Abstract
Nevus comedonicus syndrome (NCS) is a rare epidermal nevus syndrome characterized by ocular, skeletal, and central nervous system anomalies. We present a 23-month-old boy with a history of a congenital pulmonary airway malformation (CPAM) of the lung and a congenital cataract who developed progressive linear and curvilinear plaques of dilated follicular openings with keratin plugs (comedones) on parts of his scalp, face, and body consistent with nevus comedonicus. MRI of the brain demonstrated an aneurysm of the right internal carotid artery. Genetic testing identified NEK9 c.1755_1757del (p.Thr586del) at mean allele frequency of 28% in the nevus comedonicus. This same mutation was present in the CPAM tissue. This is the first case of a CPAM in a patient with an epidermal nevus syndrome. This case expands the phenotype of nevus comedonicus syndrome to include CPAM and vascular anomalies.
Keywords: congenital pulmonary airway malformation of the lung, epidermal nevus, NEK9, nevus comedonicus
1 |. INTRODUCTION
Epidermal nevi are benign areas of skin overgrowth involving the epidermis that may be present at birth or develop later in childhood and usually follow lines of Blaschko. These may be syndromic and occur with brain, eye, and skeletal manifestations (Asch & Sugarman, 2018). Nevus comedonicus syndrome is a rare variant of epidermal nevus that may begin as a linear shiny patch, sometimes noted at birth, and develops into plaques of dilated follicular openings filled with keratin plugs early in life, usually before the age of 10 years old (Tchernev et al., 2013). Nevus comedonicus can be isolated or occur in association with ocular, skeletal and/or central nervous system abnormalities in nevus comedonicus syndrome and is estimated to affect 1 in 45,000 to 1 in 100,000 people (Engber, 1978; Tchernev et al., 2013). The most common of associated features are ipsilateral cataracts, scoliosis, fused vertebrae, spina bifida, and developmental delay (Tchernev et al., 2013). Patients have also been seen with dysgenesis of the corpus callosum, limb deformities, and other skin disorders.
Nevus comedonicus is caused by somatic activating mutations in Never In Mitosis Gene A-Related Kinase 9 (NEK9), a serine/threonine kinase (Levinsohn et al., 2016). NEK9 functions as a checkpoint control and regulator of the cell cycle (Levinsohn et al., 2016). We present a toddler with molecularly confirmed nevus comedonicus syndrome, including the novel association of congenital pulmonary airway malformation (CPAM) of the lung, and aneurysm.
1.1 |. Informed consent and data sharing
The patient’s mother provided informed consent for the publication of identifiable photographs. The data are not publicly available due to privacy restrictions.
2 |. CLINICAL HISTORY
The infant was the product of a natural conception and was the first pregnancy between his biological parents. Fetal ultrasound and prenatal screening were normal until 31 weeks gestation when a right-sided lung lesion was noted on ultrasound at which point the patient was referred to the Center for Fetal Diagnosis and Treatment at the Children’s Hospital of Philadelphia (CHOP). Fetal ultrasound at 32 weeks demonstrated a large CPAM measuring 5.5 × 6.5 × 6.4 cm for a volume of 11.9 cc and a CCAM-volume-ratio (CVR) of 3.8 (Crombleholme et al., 2002). The CPAM encompassed the majority of the right lung, causing significant mediastinal shift and nonimmune hydrops as evidenced by the presence of fetal ascites, small bilateral pleural effusions, and skin/scalp edema. Polyhydramnios with an amniotic fluid index (AFI) of 40.6 cm and a deepest vertical pocket of 11.6 cm was also present. Fetal MRI was consistent with the ultrasound and demonstrated a large heterogeneous pulmonary mass occupying the majority of the right hemithorax resulting in severe shift of the heart and aorta to the contralateral side. Fetal lung volumes were markedly deceased with an observed to expected (O/E) lung ratio of 19%. Fetal echocardiogram showed normal cardiac structure and function. The family history was noncontributory. The mother declined all prenatal genetic testing. Given the size of the lung lesion and the presence of hydrops, the patient was given two courses of maternal betamethasone, which have been demonstrated to stop the growth of CPAMs in some cases (Peranteau et al., 2007; Peranteau et al., 2016). Ten days after the initial evaluation at CHOP, the patient presented in labor and ruptured membranes.
The infant was born by Cesarean delivery at 34 weeks 0 days gestational age due to preterm premature rupture of membranes. His APGAR scores were 8 at 1 min and 5 at 5 min. His birth length, weight, and head circumference were within normal limits when corrected for prematurity (76, 86, and 59%, respectively). Immediately after delivery, a right thoracotomy and resection of the right lung lower lobe, which contained the CPAM, was performed. Following surgery, the patient was admitted to the NICU where he remained intubated with ventilatory support until the day of life 31. He was discharged home without additional respiratory support at 3 months of age. Pathologic evaluation of the resected right lower lobe revealed a lobulated, predominantly solid mass with central branching airway structures that occupied the majority of the lobe (Figure 1a). Histologically, the lobular nature of the mass was maintained with central malformed airway surrounded by parenchyma with short columnar epithelium lining relatively abundant mesenchyme and a peripheral rim of abnormal but more mature parenchyma characterized by decreased mesenchyme and flattened epithelium (Figure 1b,c). Goblet cells were not present. This pattern is most suggestive of a Stocker type 3 CPAM, which is a very rare form of CPAM (Victoria et al., 2018). After birth, he was noted to have several areas of hypopigmentation including an area along the sternum, along with the left upper back/shoulder extending in a linear fashion down the left arm and a larger hypopigmented region on left lower back below a surgical scar in a Blaschkoid distribution. Dermatological evaluation concluded these areas to be epidermal nevi. Ophthalmology was consulted due to the epidermal nevi and identified a right congenital cataract. Genetic evaluation at that time included SNP microarray, which was a normal male. His evaluation was also notable for an aberrant right subclavian artery identified on chest CT and glucose-6-phosphate-dehydrogenase deficiency identified by newborn screening.
FIGURE 1.
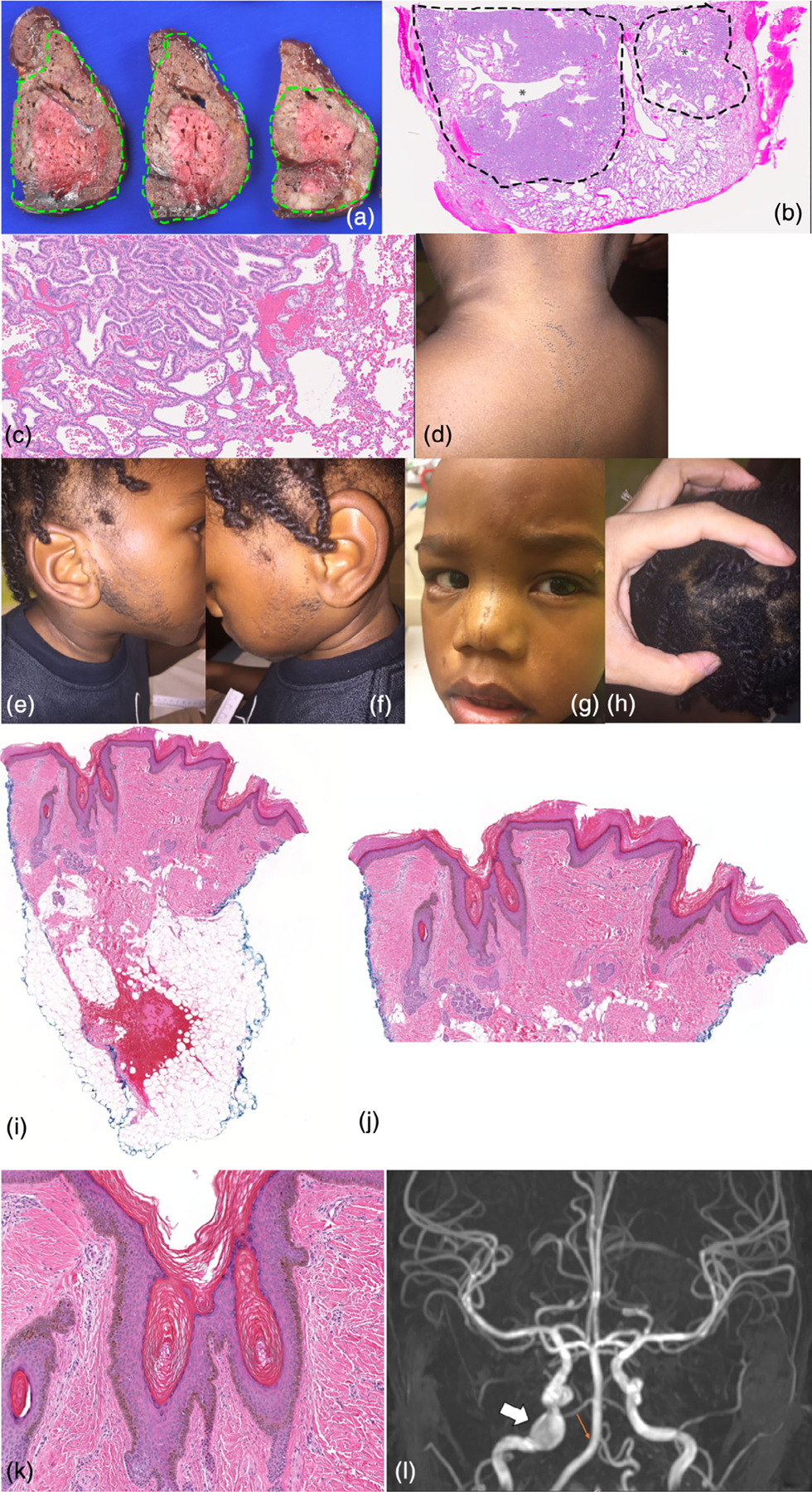
Clinical and pathologic patient features. (a–c) CPAM. (a) Gross pathology shows a solid, lobulated mass occupying the majority of the lobe of lung (outlined in green). The peripheral, better-fixed part of the lesion is tan-brown while the center unfixed area is red. There are multiple central branching airway structures without prominent cystic change. (b) The low power view of a lobule shows a central branching airway-like structure (*) surrounded by malformed parenchyma with increased mesenchyme (outlined by dotted line) with peripheral malformed lung with a more mature appearance (H&E, 0.8×). (c) High power view shows the junction between the two areas with low columnar epithelium lining abundant mesenchyme in septa between small airspaces (left) and (d) more mature but still enlarged alveoli with decreased mesenchyme and flattened epithelium (right; H&E, 13.4×). (e,f) Beard like distribution of comedones. (g) Comedones at midline face. (h) Area of wooly hair. (i–k) Nevus comedonicus biopsy specimen. (i) Scanning view of this punch biopsy specimen demonstrates a sparse inflammatory infiltrate, and the epidermis shows hyperkeratosis and papillomatosis. There are multiple areas consistent with comedones, with small cystic areas that contain hyperkeratosis. The focal hemorrhage in the adipose tissue is related to the surgical procedure to obtain the specimen (H&E, 20×). (j) Medium power view demonstrates the epidermal changes consistent with an epidermal nevus, with epidermal papillomatosis and hyperkeratosis. The areas consistent with comedones are also shown, with cystic areas containing hyperkeratotic keratin (H&E, 50×). (k) This high power view demonstrates an area of the specimen with features of comedones. There are areas of cystic epithelium which contain hyperkeratotic keratin (H&E, 100×). (l) Brain MRI demonstrating a 10 mm aneurysm of the right internal carotid artery lacerum segment (white arrow) and absent right posterior inferior cerebellar artery (small orange arrow) [Color figure can be viewed at wileyonlinelibrary.com]
He represented to dermatology at 16 months of age with widespread linear and curvilinear patches of open comedones and mild hypopigmentation on the central face, right cheek, trunk, arms, right leg and groin that had been present for 4 months. These lesions had increased in size since they first appeared and had been asymptomatic. There were no prior evaluations or treatments for this condition.
Given the clinical suspicion for nevus comedonicus syndrome, he was referred back to genetics at 20 months of age. He also had a history of gastroesophageal reflux, constipation, limited and inconsistent oral acceptance, and nasogastric-tube feeds. At that time, he had delayed fine motor and language development. He spoke five words and was receiving early intervention. On examination, height was 84.5 cm (46%), weight was 10.4 kg (20%), and head circumference was 49 cm (80%). He was a nondysmorphic child with comedones present on the face at midline and right cheek extending to hairline, trunk and back, bilateral arms and legs (Figure 1d–g). There were areas of scant hair, especially on the lateral forehead. There was an area of wooly hair with lighter color on the occiput confirmed on light microscopy (Figure 1h). He also had a scar on his flank. Punch biopsy of a nevus demonstrated papillomatosis and hyperkeratosis in the epidermis (Figure 1i–k). There were multiple areas consistent with comedones, including epidermal invaginations with associated hyperkeratotic keratin, consistent with the nevus comedonicus type of epidermal nevus. Clinical single gene testing by next-generation sequencing from the nevus identified a heterozygous variant in NEK9 c.1755_1757delAAC (p.Thr586del) with mean allele frequency of 28% in the skin. Sanger sequencing demonstrated this variant was also present in CPAM tissue. The p.Thr586del variant in exon 15 of the NEK9 gene is a deletion from nucleotides 1,755–1,757. This is predicted to result in an inframe single amino acid deletion within the regulator of chromosome condensation (RCC1) domain of this gene. The variant has not been reported in the scientific literature, ClinVar or the Genome Aggregation Database (gnomAD).
Follow up at 23 months old showed progressive comedones in a beard-like distribution. He has been treated for six months with 0.1% adapalene gel and for 1 month with 0.025% tretinoin cream with minimal success and continued progression of the areas of nevus comedonicus. He continued to have no history of seizures.
Brain MRI was recommended due to the structural brain differences associated with nevus comedonicus syndrome and demonstrated a 10 mm aneurysm of the right internal carotid artery lacerum segment, mild central volume loss, thickening of the sphenoid bone and clivus as well as focal thickening of the right orbital roof, and the right posterior inferior cerebellar artery was not visualized and thought to be an anatomic variant (Figure 1l).
3 |. DISCUSSION
Nevus comedonicus syndrome is a rare variant of epidermal nevus syndrome characterized by nevus comedonicus, ocular, skeletal, and/or central nervous system abnormalities (reviewed in Table 1). This patient’s syndrome was characterized by nevus comedonicus, congenital cataract, CPAM, developmental delay, brain anomalies, and aneurysm. CPAM has never been reported in association with any epidermal nevus syndrome. This patient’s CPAM and aneurysm may be an expansion of the phenotype of this syndrome.
TABLE 1.
Comparison of patient presented here with others in the literature
| Patient in this report | Kaliyadan Patient | Patrizi Patient | Pavithra Patient | Ferrari Patient | Suite Patient | Seo Patient | Yadav Patient | Qian Patient | |
|---|---|---|---|---|---|---|---|---|---|
| Age at Presentation | Birth | 7 years | 9 years | 8 years | 8 years | 9 years | 12 years | 13 years | 19 years |
| Sex | Male | Male | Male | Male | Male | Male | Male | Male | Female |
| Genetics |
NEK9 c.1755_1757del (p.Thr586del) at mean allele frequency of 28% in skin sample |
None | Unk | Unk | N/A | Unk | Unk | Unk | Unk |
| Cutaneous Features | |||||||||
| Comedones | + | + | + | + | + | + | + | + | + |
| Pigmentary differences | − | − | − | − | − | − | − | − | − |
| Musculoskeletal features | |||||||||
| Syndactyly | − | + (unilateral preaxial polysyndactyly of hand and foot) | + (second and third toes) | − | − | − | − | − | − |
| Polydactyly | − | + | + (preaxial polydactyly of send and third toes and right hand) | − | + (supernumerary toe) | − | − | − | − |
| Scoliosis | − | − | − | − | − | − | − | − | + |
| Other | Thickening of sphenoid bone, clivus, and right orbital roof | Radial deviation of the right thumb; medial deviation of the right great toe; short index finger on right hand | Clinodactyly; broad thumb | Bowing deformity of right third finger; ulnar drift deformity of right middle finger | Shortening and flexion deformities of first digit of left hand; small vilateral pisiform bones and scapho-lunate joint space in right hand; adduction deformity involving bilateral metatarsal along with medial deviation at the level of tarsometatarsal joint | Spina bifida | |||
| Ophthalmologic features | |||||||||
| Cataract | + (unilateral; congenital) | − | − | + (bilateral) | − | + (bilateral) | − | − | + (unilateral) |
| Nystagmus | − | − | − | + | − | − | − | − | + |
| Other | Telecanthus | Lenticular opacities in left eye | |||||||
| Neurologic features | |||||||||
| Seizures | − | − | − | − | − | − | − | + | − |
| Brain abnormality | + (mild central volume loss) | − | − | − | − | − | + (dysgenesis of corpus callosum) | + (agenesis of corpus callosum with an interhemispheric cyst) | − |
| Developmental delay | + | Unk | − | − | − | − | + | + | + |
| Learning disability | N/A | + | − | + (poor scholastic performance) | − | − | – (Intelligence Quotient-94) | + | + |
| Other | Unclear speech | Initial language delay; difficulty in calculation | |||||||
| Other features | Wooly hair; 10 mm aneurysm of the right internal carotic artery lacerum segment | Oligodontia | Depigmented hairs on scalp | Pancreatic cyst | Recurrent and intolerable painful nodules, abscesses, intercommunicating sinus tracts and hypertrophic scars involving her axillary regions, groin and mons pubis |
| Engber Patient 1 | Engber Patient 2 | Whyte Patient | ElGhelbazou Patient | Alpsoy Patient | Ito Patient | Vidaurri-de la Cruz Patient 16 | Vidaurri-de la Cruz Patient 17 | Vidaurri-de la Cruz Patient 18 | Martinez Patient | Filosa Patient | Totals | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at presentation | 14 years | 42 years | 15 years | 20 years | 28 years | 62 years | Unk | Unk | Unk | 48 years | 40 years | |
| Sex | Male | Female | Female | Female | Male | Male | Male | Female | Female | Female | ||
| Genetics | Unk | Unk | Unk | Unk | Unk | Unk | Unk | Unk | Unk | Unk | ||
| Cutaneous features | ||||||||||||
| Comedones | + | + | + | + | + | + | + | + | + | + | + | 20/20 |
| Pigmentary differences | − | − | − | − | + | − | − | − | − | − | − | 1/20 |
| Musculoskeletal features | ||||||||||||
| Syndactyly | − | − | − | − | − | − | − | − | − | − | − | 2/20 |
| Polydactyly | − | − | − | − | + | − | − | − | − | − | − | 4/20 |
| Scoliosis | + | − | − | − | − | + | + | − | + | − | − | 5/20 |
| Other | Spina bifida occulta; lanovalgus deformity of right foot; gait abnormality; hemivertebrae from L2 to L5 | Sclerodactyly | Hemicorporeal hypertrophy | Paget bone disease | Ankylosis of both sacroiliac joints, extensive lumbar spine syndesmophytic formation and large skhysis of the sacrum | |||||||
| Ophthalmologic features | ||||||||||||
| Cataract | − | − | + (unilateral) | + (unilateral; congenital) | − | − | + | − | − | + (unilateral) | Unk | 8/19 |
| Nystagmus | − | − | − | − | − | − | − | − | − | − | Unk | 2/19 |
| Other | Partial amaurosis at birth | |||||||||||
| Neurologic features | ||||||||||||
| Seizures | − | − | − | − | − | − | − | − | − | Unk | Unk | 1/18 |
| Brain abnormality | − | − | − | − | − | − | − | − | − | Unk | Unk | 3/18 |
| Developmental delay | − | − | − | − | − | − | − | − | − | Unk | Unk | 4/17 |
| Learning disability | − | − | − | − | − | − | − | − | − | Unk | Unk | 4/17 |
| Other | Transverse myelitis | Microcephaly | ||||||||||
| Other features | Mild shortness of breath and dysphagia; Raynaud’s phenomenon; pulmonary fibrosis | Cervical spondylosis; atrial fibrillation; thyroid cancer; schwannoma in the cauda equina | Widely spaced nipples | Depigmented hair on scalp; breast cancer | Lipoma |
Note: Search details included: (“naevus”[All Fields] OR “nevus, pigmented”[MeSH Terms] OR (“nevus”[All Fields] AND “pigmented”[All Fields]) OR “pigmented nevus”[All Fields] OR “nevus”[All Fields] OR “nevus”[MeSH Terms]) AND comedonicus[All Fields] AND (“syndrome”[MeSH Terms] OR “syndrome”[All Fields]). References: (Kaliyadan, Nampoothiri, Sunitha, & Kuruvilla, 2010; Patrizi, Neri, Fiorentini, & Marzaduri, 1998; Pavithra, Pai, Mallya, & Pai, 2011; Ferrari et al., 2015; Suite and Mahabir, 1994; Seo, Piao, Suhr, Lee, & Park, 2001; Yadav, Mendiratta, Rana, & Chander, 2015; Qian, Liu, Zhou, & Zhang, 2015; Engber, 1978, 1982; Whyte, 1968; Ghelbazouri et al., 2007; Alpsoy, Durusoy, Ozbilim, Karpuzoğlu, & Yilmaz, 2005; Ito, Mitamura, Tsuji, Harada, & Urabe, 2013; Vidaurri-de la Cruz, Tamayo-Sánchez, Durán-McKinster, de la Luz Orozco-Covarrubias, & Ruiz-Maldonado, 2004; Martinez, Levrero, Bazzano, Larre Borges, & De Anda, 2006; Filosa, Bugatti, Ciattaglia, Salaffi, & Carotti, 1997). NC has been seen in patients with dual diagnoses such as orofacial digital syndrome (Baker & Agim, 2014) and Alagille syndrome (Woods, Larcher, & Harper, 1994).
Abbreviation: Unk, unknown.
Exome sequencing of nevus comedonicus in unrelated patients identified somatic, heterozygous mutations in NEK9 as the molecular etiology (Levinsohn et al., 2016). These activating mutations are associated with loss of follicular differentiation, demonstrated by expansion of keratin-15 positive cells and ectopic expression of keratin 10 (Levinsohn et al., 2016). Although the genetic etiology was reported in 2016, no molecularly confirmed cases have since been reported. Our patient’s mutation is novel but localizes to the same RCC1 repeat domain found in two of three patients previously reported (Levinsohn et al., 2016). This case provides additional support for NEK9 as the molecular etiology for nevus comedonicus syndrome.
Congenital pulmonary airway malformations are developmental abnormalities of the lung that consist of a spectrum of cystic and noncystic lung lesions. The incidence of CPAMs is estimated at 1 in 11,000 to 1 in 35,000 live births (Gornall, Budd, Draper, Konje, & Kurinczuk, 2003; Laberge et al., 2001; Leblanc et al., 2017). CPAMs are usually sporadic and do not recur in families. Previously, it was thought that CPAMs were associated with other structural birth defects (up to 15–20% of cases had been associated with anomalies including cardiac and renal malformations; Sfakianaki & Copel, 2012). However, advances in prenatal imaging now allow for the diagnosis of CPAMs that would have otherwise been asymptomatic after birth; demonstrating that CPAMs are more common than previously thought and have few associated developmental anomalies. A recent study of tissue from 58 infants with surgically removed CPAMs demonstrated dysregulation of the Ras and PI3K–AKT–mTOR pathways (Swarr et al., 2018). CPAM has not been described with an epidermal nevus syndrome previously. The presence of the mutation in the affected lung tissue suggests this mutation may play a role in the etiology of this malformation in this patient. NEK9 may interact with any of the known pathways important in lung morphogenesis or perhaps this could be due to its role in cell cycle regulation (Fry, Bayliss, & Roig, 2017). However, given the lack of any obvious normal lung tissue in the resected specimen, we were unable to perform genetic analysis in normal lung tissue for direct comparison to abnormal tissue.
There is no uniformly effective treatment for nevus comedonicus. Previously reported treatments to include topical medications such as keratolytics and/or retinoids, sometimes used in combination with topical corticosteroids, a combination of a 1,450-nm diode laser and a 1,550-nm erbium-doped fiber laser, or surgical removal (Tchernev et al., 2013). Activated vitamin D3 ointment did not lead to improvement (Ito et al., 2013). Although there was previously reported successful treatment of nevus comedonicus with adapalene, it did not lead to an improvement in this patient (Mahran, Abdelsamea, & Mekkawy, 2017). Further clinical studies are needed to determine the best treatment.
In conclusion, we present an infant with molecularly confirmed nevus comedonicus syndrome characterized by nevus comedonicus, congenital cataract, developmental delay, skeletal involvement, and expand the phenotype of this syndrome to include CPAM and aneurysm. The novel finding of the aneurysm, suggests MR angiogram should be considered when imaging the brain for congenital malformations in nevus comedonicus syndrome. This case emphasizes the importance of a multidisciplinary team including dermatology, genetics, ophthalmology, and neurology in caring for patients with epidermal nevus syndromes, which often involve neurocutaneous and ophthalmologic phenotypes.
ACKNOWLEDGEMENTS
We thank the patient and his family for allowing us to share his story. We also thank the other clinicians that have cared for the patient including James Treat, Scarlet Boulos, Cara Skraban, Amanda Pritchard, and Rebecca Ahrens-Nicklas, as well as the Division of Genomic Diagnostics at CHOP. SES is supported in part by TL1TR001880. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR001880. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding information
National Center for Advancing Translational Sciences, Grant/Award Number: TL1TR001880
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
DATA AVAILABILITY STATEMENT
The data are not publicly available due to privacy restrictions.
REFERENCES
- Alpsoy E, Durusoy C, Ozbilim G, Karpuzoğlu G, & Yilmaz E. (2005). Nevus comedonicus syndrome: A case associated with multiple basal cell carcinomas and a rudimentary toe. International Journal of Dermatology, 44, 499–501. [DOI] [PubMed] [Google Scholar]
- Asch S, & Sugarman JL (2018). Epidermal nevus syndromes: New insights into whorls and swirls. Pediatric Dermatology, 35, 21–29. [DOI] [PubMed] [Google Scholar]
- Baker LA, & Agim NG (2014). Nevus comedonicus in oral-facial-digital syndrome type 1: A new finding or overlapping syndromes? Pediatric Dermatology, 31, e48–e51. [DOI] [PubMed] [Google Scholar]
- Crombleholme TM, Coleman B, Hedrick H, Liechty K, Howell L, Flake AW, … Adzick NS (2002). Cystic adenomatoid malformation volume ratio predicts outcome in prenatally diagnosed cystic adenomatoid malformation of the lung. Journal of Pediatric Surgery, 37, 331–338. [DOI] [PubMed] [Google Scholar]
- Engber PB (1978). The nevus comedonicus syndrome: A case report with emphasis on associated internal manifestations. International Journal of Dermatology, 17, 745–749. [DOI] [PubMed] [Google Scholar]
- Engber PB (1982). Nevus comedonicus syndrome. Archives of Dermatology, 118(1), 1. [PubMed] [Google Scholar]
- Ferrari B, Taliercio V, Restrepo P, Luna P, Abad ME, & Larralde M. (2015). Nevus comedonicus: A case series. Pediatric Dermatology, 32, 216–219. [DOI] [PubMed] [Google Scholar]
- Filosa G, Bugatti L, Ciattaglia G, Salaffi F, & Carotti M. (1997). Naevus comedonicus as dermatologic hallmark of occult spinal dysraphism. Acta Dermato-Venereologica, 77, 243. [DOI] [PubMed] [Google Scholar]
- Fry AM, Bayliss R, & Roig J. (2017). Mitotic Regulation by NEK Kinase Networks. Frontiers in Cell and Development Biology, 5, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelbazouri E, Ismaili N, Ahrich N, Benarafa N, Senouci A, Mansouri K, & Hassam FB (2007). Nevus comedonicus syndrome. Annales de Dermatologie et de Vénéréologie, 134, 663–666. [DOI] [PubMed] [Google Scholar]
- Gornall AS, Budd JLS, Draper ES, Konje JC, & Kurinczuk JJ (2003). Congenital cystic adenomatoid malformation: Accuracy of prenatal diagnosis, prevalence and outcome in a general population. Prenatal Diagnosis, 23, 997–1002. [DOI] [PubMed] [Google Scholar]
- Ito T, Mitamura Y, Tsuji Y, Harada K, & Urabe K. (2013). Bilateral nevus comedonicus syndrome. Yonago Acta Medica, 56, 59–61. [PMC free article] [PubMed] [Google Scholar]
- Kaliyadan F, Nampoothiri S, Sunitha V, & Kuruvilla VE (2010). Nevus comedonicus syndrome—Nevus comedonicus associated with ipsilateral polysyndactyly and bilateral oligodontia. Pediatric Dermatology, 27, 377–379. [DOI] [PubMed] [Google Scholar]
- Laberge JM, Flageole H, Pugash D, Khalife S, Blair G, Filiatrault D, … Wilson RD (2001). Outcome of the prenatally diagnosed congenital cystic adenomatoid lung malformation: A Canadian experience. Fetal Diagnosis and Therapy, 16, 178–186. [DOI] [PubMed] [Google Scholar]
- Leblanc C, Baron M, Desselas E, Phan MH, Rybak A, Thouvenin G, … Irtan S. (2017). Congenital pulmonary airway malformations: State-of-the-art review for pediatrician’s use. European Journal of Pediatrics, 176, 1559–1571. [DOI] [PubMed] [Google Scholar]
- Levinsohn JL, Sugarman JL, Yale Center for Mendelian Genomics, McNiff JM, Antaya RJ, & Choate KA (2016). Somatic Mutations in NEK9 Cause Nevus Comedonicus. American Journal of Human Genetics, 98, 1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahran AM, Abdelsamea GM, & Mekkawy MM (2017). Bilateral nevus comedonicus successfully treated with topical adapalene: A case report. Clinical Dermatology Open Access Journal, 2, 000136. [Google Scholar]
- Martinez M, Levrero P, Bazzano C, Larre Borges A, & De Anda G. (2006). Nevus comedonicus syndrome in a woman with Paget bone disease and breast cancer: A mere coincidence? European Journal of Dermatology, 16, 697–698. [PubMed] [Google Scholar]
- Patrizi A, Neri I, Fiorentini C, & Marzaduri S. (1998). Nevus comedonicus syndrome: A new pediatric case. Pediatric Dermatology, 15, 304–306. [DOI] [PubMed] [Google Scholar]
- Pavithra S, Pai H, Mallya H, & Pai GS (2011). Nevus comedonicus syndrome. Indian Journal of Dermatology, 56, 771–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peranteau WH, Boelig MM, Khalek N, Moldenhauer JS, Martinez-Poyer J, Hedrick HL, … Adzick NS (2016). Effect of single and multiple courses of maternal betamethasone on prenatal congenital lung lesion growth and fetal survival. Journal of Pediatric Surgery, 51, 28–32. [DOI] [PubMed] [Google Scholar]
- Peranteau WH, Wilson RD, Liechty KW, Johnson MP, Bebbington MW, Hedrick HL, … Adzick NS (2007). Effect of maternal betamethasone administration on prenatal congenital cystic adenomatoid malformation growth and fetal survival. Fetal Diagnosis and Therapy, 22, 365–371. [DOI] [PubMed] [Google Scholar]
- Qian G, Liu T, Zhou C, & Zhang Y. (2015). Naevus comedonicus syndrome complicated by hidradenitis suppurativa-like lesions responding to acitretin treatment. Acta Dermato-Venereologica, 95, 992–993. [DOI] [PubMed] [Google Scholar]
- Seo YJ, Piao YJ, Suhr KB, Lee JH, & Park JK (2001). A case of nevus comedonicus syndrome associated with neurologic and skeletal abnormalities. International Journal of Dermatology, 40, 648–650. [DOI] [PubMed] [Google Scholar]
- Sfakianaki AK, & Copel JA (2012). Congenital cystic lesions of the lung: Congenital cystic adenomatoid malformation and bronchopulmonary sequestration. Reviews in Obstetrics and Gynecology, 5, 85–93. [PMC free article] [PubMed] [Google Scholar]
- Suite M, & Mahabir R. (1994). Unilateral comedo nevus associated with ipsilateral congenital cataract. International Journal of Dermatology, 33(5), 390–391. [DOI] [PubMed] [Google Scholar]
- Swarr DT, Peranteau WH, Pogoriler J, Frank DB, Adzick NS, Hedrick HL, … Morrisey EE (2018). Novel molecular and phenotypic insights into congenital lung malformations. American Journal of Respiratory and Critical Care Medicine, 197, 1328–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernev G, Ananiev J, Semkova K, Dourmishev LA, Schönlebe J, & Wollina U. (2013). Nevus comedonicus: An updated review. Dermatology and Therapy (Heidelberg), 3, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria T, Srinivasan AS, Pogoriler J, Kreiger PA, Laje P, Oliver ER, … Adzick NS (2018). The rare solid fetal lung lesion with T2-hypointense components: Prenatal imaging findings with postnatal pathological correlation. Pediatric Radiology, 48, 1556–1566. [DOI] [PubMed] [Google Scholar]
- Vidaurri-de la Cruz H, Tamayo-Sánchez L, Durán-McKinster C, de la Luz Orozco-Covarrubias M, & Ruiz-Maldonado R. (2004). Epidermal nevus syndromes: Clinical findings in 35 patients. Pediatric Dermatology, 21, 432–439. [DOI] [PubMed] [Google Scholar]
- Whyte HJ (1968). Unilateral Comedo Nevus and Cataract. Archives of Dermatology, 97, 533–535. [PubMed] [Google Scholar]
- Woods KA, Larcher VF, & Harper JL (1994). Extensive naevus comedonicus in a child with Alagille syndrome. Clinical and Experimental Dermatology, 19, 163–164. [DOI] [PubMed] [Google Scholar]
- Yadav P, Mendiratta V, Rana S, & Chander R. (2015). Nevus Comedonicus Syndrome. Indian Journal of Dermatology, 60, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available due to privacy restrictions.


