Abstract
BACKGROUND
There is uncertainty about how much positive end-expiratory pressure (PEEP) should be used in patients with acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19).
OBJECTIVE
To investigate whether a higher PEEP strategy is superior to a lower PEEP strategy regarding the number of ventilator-free days (VFDs).
DESIGN
Multicentre observational study conducted from 1 March to 1 June 2020.
SETTING AND PATIENTS
Twenty-two ICUs in The Netherlands and 933 invasively ventilated COVID-19 ARDS patients.
INTERVENTIONS
Patients were categorised retrospectively as having received invasive ventilation with higher (n=259) or lower PEEP (n=674), based on the high and low PEEP/FiO2 tables of the ARDS Network, and using ventilator settings and parameters in the first hour of invasive ventilation, and every 8 h thereafter at fixed time points during the first four calendar days. We also used propensity score matching to control for observed confounding factors that might influence outcomes.
MAIN OUTCOMES AND MEASURES
The primary outcome was the number of VFDs. Secondary outcomes included distant organ failures including acute kidney injury (AKI) and use of renal replacement therapy (RRT), and mortality.
RESULTS
In the unmatched cohort, the higher PEEP strategy had no association with the median [IQR] number of VFDs (2.0 [0.0 to 15.0] vs. 0.0 [0.0 to 16.0] days). The median (95% confidence interval) difference was 0.21 (−3.34 to 3.78) days, P = 0.905. In the matched cohort, the higher PEEP group had an association with a lower median number of VFDs (0.0 [0.0 to 14.0] vs. 6.0 [0.0 to 17.0] days) a median difference of −4.65 (−8.92 to −0.39) days, P = 0.032. The higher PEEP strategy had associations with higher incidence of AKI (in the matched cohort) and more use of RRT (in the unmatched and matched cohorts). The higher PEEP strategy had no association with mortality.
CONCLUSION
In COVID-19 ARDS, use of higher PEEP may be associated with a lower number of VFDs, and may increase the incidence of AKI and need for RRT.
TRIAL REGISTRATION
Practice of VENTilation in COVID-19 is registered at ClinicalTrials.gov, NCT04346342.
Introduction
In patients with acute respiratory distress syndrome (ARDS), higher positive end-expiratory pressure (PEEP) may prevent atelectasis, improve oxygenation and reduce ventilator-induced lung injury.1 However, mortality benefit was not found in three randomised clinical trials,2–4 and in one study, higher PEEP with aggressive recruitment manoeuvres worsened outcome.5 A more recent study suggests benefit from higher PEEP in patients with ‘recruitable’ lung tissue, but harm in patients who have ‘nonrecruitable’ lung lesions.6
Patients with ARDS due to coronavirus disease 2019 (COVID-19) typically suffer from severe and often refractory hypoxaemia, and have a low respiratory system compliance.7–9 There is substantial debate as to whether COVID-19 ARDS differs from ARDS from other causes,10 and also whether COVID-19 patients have ‘recruitable’ or ‘nonrecruitable’ lung lesions.11,12 As COVID-19 patients frequently have microthrombi and pulmonary embolism as one other cause of hypoxaemia,13 a higher PEEP strategy may not necessarily correct hypoxaemia, while it can increase the risks of barotrauma and haemodynamic deterioration.14
It remains uncertain how much PEEP should be used in COVID-19 ARDS patients. In the absence of randomised clinical trial evidence, it has been recommended to use higher PEEP, according to the high PEEP/FiO2 table of the ARDS Network.15–17 We undertook the ‘Practice of VENTilation in COVID-19’ (PRoVENT-COVID) study to investigate ventilation management over the first 4 calendar days of invasive ventilation, and epidemiological characteristics and outcomes in invasively ventilated COVID-19 ARDS patients in the Netherlands.18,19 The current analysis investigates the association of early PEEP settings with outcomes, including duration of invasive ventilation, distant organ failures and mortality. We hypothesised that a higher PEEP strategy would be superior to a lower PEEP strategy.
Box 1.
no caption available
Methods
The PRoVENT-COVID study is an investigator-initiated, multicentre, observational cohort study undertaken in 22 ICUs in the Netherlands. The protocol including the statistical analysis plan has been published18 and the final protocol is available in Supplement 1. A statistical analysis plan for the current analysis was written before assessing the database, which is available in Supplement 2. Study sites were recruited through direct contact by members of the steering committee of the PRoVENT-COVID study. Study coordinators contacted the local doctors, trained data collectors to assist the local doctors and monitored the study according to the International Conference on Harmonisation Good Clinical Practice guidelines. Integrity and timely completion of data collection were ensured by the study coordinators.
The study was conducted in accordance with the Declaration of Helsinki and was registered at ClinicalTrials.gov (trial identification number NCT04346342). The Institutional Review Board of the Amsterdam UMC (Location AMC), Amsterdam, The Netherlands (Chairperson Prof Dr J.A. Swinkels) approved the study protocol on 7 April 2020 (W20_157 # 20.171) and need for patient informed consent was waived.
Patients
Consecutive patients aged at least 18 years were eligible for participation in the PRoVENT-COVID study if they were admitted to one of the participating ICUs and had received invasive ventilation for COVID-19 ARDS. The study itself had no exclusion criteria. For the current analysis, we excluded patients transferred from or to a nonparticipating centre within the first 2 calendar days of invasive ventilation, as it was impossible to collect ventilation variables, including early PEEP settings and other parameters in the nonparticipating centres.
Patient characteristics and data regarding premorbid conditions and medication were collected at baseline. In the first hour of invasive ventilation and every 8 h thereafter, at fixed time points during the first 4 calendar days, ventilator settings and parameters were collected. Thereafter, patients were categorised retrospectively as having received invasive ventilation according to a higher or lower PEEP strategy, using the high and low PEEP/FiO2 tables of the ARDS Network. We used the 8-hourly collected PEEP/FiO2 combinations to classify the applied PEEP strategy as ‘higher PEEP’ if it fitted the high PEEP/FiO2 ARDS Network table, or as ‘lower PEEP’ if it fitted the low PEEP/FiO2 ARDS Network table. For combinations that did not fit in either table, we classified them as follows: in case PEEP was higher than in the high PEEP/FiO2 table, it was classified as higher PEEP; in case PEEP was lower than in the high PEEP/FiO2 table, it was classified as lower PEEP (see Supplemental eFigure 1). Each patient was then categorised according to the majority of the available PEEP/FiO2 combinations as a higher PEEP or lower PEEP patient; the first available PEEP/FiO2 combination, that is, directly after intubation and start of invasive ventilation, was not used for patient categorisation.
Outcomes
The primary outcome was the number of days free from invasive ventilation and alive at day 28 [ventilator-free days (VFDs)], calculated from the moment of start of invasive ventilation, even if the period of unassisted breathing lasted longer than 24 consecutive hours and considering the last date of successful extubation. Patients who died within 28 days had 0 VFDs, even if extubated before day 28. A day on noninvasive ventilation (NIV) also counted in the number of VFDs.20
Secondary outcomes were acute kidney injury (AKI) defined by ‘Kidney Disease: Improving Global Outcome’21; use of renal replacement therapy (RRT) for AKI; and mortality rates in the ICU, the hospital and at 28 and 90 days. Other endpoints were duration of invasive ventilation in survivors; ICU and hospital length of stay; need for adjunctive treatments for refractory hypoxaemia, including prone positioning, lung recruitment manoeuvres, muscle paralysis and extracorporeal membrane oxygenation in the first 4 calendar days of invasive ventilation; the number of days with continuous sedation, vasopressor or inotrope administration; occurrence of reintubation or tracheostomy; pneumothorax and thromboembolic complications.
Statistical analysis
The amount of missing data was low for most of the variables (Supplementary eTable 1). Continuous variables are presented as median [IQR], and categorical variables as numbers and percentages. The PEEP groups were compared using Wilcoxon rank-sum test for continuous variables and Fisher exact tests for categorical variables.
Ventilatory variables and parameters over the first 4 calendar days are presented in line plots. The trend over time of ventilatory variables was assessed with mixed-effect linear models with centre and patients treated as random effects to account for clustering and repeated measurements, and with PEEP strategy, time as a continuous variable, and an interaction of PEEP and time as fixed effect. Overall P values from this analysis represent the overall difference among groups over time and P values from interaction represent a statistical assessment of whether the trend over time differed among the groups. All daily measurements of ventilatory variables, collected every 8 h, were aggregated as the mean per day. To further expand the analyses, the highest and lowest values each day were also compared between the groups using the same strategy. In addition, to compare variables at each day, the collection day was entered as a categorical variable in the model described above, and the P value for the daily difference was extracted using pairwise comparisons after Bonferroni correction.
The primary analysis was based on the unmatched cohort. The PEEP groups were compared for the primary outcome using median differences and 95% confidence intervals (CIs) from a mixed-effect quantile model considering T = 0.50 and an asymmetrical Laplace distribution. P values were extracted after 1000 bootstrap samplings. Binary outcomes were compared using mixed-effect logistic regression models and presented as odds ratio and 95% CIs. ICU and hospital length of stay, and 28-day and 90-day mortality, were compared using a (shared-frailty) Cox proportional hazard model and presented as hazard ratio and 95% CIs. The comparison was presented graphically through Kaplan–Meier curves. For the ICU and hospital length of stay analyses, all patients who died prior to discharge were assigned the maximum length of stay to account for death as a competing risk in this model. The proportional hazard assumption was assessed through Schoenfeld residuals. Duration of invasive ventilation was compared through a clustered Fine–Gray competing risk model, with death before extubation treated as a competing risk, and presented as subdistribution hazard ratio and 95% CIs in cumulative incidence plots. All models considered the centre as a random effect.
The secondary analysis was based on the matched cohort. A complete description is described in the Supplementary eMethods. We used a covariate-balancing propensity score (CBPS) for matching.22 The CBPS was estimated for each patient with logistic regression using relevant prognostic variables. The following baseline variables (measured at baseline or within 1 h after intubation) were considered in the matching process: age, sex, BMI, PaO2/FiO2, plasma creatinine concentration, hypertension, diabetes, use of angiotensin converting enzyme inhibitors, use of angiotensin II receptor blockers, use of a vasopressor or an inotrope, fluid balance, pH, mean arterial pressure, heart rate and respiratory system compliance. All variables were selected a priori and based on clinical relevance and known association with outcomes in this group of patients.
All analyses were conducted in R v.4.0.2 (R Foundation, Vienna, Austria) and significance level was set at 0.05.
Results
Study population
Thirty-one ICUs were invited to participate in the PRoVENT-COVID study, of which 22 met inclusion criteria. From 1 March to 1 June 2020, 1340 patients were screened. Final follow-up was completed on 1 September 2020. A total of 218 patients were not enrolled, of whom 62 (4.6%) had an alternative diagnosis, and 150 (11.1%) never received invasive ventilation (Supplementary eFigure 2). An additional 189 patients were excluded, mainly because they had received invasive ventilation in a nonparticipating hospital in the first 4 calendar days of ventilation. Of the enrolled 933 patients, 259 (27.8%) were categorised as higher PEEP patients, and 674 (72.2%) as lower PEEP patients. Of all patients, 468 could be matched, 234 (50%) in the higher PEEP group and 234 (50%) in the lower PEEP group. Details on the matching process are shown in Supplementary eResults and eFigures 3 and 4.
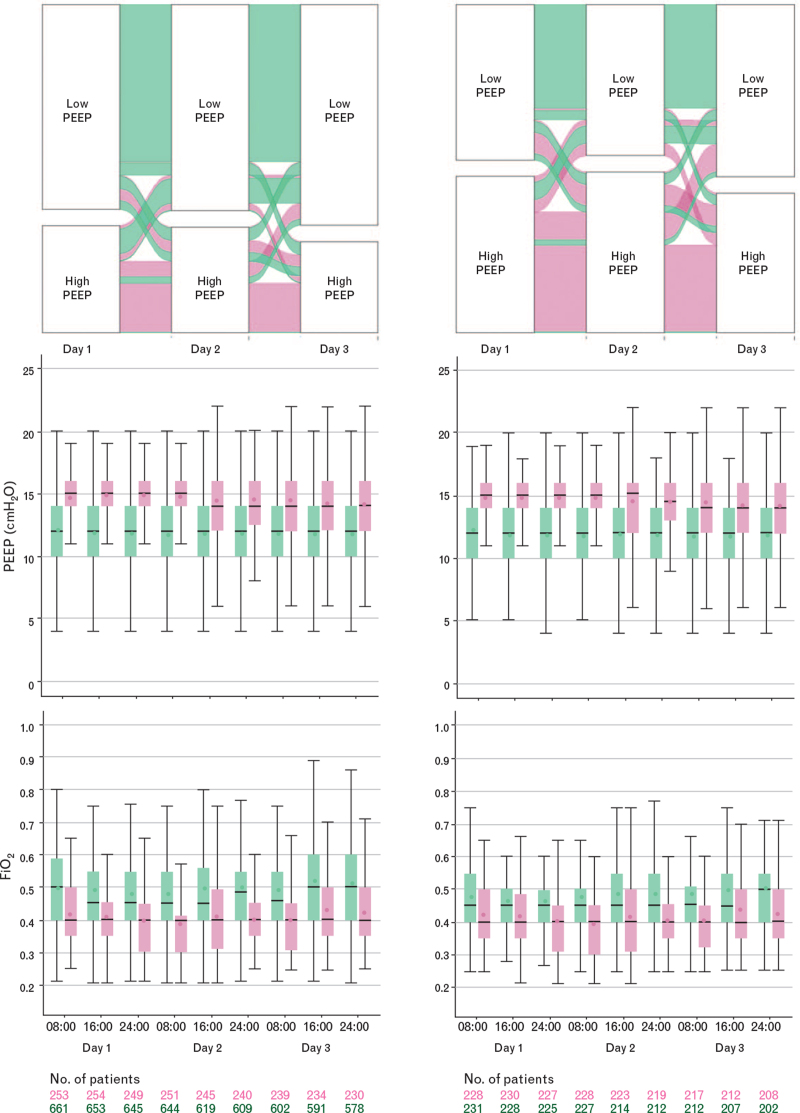
Figure 1, supplementary eFigure 1, eTable 2 and eFigures 5 to 11 show group assignment of patients in the unmatched and the matched cohorts, and PEEP and FiO2. Table 1 shows the baseline characteristics of the PEEP groups in the unmatched and matched cohorts. The main difference between the two PEEP groups in the unmatched cohort was ARDS severity. In the matched cohort, the main difference was the proportion of patients on inhaled steroids at baseline. Ventilation management is detailed in Table 2, Supplementary eTables 3 and 4, and eFigures 12 and 13. Respiratory system compliance was not different between the higher and lower PEEP groups, neither in the unmatched nor in the matched cohorts (eFigure 14). PaO2/FiO2 and plasma creatinine concentration were higher in the higher PEEP group; use of vasopressors, prone positioning, daily urine output and fluid balance were not different (Supplementary eFigures 15 to 17).
Fig. 1.
Group assignment, positive end-expiratory pressure and FiO2 in the higher (pink) and lower (green) positive end-expiratory pressure groups, before (left panels) and after (right panels) matching. The figure shows the median, lowest and highest positive end-expiratory pressure and FiO2 values. Horizontal bars inside boxes represent medians; box tops and bottoms, interquartile ranges. Whiskers extend to 1.5 times the interquartile range beyond the first and third quartiles per the conventional Tukey method. Circles represent means.
Table 1.
Baseline characteristics of the patients before and after matching
| Unmatched cohort (n=933) | Matched cohort (n=468) | |||||||
| High PEEP, n=259 | Low PEEP, n=674 | SMD | P | High PEEP, n=234 | Low PEEP, n=234 | SMD | P | |
| Age (years) | 64.0 [56.0 to 71.0] | 66.0 [58.0 to 73.0] | 0.201 | 0.018 | 65.0 [59.0 to 71.0] | 64.0 [55.2 to 71.0] | 0.134 | 0.268 |
| Male sex | 192 (74.1) | 483 (71.7) | 0.056 | 0.463 | 175 (74.8) | 176 (75.2) | 0.010 | 0.999 |
| BMI (kg m−2) | 27.8 [25.8 to 31.1] | 27.6 [25.2 to 30.7] | 0.105 | 0.327 | 27.7 [25.4 to 30.4] | 28.1 [25.7 to 31.3] | 0.105 | 0.303 |
| Transferred under invasive ventilation | 29 (11.2) | 59 (8.8) | 0.082 | 0.261 | 23 (9.8) | 22 (9.4) | 0.014 | 0.999 |
| Days between intubation and admission | 0 [0 to 0] | 0 [0 to 0] | 0.047 | 0.514 | 0 [0 to 0] | 0 [0 to 0] | 0.039 | 0.675 |
| Use of noninvasive ventilation | 24/242 (9.9) | 52/630 (8.3) | 0.058 | 0.424 | 23/220 (10.5) | 20 (9.3) | 0.037 | 0.749 |
| Duration of noninvasive ventilation (h) | 8.0 [5.5 to 14.0] | 7.0 [2.0 to 20.0] | 0.036 | 0.976 | 8.0 [4.4 to 14.0] | 5.5 [2.2 to 9.0] | 0.135 | 0.749 |
| Chest CT scan performed | 94/253 (37.1) | 229/660 (34.7) | 0.053 | 0.486 | 83/228 (36.4) | 82/228 (36.0) | 0.009 | 0.999 |
| Lung parenchyma affected | 0.071 | 0.987 | 0.266 | 0.610 | ||||
| 0% | 3/94 (3.2) | 8/229 (3.5) | 1/83 (1.2) | 5/82 (6.1) | ||||
| 25% | 28/94 (29.8) | 75/229 (32.8) | 27/83 (32.5) | 24/82 (29.3) | ||||
| 50% | 29/94 (30.9) | 68/229 (29.7) | 24/83 (28.9) | 23/82 (28.0) | ||||
| 75% | 28/94 (29.8) | 65/229 (28.4) | 26/83 (31.3) | 25/82 (30.5) | ||||
| 100% | 6/94 (6.4) | 13/229 (5.7) | 5/83 (6.0) | 5/82 (6.1) | ||||
| Chest radiograph performed | 142/163 (87.1) | 384/431 (89.1) | 0.054 | 0.567 | 132/148 (89.2) | 133/149 (89.3) | 0.019 | 0.999 |
| Quadrants affected | 0.184 | 0.306 | 0.287 | 0.151 | ||||
| 1 | 13/139 (9.4) | 24/384 (6.2) | 13/130 (10.0) | 5/133 (3.8) | ||||
| 2 | 37/139 (26.6) | 85/384 (22.1) | 35/130 (26.9) | 30/133 (22.6) | ||||
| 3 | 32/139 (23.0) | 110/384 (28.6) | 29/130 (22.3) | 35/133 (26.3) | ||||
| 4 | 57/139 (41.0) | 165/384 (43.0) | 53/130 (40.8) | 63/133 (47.4) | ||||
| Severity of ARDS | 0.447 | <0.001 | 0.158 | 0.246 | ||||
| Mild | 81/256 (31.6) | 103/663 (15.5) | 63 (26.9) | 52 (22.2) | ||||
| Moderate | 161/256 (62.9) | 469/663 (70.7) | 157 (67.1) | 160 (68.4) | ||||
| Severe | 14/256 (5.5) | 91/663 (13.7) | 14 (6.0) | 22 (9.4) | ||||
| Co-existing disorders | ||||||||
| Hypertension | 86 (33.2) | 227 (33.7) | 0.010 | 0.938 | 77 (32.9) | 81 (34.6) | 0.036 | 0.769 |
| Heart failure | 8 (3.1) | 31 (4.6) | 0.079 | 0.364 | 8 (3.4) | 14 (6.0) | 0.121 | 0.275 |
| Diabetes | 56 (21.6) | 154 (22.8) | 0.030 | 0.727 | 54 (23.1) | 51 (21.8) | 0.031 | 0.825 |
| Chronic kidney disease | 14 (5.4) | 28 (4.2) | 0.059 | 0.480 | 11 (4.7) | 10 (4.3) | 0.021 | 0.999 |
| Baseline creatininea (μmol l−1) | 78.0 [65.0 to 98.8] | 77.0 [63.0 to 96.0] | 0.025 | 0.305 | 80.0 [67.0 to 99.0] | 77.0 [66.0 to 94.8] | 0.052 | 0.337 |
| Liver cirrhosis | 1 (0.4) | 2 (0.3) | 0.015 | 0.999 | 1 (0.4) | 1 (0.4) | <0.001 | 0.999 |
| Chronic obstructive pulmonary disease | 13 (5.0) | 63 (9.3) | 0.168 | 0.032 | 12 (5.1) | 23 (9.8) | 0.179 | 0.078 |
| Active haematological neoplasia | 5 (1.9) | 10 (1.5) | 0.034 | 0.574 | 5 (2.1) | 5 (2.1) | <0.001 | 0.999 |
| Active solid neoplasia | 8 (3.1) | 16 (2.4) | 0.044 | 0.498 | 8 (3.4) | 5 (2.1) | 0.078 | 0.576 |
| Neuromuscular disease | 4 (1.5) | 4 (0.6) | 0.093 | 0.228 | 4 (1.7) | 3 (1.3) | 0.035 | 0.999 |
| Immunosuppression | 7 (2.7) | 16 (2.4) | 0.021 | 0.814 | 7 (3.0) | 8 (3.4) | 0.024 | 0.999 |
| Previous medication | ||||||||
| Systemic steroids | 13 (5.0) | 23 (3.4) | 0.080 | 0.258 | 12 (5.1) | 7 (3.0) | 0.108 | 0.349 |
| Inhalation steroids | 22 (8.5) | 88 (13.1) | 0.148 | 0.054 | 18 (7.7) | 38 (16.2) | 0.266 | 0.006 |
| Angiotensin converting enzyme inhibitor | 49 (18.9) | 112 (16.6) | 0.060 | 0.439 | 44 (18.8) | 52 (22.2) | 0.085 | 0.423 |
| Angiotensin II receptor blocker | 26 (10.0) | 78 (11.6) | 0.049 | 0.562 | 25 (10.7) | 19 (8.1) | 0.088 | 0.429 |
| Beta-blockers | 34 (13.1) | 145 (21.5) | 0.223 | 0.004 | 30 (12.8) | 46 (19.7) | 0.186 | 0.060 |
| Insulin | 15 (5.8) | 50 (7.4) | 0.066 | 0.473 | 14 (6.0) | 18 (7.7) | 0.068 | 0.583 |
| Metformin | 44 (17.0) | 105 (15.6) | 0.038 | 0.618 | 42 (17.9) | 35 (15.0) | 0.081 | 0.455 |
| Statins | 67 (25.9) | 217 (32.2) | 0.140 | 0.068 | 61 (26.1) | 81 (34.6) | 0.187 | 0.056 |
| Calcium channel blockers | 50 (19.3) | 118 (17.5) | 0.046 | 0.568 | 47 (20.1) | 40 (17.1) | 0.077 | 0.476 |
| Vital signs | ||||||||
| Heart rate (bpm) | 82.5 [72.0 to 95.5] | 84.5 [75.0 to 98.0] | 0.130 | 0.215 | 83.0 [72.0 to 96.0] | 83.4 [70.5 to 98.6] | 0.025 | 0.820 |
| Mean arterial pressure (mmHg) | 79.5 [73.7 to 86.2] | 81.0 [73.5 to 88.5] | 0.061 | 0.740 | 79.9 [74.2 to 86.1] | 79.9 [73.0 to 87.5] | 0.062 | 0.317 |
| Organ support | ||||||||
| Continuous sedation | 249 (96.1) | 646 (96.1) | <0.001 | 0.457 | 225 (96.2) | 224 (96.1) | 0.001 | 0.544 |
| Inotropic or vasopressor | 206 (79.5) | 526 (78.3) | 0.031 | 0.488 | 189 (80.8) | 188 (80.7) | 0.002 | 0.872 |
| Vasopressor | 206 (79.5) | 525 (78.1) | 0.035 | 0.507 | 189 (80.8) | 187 (80.3) | 0.013 | 0.870 |
| Inotropic | 6 (2.3) | 33 (4.9) | 0.139 | 0.724 | 6 (2.6) | 16 (6.9) | 0.204 | 0.884 |
| Fluid balance (ml) | 584.0 [22.0 to 1227.0] | 648.0 [41.5 to 1492.0] | 0.105 | 0.623 | 605.0 [9.6 to 1298.0] | 635.0 [30.0 to 1428.5] | 0.091 | 0.320 |
| Urine output (ml) | 720.0 [362.5 to 1125.0] | 710.0 [383.8 to 1165.0] | 0.053 | 0.836 | 720.0 [362.5 to 1107.5] | 700.0 [400.0 to 1190.0] | 0.070 | 0.997 |
Data are median [IQR] or number (%). Percentages may not total 100 because of rounding. CT, computed tomography; PEEP, positive end-expiratory pressure; SMD, standardised mean difference.
Most recent measurement in 24 h before intubation, or at ICU admission under invasive ventilation.
Table 2.
Ventilatory variables and rescue therapy at start of ventilation before and after matching
| Unmatched cohort, n=933 | Matched cohort, n=468 | |||||||
| High PEEP, n=259 | Low PEEP, n=674 | SMD | P ∗ | High PEEP, n=234 | Low PEEP, n=234 | SMD | P ∗ | |
| Ventilation support | ||||||||
| Assisted ventilation | 84/257 (32.7) | 185/671 (27.6) | 0.112 | 0.092 | 77/232 (33.2) | 61/233 (26.2) | 0.154 | 0.265 |
| Tidal volume (ml kg−1 PBW) | 6.4 [5.9 to 7.1] | 6.5 [5.9 to 7.0] | 0.043 | 0.296 | 6.4 [5.9 to 7.1] | 6.4 [5.9 to 7.0] | 0.028 | 0.642 |
| PEEP (cmH2O) | 14.2 [13.0 to 16.0] | 12.0 [10.0 to 14.0] | 0.911 | <0.001 | 14.3 [13.0 to 16.0] | 12.0 [10.1 to 14.0] | 0.927 | <0.001 |
| Peak pressure (cmH2O) | 28.0 [25.5 to 30.5] | 26.0 [23.0 to 29.3] | 0.391 | <0.001 | 28.0 [25.5 to 30.5] | 26.3 [23.0 to 29.5] | 0.346 | 0.007 |
| Driving pressure (cmH2O) | 13.8 [12.0 to 15.7] | 14.0 [11.9 to 16.2] | 0.130 | 0.180 | 13.7 [11.8 to 15.5] | 14.0 [11.5 to 16.7] | 0.158 | 0.425 |
| Mechanical power (J min−1) | 19.4 [16.4 to 23.8] | 18.0 [14.8 to 21.5] | 0.294 | 0.009 | 19.4 [16.4 to 23.9] | 18.2 [15.0 to 21.4] | 0.267 | 0.061 |
| Compliance (ml cmH2O−1) | 33.5 [27.5 to 39.5] | 33.1 [26.5 to 41.1] | 0.037 | 0.807 | 33.7 [28.5 to 40.1] | 33.8 [26.1 to 42.2] | 0.007 | 0.924 |
| Total respiratory rate (min−1) | 21.7 [19.7 to 24.0] | 21.5 [19.1 to 24.0] | 0.027 | 0.835 | 21.7 [19.8 to 24.0] | 21.5 [19.0 to 24.0] | 0.087 | 0.480 |
| FiO2 | 0.52 [0.45 to 0.63] | 0.60 [0.50 to 0.70] | 0.385 | <0.001 | 0.53 [0.45 to 0.65] | 0.57 [0.48 to 0.68] | 0.163 | 0.079 |
| etCO2 (kPa) | 4.9 [4.4 to 5.6] | 4.9 [4.3 to 5.5] | 0.086 | 0.799 | 4.9 [4.4 to 5.6] | 4.9 [4.3 to 5.5] | 0.067 | 0.662 |
| Fractional dead space, Enghoff | 0.14 [0.07 to 0.23] | 0.19 [0.10 to 0.29] | 0.424 | <0.001 | 0.15 [0.08 to 0.24] | 0.18 [0.09 to 0.26] | 0.225 | 0.063 |
| Laboratory tests | ||||||||
| pH | 7.37 [7.32 to 7.41] | 7.36 [7.30 to 7.40] | 0.195 | 0.046 | 7.37 [7.32 to 7.40] | 7.37 [7.32 to 7.41] | 0.084 | 0.115 |
| PaO2 (kPa) | 11.5 [10.0 to 13.5] | 10.7 [9.6 to 12.5] | 0.267 | <0.001 | 11.4 [9.9 to 13.1] | 11.0 [9.7 to 13.1] | 0.035 | 0.311 |
| PaO2/FiO2 | 19.0 [14.2 to 25.7] | 16.9 [12.7 to 22.3] | 0.193 | <0.001 | 17.9 [14.0 to 23.9] | 18.2 [14.2 to 24.8] | 0.083 | 0.587 |
| PaCO2 (kPa) | 5.7 [4.9 to 6.3] | 6.0 [5.3 to 6.7] | 0.319 | 0.004 | 5.8 [5.1 to 6.4] | 5.8 [5.2 to 6.4] | 0.060 | 0.730 |
| Lactate (mmol l−1) | 1.1 [0.9 to 1.4] | 1.2 [0.9 to 1.5] | 0.151 | 0.073 | 1.1 [0.9 to 1.4] | 1.2 [1.0 to 1.4] | 0.150 | 0.132 |
| Creatinine (μmol l−1) | 74.0 [62.8 to 93.5] | 74.0 [61.0 to 97.0] | 0.013 | 0.961 | 76.0 [64.0 to 96.2] | 74.0 [63.0 to 95.2] | 0.070 | 0.658 |
| Rescue therapy | ||||||||
| Prone positioning | 58 (22.9) | 231 (34.8) | 0.265 | 0.048 | 55 (24.0) | 75 (32.8) | 0.195 | 0.175 |
| Duration (ha) | 8.0 [5.0 to 12.0] | 8.0 [4.0 to 14.0] | 0.169 | 0.739 | 8.0 [6.0 to 12.0] | 9.0 [4.0 to 14.0] | 0.143 | 0.651 |
| Recruitment manoeuvre | 5 (2.6) | 15 (2.7) | 0.007 | 0.964 | 5 (2.9) | 5 (2.6) | 0.018 | 0.903 |
| ECMO | 0 (0.0) | 0 (0.0) | <0.001 | 0.999 | 0 (0.0) | 0 (0.0) | <0.001 | 0.999 |
| Use of NMBA | 77 (30.0) | 175 (26.0) | 0.088 | 0.111 | 75 (32.2) | 59 (25.2) | 0.155 | 0.052 |
| Hours of usea | 0.0 [0.0 to 8.0] | 0.0 [0.0 to 8.0] | 0.046 | 0.241 | 0.0 [0.0 to 8.0] | 0.0 [0.0 to 6.0] | 0.085 | 0.101 |
Data are median [IQR] or number (%). Percentages may not total 100 because of rounding. ECMO, extracorporeal membrane oxygenation; NMBA, neuromuscular blocking agent; PBW, predicted body weight; PEEP, positive end-expiratory pressure; SMD, standardised mean difference.
In patients who received it.
Calculated using pairwise contrasts in a mixed-effect generalised linear model with day, group and an interaction day group as fixed effect, and with patients and centre as random effect. A binomial distribution was used for binary variables and a Gaussian distribution for continuous.
Outcomes in the unmatched cohort and matched cohort
Outcomes in the matched and unmatched cohorts are summarised in Table 3, Fig. 2 and Supplementary eFigure 18.
Table 3.
Clinical outcomes according to groups before and after matching
| Unmatched cohort, n=933 | Matched cohort, n=468 | |||||||
| High PEEP, n=259 | Low PEEP, n=674 | Effect estimate (95% CI) | P | High PEEP, n=234 | Low PEEP, n=234 | Effect estimate (95% CI) | P | |
| Primary outcome | ||||||||
| Ventilator-free days at day 28 (days) | 2.0 [0.0 to 15.0] | 0.0 [0.0 to 16.0] | 0.21 (−3.34 to 3.78)a | 0.905 | 0.0 [0.0 to 14.0] | 6.0 [0.0 to 17.0] | −4.65 (−8.92 to −0.39)a | 0.032 |
| Mean ± SD | 7.5 ± 8.7 | 7.6 ± 8.8 | 6.9 ± 8.5 | 8.5 ± 8.8 | ||||
| Secondary outcomes | ||||||||
| Duration of ventilation (days) | 14.5 [7.0 to 24.0] | 13.0 [8.0 to 22.3] | 1.03 (0.75 to 1.42)b | 0.850 | 15.0 [7.0 to 25.0] | 13.0 [9.0 to 22.0] | 0.79 (0.58 to 1.09)b | 0.150 |
| In survivors at day 28 (days) | 17.0 [9.0 to 27.8] | 15.0 [9.0 to 28.0] | 18.0 [10.0 to 29.0] | 14.0 [9.0 to 24.5] | ||||
| Tracheostomy | 42/257 (16.3) | 105 (15.6) | 0.95 (0.61 to 1.48)c | 0.830 | 39/233 (16.7) | 34 (14.5) | 0.80 (0.44 to 1.48)c | 0.486 |
| Reintubation | 29/256 (11.3) | 88/671 (13.1) | 0.90 (0.56 to 1.45)c | 0.673 | 26/232 (11.2) | 30/232 (12.9) | 0.87 (0.48 to 1.57)c | 0.646 |
| Pneumothorax | 4/252 (1.6) | 3/643 (0.5) | 3.97 (0.76 to 20.70)c | 0.102 | 4/228 (1.8) | 1/225 (0.4) | 4.02 (0.35 to 46.51)c | 0.265 |
| Thromboembolic complications | 72 (27.8) | 202 (30.0) | 0.80 (0.57 to 1.15)c | 0.227 | 68 (29.1) | 72 (30.8) | 0.87 (0.56 to 1.36)c | 0.544 |
| Pulmonary embolism | 55 (21.2) | 155 (23.0) | 0.81 (0.54 to 1.19)c | 0.281 | 53 (22.6) | 56 (23.9) | 0.89 (0.54 to 1.46)c | 0.643 |
| Deep vein thrombosis | 9 (3.5) | 41 (6.1) | 0.77 (0.32 to 1.84)c | 0.559 | 9 (3.8) | 19 (8.1) | 0.54 (0.21 to 1.39)c | 0.201 |
| Ischaemic stroke | 9 (3.5) | 21 (3.1) | 1.33 (0.56 to 3.17)c | 0.519 | 8 (3.4) | 3 (1.3) | 2.99 (0.73 to 12.26)c | 0.128 |
| Myocardial infarction | 3 (1.2) | 13 (1.9) | 0.61 (0.17 to 2.26)c | 0.460 | 2 (0.9) | 4 (1.7) | 0.50 (0.09 to 2.73)c | 0.420 |
| Systemic arterial embolism | 0 (0.0) | 4 (0.6) | – | – | 0 (0.0) | 1 (0.4) | – | – |
| Acute kidney injury | 134/257 (52.1) | 303/672 (45.1) | 1.36 (0.99 to 1.86)c | 0.056 | 129/232 (55.6) | 107/233 (45.9) | 1.49 (1.02 to 2.18)c | 0.039 |
| Need for RRT | 62 (23.9) | 113 (16.8) | 1.54 (1.05 to 2.27)c | 0.027 | 60 (25.6) | 42 (17.9) | 1.58 (1.01 to 2.46)c | 0.045 |
| Need of rescue therapyd | 188/258 (72.9) | 526/670 (78.5) | 0.86 (0.59 to 1.26)c | 0.444 | 175/233 (75.1) | 183/232 (78.9) | 0.83 (0.51 to 1.35)c | 0.450 |
| Prone positioning | 129/257 (50.2) | 426/670 (63.6) | 0.66 (0.46 to 0.94)c | 0.023 | 121/232 (52.2) | 141/233 (60.5) | 0.71 (0.44 to 1.12)c | 0.142 |
| Recruitment manoeuvre | 16/196 (8.2) | 40/575 (7.0) | 1.22 (0.60 to 2.5)c | 0.588 | 15/175 (8.6) | 13/202 (6.4) | 1.45 (0.59 to 3.61)c | 0.420 |
| Use of NMBA | 125 (48.3) | 337 (50.0) | 0.98 (0.70 to 1.39)c | 0.931 | 116 (49.6) | 124 (53.0) | 0.95 (0.61 to 1.48)c | 0.825 |
| ECMO | 1/258 (0.4) | 7 (1.1) | 0.37 (0.04 to 2.99)c | 0.348 | 1/233 (0.4) | 3/229 (1.3) | 0.32 (0.03 to 3.14)c | 0.332 |
| Use of continuous sedationd | 259 (100.0) | 669 (99.3) | – | – | 234 (100.0) | 233 (99.6) | – | – |
| Use of inotrope or vasopressord | 250 (96.5) | 640 (95.0) | 1.40 (0.64 to 3.07)c | 0.402 | 227 (97.0) | 222 (94.9) | 1.78 (0.67 to 4.74)c | 0.250 |
| Use of vasopressor | 250 (96.5) | 639 (94.8) | 1.46 (0.67 to 3.17)c | 0.342 | 227 (97.0) | 221 (94.4) | 1.91 (0.75 to 4.87)c | 0.177 |
| Use of inotrope | 12 (4.6) | 84 (12.5) | 0.59 (0.28 to 1.27)c | 0.177 | 11 (4.7) | 33 (14.1) | 0.65 (0.26 to 1.65)c | 0.369 |
| ICU length of stay (days) | 16.0 [9.0 to 27.0] | 15.0 [9.0 to 26.0] | 1.15 (0.95 to 1.40)e | 0.140 | 16.0 [9.0 to 28.0] | 15.0 [10.0 to 25.0] | 0.86 (0.68 to 1.09)e | 0.230 |
| In survivors (days) | 19.5 [11.8 to 30.3] | 18.0 [11.0 to 30.8] | 20.0 [12.0 to 31.8] | 16.0 [11.0 to 29.0] | ||||
| Hospital length of stay (days) | 24.0 [13.0 to 39.0] | 23.0 [15.0 to 37.0] | 1.14 (0.93 to 1.40)e | 0.190 | 23.5 [13.0 to 40.0] | 24.5 [17.0 to 36.0] | 0.88 (0.69 to 1.13)e | 0.310 |
| In survivors (days) | 31.0 [20.0 to 47.0] | 30.0 [21.0 to 45.0] | 32.0 [20.0 to 48.0] | 28.0 [21.0 to 41.3] | ||||
| ICU mortality | 77/254 (30.3) | 239/664 (36.0) | 0.75 (0.53 to 1.05)c | 0.097 | 77/231 (33.3) | 69/232 (29.7) | 1.24 (0.80 to 1.91)c | 0.332 |
| Hospital mortality | 79/242 (32.6) | 246/647 (38.0) | 0.76 (0.54 to 1.08)c | 0.124 | 79/223 (35.4) | 74/226 (32.7) | 1.18 (0.77 to 1.82)c | 0.451 |
| 28-Day mortality | 71/255 (27.8) | 216/668 (32.3) | 0.85 (0.63 to 1.14)e | 0.270 | 71/230 (30.9) | 63/233 (27.0) | 1.30 (0.90 to 1.88)e | 0.170 |
| 90-Day mortality | 79/242 (32.6) | 259/625 (41.4) | 0.77 (0.58 to 1.02)e | 0.067 | 79/220 (35.9) | 78/214 (36.4) | 1.15 (0.81 to 1.64)e | 0.430 |
Data are median [IQR], mean ± SD or number (%). Percentages may not total 100 because of rounding. CI, confidence interval; ECMO, extracorporeal membrane oxygenation; NMBA, neuromuscular blocking agent; PEEP, positive end-expiratory pressure; RRT, renal replacement therapy.
Median difference from a mixed-effect quantile model considering a Τ = 0.50 and an asymmetric Laplace distribution. P values were extracted after 1000 bootstrap samplings.
Subdistribution hazard ratio from a clustered Fine–Gray competing risk model, with death before extubation treated as competing risk.
Odds ratio from mixed-effect logistic regression models.
Assessed in the first 4 days of ventilation.
Hazard ratio from a (shared-frailty) Cox proportional hazard model. For the ICU and hospital length of stay analyses, all patients who died prior to discharge were assigned the maximum length of stay to account for death as a competing risk in this model. P value for Schoenfeld residuals: ICU length of stay (P = 0.420 in the unmatched cohort and P = 0.330 for the matched cohort); hospital length of stay (P = 0.830 in the unmatched cohort and P = 0.770 for the matched cohort); 7-day mortality (P = 0.380 in the unmatched cohort and P = 0.780 in the matched cohort); 28-day mortality (P = 0.260 in the unmatched cohort and P = 0.110 in the matched cohort); 90-day mortality (P = 0.100 in the unmatched cohort and P = 0.055 in the matched cohort).
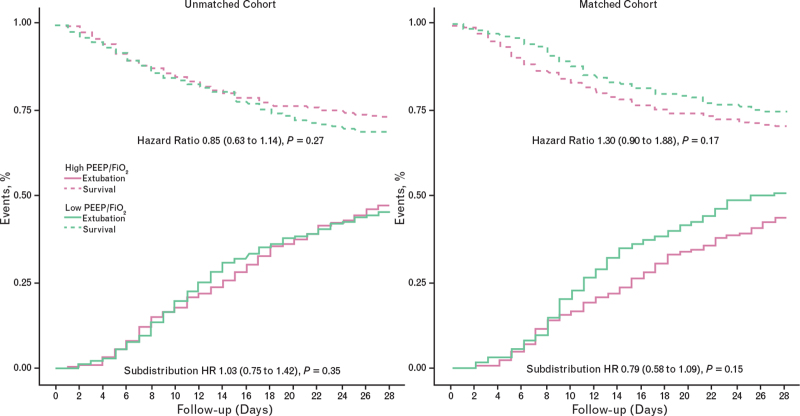
Fig. 2.
Mortality and pattern of extubation in the higher (pink) and lower (green) positive end-expiratory pressure groups, before (left panels) and after (right panels) matching. HR, hazard ratio.
In the unmatched cohort, 28 days after the start of invasive ventilation, patients in the higher PEEP group had a median of 2 [IQR 0 to 15] VFDs compared with 0 [0 to 16] VFDs in the lower PEEP group [median difference 0.21 (95% CI −3.34 to 3.78) days, P = 0.905]. Patients in the higher PEEP group needed RRT more often than patients in the lower PEEP group. Mortality rates and other endpoints were not different between the higher and lower PEEP groups.
In the matched cohort, 28 days after the start of invasive ventilation, patients in the higher PEEP group had median of 0 [0 to 14] VFDs days compared with 6 [0 to 17] VFDs in the lower PEEP group [median difference −4.65 (95% CI −8.92 to −0.39) days, P = 0.032]. Patients in the higher PEEP group developed AKI and received RRT more often than patients in the lower PEEP group. Mortality rates and other endpoints were not different between the higher and lower PEEP groups in the matched cohort.
Discussion
The findings of this study can be summarised as follows: in an unmatched cohort of COVID-19 ARDS patients, a higher PEEP strategy had no association with the number of VFDs but this strategy was associated with a greater use of RRT; in a matched cohort, a higher PEEP strategy was associated with a lower number of VFDs, more AKI and a greater use of RRT; and neither in the unmatched nor in the matched cohort did use of higher PEEP have an association with mortality.
To our knowledge, this is the first study to investigate associations between early PEEP settings and outcome in COVID-19 ARDS patients using the ARDS Network tables for patient classification. A composite endpoint was chosen as the primary endpoint because it reflects duration of invasive ventilation in surviving patients, but also mortality. The study was designed to minimise bias by strictly adhering to a predefined statistical analysis plan, and there was minimal loss to follow-up. The study involved one-third of all COVID-19 ARDS patients receiving invasive ventilation in the first months of the national outbreak in the Netherlands, and patients were enrolled in 22 university hospitals, nonuniversity teaching and nonteaching hospitals, contributing to its generalisability. In addition, patients were enrolled in the trial over a period of 3 months, during which general care for COVID-19 patients did not change, that is, before treatment with dexamethasone became standard practice. As such, the findings of this study extend our understanding of the effects of higher PEEP in ARDS patients in general,2–5 and in COVID-19 ARDS in particular.
The, at times, extreme hypoxaemia may have triggered the use of higher PEEP in COVID-19 ARDS patients. Early in the global pandemic, it was presumed that there could be two phenotypes of COVID-19 ARDS, with different pulmonary compliances and shunt fractions, and also dissimilarities in recruitability. However, in the current cohort, respiratory system compliance was consistently low,20 in line with the findings in other studies.7,23,24 Furthermore, it has been shown that recruitment manoeuvres neither reduced shunt fraction nor increased systemic oxygenation.25 Of note, PEEP in the lower PEEP group in our study was higher than that in one large observational study in patients with ARDS from another origin,26 and also higher than in various studies in patients with ARDS related to COVID-19.7,23,24,27 By now, this preference for higher PEEP may have waned for two reasons; healthcare providers may have realised increasingly that higher PEEP may not improve oxygenation, at least not in all patients, and that other measures such as prone positioning are more effective in achieving this goal. Also, it may be that healthcare providers increasingly accept lower oxygen levels, realising that any attempt to ‘normalise’ physiology as much as possible could come at the cost of harm. One recent report even suggests that higher PEEP should be avoided, limiting PEEP strictly to values necessary to maintain sufficient oxygenation.28 Prospective interventional studies remain needed to determine the best PEEP strategy in COVID-19 ARDS.
Invasive ventilation with higher PEEP was associated with higher incidences of AKI and use of RRT. In critically ill patients, invasive ventilation is associated with an increased risk of AKI, but it is uncertain if the amount of PEEP modifies this risk.29 Higher PEEP may affect kidney function by reducing renal perfusion or increasing venous congestion through its effects on the heart.30 The need for RRT for AKI increases workload and costs31 and is associated with worse outcomes.32 In addition, in COVID-19 patients, efficient RRT is hampered by the high occurrence of circuit thrombosis,33–36 leading to long down-times. Last but not least, due to the overwhelming numbers of COVID-19 patients and the frequent need for replacement of clotted circuits, many ICUs could quickly run out of the medical supplies needed for this therapy.
All models considered the centres as a random effect to account for the clustering of the patients within centres. It was not our plan to compare PEEP level across centres or the reason why centres were not included as a fixed effect. While differences in PaO2/FiO2 between the groups in the matched cohort may suggest differences in disease severity, matching was also based on Sequential Organ Failure Assessment and Acute Physiology and Chronic Health Evaluation-II or IV scores, making it very unlikely that patients in the higher PEEP group were sicker than patients in the lower PEEP group. Of note, the standardised mean difference was higher than 10% for some variables, but if stricter calipers were used, the groups became too small to provide meaningful analysis. While in the unmatched cohort there was no difference in steroid use at baseline, after matching there was a difference in use of inhalational steroids between the two PEEP groups.
The PRoVENT-COVID study had limitations. We did not collect data regarding the degree to which local protocols for prone positioning and lung recruitment were in place, or whether other treatments, such as antiviral or antimalarial treatments, were used. However, none of the randomised clinical trials of these treatments showed an effect on the two components of the primary outcome used in the current study. We also did not collect reasons for changes in PEEP settings, which could have been based on changes of PaO2 or SpO2, responses to therapies such as prone positioning, or haemodynamic side effects such as hypotension. Recruitment manoeuvres were used infrequently, but it is possible that this was reported incompletely in the patient records, and therefore could not be captured in retrospect. Ventilation variables and adjunctive treatments data were collected for the first 4 calendar days of invasive ventilation, to keep the workload of the study acceptable. We cannot exclude, however, that ventilation practices and use of adjunctive treatments beyond day 4 also have an impact on outcome. It is not implausible that concerns about aerosol formation and spreading, for example, with use of NIV, influenced decisions on extubation. If this happened, however, it probably affected duration of invasive ventilation in both groups but it does hamper generalisation to cohorts of ARDS patients in which there is less concern about the risk of infection of personnel. Because it is common practice to extubate at lower PEEP, use of higher PEEP at least in theory could also delay weaning, thus reducing the number of VFDs. Finally, due to the observational nature of the study, no causal relationship can be determined and the findings should be seen only as exploratory and can only provide the statistical underpinning and rationale for further investigations.
In conclusion, in COVID-19 ARDS patients, a higher PEEP strategy may reduce the number of VFDs and increase the incidences of AKI and use of RRT. A randomised clinical trial in patients with COVID-19 ARDS is needed to better define the effects of a higher PEEP strategy versus a lower PEEP strategy on outcomes.
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: none.
Financial support and sponsorship: this work was supported by the Department of Intensive Care, Amsterdam UMC, Location AMC, Amsterdam.
Conflicts of interest: none.
Presentation: none.
PRactice of VENTilation in COVID-19.
Collaborative Group members are as follows: J.P. van Akkeren; A.G. Algera; C.K. Algoe; R.B. van Amstel; O.L. Baur; P. van de Berg; A.E. van den Berg; D.C.J.J. Bergmans; D.I. van den Bersselaar; F.A. Bertens; A.J.G.H. Bindels; M.M. de Boer; S. den Boer; L.S. Boers; M. Bogerd; LDJB; MB; J.S. Breel; H. de Bruin; S. de Bruin; C.L. Bruna; L.A. Buiteman-Kruizinga; O. Cremer; R.M. Determann; W. Dieperink; D.A. Dongelmans; H.S. Franke; M.S. Galek-Aldridge; M.J. de Graaff; L.A. Hagens; J.J. Haringman; S.T. van der Heide; P.L.J. van der Heiden; N.F.L. Heijnen; S.J.P. Hiel; L.L. Hoeijmakers; L. Hol; M. W. Hollmann; M.E. Hoogendoorn; J. Horn; R. van der Horst; E.L.K. Ie; D. Ivanov; N.P. Juffermans; E. Kho; E.S. de Klerk; A.W.M.M Koopman-van Gemert; M. Koopmans; S. Kucukcelebi; M.A. Kuiper; D.W. de Lange; N. van Mourik; S.G. Nijbroek; M. Onrust; E.A.N. Oostdijk; FP; C.J. Pennartz; JP; L. Pisani; I.M. Purmer; T.C.D. Rettig; J.P Roozeman; M.T.U. Schuijt; MJS; ASN; M.E. Sleeswijk; M.R. Smit; P.E. Spronk; W. Stilma; A.C. Strang; AMT; P.R Tuinman; CMAV; F.L. Veen-Schra; L.I. Veldhuis; P. van Velzen; W.H. van der Ven; A.P.J. Vlaar; P. van Vliet; P.H.J. van der Voort; L. van Welie; H.J.F.T. Wesselink; H.H. van der Wier-Lubbers; B. van Wijk; T. Winters; W.Y. Wong; A.R.H. van Zanten.
Published online 7 July 2021
Supplemental digital content is available for this article.
References
- 1.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2014; 370:980. [DOI] [PubMed] [Google Scholar]
- 2.Brower RG, Matthay MA, Morris A, et al. Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 3.Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299:646–655. [DOI] [PubMed] [Google Scholar]
- 4.Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299:637–645. [DOI] [PubMed] [Google Scholar]
- 5.Cavalcanti AB, Suzumura EA, Laranjeira LN, et al. Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial I. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 2017; 318:1335–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Constantin JM, Jabaudon M, Lefrant JY, et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med 2019; 7:870–880. [DOI] [PubMed] [Google Scholar]
- 7.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med 2020; 46:2200–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med 2020; 8:1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haudebourg AF, Perier F, Tuffet S, et al. Respiratory mechanics of COVID-19- versus non-COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med 2020; 202:287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020; 46:1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bos LD, Paulus F, Vlaar APJ, et al. Subphenotyping ARDS in COVID-19 patients: consequences for ventilator management. Ann Am Thorac Soc 2020; 17:1161–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beenen LFM, Bos LD, Scheerder MJ, et al. Extensive pulmonary perfusion defects compatible with microthrombosis and thromboembolic disease in severe Covid-19 pneumonia. Thromb Res 2020; 196:135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan E, Beitler JR, Brochard L, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med 2020; 8:816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alhazzani W, Moller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med 2020; 48:e440–e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsolaki V, Siempos I, Magira E, et al. PEEP levels in COVID-19 pneumonia. Crit Care 2020; 24:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rello J, Storti E, Belliato M, et al. Clinical phenotypes of SARS-CoV-2: implications for clinicians and researchers. Eur Respir J 2020; 55:2001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boers NS, Botta M, Tsonas AM, et al. PRactice of VENTilation in Patients with Novel Coronavirus Disease (PRoVENT-COVID): rationale and protocol for a national multicenter observational study in The Netherlands. Ann Transl Med 2020; 8:1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botta M, Tsonas AM, Pillay J, et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med 2020; 9:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yehya N, Harhay MO, Curley MAQ, et al. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med 2019; 200:828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120:c179–c184. [DOI] [PubMed] [Google Scholar]
- 22.Imai K, Ratkovic M. Covariate balancing propensity score. J R Stat Soc B 2014; 76:243–263. [Google Scholar]
- 23.Grasselli G, Cattaneo E, Florio G, et al. Mechanical ventilation parameters in critically ill COVID-19 patients: a scoping review. Crit Care 2021; 25:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med 2021; 47:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robba C, Ball L, Battaglini D, et al. Early effects of ventilatory rescue therapies on systemic and cerebral oxygenation in mechanically ventilated COVID-19 patients with acute respiratory distress syndrome: a prospective observational study. Crit Care 2021; 25:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800. [DOI] [PubMed] [Google Scholar]
- 27.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ball L, Robba C, Maiello L, et al. Computed tomography assessment of PEEP-induced alveolar recruitment in patients with severe COVID-19 pneumonia. Crit Care 2021; 25:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Akker JP, Egal M, Groeneveld AB. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care 2013; 17:R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joannidis M, Forni LG, Klein SJ, et al. Lung-kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med 2020; 46:654–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srisawat N, Lawsin L, Uchino S, et al. Cost of acute renal replacement therapy in the intensive care unit: results from The Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) study. Crit Care 2010; 14:R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabarre P, Dumas G, Dupont T, et al. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med 2020; 46:1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts LN, Bramham K, Sharpe CC, et al. Hypercoagulability and anticoagulation in patients with COVID-19 requiring renal replacement therapy. Kidney Int Rep 2020; 5:1377–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 2020; 18:1738–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 2020; 18:1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maier CL, Truong AD, Auld SC, et al. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet 2020; 395:1758–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.