Abstract
Purpose:
Since eyes with center-involved diabetic macular edema (CI-DME) and good baseline visual acuity (VA) showed no difference in VA loss when managed initially with observation, laser, or aflibercept, understanding the estimated costs of these strategies to the US population is relevant for health care planning.
Methods, Setting, and Participants:
Total costs for managing participants with CI-DME and good baseline VA assigned to aflibercept (n= 236), laser (n=240), or observation (n = 236) during the 2-year trial were calculated. Using epidemiological data and extrapolating costs, 10-year costs for caring for persons with CI-DME and good baseline VA throughout the US was estimated.
Interventions:
Observation or laser groups initiated aflibercept if VA decreased. Aflibercept group received injections up to every 4 weeks.
Main Outcomes and Measures:
Estimated 10-year U.S. population costs to manage CI-DME with good VA.
Results:
Assuming all patients in the US with CI-DME and good baseline VA received aflibercept initially, 10-year costs were projected to be $28.80 billion compared with $14.42 billion if initially receiving laser treatment or $15.70 billion if initially observed, with aflibercept added if VA worsened in the laser or observation arms.
Conclusions and Relevance:
Similar VA outcomes on average are obtained by initially managing CI-DME and good baseline VA with laser or observation strategies instead of immediately using aflibercept. While any one of these three strategies might be warranted depending on an individual’s specific circumstances, on a societal level, cost savings might be achieved with these first two approaches.
Trial Registration:
ClinicalTrials.gov Identifier: NCT01909791
Graphical Abstract
Similar visual acuity outcomes on average are obtained by initially managing center-involved diabetic macular edema and good baseline visual acuity with laser or observation strategies instead of immediately using aflibercept. While any one of these three strategies might be warranted depending on an individual’s specific circumstances, on a societal level, cost savings might be achieved with these first two approaches.
Introduction
The DRCR Retina Network’s Protocol V trial showed no statistically significant differences in vision loss at two years comparing three different treatment strategies for eyes with center involved diabetic macular edema (CI-DME) and good baseline visual acuity (VA) (approximate Snellen equivalent 20/25 or better). The strategies included immediate receipt of intravitreous aflibercept compared with initial observation or initial focal/grid laser, each followed by aflibercept therapy only if VA declined by a pre-specified amount. Mean VA in each of the three treatment groups at two years was 20/20 and no substantial differences were identified in frequency of VA loss (16%-19%) at 2 years among the groups.
Since each treatment approach in Protocol V was effective and safe, we believe it is useful to evaluate the potential difference in costs to individual patients and the entire US population for employing these 3 strategies. This report provides estimates of costs for employing the DRCR Retina Network Protocol V strategies of initial aflibercept, laser, or observation for patients with CI-DME and good baseline VA to an individual patient and for the overall U.S. population over a 2-year period for which definitive data are known, and then extrapolated based on a range of assumptions to a 10-year time horizon as is typically done for policy planning.
Methods
The Protocol V randomized controlled trial was conducted at 91 clinical sites in the US and Canada. The protocol and statistical analysis plan are available with the primary outcome report.1 The study adhered to the tenets of the Declaration of Helsinki.2 The ethics board associated with each site provided approval. Participants provided written informed consent. Eligible participants had baseline best corrected VA of 20/25 or better using Electronic-Early Treatment Diabetic Retinopathy Study (E-ETDRS) testing3 and a thickened central subfield on optical coherence tomography4,5 with definite retinal thickening due to DME involving the center of the macula confirmed by the investigator on clinical exam.
The details of the treatment approaches for the three strategies have been reported elsewhere. 1 Eyes in the laser photocoagulation group received laser photocoagulation treatment at baseline with retreatment at 13-week intervals if indicated. Aflibercept injections were initiated for eyes in the laser photocoagulation or observation group if VA decreased from baseline by at least 10 letters (≥ 2 lines on an eye chart) at any visit or by 5-9 letters (1 line) at 2 consecutive visits. Eyes in the initial aflibercept group received aflibercept at baseline. The anti-VEGF retreatment regimen was identical in the aflibercept, laser, and observation groups (following loss of VA in the laser and observation groups). Aflibercept was given as frequently as every 4 weeks using protocol specified criteria based on changes in VA and central subfield thickness.
In the laser photocoagulation group and observation groups, follow-up occurred at 8 and 16 weeks and then every 16 weeks unless VA or CST worsened. If there was worsening, the visit schedule was reduced to 8 and then 4 weeks if worsening continued. In all groups, visits occurred every 4 weeks while injections were being given. Once injections were deferred twice, follow-up could be extended to 8 weeks and then 16 weeks provided VA and CST remained stable.
Population Modeling
Since Protocol V did not show superior outcomes when starting with aflibercept, laser, or observation, analyses were undertaken to evaluate all three scenarios for individuals with CI-DME and VA of 20/25 or better, corresponding to the treatment arms of Protocol V: 1) all individuals received aflibercept initially, 2) all received laser therapy initially with aflibercept for subsequent VA loss as occurred in Protocol V, and 3) all received observation initially with aflibercept for subsequent VA loss as occurred in Protocol V. Costs were calculated based on the Protocol V costs and extrapolated to population-wide longer-term outcomes. Resource utilization data were extracted from Protocol V outcomes. Number of visits, number of injections, number of laser treatments, and other diagnostic and therapeutic ophthalmic procedures at one and two years were determined based on data from the actual Protocol V trial. Medicare reimbursement was used to determine costs for injectables, procedures, facilities (when appropriate), and anesthesia (when appropriate) for procedures as unit costs as listed in Supplemental Table 1, then multiplied them by resource utilization to get total costs.
The trial collected outcomes for study participants for two years with extrapolations based on a range of plausible assumptions for years 3 through 10. For the main analyses, the only costs included beyond 2 years were clinic visits, OCTs, and aflibercept injections for CI-DME based on costs in year 2, since other trials in DME with follow-up beyond two years showed that visits, OCTs and anti-VEGF injections typically were closer in frequency to those noted in year 2 than in year 1.6 Thus, the extrapolated data assumed an average of 3 visits, 3 OCTs, and 0.5 injections per year in years 3 through 10 in each of the three treatment groups. Since these assumptions may be incorrect, and since the anti-VEGF injections account for most of the costs, this number of injections was varied in a sensitivity analysis to provide a range of costs when extrapolating data beyond two years through 10 years. Costs in the future were discounted by 3% annually in accordance with standards for economic analysis.7
Next, costs were extrapolated for the U.S. population from 2020-2029 by employing each of the 3 strategies to all patients with CI-DME with good VA. To do this, the prevalence of persons with diabetes in the US was estimated based on prevalence and incidence data from the 2020 CDC National Diabetes Statistics Report.8 We use the ranges (95% confidence intervals) on incidence and prevalence from that report to explore uncertainty in diabetes prevalence in sensitivity analysis. The future prevalence was based on initial prevalence in each year plus forecasted incident cases and subtracting mortality. Mortality is based on US mortality and adjusted for the relative risk of mortality in persons with diabetes (Supplemental Table 2). Supplemental Figure 1 shows the forecast of cases of prevalent, diagnosed diabetes from 2020-2029.
We then estimate the number of relevant persons who might receive treatment. To obtain prevalence estimates of all persons with CI-DME with good baseline VA, we estimate the number of persons with clinically significant DME, then estimate the fraction with CI-DME, and then the fraction with visual acuity 20/25 or better. We assumed that 3.8% of people with diabetes in 2020 started with clinically significant DME as defined in those population-based studies.9 Then, based on the LALES (a Latino population) and WESDR (a White population) studies,10,11 1.4% of people with diabetes were assumed to develop clinically-significant DME each year. Of those with clinically-significant DME, we assumed 70% had CI-DME. This percentage was based on prevalence from the ETDRS cohort demonstrating that of 3199 eyes with clinically-significant DME, 2253 (70.4%) had CI-DME on fundus photos (personal communication A. Glassman), and that other investigations have suggested that the proportion of eyes diagnosed as having DME or CSME on monocular fundus photographs with no DME based on OCT CST are balanced by the number of eyes diagnosed as not having DME or CSME on monocular fundus photographs having DME on OCT.12 Among eyes with CI-DME, 40% were assumed to have visual acuity 20/25 or better, based on the ETDRS cohort having 896 of 2253 eyes (39.7%) with CI-DME had VA 20/25 or better (personal communication A. Glassman). See Supplemental Table 1 for additional assumptions for population long-term modeling.
Results
Eyes in the initial aflibercept group completed a mean of 5.81 injections, 0.04 lasers, and 4.21 additional visits at which treatment did not occur over the 2 years of the trial. Eyes in the initial laser group completed an average of 0.70 injections, 1.30 lasers, and 3.80 additional visits at which treatment did not occur; and eyes in the initial observation group completed an average of 1.32 injections, 0.01 lasers, and 5.06 additional visits at which treatment did not occur visits.
Per-Person Costs
Combining costs for visits, aflibercept injections, laser treatment, OCTs, and costs of other ophthalmic procedures, the 2-year cost for an individual with CI-DME and good VA is $17,330 when initiating management with aflibercept, $6,917 when initiating management with observation, and $5,885 when initiating management with laser (Table 1). In the initial aflibercept injection group, 92% of the cost incurred over 2 years was the cost associated with injections (OCT, aflibercept, plus injection fee); 80% of the observation group cost and 64% of the laser group costs were injection-associated costs because of decreased VA.
Table 1:
Annual Per-Person Costs by Treatment Group and Cost Category 2019 US Dollars (Standard Error)
| Intravitreous Injection Procedure + Drug* | Laser | Clinic Visits without Treatment | Other Costs | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Group | Year 1* | Year 2 | Total‡ | Year 1 | Year 2 | Total* | Year 1 | Year 2 | Total* | Year 1 | Year 2 | Total | Year 1 | Year 2 | Total* |
| Aflibercept | $11596 (335) | $4460 (360) | $15926 (566) | $23 (8) | $25 (8) | $47 (13) | $554 (16) | $618 (16) | $1154 (24) | $110 (55) | $96 (41) | $203 (203) | $12283 (338) | $5199 (363) | $17330 (568) |
| Laser | $1397 (266) | $2412 (336) | $3739 (488) | $744 (19) | $107 (17) | $848 (28) | $500 (12) | $595 (16) | $1077 (22) | $147 (90) | $75 (30) | $221 (221) | $2788 (280) | $3189 (338) | $5885 (509) |
| Observation | $2630 (324) | $2994 (368) | $5537 (595) | $7 (4) | $15 (6) | $21 (10) | $666 (14) | $609 (18) | $1256 (25) | $27 (16) | $77 (31) | $102 (102) | $3330 (322) | $3694 (372) | $6917 (600) |
7% of the introvitreous injection procedure cost is for the procedure and optical coherence tomography and 93% is for the drug.
Other costs include diagnostic and therapeutic ophthalmic procedures not included as part of visits, optical coherence tomography, study anti-vascular endothelial growth factos injections, or laser treatments.
Two-year totals combine the years but discounting costs in year 2 by 3%.
Given the reduction in number of injections from year 1 to year 2 in the aflibercept group, the overall costs (standard error) decreased from the first year from $12,283 ($338) to $5,199 ($363) in the second year. Given the increase in number of injections between years 1 and 2 in the observation and laser groups, the costs increased by approximately $400 in each group between years 1 and 2 of the actual trial (Table 1).
Population Costs
If all patients in the US with CI-DME and 20/25 or better VA over the next ten years were to initiate treatment with aflibercept as used in the trial, the 10-year costs from 2020 through 2029 are projected to be $23.61 billion (Table 2, Figure 1). This compares with $12.25 billion if these patients initiated management with laser or $13.28 billion if they initiated management with observation, assuming aflibercept was added for vision loss in these latter two groups.
Table 2:
U.S. Population Costs
| Total Annual Costs (billions) | Differences (billions) | |||||
|---|---|---|---|---|---|---|
| Aflibercept | Laser | Observation | A-L | O-L | A-O | |
| 2020 | $2.03 billion | $0.80 billion | $0.91 billion | $1.24 billion | $0.11 billion | $1.12 billion |
| 2021 | $2.10 billion | $0.92 billion | $1.03 billion | $1.18 billion | $0.11 billion | $1.07 billion |
| 2022 | $2.25 billion | $1.05 billion | $1.16 billion | $1.20 billion | $0.11 billion | $1.09 billion |
| 2023 | $2.44 billion | $1.20 billion | $1.31 billion | $1.24 billion | $0.11 billion | $1.13 billion |
| 2024 | $2.62 billion | $1.35 billion | $1.46 billion | $1.27 billion | $0.11 billion | $1.16 billion |
| 2025 | $2.80 billion | $1.50 billion | $1.61 billion | $1.31 billion | $0.12 billion | $1.19 billion |
| 2026 | $2.99 billion | $1.65 billion | $1.77 billion | $1.34 billion | $0.12 billion | $1.22 billion |
| 2027 | $3.17 billion | $1.80 billion | $1.92 billion | $1.37 billion | $0.12 billion | $1.25 billion |
| 2028 | $3.35 billion | $1.95 billion | $2.08 billion | $1.40 billion | $0.13 billion | $1.28 billion |
| 2029 | $3.54 billion | $2.10 billion | $2.23 billion | $1.44 billion | $0.13 billion | $1.31 billion |
| Cumulative Undiscounted* | $27.29 billion | $14.30 billion | $15.48 billion | $12.99 billion | $1.17 billion | $11.82 billion |
| Cumulative Discounted** | $23.61 billion | $12.25 billion | $13.28 billion | $11.35 billion | $1.03 billion | $10.33 billion |
A: Aflibercept; L: Laser; O: Observation
Sum of the costs from 2020 through2029.
Discounts cost from 2021 through 2029 by 3% for each year in the future and then sums them together.
Figure 1:
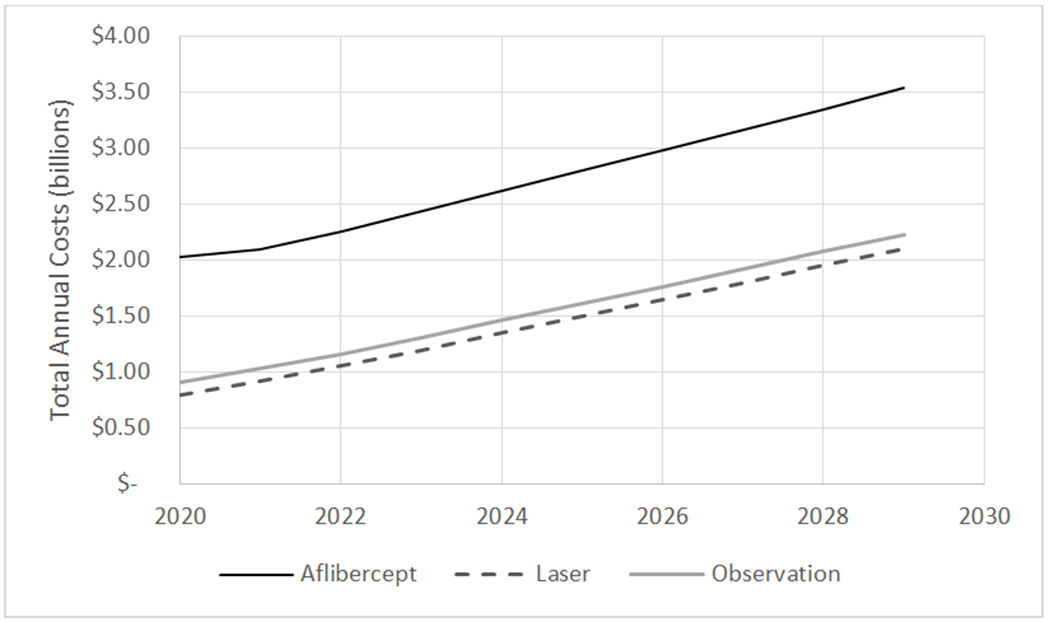
The figure represents the total annual cost in billions if all patients in the U.S. with center-involved DME (diabetic macular edema) and good visual acuity were managed initially with aflibercept, laser, or observation. Annual costs are estimated for the 10 year period from 2020 through 2029 based on assumptions about diabetes, center-involved DME with good visual acuity, and treatment patterns similar to the Protocol V trial. Supplemental Table 2 includes assumptions about prevalence and incidence of diagnosed diabetes, the prevalence and incidence of those with diabetes having center-involved DME, and the proportion of center-involved DME that is 20/32 or worse. Table 1 includes information on per-person treatment costs from Prtocol V.
Sensitivity Analyses
In sensitivity analyses, if aflibercept injections (cost of injection plus drug) theoretically become less costly, the overall societal cost of initial management with aflibercept becomes less costly (Figure 2). The cost of initial management with laser or observation also decreases because patients undergoing those management strategies also receive aflibercept injections when VA decreases. The price per aflibercept injection would have to be less than $0 (costs of drug alone) for initial management of CI-DME with aflibercept to be less costly than initial management with laser.
Figure 2:
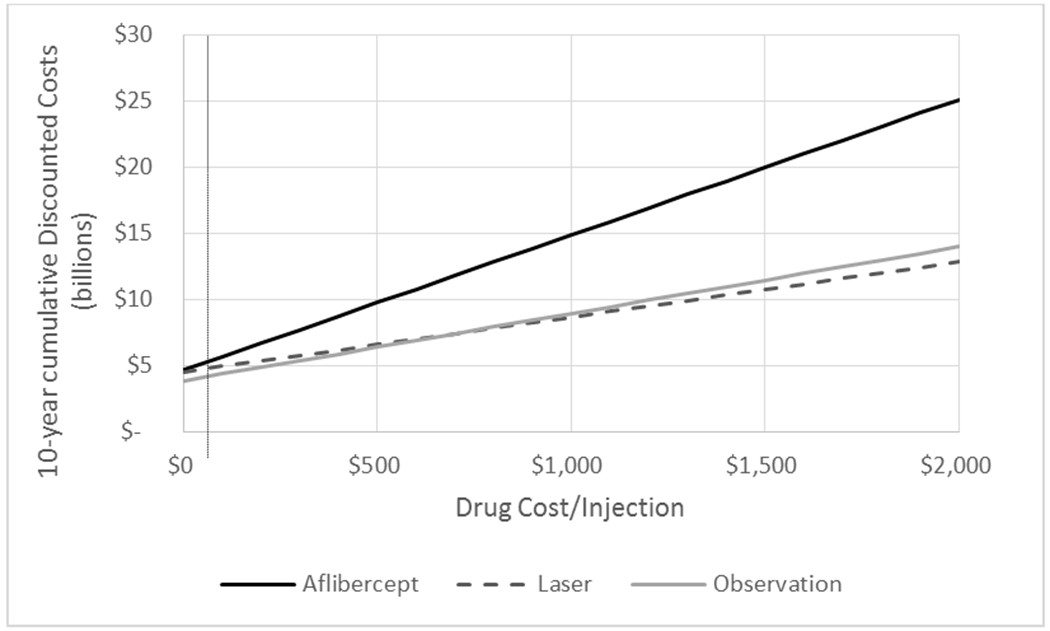
The figure represents the 10-year total cumulative costs in billions for different assumptions of costs of aflibercept, including if aflibercept cost $0. The vertical dotted line represents a cost per injection similar to to bevacizumab ($63.72). The total cost is based on assumptions about diabetes, center-involved DME with good visual acuity, and treatment patters similar to the Protocol V trial. It assumes all patients in the U.S. with center-involved DME and good visual acuity were managed initially with aflibercept, laser, or observation respectively. Future costs are discounted by 3% annually.
Varying the number of aflibercept injections in years 3 through 10 could make a substantial difference in societal costs (Supplemental Figure 2). Although costs dropped, even if there were 0 annual injections beyond 3 years, aflibercept still had a $19.01 billion in cumulative discounted costs over a ten year period. Initial management with laser and observation were still less costly than initial management with aflibercept as long as laser or observation did not require substantial increases in the number of annual aflibercept injections in the long-term (>1.25 injections more/year in 3 through 10) (Supplemental Figure 2).
The number of visits and OCTs in years 3 through 10 did not make a substantial difference on societal costs (Supplemental Figure 3).
If we use the lower and higher estimates of diabetes incidence and prevalence, the total costs are slightly lower and higher (Supplemental Tables 3a and 3b). We see the 10-year cumulative costs of aflibercept range between 9.62 and 13.11 billion more expensive than laser.
Discussion
The DRCR Retina Network Protocol V trial demonstrated that initiating management with either aflibercept, laser, or observation are all reasonable approaches from the perspective of vision outcomes in an eye with CI-DME and good baseline VA. Therefore, given that each aflibercept injection costs $1,850, not unexpectedly, this report demonstrates that initial management with aflibercept is more expensive for an individual participant than initial management with either laser or observation. This report, also, provides estimates when that cost is extrapolated to the overall U.S. population with CI-DME and good VA not just for 2 years but out to 10 years, estimating that initial treatment with aflibercept costs approximately $23.61 billion compared with approximately $13.28 billion for observation and $12.25 billion for laser.
Since costs across countries can vary, these analyses include supplemental tables to facilitate making estimates when varying costs. In sensitivity analyses, as aflibercept injections theoretically are made less costly, while the overall societal cost of initial management of CI-DME with aflibercept becomes less costly (Figure 2), the cost of initial management of CI-DME with laser or observation also decreases because patients undergoing those management strategies also receive aflibercept injections when VA decreases. If bevacizumab was used at a cost of $63.72 per injection (not including costs for OCT and injection procedure) instead of aflibercept, and the outcomes were similar to the results of protocol V, the 10 year projected costs would be $5.34 billion for anti-VEGF treatment compared to $4.82 billion for laser and $4.23 billion for observation (Figure 2). In addition, even if aflibercept injections were free, when considering the other costs associated with the aflibercept treatment strategy such as for the injection procedure, the aflibercept strategy remains more expensive than observation alone.
Of interest is whether the initial laser strategy, which had fewer eyes develop worsening VA with CI-DME to warrant initiating aflibercept over 2 years of follow-up in the trial compared with the observation arm, is a less costly strategy than the strategy of initial observation only for these eyes. In fact, these projections suggest that over 10 years the strategy of initially employing laser could result in savings of $1.03 billion compared with initial observation. However, given the various assumptions used for these calculations, there is uncertainty in assuming the laser strategy would provide substantial cost savings compared with the observation strategy.
Study Limitations
These results are limited by the many assumptions needed to estimate the number of individuals with center-involved DME from U.S. population studies (e.g., NHANES or LALES) or from U.S. multicenter clinical trials from the 1980s (e.g., the ETDRS) as well as the many assumptions needed to extrapolate data through two years out to ten years. Furthermore, the ETDRS used fundus photography to diagnosis DME, which may underestimate the prevalence of center-involved DME detectable by OCT.12 Also, population studies in the U.S. may not generalize throughout the rest of the world. The modeling of the population outcomes over 10 years also involves making assumptions about the longer-term prevalence and incidence of diabetes and center-involved DME as well as making assumptions about the longer-term (beyond 2 years) patterns of resource utilization. If those assumptions are incorrect, the actual population costs will be different. To try to address these limitations, several sensitivity analyses (supplemental figure 2–3) were conducted to understand how some of these assumptions might affect our conclusions. While the overall magnitude of costs could be substantially different from our base case results, when considering the uncertainty of the assumptions used by evaluating the boundaries of the sensitivity analyses, the overall conclusion still appear to hold. Specifically, giving aflibercept injections to patients with center-involved DME and good vision may lead to billions of additional population costs over the next 10 years. Nevertheless, the costs of any of these strategies may be justified within an individual with center-involved DME and good visual acuity depending on individual circumstances. Finally, this study is not a cost-effectiveness analysis. As such, it does not examine for potential improvements in visual acuity for which higher associated costs might be worthwhile.
Conclusion
Similar visual acuity outcomes on average are obtained by initially managing patients with CI-DME and good baseline VA with laser or observation strategies instead of immediately using aflibercept. While any one of these three strategies might be warranted depending on an individual’s specific circumstances, on a societal level, cost savings might be achieved with these first two approaches.
Funding/Support:
Research reported in this publication was supported by the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers UG1EY014231 and UG1EY023207. Regeneron provided aflibercept for the study and funds to DRCR Retina Network to defray the study’s clinical site costs. As per the DRCR.net Industry Collaboration Guidelines (available at www.drcr.net), the DRCR.net had complete control over the design of the protocol, ownership of the data, all editorial content of presentations and publications related to the protocol, and the decision to submit for publication.
Role of the Sponsor:
The funding organization (National Institutes of Health) participated in oversight of the conduct of the study and review of the manuscript but not directly in the design or conduct of the study, nor in the collection, management, analysis, or interpretation of the data, or in the decision to submit for publication or the preparation of the manuscript.
Conflict of Interest Disclosures:
Potential financial conflicts of interests and other financial disclosures:
Mr. Glassman reports grants from the National Eye Institute and Regeneron; Grants from Genentech outside of the submitted work.
Dr. Bressler reports grants from American Medical Association, Bayer, Novartis, Roche, and Samsung
Dr Sun reports grants from Roche Genentech, Juvenile Diabetes Research Foundations, Kalvista; Personal fees from Current Diabetes Reports, JAMA Ophthalmology, and Merck; as well as nom-financial support from Optovue, Roche Genentech and KalVista outside the submitted work.
Footnotes
Data Sharing Statement: The data collected for this study, including de-identified participant data and a data dictionary defining each field in the data set will be made available at https://public.jaeb.org/drcrnet/stdy.
Supplementary Material
References
- 1.DRCR Retina Network. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: A randomized clinical trial. JAMA. 2019;321(19):1880–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. [DOI] [PubMed] [Google Scholar]
- 3.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the Early Treatment of Diabetic Retinopathy Study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. [DOI] [PubMed] [Google Scholar]
- 4.Chalam KV, Bressler SB, Edwards AR, et al. Retinal thickness in people with diabetes and minimal or no diabetic retinopathy: Heidelberg Spectralis optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(13):8154–8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA. 2015;314(20):2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elman MJ, Ayala A, Bressler NM, et al. Intravitreal Ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122(2):375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-effectiveness in health and medicine. Oxford University Press; 2016. [Google Scholar]
- 8.Centers for Disease Control and Prevention. National diabetes statistics report 2020, Estimates of diabetes and its burden in the Unites States. 2020; https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf.
- 9.Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132(11):1334–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varma R, Paz SH, Azen SP, et al. The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmalogy. 2004;111:1121–1131. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy IV. Diabetic macular edema. Ophthalmology. 1984;91(12):1464–1474. [DOI] [PubMed] [Google Scholar]
- 12.Wang YT, Tadarati M, Wolfson Y, Bressler SB, Bressler NM. Comparison of Prevalence of Diabetic Macular Edema Based on Monocular Fundus Photography vs Optical Coherence Tomography. JAMA Ophthalmol. 2016;134(2):222–228. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Statistics About Diabetes. 2020; https://www.diabetes.org/resources/statistics/statistics-about-diabetes. Accessed 1/27/2020.
- 14.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Population health metrics. 2010;8(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol. 1989;107(2):244–249. [DOI] [PubMed] [Google Scholar]
- 16.Leske MC, Wu SY, Hennis A, et al. Incidence of diabetic retinopathy in the Barbados Eye Studies. Ophthalmology. 2003;110(5):941–947. [DOI] [PubMed] [Google Scholar]
- 17.Varma R, Choudhury F, Klein R, et al. Four-year incidence and progression of diabetic retinopathy and macular edema: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149(5):752–761 e751-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arias E, Xu J. United States Life Tables, 2017. National Vital Statistics Reports. 2017;68(7). [PubMed] [Google Scholar]
- 19.Gregg EW, Gu Q, Cheng YJ, Narayan KV, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Annals of internal medicine. 2007;147(3):149–155. [DOI] [PubMed] [Google Scholar]
- 20.Baker CW, Glassman AR, Beaulieu WT, et al. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. Jama. 2019;321(19):1880–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


