of their anti-CDI therapy to assess for recurrence. Patients were assessed clinically at each time point. Stool was collected at CDI diagnosis (prior to antibiotic initiation), up to bi-weekly in the first 2 weeks post CDI diagnosis, and then weekly through 8 weeks following treatment. Recurrence was defined as diarrhea (Bristol stool scale 6 or 7) and at least 3 BMs daily for 3 days. If these criteria were met, stool was sent for C. difficile testing via GHD/EIA and if samples were positive for both the patient was considered to have a recurrence.
Univariate analysis using Fisher’s exact test for binary variables and student’s t-test for continuous variables were performed for statistical analyses. To assess clinical and diagnostic risks for recurrent CDI, we developed a Cox proportional hazard model to evaluate a priori risk factors as well as covariates found to be significant on univariate analysis. Once our final model was constructed, we tested the proportional hazards assumption using Martingale residuals. Unadjusted survival curves, based on the outcome of CDI recurrence, were constructed on significant covariates associated with CDI recurrence.
This protocol was approved by the institutional review board (IRB) at Brigham and Women’s Hospital.
Results:
75 patients with confirmed primary CDI were enrolled between Aug 1, 2015 and Sept 1, 2018. Among the cohort mean age was 58.1 years +/− 15.5, 69.3% (52) were female, the majority were white 74.7% (56), 52% were lifelong nonsmokers, 11.3% (8) had baseline irritable bowel syndrome, and 54.7% (41) were actively using PPIs (Table 1).
Table 1:
Patient Characteristics
| Variable Name | n = 75 |
|---|---|
| Age (Mean ± SD) | 58.1 ± 15.5 |
|
| |
| Female N,% | 52 (69.3%) |
|
| |
| Race N,% | |
| Black | 11 (14.7%) |
| White | 56 (74.7%) |
|
| |
| BMI (Mean ± SD) | 28.5 ± 6.5 |
|
| |
| Received Antibiotics prior to Diagnosis N,% | 56 (74.7%) |
|
| |
| Prior PPI use N,% | 41 (54.7%) |
|
| |
| History of Cirrhosis N,% | 5 (6.7%) |
|
| |
| Dietary restrictionsN,% | |
| No | 68 (90.7%) |
| Vegan | 1 (1.3%) |
| Vegetarian | 3 (4.0%) |
| Gluten Free | 1 (1.3%) |
| Lactose Free | 2 (2.7%) |
|
| |
| Smoking status | |
| Never | 39 (52.7%) |
| Former | 31 (41.9%) |
| Current | 4 (5.4%) |
|
| |
| Diagnosis of Irritable Bowel Syndrome N,% | 8 (11.3%) |
|
| |
| Baseline diarrhea or constipation N,% | |
| No | 54 (74.0%) |
| Diarrhea | 8 (11.0%) |
| Constipation | 9 (12.3%) |
| Both | 2 (2.7%) |
|
| |
| Baseline Bristol Score (Mean ± SD) | 3.1 ± 1.2 |
|
| |
| Ursodiol Use N,% | 0 (0.0%) |
|
| |
| Cholestyramine Use N,% | 2 (2.7%) |
|
| |
| Cholestid Use N,% | 1 (1.4%) |
|
| |
| CDI Treatment Regimen | |
| Metronidazole | 17 (22.7%) |
| Vancomycin | 58 (77.3%) |
|
| |
| Other current antibiotics (not for C diff) | 8 (10.7%) |
|
| |
| Test Used for Diagnosis N,% | |
| PCR | 35 (46.7%) |
| EIA Toxin | 40 (53.3%) |
|
| |
| Baseline Lab Values (Mean ± SD) | |
|
| |
| WBC | 12.9 ± 16.4 |
|
| |
| Hct | 40.6 ± 4.5 |
|
| |
| Plts | 252.7 ± 108.0 |
|
| |
| ALT | 42.3 ± 80.0 |
|
| |
| AST | 38.3 ± 75.9 |
|
| |
| Alkaline Phosphatase | 85.5 ± 46.5 |
|
| |
| T. Bilirubin | 0.6 ± 0.5 |
|
| |
| BUN | 20.9 ± 22.6 |
|
| |
| Cr | 1.7 ± 4.3 |
|
| |
| PT | 20.5 ± 23.9 |
|
| |
| INR | 1.3 ± 0.4 |
Over the 8-week follow up period 22 (29.3%) patients developed a confirmed CDI recurrence. On univariate analysis significant clinical predictors of recurrence included treatment with metronidazole compared to vancomycin (40.9% vs 15.1%, p=0.03), initial confirmed diagnosis by GDH a nd EIA toxin testing compared to PCR (77.3% vs 43.4%, p=0.007) and lower platelet count (206 +/− 72.1 vs 270.9 +/− 114.8, p=0.03). Notably, PPI use, a known risk factor for CDI, did not increase risk (45.5% vs 58.5%, p=0.3) (Table 2). Overall 15/22 (68.2%) patients who experienced recurrent infection had received systemic antibiotics prior to their initial CDI episode compared to 41/53 of those who did not recur (77.4%)(Table 2). The relative risk for prior systemic antibiotic use is .89 (.42, 1.87).
Table 2:
Univariate Risk Factors for Recurrence of CDI
| CDI Recurrence | |||
|---|---|---|---|
|
| |||
| YES N= 22 | NO N=53 | P value | |
| Mean Age (±SD) | 57.1 ± 18.0 | 58.5 ± 14.4 | 0.730 |
|
| |||
| Female (N,%) | 15 (68.2%) | 37 (69.8%) | 0.889 |
|
| |||
| Race (N,%) | 0.249 | ||
| Black | 1 (4.5%) | 10 (18.9%) | |
| White | 18 (81.8%) | 38 (71.7%) | |
|
| |||
| Mean BMI (±SD) | 28.5 ± 6.6 | 28.4 ± 6.6 | 0.969 |
|
| |||
| Received Antibiotics prior to Diagnosis (N,%) | 15 (68.2%) | 41 (77.4%) | 0.405 |
|
| |||
| Antibiotic Type | 0.076 | ||
| Beta- Lactam | 8 (36%) | 10 (18.8%) | |
| Fluoroquinolone | 1 (4.5%) | 14 (26.4%) | |
| Lincosamide | 3 (13.6%) | 2 (3.7%) | |
| Macrolide | 0 | 2 (3.7%) | |
| Metronidazole | 2 (9%) | 1 (1.8%) | |
| Nitrofurantoin | 0 | 1 (1.8%) | |
| Peptide Antibiotic | 0 | 5 (9.4%) | |
| Rifamycin | 0 | 1 (1.8%) | |
| Sulfa Drug | 1 (4.5%) | 2 (3.7%) | |
| None | 7 (32%) | 15 (28.3%) | |
|
| |||
| Prior PPI use (N, %) | 10 (45.5%) | 31 (58.5%) | 0.301 |
|
| |||
| History of Cirrhosis (N,%) | 2 (9.1%) | 3 (5.7%) | 0.626 |
|
| |||
| Dietary restrictionsN,% | 0.248 | ||
| No | 19 (86.4%) | 49 (92.5%) | |
| Vegan | 1 (4.5%) | 0 (0.0%) | |
| Vegetarian | 2 (9.1%) | 1 (1.9%) | |
| Gluten Free | 0 (0.0%) | 1 (1.9%) | |
| Lactose Free | 0 (0.0%) | 2 (3.8%) | |
|
| |||
| Smoking status (N,%) | 0.220 | ||
| Never | 15 (68.2%) | 24 (46.2%) | |
| Former | 6 (27.3%) | 25 (48.1%) | |
| Current | 1 (4.5%) | 3 (5.8%) | |
|
| |||
| Diagnosis of Irritable Bowel Syndrome (N,%) | 0 (0.0%) | 8 (16.3%) | 0.051 |
|
| |||
| Baseline diarrhea or constipation (N,%) | 0.575 | ||
| No | 18 (81.8%) | 36 (70.6%) | |
| Diarrhea | 1 (4.5%) | 7 (13.7%) | |
| Constipation | 2 (9.1%) | 7 (13.7%) | |
| Both | 1 (4.5%) | 1 (2.0%) | |
|
| |||
| Baseline Bristol Score (±SD) | 2.7 ± 1.2 | 3.3 ± 1.2 | 0.084 |
|
| |||
| Ursodiol Use (N,%) | 0 (0.0%) | 0 (0.0%) | 0.0 |
|
| |||
| Cholestyramine Use (N,%) | 2 (9.1%) | 0 (0.0%) | 0.085 |
|
| |||
| Colestipol Use (N,%) | 0 (0.0%) | 1 (1.9%) | 1.0 |
|
| |||
| CDI Treatment Regimen (N,%) | 0.030 | ||
| Metronidazole | 9 (40.9%) | 8 (15.1%) | |
| Vancomycin | 13(59.1%) | 45 (84.9%) | |
|
| |||
| Test Used for Diagnosis (N,%) | 0.007 | ||
| PCR | 5 (22.7%) | 30 (56.6%) | |
| EIA Toxin | 17 (77.3%) | 23 (43.4%) | |
|
| |||
| Mean White Blood Cell Count (±SD) | 8.2 ± 3.3 | 14.8 ± 18 | 0.147 |
|
| |||
| Mean Platelet Count (±SD) | 206.3 ± 72. | 270.9 ± 114.8 | 0.030 |
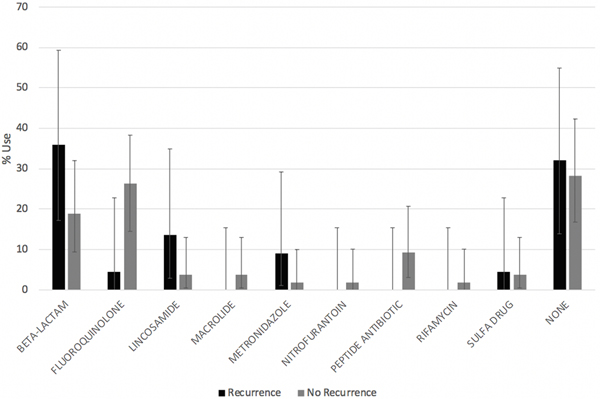
No significant differences were found among the type of antibiotic used prior to the development of primary C.difficile infection (Figure 1).
Figure 1:
Antibiotic Use by Recurrence Status (Percentage use, 95% CI intervals)
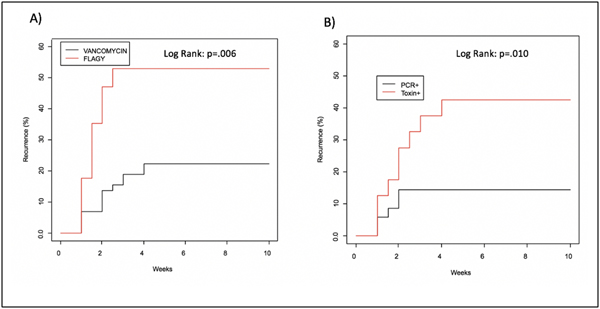
A Cox proportional hazard model was employed to further evaluate the significant variables identified from the univariate analysis as well as PPI use. The model revealed that diagnosis of primary CDI with GDH/EIA toxin, as opposed to PCR alone, (HR 3.39, 95%CI 1.23, 9.31, p=0.018; Kaplan Meier log rank p=0.01; Figure 2), and treatment of the primary CDI infection with metronidazole (HR 3.27 95% CI 1.31–8.19, p=0.01) were the most significant predictors of CDI recurrence; Kaplan Meier log rank p=0.006; Figure 2).
Figure 2:
Kaplan Meier Curves for Recurrence of CDI A) Primary Antibiotic Use and B) Primary Diagnostic Test.
Conclusion:
In a large prospective and longitudinal cohort of uncomplicated CDI patients, diagnosis of primary CDI via GDH/EIA toxin testing, and treatment with metronidazole were the strongest risk factors for developing recurrent infection. Importantly, both identified risk factors have ready applications to clinical care and can be modified to reduce patients’ risks for recurrent infections.
Active infection is driven by the pathogen’s elaboration of toxin. Diagnostic methods measuring antigenic toxin in gut contents detects the primary driver behind disease, as opposed to molecular methods which evaluate the presence or absence of specific genes but not their expression. PCR-only testing has increased rates for false positive results in patients harboring non-toxigenic strains or who may be colonized with a toxigenic strain that is not elaborating substantive toxin in the face of GI symptoms that may mirror primary CDI. [5,6]
The IDSA’s updated CDI Treatment Guidelines for 2018[4] now recommend a two-step testing method for CDI testing, using an initial sensitive screening test for C. difficile’s presence such as GDH or PCR, followed by confirmation of toxin elaboration in vivo with the EIA toxin test.[6] This recommendation was developed due to the increased risk of false positive results using only PCR-based testing, which cannot distinguish colonization with C. difficile from active infection related to active toxin production. It is therefore not surprising that in our cohort of patients with primary CDI infection, those diagnosed by EIA toxin were more likely to have true CDI and were this at higher risk for developing recurrent infections. Additionally, the guidelines removed metronidazole as a first line therapy for non-severe primary CDI, given higher failure rates as compared to treatment with vancomycin. Among our cohort we noted a considerable percentage of patients received metronidazole, and not surprisingly this treatment significantly increased risks of recurrence, supporting the updated guidelines. It is interesting to note that the American College of Gastroenterology Guidelines were last published in 2013.[7] In these guidelines metronidazole is still considered first line therapy and PCR testing is recommended over toxin based testing. This field has evolved considerably since the 2013 ACG guidelines, stressing the need for an update.
Other studies have previously assessed clinical risk factors for recurrence of CDI. Recently Cobo et al. assessed 274 patients diagnosed with CDI. They created a clinical tool that utilized four factors including age, history of CDI within the previous year, toxin in the stool and persistence of diarrhea on the fifth day of treatment.[3] Additionally, a cohort of veterans similarly included PPI use, cephalosporin use, presence of non-severe, uncomplicated CDI, community onset CDI, and prior antidiarrheals into their model.[8] The microbiome has also been assessed as possible predictors of recurrence. Khanna et al. has found increases in taxa associated with Veillonella, Enterobacteriaceae, Streptococci, Parabacteroides and Lachnospiraceae in patients who recurred compared to patients without recurrence. Their risk index using these factors had a 78% prediction of recurrence or not, but did not define the timing in which testing should be performed.[9] While compelling, at this time it is not practical to use the microbiome as a real time prediction tool to assist in management of patients. In contrast, in our cohort, the most significant risk factors for recurrence could be determined from the medical record at the point of ordering diagnostic testing for primary CDI, support implementing strategies at this early stage to reduce risks for recurrent infections.
Importantly, our findings could be readily implemented to support clinical decisions at the point of ordering testing for primary CDI, including implementation of CDI-focused clinical workflows in the EHR, as well as enabling support through the testing offered by CLIA laboratories, and institutional guidelines regarding antibiotic selection for treatment of primary CDI:
Leverage a strategy for diagnostic testing that includes testing for C. difficile toxin production in vivo, such as with a CLIA-approved EIA method.
In primary-CDI confirmed patients, avoid metronidazole for primary treatment, even in patients with uncomplicated CDI.
Limitations of our study include our focus on patients with uncomplicated CDI and the exclusion of patients who are immunocompromised either due to medications or co-morbid disease states. However, the focus on patients with uncomplicated CDI and longitudinal follow-up was essential to clearly define potential clinical markers with predictive capacity for recurrent CDI.
In this large prospective longitudinal cohort of uncomplicated CDI patients, treatment with metronidazole and confirmed diagnosis of primary CDI by GDH/EIA toxin were the strongest predictors for developing recurrent CDI. In addition, recurrence was accurately identified with real time stool inspection and testing using a two-step testing method to confirm C. difficile with active toxin production. We recognize that further studies in this cohort and in additional high risk cohorts will provide further robust clinical evidence to validate these findings. This is an important first step in identifying easily accessible predictive tools to guide clinical care for these patients.
Acknowledgments
Declaration of Funding:
This study was supported by the American College of Gastroenterology Junior Faculty Development Award. ACG-JR-017-2015, the BWH Precision Medicine program.
Financial disclosures:
GKG is a Strategic Advisory Board Member of Kaleido Biosciences and has company stock options, and is a Scientific Advisory Board Member, co-founder, and shareholder of ConsortiaTX. No funding for the present work was provided by either company.
LB is founder and chair of the Scientific Advisory Board for ConsortiaTX, and SAB member of Inspirata Inc. No funding for the present work was provided by either company. JRA consults for and has research support from Finch Therapeutics and Merck and Co.
References
- 1.Fletcher JR, Erwin S, Lanzas C, Theriot CM. Shifts in the Gut Metabolome and Clostridium difficile Transcriptome throughout Colonization and Infection in a Mouse Model mSphere. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lessa FC, Mu Y, Bamberg WM et al. Burden of Clostridium difficile infection in the United States The New England journal of medicine. 2015;372:825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cobo J, Merino E, Martinez C et al. Prediction of recurrent clostridium difficile infection at the bedside: the GEIH-CDI score Int J Antimicrob Agents. 2018;51:393–398. [DOI] [PubMed] [Google Scholar]
- 4.McDonald LC, Gerding DN, Johnson S et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66:987–994. [DOI] [PubMed] [Google Scholar]
- 5.Longtin Y, Trottier S, Brochu G et al. Impact of the type of diagnostic assay on Clostridium difficile infection and complication rates in a mandatory reporting program Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;56:67–73. [DOI] [PubMed] [Google Scholar]
- 6.Crobach MJ, Planche T, Eckert C et al. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2016;22 Suppl 4:S63–81. [DOI] [PubMed] [Google Scholar]
- 7.Surawicz CM, Brandt LJ, Binion DG et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections The American Journal of Gastroenterology. 2013;108:478–498; quiz 499. [DOI] [PubMed] [Google Scholar]
- 8.Reveles KR, Mortensen EM, Koeller JM et al. Derivation and Validation of a Clostridium difficile Infection Recurrence Prediction Rule in a National Cohort of Veterans Pharmacotherapy. 2018;38:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanna S, Montassier E, Schmidt B et al. Gut microbiome predictors of treatment response and recurrence in primary Clostridium difficile infection Aliment Pharmacol Ther. 2016;44:715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]