Abstract
Background:
Pulmonary outcome of premature neonates has focused more on short-term than long-term respiratory morbidities.
Objective:
Describe risk factors/biomarkers associated with short-term [Bronchopulmonary dysplasia (BPD) (supplemental oxygen use at 36 weeks post-menstrual age (PMA))] and longer-term [Chronic Respiratory Morbidity (CRM) (respiratory related symptoms, medications, medical/emergency visits, hospitalizations at 6–12 months corrected gestational age (CGA)] respiratory outcomes in a longitudinal cohort.
Design/Methods:
Neonates born at 24–29 weeks gestation were prospectively followed to 6–12 months CGA. Associations between clinical and laboratory risk factors/biomarkers of BPD and CRM were explored.
Results:
Of 86 subjects, 94% survived. Outcomes were available for 89% at 36 weeks PMA (BPD present in 42% of infants) and 72% at 6–12 months CGA (CRM present in 47% of infants). For the 54 infants with known outcomes for both BPD and CRM, diagnoses were discordant in 41%. BPD was associated with lower birthweight and birthweight z-score for GA, lower Apgar scores, more surfactant doses, higher SNAPPE-II scores, highest day 1 inspired oxygen concentration, day 7 oxygen use, prolonged ventilatory support, bacteremia, necrotizing enterocolitis, and treated patent ductus arteriosus. CRM was associated with lower Apgar scores, day 7 oxygen use and higher urine vascular endothelial growth factor. Patterns of plasma and urine lipid oxidation products differed in the two outcomes.
Conclusion:
In this hypothesis generating and exploratory study, BPD and CRM were associated with different risk factors/biomarker patterns. Concordance between these two outcomes was weak. Strategies for reducing CRM should be studied in cohorts identified by appropriate early risk factors/biomarkers.
Keywords: Bronchopulmonary dysplasia (BPD), Chronic Respiratory Morbidity (CRM), Epidemiology, Neonatal lung disease, Neonatal pulmonary, Preterm neonates
Introduction
Bronchopulmonary dysplasia (BPD) [use of supplemental oxygen at 36 weeks post-menstrual age (PMA)] is thought to be strongly associated with abnormal longer-term respiratory outcomes in and beyond the first year of life.1,2 BPD is a significant public health problem impacting 10,000 preterm US neonates annually3,4. Despite improvements in respiratory care practices and survival and in extremely low gestational age newborns, the prevalence of BPD continues to increase5. Longer term respiratory outcomes have been studied using multiple different outcome measures and definitions including Chronic Respiratory Morbidity (CRM)6, Post-Prematurity Respiratory Disease (PRD)7 and Chronic Pulmonary Insufficiency of Prematurity (CPIP)8. While CPIP was adopted by an FDA working group in an attempt to standardize definitions and outcome measures for clinical trials8, this term has created some confusion since it was previously used to describe another type of premature lung disorder. For the purposes of this report, the CRM outcome [defined as respiratory symptoms and medication use, medical/emergency visits, and hospitalizations at 6–12 months corrected gestational age (CGA)] as reported in our prior publication was used6. While the pathogenesis of BPD and CRM are not fully understood, multiple factors such as inflammation, oxidation, infection, and airway abnormalities appear to play key roles. Defining early clinical and laboratory-based risk factors and biomarkers that are definitively associated with short- and longer-term respiratory outcomes, such as BPD and CRM, is critical to understanding preterm lung pathophysiology and for developing preventive interventions.
Early clinical and laboratory-based risk-factors/biomarkers have been associated with BPD1. BPD’s definition has changed, with severity staging modified by duration and concentration of supplemental oxygen and need for positive pressure support9,10. Neonates with BPD are thought to be at higher risk for CRM and associated morbidities including asthma, repeated respiratory infections, use of asthma medications, increased medical visits and re‐hospitalizations, and later childhood pulmonary function testing abnormalities. These morbidities maintain the need for ongoing respiratory care of former preterm infants by Pediatricians.
Recent studies suggest some BPD definitions are poor predictors of CRM2,6. Preterm neonates with or without BPD can develop CRM and not all neonates with BPD go on to develop CRM. Our goal was to better understand the relationship between these two conditions. We conducted a multisite prospective, longitudinal, observational cohort study in 86 preterm neonates to explore clinical and laboratory-based risk factors and biomarkers associated with BPD and/or CRM.
Materials and Methods
Eligibility and Enrollment:
Gestational age (GA) 24 0/7 – 28 6/7 weeks and birth weight ≥ 500 grams neonates admitted to Brigham and Women’s Hospital (BWH), Boston, MA (Site 1), Tufts Medical Center, Boston, MA (Site 2), Ohio State University, Columbus OH (Site 3) and King’s College Hospital, London, England (Site 4) were enrolled in this observational study by 72 hours of life. GA was based on early obstetrical sonography in conjunction with last menstrual period dating, or Ballard examination if dates or sonogram were unavailable or inconsistent. Potential subjects were excluded for: 5-minute Apgar score ≤ 3, major congenital anomaly, serious maternal or neonatal infection at the time of birth, enrollment in a therapeutic intervention trial, or parents/guardians were unwilling or unable to complete study activities after discharge. Informed consent was obtained after protocol approval by each site’s institutional review boards (IRB).
Data Collection:
Maternal history and delivery room course were obtained on enrollment. Medication use, vital signs, growth parameters, respiratory support (median number of days intubated on mechanical ventilation [High Frequency (HFV), Conventional Ventilation (CV)] or extubated but on positive end expiratory pressure [CPAP and Non-Invasive Positive Pressure Ventilation (NIPPV)] , daily highest inspired oxygen concentration (FiO2)), and nutritional support were prospectively collected. Initial disease severity was assessed by the Score for Neonatal Acute Physiology II (SNAPPE-II) 11. Head sonograms were obtained on days 7 and 28. At discharge, medical complications were documented. After discharge, parents were contacted monthly through 6 months and at 12 months to characterize respiratory status. Outcome data were not available on all neonates transferred to non-study site facilities for convalescent care. Data were maintained in a REDCap database12,13.
Specimen Collection for Early Biomarkers:
Placental tissue, urine, and plasma were collected by 24 hours of life. A single Pathologist (CS) blinded to the clinical course examined the placental tissue. Evidence for acute intra-amniotic infection (staining for tumor necrosis factor - alpha (TNF-α) for active infection/inflammation), chronic placental inflammation (viral infection, maternal immune dysfunction), fetal-placental vascular pathology, and maternal uteroplacental vascular pathology was recorded14,15. Given the proposed role of oxidant injury to cellular lipids, proteins, and DNA in the lung by reactive oxygen species, plasma was evaluated for biomarkers of inflammation and oxidation. Markers of lipid oxidation were measured in urine using liquid chromatography/mass spectroscopy (LC/MS) including 8-iso-prostaglandin F2α (PGF2α), 2,3-dinor-8-iso-PGF2α, PGF2α, thromboxane B2 (TXB2), 11-dehydro-TBXB2, 2,3-dinor T6XB2 and 15-deoxy-Δ12,14-prostaglandin J2 (PGJ2)16–18. Protein and DNA oxidation was assessed by LC/MS19, including o-tyrosine (o-tyr), nitrotyrosine (3-NO-tyr), chlorotyrosine (3-Cl-tyr), 3-hydroxytyroine (3-OH-tyr), 8-hydroxy dexoyguanosine, (8OHdG,) and phenylalanine. Protein carbonyls (abcam, ab126287) were assessed by enzyme linked immunosorbent assay (ELISA). Urine was analyzed for vascular endothelial growth factor (VEGF) and Bombesin-like peptide (BLP), as previously reported by our group, based on observed association of these biomarkers with BPD20,21.
BPD Definition and Physiologic O2 Challenge:
BPD was defined as supplemental oxygen use at 36 weeks PMA and by oxygen challenge testing22. Subjects requiring any ventilatory support or receiving FiO2 ≥ 0.3 to keep oxygen saturations (SpO2) ≥ 90% were considered as having BPD without further testing. Challenged subjects falling to <SpO2 90% in room air were also considered to have BPD.
Respiratory Questionnaires:
NICHD SUPPORT trial parental questionnaires assessing longer-term respiratory outcomes were administered at 6 – 12 months CGA23 [clinical status, medication use, medical and Emergency Room (ER) visits, hospital re-admissions (frequency and etiology), wheezing or coughing with intercurrent illness and environmental exposure (smoking, pets, allergens)].
Respiratory Diary:
Between the 5th and 6th months CGA, parents completed a daily respiratory diary for 28 consecutive days documenting the presence and severity of respiratory symptoms and ongoing treatments. Wheeze and/or cough or the use of asthma medications for ≥ 2 days per week, for 3 consecutive weeks out of a 4-week period defined the presence of CRM. Asthma medications included relievers (ß-agonists) and preventers (corticosteroids, leukotriene modifiers, theophylline, aminophylline, cromolyn, nedocromil)6. A similar CRM definition utilitzing other concurrently collected survey assessments (phone interview, in person interview, questionnaire) of the same clinical information between 6–12 months CGA was used as a proxy for the diary if the diary was not returned.
BPD definition:
BPD was defined as use of supplemental oxygen and/or positive pressure support at 36 weeks PMA. No distinction was made between NIH consensus definition groups ‘moderate’ and ‘severe’ and the group without BPD included NIH consensus definition groups ‘no’ and ‘mild’ BPD9.
Monthly phone interview:
Parents were questioned monthly by phone about the infant’s clinical status, medication use, medical and ER visits, and hospital re-admissions and were compensated $25 US on a debit card for each interview.
Definition of CRM:
Although in attempts to standardize definitions and outcome measures for BPD, FDA has supported the use of CPIP, as proposed by Steinhorn et al8, for longer-term respiratory assessment, in the absence of a clear consensus on definition of that term and confusion from prior CPIP definitions24, we chose to continue using the term previously described by our group, CRM. We integrated data from the respiratory diary, monthly phone interviews, and respiratory questionnaires at 6 and 12 months to assign a CRM diagnosis6,8. Data elements included respiratory symptoms, use of respiratory medications, medical or ER visits for respiratory problems, and respiratory hospitalizations. Self-reported information in respiratory diaries returned at 6 months CGA was the primary source used to establish whether CRM was present. CRM was defined as cough, wheeze, or use of respiratory medications for ≥ 2 days/week for 3 consecutive weeks of the 4 week period of diary documenetation6. If the diary was incomplete, telephone interviews or respiratory questionnaires from a minimum of 3 time points between 5 and 12 months CGA were substituted. If respiratory symptoms (cough, wheeze), use of respiratory medications, a medical or ER visit for respiratory problems, or a respiratory hospitalization occurred in 2 of the 3 time points, the subject was classified as having CRM6. The algorithm for diagnosis of CRM based on non-diary data (in subjects for whom the diary was not completed) was constructed based on the principles used for the diary data.
SNAPE-II:
Six data elements collected by 12 hours after birth were combined 11. Urine output was averaged over 12 hours. The worst score in each category was recorded. Total score range = 0 – 115.
Sample Size and Statistical Analysis:
A sample size of 85 was chosen based on the anticipated CRM rate generated by the parental diaries and questionnaires previously described6. Since the study was exploratory in nature, the sample size was not powered to detect any specific association. This is consistent with the goal of the study being hypothesis-generating and to help suggest areas of future research in this population. SAS v9.4 (Cary, NC) was used for data analysis with two-sided tests and a significance level ≤ 0.05. Comparisons of characteristics between BPD and CRM positive and negative groups were examined using chi-square or Fisher’s Exact tests for categorical variables and Student t-tests or Wilcoxon Rank Sum Test for continuous measures. Concordance between the BPD and CRM outcomes was evaluated using a kappa coefficient.
Results
Demographics
Eighty-eight subjects were consented of which 2 were withdrawn (Site 1 =16, Site 2=30, Site 3=28, Site 4 =12). Eighty-one (95%) subjects survived (shown in Figure 1). One death ocurred after 36 weeks PMA but prior to discharge. The racial composition of the cohort was 59% Caucasian, 22% African-American, 10% Asian, and 8% other. Outcome data were available on 72 of the surviving subjects (89%) at 36 weeks PMA and 58 of the surviving subjects (72%) at 6–12 months CGA. The CRM outcome was based on respiratory diary data in 43 subjects and concurrent non- diary data in 15 subjects (only 5 of which had CRM). Of the 30 subjects with a diagnosis of CRM based primarily on the diary, there was 92% concordance with a “no CRM” assignment by non-diary based data in the same subjects, supporting the use of the alternative algorithm to substitute for missing diary data. BPD was diagnosed in 42% and CRM in 47% of these subjects. Median GA, sex, multiple gestation, race and maternal education were not associated with either BPD or CRM. Lower birthweight and birthweight z-score for GA were associated with BPD but not CRM (Table 1).
Fig. 1.
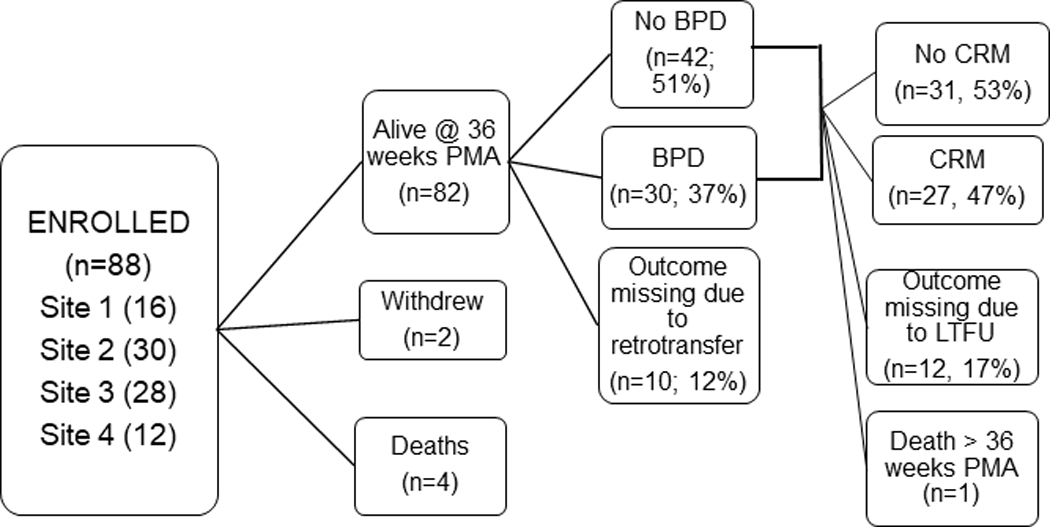
Consort Diagram
Table 1.
Demographics (bold p ≤ 0.05)
| Death (n=5) | BPD (n=72) | CRM (n=58) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Yes | No | p-value | n | Yes | No | p-value | ||
| Site and Enrollment | n (%) | n (%) | n (%) | n (%) | |||||
| Harvard (n=16) | 1 | 4 (13) | 11 (26) | 7 (26) | 6 (19) | ||||
| Tufts (n=30) | 0 | 9 (30) | 20 (48) | 14 (52) | 13 (42) | ||||
| Ohio (n=28) | 2 | 14 (47) | 8 (19) | 4 (15) | 6 (19) | ||||
| London (n=12) | 2 | 3 (10) | 3 (7) | 2 (7) | 6 (19) | ||||
| Total Enrolled (n=86) | 30(42) | 42(58) | 27(47) | 31(53) | |||||
| Female | 72 | 13 (43) | 21 (50) | 0.58 | 58 | 10 (37) | 18 (58) | 0.11 | |
| Multiple Gestation | 72 | 15 (50) | 17 (40.5) | 0.42 | 58 | 12 (44) | 19 (61) | 0.20 | |
| Caucasian | 67 | 18 (60) | 17 (46) | 0.25 | 53 | 13 (54) | 13 (45) | 0.50 | |
| Mother Completed High School | 72 | 29 (97) | 40 (95) | >0.99a | 58 | 27 (100) | 30 (97) | >0.99a | |
| Median Gestational Age (weeks) (IQR) | 72 | 26.2 (24.9, 28) | 27.3 (26.1, 28) | 0.10b | 58 | 26.2 (24.9, 28) | 27.3 (26.1, 28) | 0.41b | |
| Median Birthweight (grams) (IQR) | 72 | 769 (690, 890) | 998 (855, 1178) | 0.002b | 58 | 835 (690, 1125) | 914 (815, 1170) | 0.59b | |
| Mean Birthweight z-score for GA (SD) | 72 | 0.13 (0.78) | 0.29 (0.67) | 0.02 | 58 | 0.13 (0.81) | 0.11 (0.64) | 0.92 | |
exact p-value
Wilcoxon (non-parametric)
IQR = Interquartile Range
SD = Standard Deviation
CRM = Chronic Respiratory Morbidity
Associations of antenatal variables with short (BPD) and long-term respiratory outcomes (CRM):
Late prenatal care, illicit drug use, maternal diabetes mellitus, maternal hypertension, abrutio placenta, chorioamnionitis, maternal antibiotics within 72 hours of delivery, any antenatal corticosterioids and/or magnesium sulfate, prolonged premature rupture of membranes, and maternal BMI were not associated with either BPD or CRM. Neither smoking in the home or parental chronic respiratory disease were associated with either BPD or CRM. Labor was associated with a lower risk of BPD, but had no significant association with CRM (Table 2).
Table 2:
Antenatal and Delivery Room Risk Factors (bold = p ≤ 0.05)
| OUTCOME |
||||||||
|---|---|---|---|---|---|---|---|---|
| BPD |
CRM |
|||||||
| Yes | No | p-value | Yes | No | p-value | |||
| n | n (%)/IRQ | n (%)/IRQ | n | n (%)/IRQ | n (%)/IRQ | |||
| Received Prenatal Care | 72 | 30 (100) | 40 (95) | 0.51a | 58 | 27 (100) | 29 (94) | 0.494a |
| Illicit Drug Use During Pregnancy | 72 | 5 (17) | 7 (17) | >0.99 | 58 | 3 (11) | 3 (10) | >0.99a |
| Any Maternal Diabetes | 69 | 2 (7) | 3 (7.5) | >0.99a | 56 | 1 (4) | 2 (7) | >0.99a |
| Any Maternal Hypertension | 72 | 3 (10) | 2 (5) | 0.64a | 58 | 0 | 3 (10) | 0.241a |
| Abruptio Placentae | 71 | 4 (13) | 1 (2) | 0.15a | 57 | 0 | 1 (3) | >0.99a |
| Pre-Term Labor During Admission | 69 | 19 (63) | 34 (87) | 0.02 | 55 | 4 (16) | 6 (20) | 0.74a |
| Chorioamnionitis | 72 | 5 (17) | 8 (19) | 0.80 | 58 | 4 (15) | 8 (26) | 0.30 |
| Maternal Antibiotics 72 Hrs Prior to Delivery | 70 | 14 (48) | 24 (58.5) | 0.40 | 57 | 12 (44) | 18 (60) | 0.24 |
| PPROM | 52 | 7 (37) | 13 (39) | 0.86 | 44 | 15 (71) | 13 (57) | 0.30 |
| Any ACS | 72 | 29 (97) | 41 (98) | >0.99a | 58 | 27 (100) | 31 (100) | - |
| Most Recent Course of BMZ | 61 | 0.68a | 53 | >0.99a | ||||
| None | 1 (4) | 1 (3) | 0 | 0 | ||||
| Partial | 6 (26) | 7 (18) | 5 (21) | 6 (21) | ||||
| Complete | 16 (70) | 30 (79) | 19 (79) | 34 (79) | ||||
| Any Mag Sulfate | 67 | 13 (48) | 25 (62.5) | 0.24 | 57 | 13 (48) | 18 (60) | 0.37 |
| Any Mag Sulfate or Tocolytics | 67 | 27 (100) | 40 (100) | - | 57 | 27 (100) | 30 (100) | - |
| Median maternal pre-pregnancy BMI (IQR) | 66 | 25.8 (20.2, 30.4) | 25.5 (22.9, 29.5) | >0.99b | 53 | 28.6 (23.0, 31.4) | 25.2 (22.7, 28.6) | 0.14b |
| C-Section | 71 | 22 (76) | 31 (74) | 0.85 | 57 | 20 (77) | 20 (64.5) | 0.31 |
| Intubated in Delivery Room | 72 | 27 (90) | 36 (86) | 0.73a | 58 | 23 (85) | 27 (87) | >0.99a |
| Median 1 Minute Apgar (IQR) | 72 | 4.5 (2, 5) | 6 (4, 8) | 0.004b | 58 | 5 (2, 6) | 7 (5, 8) | 0.01b |
| Median 5 Minute Apgar (IQR) | 72 | 7 (6, 8) | 8 (7, 8) | 0.009b | 58 | 7 (5, 8) | 8 (7, 8) | 0.03b |
| Smoking in Home | 62 | 2 (8) | 1 (3) | 0.56a | 49 | 1 (4) | 1 (4) | >0.99a |
| Any Parental Chronic Respiratory Disease | 62 | 9 (36) | 13 (35) | 0.94 | 49 | 8 (32) | 9 (37.5) | 0.69 |
exact p-value
Wilcoxon (non-parametric)
BMZ = Betamethasone
IQR = Interquartile Range
BMI = Body Mass Index
CRM = Chronic Respiratory Morbidity
Delivery room management:
Lower 1 and 5 minute Apgar scores were associated with both BPD and CRM (Table 2).
Pathologic and biochemical biomarkers:
Placental or umbilical cord inflammmation, vasculopathy (fetal or maternal), or presence of iron deposits were not associated with either BPD or CRM (E -Supplemental Table 1)25. Plasma 2,3-dinor-8-iso-PGF2α, PGF2 α, 11-dehydro-TXB2, 2,3-dinor TXB2, o-tyrosine (o-tyr) and chlorotyrosine (3-Cl-tyr) and urine PGJ2 were below limits of detection. No measurable plasma or urine biomarkers of DNA, lipid, or protein oxidation correlated with BPD with the exception of urine 11-dehydro-TXB2. Higher serum 8-isoPGF2 and urine 2,3-dinor-8-iso-PGF2α, and higher urine VEGF levels were associated with CRM (Table 3, Supplemental Figures 1,2 and 3).
Table 3.
Laboratory Biomarkers of BPD and CRM (bold p ≤ 0.05)
| BPD | CRM | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Mean (SD) | Mean (SD) | |||||||
| n | Yes | No | p-value | n | Yes | No | p-value | |
| PLASMA OXIDANTS | ||||||||
| 3-NO-tyr pmol/mL | 53 | 1.54 (4.92) | 0.70 (1.12) | 0.45 | 50 | 0.50 (0.39) | 0.69 (1.18) | 0.43 |
| 8-OHdG pmol/mL | 53 | 8.04 (19.59) | 2.01 (3.27) | 0.18 | 50 | 6.76 (18.46) | 1.81 (1.46) | 0.22 |
| 3-OH-tyr nmol/mL | 53 | 0.33 (0.22) | 0.32 (0.15) | 0.89 | 50 | 0.30 (0.19) | 0.35 (0.16) | 0.39 |
| phenylalanine mmol/mL | 53 | 0.35 (0.15) | 0.42 (0.20) | 0.15 | 50 | 0.43 (0.21) | 0.38 (0.17) | 0.38 |
| 8-iso-PGF2α pmol/mL | 39 | 1.45 (1.19) | 1.25 (0.80) | 0.55 | 33 | 1.73 (1.09) | 1.06 (0.75) | 0.05 |
| TXB2 pmol/mL | 39 | 20.10 (55.79) | 14.25 (23.04) | 0.69 | 33 | 25.33 (55.63) | 15.43 (25.10) | 0.51 |
| 15-deoxy-D12,14 PGJ2 pmol/mL | 39 | 0.06 (0.07) | 0.07 (0.05) | 0.65 | 33 | 0.06 (0.05) | 0.06 (0.04) | 0.90 |
| Protein Carbonyls pmol/mg pro | 64 | 160.09 (65.80) | 152.01 (59.69) | 0.62 | 53 | 168.69 (65.18) | 141.67 (60.90) | 0.13 |
| URINE OXIDANTS | ||||||||
| o-tyr pmol/mL051220/mgCr | 55 | 54.32 (81.95) | 51.29 (90.64) | 0.90 | 43 | 44.11 (87.92) | 25.55 (31.91) | 0.38 |
| 3-NO-tyr (pmol/mL)/mgCr | 55 | 5.35 (2.72) | 7.90 (11.24) | 0.23 | 43 | 5.84 (2.34) | 4.80 (1.70) | 0.11 |
| 3-Cl-tyr (pmol/mL)/mgCr | 55 | 1.47 (1.54) | 3.59 (8.38) | 0.17 | 43 | 1.83 (0.99) | 1.69 (1.30) | 0.69 |
| 8-OHdG (pmol/mL)/mgCr | 55 | 3.28 (2.09) | 7.62 (19.50) | 0.22 | 43 | 3.42 (2.22) | 2.86 (1.83) | 0.38 |
| 3-OH-tyr (nmol/mL)/mgCr | 55 | 0.36 (0.26) | 0.34 (0.27) | 0.79 | 43 | 0.34 (0.17) | 0.29 (0.16) | 0.32 |
| phenylalanine (nmol/mL)/mgCr | 54 | 322.58 (316.54) | 373.18 (316.33) | 0.56 | 43 | 346.83 (243.08) | 312.83 (304.87) | 0.69 |
| 2,3-dinor-8-iso-PGF2α (pmol/mL)/mgCr | 50 | 4.54 (2.62) | 3.92 (2.39) | 0.41 | 41 | 5.09 (2.50) | 3.40 (2.33) | 0.04 |
| 8-iso-PGF2α(pmol/mL)/mgCr | 50 | 0.24 (0.20) | 0.23 (0.13) | 0.76 | 41 | 0.27 (0.19) | 0.20 (0.11) | 0.17 |
| PGF2α/(pmol/mL)/mgCr | 50 | 0.44 (0.38) | 0.39 (0.33) | 0.67 | 41 | 0.47 (0.45) | 0.35 (0.24) | 0.31 |
| 11-dehydro-TXB2/(pmol/mL)/mgCr | 50 | 1.49 (2.10) | 0.47 (0.51) | 0.05 | 41 | 1.02 (2.06) | 1.30 (2.37) | 0.69 |
| TXB2/(pmol/mL)/mgCr | 50 | 0.19 (0.17) | 0.25 (0.55) | 0.59 | 41 | 0.19 (0.15) | 0.28 (0.62) | 0.51 |
| 2,3-dinor TXB2/(pmol/mL)/mgCr | 50 | 0.54 (0.49) | 0.43 (0.42) | 0.42 | 41 | 0.46 (0.42) | 0.44 (0.45) | 0.87 |
| URINE FACTORS/PEPTIDES | ||||||||
| VEGF pg/mgCr | 68 | 47.15 (47.57) | 33.20 (23.51) | 0.15 | 55 | 39.43 (23.13) | 26.26 (22.29) | 0.04 |
| GRP ng/mgCr | 68 | 2.51 (4.96) | 1.20 (1.02) | 0.17 | 55 | 1.47 (1.42) | 2.01 (2.91) | 0.38 |
Postnatal care and complications:
The number of surfactant doses, pharmacologic PDA treatment, culture proven sepsis, necrotizing enterocolitis, higher SNAPPE-II scores, highest FiO2 on day of life 1, need for oxygen on day of life 7, and higher median number of days needing ventilatory support were associated with an increased risk of BPD. In contrast, only day of life 7 oxygen was associated with CRM. Use of supplemental oxygen at 28 days was not associated with either BPD or CRM (Table 4).
Table 4.
Postnatal Care and Complications (bold = p ≤ 0.05)
| OUTCOME | ||||||||
|---|---|---|---|---|---|---|---|---|
| BPD | CRM | |||||||
| Yes | No | p-value | Yes | No | p-value | |||
| n | n (%) | n (%) | n | n (%) | n (%) | |||
| CPAP or Positive Pressure Ventilation DOL1 | 71 | 29 (100) | 42 (100) | - | 58 | 27 (100) | 31 (100) | - |
| Highest Type of Positive Pressure DOL1 | 71 | 58 | ||||||
| NIPPV/CPAP | 1 (3) | 7 (17) | 0.13a | 2 (7) | 4 (13) | 0.68b | ||
| HFV/CV | 28 (97) | 35 (83) | 25 (93) | 27 (87) | ||||
| Any Surfactant Given | 71 | 28 (97) | 37 (88) | 0.39b | 57 | 28 (90) | 24 (92) | >0.99a |
| Median Number of Surfactant Doses (IQR) | 71 | 2 (1, 2) | 1 (1, 2) | 0.03b | 56 | 1.5 (1, 2) | 1 (1, 1) | 0.31b |
| Received Steroids for BPD | 70 | 3 (11) | 0 | 0.06a | 57 | 1 (4) | 1 (3) | >0.99a |
| Pneumothorax | 66 | 0 | 3 (8) | 0.26a | 50 | 0 | 3 (12) | 0.23a |
| PDA | 72 | 19 (63) | 23 (55) | 0.47 | 58 | 18 (67) | 16 (53) | 0.31 |
| Treatment for PDA (among those with PDA) | 72 | 0.02a | 58 | 0.75a | ||||
| None | 0 | 6 (26) | 3 (17) | 4 (25) | ||||
| Medical | 13 (68) | 10 (43.5) | 9 (50) | 8 (50) | ||||
| Surgical | 2 (11) | 0 | 2 (11) | 0 | ||||
| Medical and Surgical | 4 (21) | 7 (30) | 4 (22) | 4 (25) | ||||
| Evidence of IVH or PVL on head US | 72 | 6 (20) | 11 (26) | 0.54 | 58 | 6 (22) | 10 (32) | 0.39 |
| Indomethacin < 24 Hours for Neuroprophylaxis | 72 | 5 (17) | 4 (9.5) | 0.48a | 58 | 4 (15) | 2 (6) | 0.40a |
| Any Culture Proven Infections | 70 | 13 (45) | 9 (22) | 0.04 | 57 | 8 (30) | 7 (23) | 0.59 |
| Baby Received Breast Milk In First 28 Days | 70 | 24 (83) | 40 (98) | 0.08a | 57 | 25 (93) | 28 (93) | >0.99a |
| Treatment of Proven NEC | 62 | 0.01a | 58 | 0.35a | ||||
| None | 25 (83) | 42 (100) | 26 (96) | 29 (94) | ||||
| Yes, Without Surgery | 3 (10) | 0 | 1 (4) | 0 | ||||
| Yes, With Surgery | 2 (7) | 0 | 0 | 2 (6) | ||||
| Median SNAPPE-II Score (IQR) | 62 | 42 (29, 52) | 24 (17, 32) | 0.0003b | 46 | 42 (24, 49) | 24 (19, 37) | 0.13b |
| Median FiO2 Highest DOL1 (IQR) | 69 | 0.40 (0.30, 0.60) | 0.30 (0.28, 0.50) | 0.05b | 57 | 0.36 (0.30, 0.50) | 0.37 (0.30, 0.65) | 0.70b |
| Supplemental Oxygen by Hood, CPAP, or Ventilator DOL1 | 72 | 30 (100) | 39 (93) | 0.26a | 58 | 27 (100) | 30 (97) | >0.99a |
| Supplemental Oxygen by Hood, CPAP, or Ventilator DOL7 | 72 | 25 (83) | 26 (62) | 0.05 | 58 | 24 (89) | 21 (68) | 0.05 |
| Median HFV and CV Days (IQR) | 71 | 25 (6, 44) | 2 (1, 11) | <0.0001b | 56 | 14 (3, 39) | 5 (1, 11) | 0.08b |
| Median NIPPV and CPAP Days (IQR) | 61 | 35.5 (28.5, 44) | 25 (11, 33) | 0.002b | 51 | 35.5 (23.5, 42) | 28 (14, 33) | 0.06b |
| Median Days of Parenteral Nutrition (IQR) | 69 | 18.5 (9.5, 27.5) | 13 (10, 16) | 0.06b | 57 | 14 (10, 23) | 15 (10, 23) | >0.99b |
| Supplemental Oxygen by Hood, CPAP, or Ventilator DOL28 | 69 | 34 (77) | 23 (59) | 0.12 | 56 | 20 (77) | 20 (67) | 0.40 |
| CPAP or Positive Pressure Ventilation at Discharge | 57 | 1 (4) | 0 | 0.48a | 45 | 1 (5) | 0 | 0.42a |
| Discharged Home | 72 | 18 (60) | 24 (57) | 0.81 | 58 | 18 (67) | 20 (64.5) | 0.86 |
exact p-value
Wilcoxon (non-parametric)
IRQ = Interquatlie Range
DOL = Day of Life
NIPPV = Non-invasive Positive Pressure Ventilation
CV= conventional ventilation
HFV = High Frequency Ventialtion
NEC=Necrotizing Enterocolitis
BPD=Bronchopulmonary Dysplasia
PDA = Patent Ductus Arteriosus
PVL=Periventricular Leukomolacia
IVH = Intraventricular Hemorrhage
CPAP = Continuous Positive Airway Pressure
CRM = Chronic Respiratory Morbidity
US = ultrasound
Concordance of BPD and CRM:
While these diagnoses were in agreement for 59% of subjects (subjects developed both BPD and CRM (37%) or neither BPD or CRM (22%)), there was substantial discordance (41%) in individual subjects between the diagnoses [i.e., BPD present but no CRM (13%); no BPD and CRM developed (28%)]. Specifically, we demonstrated that 56% of subjects who developed CRM did not have BPD, 37% of subjects with BPD did not develop CRM, and 57% of subjects without BPD ultimately developed CRM. The kappa statistic was low [0.1852 (95% CI: −0.0651, 0.4355)] suggesting only slight agreement between BPD and CRM (Table 5).
Table 5:
Concordance between BPD and CRM. There were 54 babies with values for both outcomes. The kappa (0.185) shows that agreement between the two outcomes is poor, with the lower bound of the confidence actually below 0 (i.e. agreement less than expected by chance). Simple Kappa Coefficient = 0.1852 (95% CI −0.065, 0.4355)
| CRM | ||||
|---|---|---|---|---|
|
| ||||
| No | Yes | Total | ||
| BPD | No | 20 (37%) | 15 (28%) | 35 |
| Yes | 7 (13%) | 12 (22%) | 19 | |
| Total | 27 | 27 | 54 | |
Discussion
The use of BPD as a short-term clinical predictor of CRM (the more important long term respiratory outcome) is suboptimal. These discrepancies suggest the need for a careful re-examination of the outcomes used in intervention trials for prevention and/or treatment of chronic respiratory morbidity in preterm newborns. Although reported associations between BPD and CRM have been inconsistent, respiratory outcome at 12 month CGA is most important, as it portends respiratory status in early childhood6. The unique early CRM biomarkers identified here add to a sparse literature on CRM predictors. Because inflammation and oxidation have been implicated in the pathogenesis of lung injury, serum and urine hydroperoxides and total oxidation products of proteins (carbonyl formation), DNA (8-deoxyguanine), and lipids (isoprostanes) were analyzed 18. While none of the plasma biomarkers of protein, lipid or DNA oxidation were associated with BPD, one urine lipid oxidation product was more elevated in BPD subjects, and both serum and different urine lipid oxidation biomarkers were elevated in subjects who developed CRM. Failure of BPD and CRM to share the same patterns of clinical risk factors or laboratory biomarkers suggests different etiologies or processes may be important.
Previously, we had found associations of urinary BLP and VEGF levels with BPD 20,21. Although neither analyte had a strong association with BPD in the present study, higher VEGF levels were associated with CRM, implying that the course of early vascular development could impact long-term respiratory health. Increased SNAPPE-II scores were associated with an increased likelihood of BPD, but not CRM, supporting the possibility that early overall severity of illness might not increase CRM risk11. Only low Apgar scores and day 7 oxygen use were associated with both BPD and CRM. Ideally, a BPD diagnosis would identify neonates at highest risk of CRM who might benefit from earlier therapeutic interventions. Even using oxygen challenge testing26, we did not find that oxygen use at 36 weeks PMA was strongly predictive of CRM.
We believe that more attention should be focused on the early identification of neonates who will ultimately develop CRM27. Several clinical risk factors and laboratory biomarkers were associated with CRM. These biomarkers should be validated per the FDA and NIH BEST Biomarker qualification program. The NIH Prematurity and Respiratory Outcomes (PROP) trial sought to identify predictors of CRM in extremely low GA neonates1,7. Using similar surveys to define long-term [that PROP called post-prematurity respiratory disease (PRD)], they also observed strong associations between day of life 1 biomarkers and CRM. Although we found multiple clinical factors associated with BPD, most were not associated with CRM in our cohort. We also did not find the same strength in association between BPD and one-year CGA outcomes. The PROP study’s larger cohort size allowed for modeling on multiple variables, which may explain some of the differences in findings. While their conclusion that the fetal environment plays a significant role in long-term outcome was supported by our early biomarker findings, their proposition that genetic factors also contribute to respiratory outcomes has mixed support from the literature. Our own findings from twin pairs suggests that genetics does not significantly influence BPD risk in preterm neonates of this GA28.
Limitations of our one-year trial include the small sample size and potential bias from loss to follow-up. There was no significant difference in the gestational age, birthweight, Apgar scores, or SNAPPE II scores between the 28 lost-to follow-up subjects and those on whom data were available for analysis. Although analysis of a large number of variables could have introduced Type I error through finding random associations, many of our clinical biomarker findings (Table 2 and 4) support previously published observations.20,29.
Since the report of our randomized trial of intratracheal recombinant human superoxide dismutase (rhSOD) for prevention of BPD30 there has been discussion of how well BPD predicts CRM. In that trial, while rhSOD did not alter the BPD rate, at one year CGA there were significant reductions in respiratory medication use, emergency room visits, and hospital readmissions. Clinical trials for reducing long-term respiratory morbidity in premature neonates have continued to focus on BPD as an outcome. The findings of this study highlight the need to design clinical trials that focus on the best clinical and laboratory predictors of CRM 31–33.
Conclusions
BPD and CRM were associated with different patterns of early clinical risk factors and laboratory biomarkers. BPD did not correlate well with the development of CRM and CRM developed in many neonates without BPD. While elevated lipid peroxidation products have been reported in BPD, they have not yet been associated with CRM34. We found that increased lipid peroxidation biomarkers in both plasma and urine and elevated urine VEGF levels were associated with CRM in a different pattern from BPD. CRM is a more clinically meaningful endpoint to families, clinicians, and regulators. Identifying early risk factors and biomarkers associated with CRM rather than BPD may identify the most clinically relevant neonates for intervention trials of therapies aimed at improving long-term respiratory outcomes.
Supplementary Material
Supplemental Fig. 1: Box and whisker chart of serum 8-isoPGF2α levels from day one of life in infants who develop and do not develop BPD or CRM.
Supplemental Fig. 2 Box and whisker chart of urine 2,3-dinor-8-iso-PGF2α levels from day one of life in infants who develop and do not develop BPD or CRM.
Supplemental Fig. 3 Box and whisker chart of urine VEGF levels from day one of life in infants who develop and do not develop BPD or CRM.
Bullet points:
What is the key message? BPD and CRM were associated with different risk factors/biomarkers and agreement between these two outcomes was weak.
What does it add to the existing literature? Most literature on preterm neonatal lung disease focuses on short-term outcomes (BPD). Our data add to gaps in knowledge about biomarkers associated with long-term respiratory morbidity and their differences from predictors of BPD.
What is the impact? Therapies to improve long-term respiratory outcomes should be studied in neonates with associated risk factors/biomarkers.
Acknowledgement
We acknowledge the important contributions of Dr. Robert Castile, now retired, who supported data and sample collections of subjects enrolled at the Columbus, Ohio site. The authors have no conflicts of interest to declare.
Funding Sources
This project, submitted under a collaborative effort of the Harvard, Tufts and Ohio State’s NIH Clinical and Translational Science Awards (CTSAs), was granted CTSA supplemental funding under an RFA entitled: Research on Outcome Measures for Pediatric Clinical Trials in Support of the Best Pharmaceuticals for Children Act through NCRR and NICHD in the priority area: Neonatology response outcomes (Tufts CTSA UL1RR025752 ARRA supplement 1 C76HF15064–01-00 and BWH CTSA Grant # UL1RR025758). Biostatistical support was also provided through the Tufts CTSA award UL1TR002544.
Footnotes
Data Availability Statement
The consent forms approved for this trial included a statement that assured parents that the data collected on mother and child would only be visible to the study team. If questions arise regarding the source data or there is a request for additional analysis, we will make an effort to revisit the data that will be stored at the Tufts study center.
Statement of Ethics
Institutional Review Board approval was obtained at all sites and informed consent was obtained from the parents of all participants.
References
- 1.Keller RL, Feng R, DeMauro SB, et al. Bronchopulmonary Dysplasia and Perinatal Characteristics Predict 1-Year Respiratory Outcomes in Newborns Born at Extremely Low Gestational Age: A Prospective Cohort Study. J Pediatr 2017;187:89–97.e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lefkowitz W, Rosenberg SH. Bronchopulmonary dysplasia: pathway from disease to long-term outcome. J Perinatol 2008;28(12):837–840. [DOI] [PubMed] [Google Scholar]
- 3.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: Final Data for 2017. Natl Vital Stat Rep 2018;67(8):1–50. [PubMed] [Google Scholar]
- 4.Van Marter LJ. Epidemiology of bronchopulmonary dysplasia. Semin Fetal Neonatal Med 2009;14(6):358–366. [DOI] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Bell EF, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015;314(10):1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parad RB, Davis JM, Lo J, et al. Prediction of respiratory outcome in extremely low gestational age infants. Neonatology 2015;107(4):241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maitre NL, Ballard RA, Ellenberg JH, Davis SD, Greenberg JM, Hamvas A, Pryhuber GS;Prematurity and Respiratory Outcomes Program. Respiratory consequences of prematurity: evolution of a diagnosis and development of a comprehensive approach. J Perinatol 2015. May;35(5):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinhorn R, Davis JM, Göpel W, et al. Chronic Pulmonary Insufficiency of Prematurity: Developing optimal endpoints for drug development. J Pediatr 2017;191:15–21.e11. [DOI] [PubMed] [Google Scholar]
- 9.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116(6):1353–1360. [DOI] [PubMed] [Google Scholar]
- 10.Higgins RD, Jobe AH, Koso-Thomas M, et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J Pediatr 2018;197:300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr 2001;138(1):92–100. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basu J, Agamasu E, Bendek B, et al. Placental tumor necrosis factor-α protein expression during normal human gestation. J Matern Fetal Neonatal Med 2016;29(24):3934–3938. [DOI] [PubMed] [Google Scholar]
- 15.Baker AM, Braun JM, Salafia CM, et al. Risk factors for uteroplacental vascular compromise and inflammation. Am J Obstet Gynecol 2008;199(3):256.e251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hevko JM, Bowers RC, Murphy RC. Synthesis of 5-oxo-6,8,11,14-eicosatetraenoic acid and identification of novel omega-oxidized metabolites in the mouse macrophage. J Pharmacol Exp Ther 2001;296(2):293–305. [PubMed] [Google Scholar]
- 17.R H. Eicosanoids http://www.lipidmaps.org. Published 2007. Accessed.
- 18.Rogers LK, Tipple TE, Britt RD, Welty SE. Hyperoxia exposure alters hepatic eicosanoid metabolism in newborn mice. Pediatr Res 2010;67(2):144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orhan H, Vermeulen NP, Tump C, Zappey H, Meerman JH. Simultaneous determination of tyrosine, phenylalanine and deoxyguanosine oxidation products by liquid chromatography-tandem mass spectrometry as non-invasive biomarkers for oxidative damage. J Chromatogr B Analyt Technol Biomed Life Sci 2004;799(2):245–254. [DOI] [PubMed] [Google Scholar]
- 20.Levesque BM, Kalish LA, Winston AB, et al. Low urine vascular endothelial growth factor levels are associated with mechanical ventilation, bronchopulmonary dysplasia and retinopathy of prematurity. Neonatology 2013;104(1):56–64. [DOI] [PubMed] [Google Scholar]
- 21.Cullen A, Van Marter LJ, Allred EN, Moore M, Parad RB, Sunday ME. Urine bombesin-like peptide elevation precedes clinical evidence of bronchopulmonary dysplasia. Am J Resp Crit Care Med 2002;165(8):1093–1097. [DOI] [PubMed] [Google Scholar]
- 22.Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol 2003;23(6):451–456. [DOI] [PubMed] [Google Scholar]
- 23.Stevens TP, Finer NN, Carlo WA, et al. Respiratory outcomes of the surfactant positive pressure and oximetry randomized trial (SUPPORT). J Pediatr 2014;165(2):240–249.e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krauss AN, Klain DB, Auld PA. Chronic pulmonary insufficiency of prematurity (CPIP). Pediatrics 1975. January;55(1):55–8. PMID: 234187. [PubMed] [Google Scholar]
- 25.Drachenberg CB, Papadimitriou JC. Placental iron deposits: significance in normal and abnormal pregnancies. Hum Pathol 1994;25(4):379–385. [DOI] [PubMed] [Google Scholar]
- 26.Walsh MC, Yao Q, Gettner P, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114(5):1305–1311. [DOI] [PubMed] [Google Scholar]
- 27.Group F-NBW. BEST (Biomarkers, EndpointS, and other Tools) Resource In:2016. [PubMed] [Google Scholar]
- 28.Parad RB, Winston AB, Kalish LA, et al. Role of Genetic Susceptibility in the Development of Bronchopulmonary Dysplasia. J Pediatr 2018;203:234–241.e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taglauer E, Abman SH, Keller RL. Recent advances in antenatal factors predisposing to bronchopulmonary dysplasia. Semin Perinatol 2018;42(7):413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis JM, Parad RB, Michele T, et al. Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics 2003;111(3):469–476. [DOI] [PubMed] [Google Scholar]
- 31.Villamor-Martínez E, Pierro M, Cavallaro G, Mosca F, Kramer BW, Villamor E. Donor Human Milk Protects against Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Nutrients 2018;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villamor-Martinez E, Álvarez-Fuente M, Ghazi AMT, et al. Association of Chorioamnionitis With Bronchopulmonary Dysplasia Among Preterm Infants: A Systematic Review, Meta-analysis, and Metaregression. JAMA Netw Open 2019;2(11):e1914611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis JM, Pilon AL, Shenberger J, Breeze JL, Terrin N, Mazela J, Gulczynska E, Lauterbach R, Parad R. The role of recombinant human CC10 in the prevention of chronic pulmonary insufficiency of prematurity. Pediatr Res 2019. August;86(2):254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuligowski J, Aguar M, Rook D, et al. Urinary Lipid Peroxidation Byproducts: Are They Relevant for Predicting Neonatal Morbidity in Preterm Infants? Antioxid Redox Signal 2015;23(2):178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1: Box and whisker chart of serum 8-isoPGF2α levels from day one of life in infants who develop and do not develop BPD or CRM.
Supplemental Fig. 2 Box and whisker chart of urine 2,3-dinor-8-iso-PGF2α levels from day one of life in infants who develop and do not develop BPD or CRM.
Supplemental Fig. 3 Box and whisker chart of urine VEGF levels from day one of life in infants who develop and do not develop BPD or CRM.


