Abstract
Background
Intra-articular hip injections for osteoarthritis represent a useful instrument to reduce pain and disability in the common clinical practice. Several medications can be injected locally with different level of evidence-based efficacy.
Objective
The objective of this systematic review is to investigate the effectiveness of intra-articular injections of different medications or substances for the pain treatment and the management of disability in subjects affected by hip osteoarthritis.
Methods
Two reviewers selected independently randomised controlled trials published in the last 10 years, using PubMed and Scopus databases. The risk of bias was evaluated with Cochrane library assessment tool.
Results
12 randomised controlled trials have been selected. We found 8 papers comparing hyaluronic acid with platelet rich plasma, with corticosteroids and with saline solution; 1 paper compares two types of hyaluronic acid with different molecular weights; 3 papers study the effects of corticosteroids alone or compared to ketorolac or saline solution.
Conclusions
The studies reviewed were heterogeneous regarding sample size, level of osteoarthritis, evaluated with Kellegren-Lawrence score, medications used and follow up timings. However, we have observed that intra-articular injections of platelet-rich plasma seem to decrease pain at short term and disability at long term, in patients affected by hip osteoarthritis better than hyaluronic acid. The association of hyaluronic acid and corticosteroids could give better results compared to hyaluronic acid alone, while the use of intra-articular ketorolac and saline solution requires more studies.
Keywords: Intra-articular injections, hip, osteoarthritis, hyaluronic acid, corticosteroids, platelet-rich plasma, pain, functional outcome
Background
Osteoarthritis (OA) may involve all joints and typically affects weight-bearing ones; the hip is the second most frequently affected after the knee [1]. Osteoarthritis is a degenerative articular disease that seriously and progressively invalidates patient’s quality of life, and represents one of the main cause of disability in over 45-year olds, with a number of about 400 million people affected worldwide [2–4].
Hip osteoarthritis has one of the highest financial burden [5], and the prevalence ranges from 6.7% to 9.2% among adults 45 years of age [6, 7], and increase to 25% in patients aged over 55 years, constituting a source of chronic joint pain and stiffness [8–10].
Intra-articular injections are increasingly considered useful to relief pain and to improve joint motion [11].
Several medications are locally injected with different level of evidence-based efficacy as hyaluronic acid (HA), platelet-rich plasma (PRP), corticosteroids (CS) drugs, non-steroidal anti-inflammatory drugs (NSAIDs).
In the last two decades, intra-articular HA injections became popular in clinical practice because the viscosupplementation (VS) appears to have mechanical and biological effects, restoring viscoelasticy of the synovial fluid, promoting shock absorption, lubrification and joint protection [12–14]. HA exerts also a notable anti-inflammatory and analgesic effects by reducing synovial inflammation sustained by proinflammatory cytokines such as interleukin-1 (IL-1), with a significant impact on pain relief and immunomodulatory effect on inflammatory cells [15]. In addition, HA has been shown to have a chondroprotective effect in experimental in vivo and in humans studies,through the stimulation of cartilage proliferation and proteoglycan synthesis, suppression of chondrocyte apoptosis, protection from oxidative damage by free radicals, degradation of catabolic enzymes and proteases and improvement of mitochondrial function [16–20].
Platelet-rich plasma is another injectable medication obtained by centrifuging once or twice an anticoagulated venous blood sample, in order to produce a plasma fraction containing a high concentration of platelets, activated by calcium gluconate, to release several growth factors stored in platelets’ alpha-granules, such as platelet derived growth factor (PDGF), transforming growth factor beta (TGF-ß), insulin-like growth factor 1 (IGF-1), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF) [21, 22]. These signal pathways promote bone and soft tissue joint healing [23].
The anti-inflammatory effects of intra-articular corticosteroids injections interrupt the inflammatory cascade resulting in inhibition of vasodilatation, leukocyte migration and decreasing capillary permeability. However, the alteration of gene expression and immunomodulatory effects steroid-related, are associated with inhibition of anabolic activity of chondrocytes, decreased collagen expression and additional joint damage [24].
Other intra-articular medications, such as saline solution (SS) and non-steroidal anti-inflammatory drugs, are reported in literature for hip osteoarthritis, but exiguous studies have been conducted to date about their real efficacy on pain and function; in addition very few papers compare the effectiveness of the different injectable substances in hip OA.
The objective of this systematic review is to investigate the effectiveness of intra-articular injections of different medications or substances for the pain treatment and the management of disability in subjects affected by hip osteoarthritis.
Methods
A comprehensive literature search via PubMed and Scopus databases was conducted using the following MESH terms: “intra-articular hip injections” AND “osteoarthritis” AND “hyaluronic acid” OR “steroids” OR “platelet-rich plasma” OR “saline solution”.
The inclusion criteria were randomized controlled trials (RCTs) published in the last decade up to March 2021, in English language, including adults > 18 years affected by hip osteoarthritis (Kellgren-Lawrence score I to IV), treated with intra-articular hip injections of hyaluronic acid, corticosteroids, platelet-rich plasma and saline solution and evaluated with disability and pain outcome measures.
The exclusion criteria were papers analysing adults with hip osteoarthritis treated with other conservative therapies (oral medications, physical therapies, therapeutic exercises), with surgical treatments and all those articles not connected with human medicine and not dealing with the objective of the review were excluded (Table 1).
Table 1.
Inclusion and exclusion criteria according to PICO worksheet and search strategy. US National Center for Dental Hygiene Research. Miller, S.A. (2001)
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Population | Subjects > 18 years with hip osteoarthritis (Kellgren-Lawrence score I-IV) | Subjects with hip osteoarthritis treated with other conservative therapies and/or surgical interventions. |
| Intervention | Intra-articular hip injections with hyaluronic acid of different molecular weights, corticosteroids, platelet-rich plasma and saline solution | Other conservative therapies (oral medications, physical therapies, therapeutic exercises) and surgical treatments. |
| Outcome | Disability and pain. | |
| Comparison | Intra-articular hip injections with hyaluronic acid of different molecular weights, corticosteroids, platelet-rich plasma and saline solution | |
| Date | RCTs published in the last decade up to March 2021 | |
| Language | Only studies written in English were included |
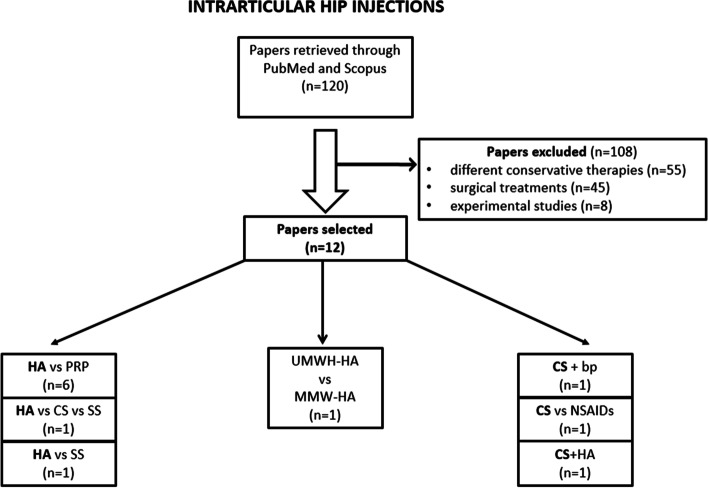
Two reviewers (SC, PEF) selected independently the articles eligible for inclusion in the review in order to reduce the risk of inter-observer bias. Any study not approved by both of the reviewers was discarded (Fig. 1). Afterwards, the reviewers extrapolated from the articles the characteristics of the sample, the intra-articular medication injected, the trial procedures, the outcome indexes, the timing of follow-up and main results of each paper selected (Table 2).
Fig. 1.
Study selection process for intra-articular hip injections. HA: hyaluronic acid; PRP: platelet-rich plasma; CS: corticosteroids; SS: saline solution; UHMW-HA: ultra-high molecular weight hyaluronic acid; MMW-HA: medium molecular weight hyaluronic acid; bp: bupivicaine; NSAIDs: non-steroidal anti-inflammatory drugs
Table 2.
Characteristics of the studies
| PAPERS | INTERVENTIONS (n. patients or hips) |
TOTAL PATIENTS | K-L GRADE | INJECTIONS CHARATHERISTICS | OUTCOME | |||
|---|---|---|---|---|---|---|---|---|
| MEASURES | FOLLOW-UP | MAIN RESULTS | ||||||
| INTRA-ARTICULAR MEDICATION | N. INJECTIONS (n/w) |
|||||||
| Jurgensmeier et al. 2021 [25] |
CS (30) KET (28) |
58 hips | >II |
Triamcinolone acetonide 80 mg VS Ketorolac 30 mg |
1 |
HOOS VAS PROMIS |
T0: baseline T1: 1 week T2: 1 months T3: 3 months |
KET comparable improvement to CS |
| Krautler et al. 2021 [26] |
HA (LMW HA): (16) PRP (LP-PRP)( (20) |
36 hips | II-III |
Supartz® (620-1170 kDa) 2.5 ml of 1% (10 mg) VS LP-PRP 1-2 ml |
3 1/W |
WOMAC ROM |
T0: baseline T1: 3 months T2: 6 months T3: 12 months T4: 24 months |
Significant improvement in WOMAC and hip internal rotation for LP-PRP group at 6 months. Better WOMAC for LP-PRP until 24 months, between groups. |
| De Rezende et al. 2020 [27] |
CS (19) CS + 2 ml HA (19) CS + 4 ml HA (22) CS + 6 ml HA (22) |
82 | II-III |
Hylan G-F 20® (6000 kDa) 6 ml ± Triamcinolone acetonide 40 mg 2 ml |
1 |
VAS ROM WOMAC Lequense |
T0: baseline T1: 1 month T2: 3 months T3: 6 months T4: 12 months |
Improvement in all groups for pain, function, and quality of life up to a year in HOA. CS+HA improve ROM up to one year. |
| Villanova et al. 2020 [28] |
HA (37) PRP (37) |
74 | I-IV |
Synvisc-One® 60 mg/6 mL (6000 kDa) VS PRP 6 ml |
1 |
VAS HHS WOMAC OARSI criteria |
T0: baseline T1: 1 week T2: 1 month T3:12 months |
Both groups showed improvements in VAS score at each follow-up. HA group showed a significative HHS score at T3 |
| Brander et al. 2019 [29] |
HA (182) SS (175) |
357 | III |
Hylan G-F 20® (6000 kDa) 48 mg/6 ml VS Saline solution 6ml |
1 | WOMAC PTGA |
T0: baseline T1: 4 weeks T2: 8 weeks T3: 16 weeks T4: 26weeks |
Significative improvements in both groups for all outcomes measures up to T4. |
| Clementi et al. 2018 [30] |
UHMW-HA (23) MMW-HA (27) |
50 | III |
Fermathron S®, UHMW-HA 69mg/3,0 ml |
1 |
Lequense index VAS WOMAC |
T0: baseline T1: 1 month T2: 3 months T3: 6 months T4: 12months |
No significant difference in the clinical outcomes between groups until T4. |
|
Hyalubrix® 60, MMW-HA (3200 kDa) |
2 | |||||||
| Doria et al. 2017 [31] |
HA (40) PRP (40) |
80 | I-II |
Hyalubrix® 15 mg/ml (3200 kDa) VS PRP 5 ml |
3 1/W |
WOMAC VAS HHS |
T0: baseline T1: 6 months T2:12 months |
PRP did not offer significantly better results compared with HA |
| Dallari et al. 2016 [32] |
PRP(44) PRP+HA (31) HA(36) |
111 | I-IV |
PRP 5 ml VS Hyalubrix® HA 30 mg/2 mL (3200 kDa) |
3 1/W |
VAS HHS WOMAC |
T0: baseline T1: 2 months T2: 6 months T3: 12 months |
At all follow-ups PRP group had the lowest VAS scores, compared with HA and PRP+HA groups The WOMAC score of the PRP group was significantly better at T1 and T2, but not at T3. |
| Di Sante et al. 2016 [33] |
HA(22) PRP (21) |
41 | II-III |
Hyaluronic acid 30 mg/2 ml, (1000-2900 kDa) VS PRP 3 ml |
3 1/W |
VAS WOMAC |
T0: baseline T1: 4 weeks T2: 16 weeks |
The functional WOMAC and VAS score in the HA were better at T2 than PRP. PRP presents significant improvement in VAS at T1 |
| Battaglia et al. 2013 [1] |
PRP (50) HA (50) |
100 | II-IV |
PRP 5 ml VS Hyalubrix®30 mg/2 ml HMW-HA (1500 kD) |
3 1/ 2 W |
HHS VAS |
T0: baseline T1: 1 month T2: 3 months T3:12 months |
PRP showed improvement in HHS and VAS as HA until T3 |
| Atchia et al. 2011 [34] |
HA (19) SS (19) CS (19) CTL (20) |
77 |
Croft I-IV |
Durolane 3 ml/60 mg ( 90.000 KDa) VS SS 3 ml VS Methylprednisolone acetate 3 ml/120 mg |
1 |
WOMAC NRS |
T0: baseline T1: 1 week T2: 4 weeks T3:8 weeks |
Significant improvement in NRS and WOMAC until T3 in CS group |
| Young et al. 2011 [35] |
CS 55 CS + sw: 55 |
110 |
Triamcinolone acetonide 40 mg + Bupivicaine 2 ml VS Triamcinolone acetonide 40 mg + Bupivicaine 2 ml + Sterile water 6 ml |
1 |
WOMAC Oxford pain chart |
T0: baseline T1: 3 months |
No differences between groups | |
K-L Kellgren-Lawrence score, W week, CS corticosteroids, KET ketorolac, HOOS Hip Osteoarthritis Outcome Scores, VAS Visual Analogic Scale; PROMIS Global Health Scores, HA hyaluronic acid, LMW-HA low molecular weight hyaluronic acid, LP-PRP leukocyte-poor platelet-rich plasma, WOMAC Western Ontario and Mc master University osteoarthritis index, ROM range of motion, PRP platelet-rich plasma, HHS Harris Hip Score, OARSI osteoarthritis research society international, SS saline solution, PTGA Patient Global Self-Assessment, UHMW-HA ultra-high molecular weight hyaluronic acid, MMW-HA medium molecular weight hyaluronic acid, HMW-HA high molecular weight hyaluronic acid, CTL control, NRS numeric rating scale, sw steril water
Results
The literature search identified 120 papers published in PubMed and Scopus databases as described in algorithm (Fig. 1). We excluded 108 papers: 55 studied conservative therapies for hip ostheoartritis not included in this review, 45 articles described surgical treatments and 8 were experimental studies.
Twelve RCTs were included: 8 papers about hip injections with HA compared to other medications (corticosteroids, platelet-rich plasma and saline solution), one study compared the effects of two different molecular weights HA injections; three papers analyzed intra-articular steroids hip injections, alone or in comparison with NSAIDs, hyaluronic acid and saline solution.
A total of 1176 patients were included in this systematic review: 123 were treated with CS injection, 452 with HA, 212 with PRP, 63 with CS + HA, 55 with CS+ sterile water 28 with ketorolac (KET), 194 with normal saline solution, 31 with PRP+HA; the others 18 patients represented RCTs control groups with standard care.
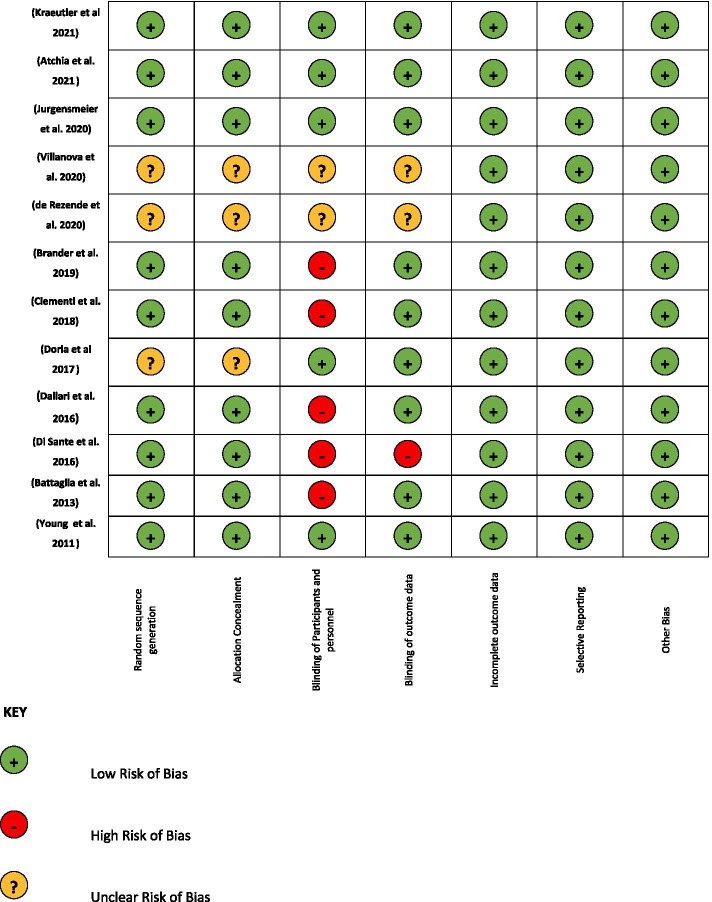
The Cochrane library assessment tool was used to evaluate risk of bias in the 12 RCTs according with PRISMA guidelines [36]. A green light was assigned to a low risk of bias, a yellow light to an unclear risk of bias and a red light to a high risk of bias (Fig. 2). Only 4 papers [25, 26, 34, 35] were found to have a low risk of bias (all green lights for the parameters considered). Regarding “Random sequence generation” and “Allocation concealment”, 25% of the articles were unclear, and 75% had a low risk of bias. Regarding “Blinding of participants and personnel” 41,7% had a low risk of bias, 16,6% were unclear and 41,7% had a high risk of bias.
Fig. 2.
Evaluation of Bias
For the evaluation of “Blinding of outcome data”, 1 paper had a high risk of bias, 24% of papers are unclear and about 75% had a low risk of bias. Considering “Incomplete outcome data”, “Selective reporting”, and “other biases”, all papers presented a low risk of bias.
Discussion
Intra-articular hip injections have been employed for some years as a safe and effective therapeutic tool in the clinical management of painful hip osteoarthritis, with a reported complication rate of between 10 to 30% of patients [13], significantly decreased by the introduction of ultrasound and fluoroscopic guidance [37].
The objective of this systematic review is to investigate the effectiveness of intra-articular injections of different medications or substances for the pain treatment and the management of disability in subjects affected by hip osteoarthritis.
In our review we found 12 RCTs about intra-articular injections of hyaluronic acid, platelet-rich plasma, corticosteroids, saline solution and ketorolac for symptomatic hip OA, as described in Table 2.
We used the Cochrane library assessment tool to evaluate risk of bias, and it results a very low risk only for 4 papers [25, 26, 34, 35]. A high risk of bias was found for the items “Blinding of participants and personnel” for 5 papers [1, 29, 30, 32, 33] and “Blinding of outcome data” for one paper [33]. The different administrative procedure of PRP compared to HA could make difficult to realize the blinding of patients and clinicians during the treatment (Fig. 2).
All the selected papers used similar protocols for the number of injections, according with drugs data sheet.
Also outcome measures were similar in the different studies: VAS NRS, Lequense index for pain, WOMAC and HHS for functional disability, Oxford pain chart for quality of life used only in one paper [35] as described in Table 2.
The selected RCTs were heterogeneous regarding sample size, that ranged from 36 hips [26] to 357 patients, reported in the unique multicenter clinical trial [29] founded in our research.
The 1176 patients of this review presented very different levels of osteoarthritis according with Kellegren-Lawrence score [38], from I (possible narrowing of joint space medially and possible osteophytes around the femoral head; or osteophytes alone) to IV (gross loss of joint space with sclerosis and cysts, marked deformity of femoral head and acetabulum and large osteophytes), with a wide variability, also, considering Croft grade in one paper [34] from I (definite osteophytes only and measurement) to Croft IV (presence of three of the following: joint space narrowing, osteophytosis, subchondral sclerosis of > 5 mm, cyst formation) [39].
Ten RCTs, selected in this review, studied the use of hip HA injections as treatment or as control groups.
Despite the widespread use of injections with hyaluronic acid, in the literature there is no consensus about the best viscosupplementation protocol for hip OA [40, 41].
Our data showed that the molecular HA weight was different in the papers selected. Two RCTs analysed the effects of high molecular weight HA, while all the other studies [1, 27–29, 31–33] used a mean molecular weight HA, except one [26], in which a lower molecular weight HA was employed. The comparison of their clinical effects is difficult for the considerable HA heterogeneity in term of molecular weight, concentration, elasticity, viscosity of products, in patients affected by different level of hip degenerations.
However, in this review, we did not observe different improvement in pain and functional outcomes between patients treated with high molecular weight and with mean molecular weight HA until 12 months after treatment. Only one paper gave different results [30] until 12 months comparing effects of high and mean molecular weight HA.
The effects of PRP versus HA for the treatment of symptomatic hip osteoarthritis was analysed in 6 randomised controlled trials [1, 26, 28, 31–33], with different dosages of intra articular PRP, ranging from 1-2 ml to 6 ml.
PRP was found better then HA both for pain decrease and for functional improvement at short and long term [26, 32, 33]. Two authors showed, conversely, that HA had better results than PRP in pain relief until 16 weeks [33] and better functional scores at 12 months after injection [28].
We can suggest a PRP short-term efficacy for pain decrease and a HA long-term effectiveness for the improvement of function. According with literature it could be related to HA interaction with the CD44 synoviocytes receptors [42].
However there are few high quality clinical studies about the effects of intra-articular PRP hip injections [43] on pain and disability and we underline that the standard procedures for PRP production and administration protocols varies widely among studies [33, 44, 45].
The effects of corticosteroids intra-articular hip injection in hip osteoarthritis are reported only in four studies selected. Authors used two different type of steroids: methylprednisone acetate [34] and triamcinolone from 40 mg to 80 mg [25, 27, 35], which is recommended over other steroids for osteoarthritis, due to a longer lasting and more effective pain relief [46].
Our results showed that the association of HA and CS gave a better improvement for pain, function and quality of life compared to HA alone until 1 year follow-up [27]. The combined use of HA and CS may be probably more effective than HA alone in longer-lasting analgesic effects. But, to our knowledge, the RCT of De Rezende et al. 41] is the only paper of the last decade, that analysed this association. Other studies are necessary to confirm this hypothesis.
HA seemed to give less improvements than CS in pain and function until 8 weeks after treatment [34]. According to literature data [47], this review confirmed that intra-articular CS injections improve hip OA symptoms in the short- and mid-term, and the duration of pain relief is shorter compared with HA.
The study of Young et al. [35] demonstrated the effectiveness of different injected volumes (3 to 9 ml) of triamcinolone acetonide (TA) with bupivacaine and sterile water, in improving pain and function until 3 months after treatment.
Corticosteroids injections were found comparable for pain relief and functional improvement to ketorolac intra-articular injections until 3 months from the treatment [25].
Brander et al. [38] showed that saline solution could improve pain and function until 26 weeks as hyaluronic acid but additional studies are necessary to determine if the effect is due to mechanical or biological mechanism.
Despite the widespread use of intra-articular injections we founded few randomised controlled trials, published in the last 10 years. The limits of this review are the heterogeneity of papers selected regarding level of patients OA and drugs studied. It was not possible to compare the outcomes in patients’ subgroups with similar grade of hip osteoarthritis. The RCTs selected presented low sample size. The absence of blinding was a papers’ protocol bias and gave them a low quality score.
Conclusion
Intra-articular hip injections can be a useful instrument to reduce pain and improve function in hip osteoarthrosis, however structured studies of high quality about this topic are still lacking. Although this review does not allow us to provide strong recommendations, we can observe that there is a short-term efficacy of PRP for pain decrease and a long-term effectiveness for the improvement of function in patients affected by hip osteoarthrosis. The association of hyaluronic acid and corticosteroid can give better results compared to hyaluronic acid alone, while the use of intra-articular ketorolac and saline solution require more studies.
However, more high-quality multicentric studies with higher sample size are still needed to further define evidence-based best practice for intra-articular treatment of patients with hip osteoarthrosis.
Acknowledgements
Not applicable
About this supplement
This article has been published as part of BMC Musculoskeletal Disorders Volume 22 Supplement 2 2021: All about the hip. The full contents of the supplement are available at https://bmcmusculoskeletdisord.biomedcentral.com/articles/supplements/volume-22-supplement-2.
Abbreviations
- CS
Corticosteroids
- Dex
Dexamethasone
- EGF
Epidermal growth factor
- FGF
Fibroblast growth factor
- GAG
Glycosaminoglycan
- HA
Hyaluronic acid
- HHS
Hip Harris Score
- KET
Ketorolac
- IL-1
Interleukin-1
- IGF-1
Insulin-like growth factor 1
- MW
Molecular weight
- NRS
Numeric Rating Scale
- NSAIDs
Non-steroidal anti-inflammatory drugs
- OA
Osteoarthritis
- PDGF
Platelet derived growth factor
- PRP
Platelet-rich plasma
- Qol
Quality of life
- RCT
Randomized controlled trial
- ROM
Range of motion
- SS
Saline solution
- TA
Triamcinolone acetonide
- TGF-ß
Transforming growth factor beta
- VAS
Visual analogic scale
- VEGF
Vascular endothelial growth factor
- VS
Viscosupplementation
- WOMAC
Western Ontario and Mc master University osteoarthritis index
Authors’ contributions
All the authors equally contributed, read and approved the final manuscript. PEF: contribution in conception acquisition, analysis of data and drafted paper. SC: contribution in acquisition, analysis of data and drafted work. DC: contribution in acquisition of data and revision of paper. GM: contribution in revision of paper. GF: contribution in data analysis and interpretation of data. GR: contribution in conception, interpretation and revision of paper
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. There were no publication costs.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Battaglia M, Guaraldi F, Vannini F, et al. Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics. 2013;36:e1501–e1508. doi: 10.3928/01477447-20131120-13. [DOI] [PubMed] [Google Scholar]
- 2.Kloppenburg M, Berenbaum F. Osteoarthritis year in review 2019: Epidemiology and therapy. Osteoarthr Cartil. 2020;28:242–248. doi: 10.1016/j.joca.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Emery CA, Roos EM, Verhagen E, Finch CF, Bennell KL, Story B, Spindler K, Kemp J, Lohmander LS. Oarsi clinical trials recommendations: Design and conduct of clinical trials for primary prevention of osteoarthritis by joint injury prevention in sport and recreation. Osteoarthr Cartil. 2015;23:815–825. doi: 10.1016/j.joca.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Mokdad AH, Ballestros K, Echko M, et al. US Burden of Disease Collaborators. The state of US health, 1990-2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319(14):1444–1472. doi: 10.1001/jama.2018.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15(8):S230–S235. [PubMed] [Google Scholar]
- 6.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and otherrheumaticconditions in the UnitedStates. Part II. Arthritis Rheum. 2008;58:26e35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective: a population based review of the fourth most common cause of hospitalization in U.S. adults. Orthop Nurs. 2012. [DOI] [PubMed]
- 8.Iagnocco A, Naredo E. Osteoarthritis: research update and clinical applications. Rheumatology. 2012;51(suppl 7):vii2–vii5. doi: 10.1093/rheumatology/kes328. [DOI] [PubMed] [Google Scholar]
- 9.Iagnocco A. Ultrasound in osteoarthritis. Clin Exp Rheumatol. 2014;32(1 Suppl 80):S48–S52. [PubMed] [Google Scholar]
- 10.Iagnocco A. Imaging the joint in osteoarthritis: a place for ultrasound? Best Pract Res Rheumatol. 2010;24:27–38. doi: 10.1016/j.berh.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Mora JC, Przkora R, Cruz-Almeida Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J Pain Res. 2018;11:2189–2196. doi: 10.2147/JPR.S154002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27:1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Henrotin Y, Raman R, Richette P, et al. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin Arthritis Rheum. 2015;45(2):140–149. doi: 10.1016/j.semarthrit.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Legre-Boyer V. Viscosupplementation: Techniques, indications, results. Orthop Traumatol Surg Res. 2015;101(1 Suppl):S101–S108. doi: 10.1016/j.otsr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 15.A, Miralles A, Schmidt RF, Belmonte C. Intra-articular injections of hyaluronan solutions of different elastoviscosity reduce nociceptive nerve activity in a model of osteoarthritic knee joint of the guinea pig. Osteoarthr Cartil. 2009;17:798–804. doi: 10.1016/j.joca.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K, Hashimoto S, Kubo T, Hirasawa Y, Lotz M, Amiel D. Effect of hyaluronan on chondrocyte apoptosis and nitric oxide production in experimentally induced osteoarthritis. J Rheumatol. 2000;27:1713–1720. [PubMed] [Google Scholar]
- 17.Zhou PH, Liu SQ, Peng H. The effect of hyaluronic acid on IL-1βinduced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop Res. 2008;26:1643–1648. doi: 10.1002/jor.20683. [DOI] [PubMed] [Google Scholar]
- 18.Barreto RB, Sadigursky D, de Rezende MU, Hernandez AJ. Effect of hyaluronic acid on chondrocyte apoptosis. Acta OrtopBras. 2015;23:90–93. doi: 10.1590/1413-785220152302144341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grishko V, Xu M, Ho R, Mates A, Watson S, Kim JT, Wilson GL, Pearsall AW., 4th Effects of hyaluronic acid on mitochondrial function and mitochondria-driven apoptosis following oxidative stress in human chondrocytes. J BiolChem. 2009;284:9132–9139. doi: 10.1074/jbc.M804178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawasaki K, Ochi M, Uchio Y, Adachi N, Matsusaki M. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J Cell Physiol. 1999;179:142–148. doi: 10.1002/(SICI)1097-4652(199905)179:2<142::AID-JCP4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Anitua E, Andia I, Ardanza B, Nurden P, NurdenAT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 22.Song SU, Cha YD, Han JU, et al. Hyaline cartilage regeneration using mixed human chondrocytes and transforming growth factor beta 1–producing chondrocytes. Tissue Eng. 2005;11:1516–1526. doi: 10.1089/ten.2005.11.1516. [DOI] [PubMed] [Google Scholar]
- 23.Gato-Calvo L, Magalhaes J, Ruiz-Romero C, Blanco FJ, Burguera EF. Platelet-rich plasma in ostheoartritis treatment: review of current evidence. Ther Adv Chronic Dis. 2019;10:2040622319825567. doi: 10.1177/2040622319825567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittal N, Bhandari M, Kumbhare D. Are corticosteroid injections safe to injectinto knees with osteoarthritis? Am J Phys Med Rehabil. 2018;97:461e4. doi: 10.1097/PHM.0000000000000909. [DOI] [PubMed] [Google Scholar]
- 25.Jurgensmeier K, Jurgensmeier D, Kunz DE, Fuerst PG, Warth LC, Daines SB. Intra-articular Injections of the Hip and Knee With Triamcinolone vs Ketorolac: A Randomized Controlled Trial. J Arthroplast. 2021;36:416e422. doi: 10.1016/j.arth.2020.08.036. [DOI] [PubMed] [Google Scholar]
- 26.Kraeutler MJ, Houck DA, GarabekyanT MSL, Dragoo JL, Mei-Dan O. Comparing Intra-articular Injections of Leukocyte-Poor Platelet-Rich Plasma Versus Low–Molecular Weight Hyaluronic Acid for the Treatment of Symptomatic Osteoarthritis of the Hip. Orthop J Sports Med. 2021;9(1):2325967120969210. doi: 10.1177/2325967120969210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Rezende MU, Campos Gurgel HM, Pereira Ocampos G, de Campos GC, Frucchi R, Pailo AF, Pasqualin T, Negreiros Vicente JR, de Camargo OP. Improvements in hip osteoarthritis with lavage, triamcinolone and Hylan G-F20. Acta Ortop Bras. 2020;28(6):280–286. doi: 10.1590/1413-785220202806240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villanova-López MM, Núnez-Núnez M, Fernández-Prieto D, González-López C, García-Donaire J, Pérez-Pérez A, Fernández S, del Castillo S, Murillo-Izquierdo M, Camean-Fernández M, Gutiérrez-Pizarraya A, Navas-Iglesias N, Roca-Ruiz LJ, Calleja-Hernández MÁ, Ballester-Alfarog JJ. Randomized, double-blind, controlled trial, phase III, to evaluate the use of platelet-rich plasma versus hyaluronic acid in hip coxarthrosis. Rev Esp Cir OrtopTraumatol. 2020;2(64):134–142. doi: 10.1016/j.recot.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Brander V, Skrepnik N, Petrella RJ, et al. Evaluating the use of intra-articular injections as a treatment for painful hip osteoarthritis: a randomized, double-blind, multicenter, parallel-group study comparing a single 6-mL injection of hylan G-F 20 with saline. Osteoarthr Cartil. 2019;27:59–70. doi: 10.1016/j.joca.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Clementi D, D’Ambrosi R, BertoccoP BMS, Cardile C, Ragni P, Giafreda G, Ragone V. Efficacy of a single intra-articular injection of ultra-high molecular weight hyaluronic acid for hip osteoarthritis: a randomized controlled study. Eur J Orthop Surg Traumatol. 2018;28:915–922. doi: 10.1007/s00590-017-2083-9. [DOI] [PubMed] [Google Scholar]
- 31.Doria C, Mosele G, Caggiari G, et al. Treatment of early hip osteoarthritis: ultrasoundguided platelet rich plasma versus hyaluronic acid injections in a randomized clinical trial. Joints. 2017;05:152–155. doi: 10.1055/s-0037-1605584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dallari D, Stagni C, Rani N, et al. Ultrasound-Guided injection of platelet-rich plasma and hyaluronic acid, separately and in combination, for hip osteoarthritis: a randomized controlled study. Am J Sports Med. 2016;44:664–671. doi: 10.1177/0363546515620383. [DOI] [PubMed] [Google Scholar]
- 33.Di Sante L, Villani C, Santilli V, et al. Intra-Articular hyaluronic acid vs platelet-rich plasma in the treatment of hip osteoarthritis. Med Ultrason. 2016;18:463. doi: 10.11152/mu-874. [DOI] [PubMed] [Google Scholar]
- 34.Atchia I, Kane D, Reed MR, et al. Efficacy of a single ultrasound-guided injection for the treatment of hip osteoarthritis. Ann Rheum Dis. 2011;70:110–116. doi: 10.1136/ard.2009.127183. [DOI] [PubMed] [Google Scholar]
- 35.Young R, Harding J, Kingsly A, Bradley M. Therapeutic hip injections: Is the injection volume important? ClinicalRadiology. 2012;67:55e60. doi: 10.1016/j.crad.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 36.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Migliore A, Tormenta S, Laganà B, Piscitelli P, Granata M, Bizzi E, Massafra U, Giovannangeli F, Maggi C, De Chiara R, Iannessi F, Sanfilippo A, Camminiti M, Pagano MG, Bagnato G, Iolascon G. Safety of intra-articular hip injection of hyaluronic acid products by ultrasound guidance: an open study from ANTIAGE register. Eur Rev Med PharmacolSci. 2015;17:1752–1759. [PubMed] [Google Scholar]
- 38.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reijman M, HazesJMW PHAP, BernsenRMD,KoesBW, Bierma-ZeinstraSMA Validity and reliability of three definitions of hip osteoarthritis: cross sectional and longitudinal approach. Ann Rheum Dis. 2004;63(11):1427–1433. doi: 10.1136/ard.2003.016477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of non- pharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64(4):465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, et al. Oarsi recommendations for the management of hip and kneeosteoarthritis, part II: Oarsi evidence-based, expert consensus guidelines. Osteoarthr Cartil. 2008;16:137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Yasuda T. Hyaluronan inhibits prostaglandin E2 production via CD44 in U937 human macrophages. Tohoku J ExpMed. 2010;220:229–235. doi: 10.1620/tjem.220.229. [DOI] [PubMed] [Google Scholar]
- 43.Medina-Porqueres I, Ortega-Castillo M, Muriel-Garcia. Effectiveness of platelet-rich plasma in the management of hip osteoarthritis: a systematic review and meta-analysis. Clin Rheumatol. 2021;40:53–64. doi: 10.1007/s10067-020-05241-x. [DOI] [PubMed] [Google Scholar]
- 44.O’Connel B, Wragg NM, Wilson SL. The use of PRP injections in the management of knee osteoarthritis. Cell Tissue Res. 2019;376:143–152. doi: 10.1007/s00441-019-02996-x. [DOI] [PubMed] [Google Scholar]
- 45.Del Buono A, Papalia R, Denaro V, Maccauro G, Maffulli N. Platelet rich plasma and tendinopathy: state of the art. Int J Immunopathol Pharmacol. 2011;24(1 Suppl 2):79–83. doi: 10.1177/03946320110241S215. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh P, Read R, Numata Y, Smith S, Armstrong S, Wilson D. The effects of intraarticular administration of hyaluronan in a model of earlyosteoarthritis in sheep: II. Cartilagecomposition and proteoglycanmetabolism. Semin Arthritis Rheum. 1993;22(6 Suppl 1):31–42. doi: 10.1016/S0049-0172(10)80017-4. [DOI] [PubMed] [Google Scholar]
- 47.Zhong H, Zhao G, LinT ZX, Li X, Lin J, Zhao S, et al. Intra-Articular Steroid Injection for Patients with Hip Osteoarthritis: A Systematic Review and Meta-Analysis. Biomed Res Int. 2020(Article ID):6320154. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.