Abstract
BACKGROUND:
Critically ill coronavirus disease 2019 (COVID-19) patients have frequent thrombotic complications and laboratory evidence of hypercoagulability. The relationship of coagulation tests and thrombosis requires investigation to identify best diagnostic and treatment approaches. We assessed for hypercoagulable characteristics in critically ill COVID-19 patients using rotational thromboelastometry (ROTEM) and explored relationships of D-dimer and ROTEM measurements with thrombotic complications.
METHODS:
Critically ill adult COVID-19 patients receiving ROTEM testing between March and April 2020 were analyzed. Patients receiving therapeutic anticoagulation before ROTEM were excluded. Rotational thromboelastometry measurements from COVID-19 patients were compared with non−COVID-19 patients matched by age, sex, and body mass index. Intergroup differences in ROTEM measurements were assessed using t tests. Correlations of D-dimer levels to ROTEM measurements were assessed in COVID-19 patients who had available concurrent testing. Intergroup differences of D-dimer and ROTEM measurements were explored in COVID-19 patients with and without thrombosis.
RESULTS:
Of 30 COVID-19 patients receiving ROTEM, we identified hypercoagulability from elevated fibrinogen compared with non- COVID-19 patients (fibrinogen assay maximum clot firmness [MCF], 47 ± 13 mm vs. 20 ± 7 mm; mean intergroup difference, 27.4 mm; 95% confidence interval [CI], 22.1−32.7 mm; p < 0.0001). In our COVID-19 cohort, thrombotic complications were identified in 33%. In COVID-19 patients developing thrombotic complications, we identified higher D-dimer levels (17.5 ± 4.3 qg/mL vs. 8.0 ± 6.3 qg/mL; mean difference, 9.5 qg/mL; 95% CI, 13.9−5.1; p < 0.0001) but lower fibrinogen assay MCF (39.7 ± 10.8 mm vs. 50.1 ± 12.0 mm; mean difference, −11.2 mm; 95% CI, −2.1 to −20.2; p = 0.02) compared with patients without thrombosis. We identified negative correlations of D-dimer levels and ROTEM MCF in these patients (r = −0.61; p = 0.001).
CONCLUSION:
We identified elevated D-dimer levels and hypercoagulable blood clot characteristics from increased fibrinogen on ROTEM testing in critically ill COVID-19 patients. However, we identified lower, albeit still hypercoagulable, ROTEM measurements offibrino- gen in COVID-19 patients with thrombotic complications compared with those without. Further work is required to externally validate these findings and to investigate the mechanistic drivers for these relationships to identify best diagnostic and treatment approaches for these patients.
LEVEL OF EVIDENCE:
Epidemiologic, level IV.
Keywords: COVID-19, rotational thromboelastometry, hypercoagulability, fibrinogen, thrombosis
Critically ill patients with coronavirus disease 2019 (COVID-19) encounter marked elevations in D-dimer levels suggesting a hypercoagulable state.1,2 Higher mortality has been seen in severe cases with elevated D-dimer levels,3 which is hypothesized to be due in part to thrombotic complications. While D-dimer is sensitive for thrombosis, it is an acute phase reactant, and it is unclear whether elevations in D-dimer alone have adequate specificity for thrombosis in postinfectious settings of inflammation.4 In addition, D-dimer reflects the end product of fibrin clot degradation, rather than an initiating substrate/trigger for clot formation. While emerging evidence using viscoelastic coagulation assays like rotational thromboelastometry (ROTEM) have identified hypercoagulable blood clot characteristics from fibrinogen in COVID-19 patients,5 the relationship of hypercoagulable ROTEM measurements with thrombotic complications in these patients is unclear. Because of these limitations and uncertainties, current guidelines do not yet endorse empiric treatment intensity anticoagulation approaches for these patients.6
Clinical trials are currently underway investigating the utility of empiric antithrombotic7 and even fibrinolytic treatment8 approaches for COVID-19 patients. However, parallel investigations are required to identify whether laboratory assessments of hypercoagulability can risk stratify those who will go on to develop thrombotic complications. Consequently, we performed a pilot study to evaluate for hypercoagulable blood clot characteristics in critically ill COVID-19 patients using a whole blood viscoelastic coagulation assay: ROTEM (Instrumentation Laboratory, Bedford, MA). We compared these measurements to D-dimer levels and other traditional coagulation tests and additionally sought to explore the relationships of these measurements with thrombotic complications.
PATIENTS AND METHODS
Study Design and Participants
Consecutive, critically ill patients admitted with respiratory failure secondary to COVID-19 receiving clinical ROTEM testing at NewYork-Presbyterian Hospital: Columbia University Irving Medical Center between March and April 2020 were assessed. Patients younger than 18 years, those on therapeutic anticoagulation preceding or at the time of ROTEM testing, and those with a known history of thrombosis before ROTEM testing were excluded. This observational study was approved by the institutional review board. A separate institutional review board−approved study of non−COVID-19 patients admitted to the same hospital undergoing ROTEM testing was assessed as a comparator group.9 Patients from the non−COVID-19 cohort were surgical patients who had no known coagulation abnormalities. Non−COVID-19 patients were matched to COVID-19 patients based on age (±5 years), sex, and body mass index (±3 kg/m2).
Rotational Thromboelastometry
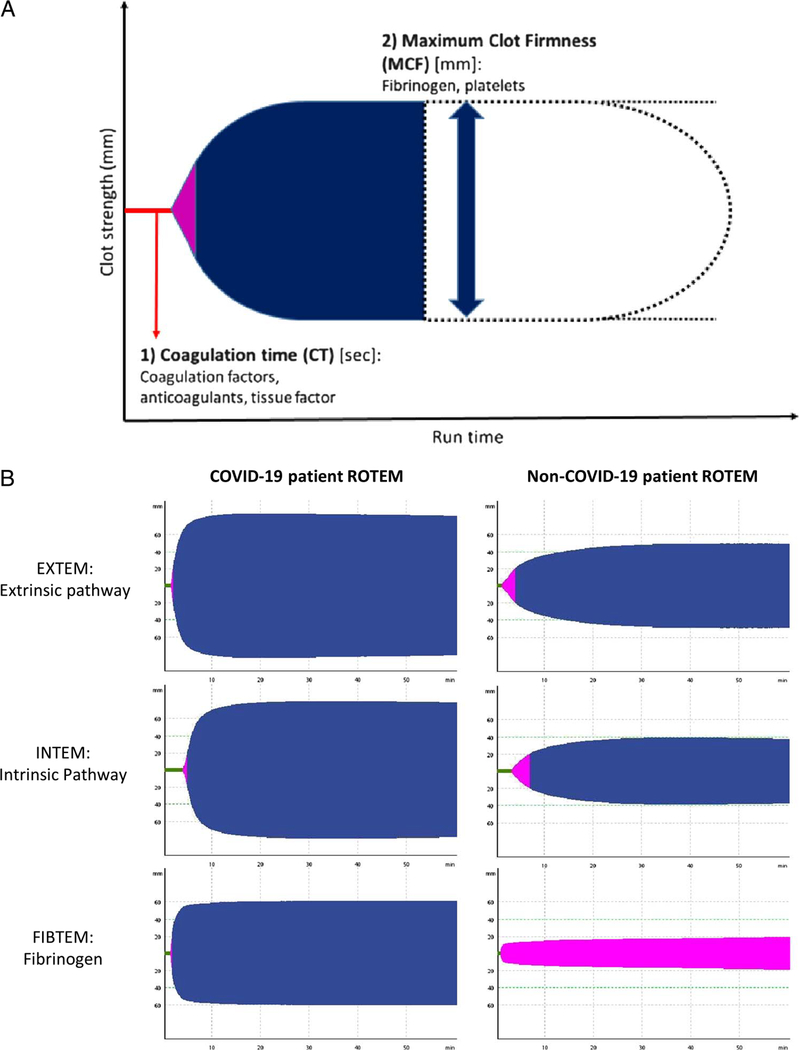
Rotational thromboelastometry is a Food and Drug Administration−approved, clinical functional coagulation test that uses whole blood. Unlike traditional plasma coagulation tests, which removes cellular components from testing, ROTEM evaluates cellular components (platelets, fibrinogen, erythrocytes), coagulation factors, and their interactions required to initiate, strengthen, and stabilize blood clotting. Subsequently, ROTEM assesses clot initiation kinetics (coagulation time [CT]) and clot strength characteristics (maximum clot firmness [MCF]) via developing blood clot under rotational shear conditions (Fig. 1A). Clinically relevant hypercoagulability can be identified in patients with elevated ROTEM MCF parameters two standard deviations above normal healthy control testing (above reference range), which has previously been associated with thrombotic clinical outcomes in non-COVID-19 patients.9
Figure 1.
Fibrinogen driven hypercoagulability in critically ill COVID-19 patients compared with non−COVID-19 patients detectable using ROTEM. (A) Rotational thromboelastometry assesses developing blood clot strength (y axis; millimeters) over time (x axis; seconds) via rotational shear conditions on a whole blood sample. Time for initial blood clot formation is assessed using CT. This is reflective of coagulation factor contribution to clotting kinetics in the extrinsic (EXTEM) or intrinsic (INTEM) pathway. Clot strength is assessed using MCF. This is reflective of fibrinogen and platelet contribution to clot strength. Increased MCF is indicative of hypercoagulable clot characteristics. A separate FIBTEM will assess whether clot strength abnormalities are driven by fibrinogen contribution to clot formation. (B) Rotational thromboelastometry tracing revealing evidence of significant hypercoagulabile clot characteristics driven by elevated fibrinogen contribution to blood clot strength in COVID-19 compared with non−COVID-19 patients.
Rotational thromboelastometry testing was performed on intensive care unit admission using 3 mL of whole blood drawn into a citrated tube and processed within 60 minutes of collection. Primary ROTEM measurements included clot strength characteristics (MCF) among the extrinsic pathway assay (EXTEM), intrinsic pathway assay (INTEM), and fibrinogen assay (FIBTEM). Secondary ROTEM measurements of interest included clot formation kinetics (CT), clot lysis (maximum lysis), and plasma/serum coagulation tests (prothrombin time, partial thromboplastin time [PTT], international normalized ratio [INR], D-dimer) drawn concurrently with ROTEM. Daily ROTEM calibration, verification, and operational checks were run to ensure validity.
Thrombotic Complications
Incident thrombosis was defined as a composite event comprising of deep vein thrombosis (DVT), pulmonary embolism (PE), ischemic stroke, and myocardial infarction (MI). Deep vein thrombosis, PE, and stroke were diagnosed via radiologic studies. Incident MI was diagnosed by the clinical treating team using troponin and electrocardiographic data. Presumptive diagnoses without radiologic confirmation were not included. All studies were performed at the clinical discretion of the treating team. All COVID-19 patients at the time of ROTEM testing were on thromboprophylaxis using either heparin or enoxaparin.
Statistical Analysis
Intergroup differences and 95% confidence intervals (CIs) were determined applying t tests for continuous variables and χ2 for categorical variables. Intergroup differences in ROTEM measurements of hypercoagulability (MCF) between COVID-19 compared with matched non−COVID-19 patients were investigated as primary outcomes. Intergroup differences of ROTEM measurements of coagulation kinetics (CT) and standard coagulation tests were investigated as secondary outcomes. Pearson correlation tests were performed to assess the relationship of D-dimer levels with ROTEM assessments of hypercoagulability (MCF) in COVID-19 patients with available concurrent laboratory data. Exploratory analyses were performed to assess intergroup differences and 95% CIs of ROTEM measurements of hypercoagulability (MCF) in COVID-19 patients with incident thrombosis compared with those without. Statistical significance was evaluated at p < 0.05. Analyses were performed using SPSS version 26 (IBM Corp., Armonk, NY).
RESULTS
A total of 30 critically ill COVID-19 patients were included in the analyses. The COVID-19 patient cohort had a mean ± SD age of 63 ± 12 years and a mean ± SD body mass index of 33 ±8.1 kg/m2. The COVID-19 and non-COVID-19 patients were well matched based on baseline characteristics. There were notable intergroup differences in nonmatched baseline medical comorbidities (Table 1). Using ROTEM, we identified that COVID-19 patients had hypercoagulable blood clot characteristics (ROTEM MCF) more frequently compared with non−COVID-19 patients (97% vs. 10%). When assessing ROTEM measurements as continuous variables, we identified significantly elevated clot strength measurements (MCF) in COVID-19 patients compared with non−COVID-19 patients (Table 1) with the largest intergroup differences seen in assays for fibrinogen contribution to clot strength (FIBTEM MCF: mean intergroup difference, 27.4 mm; 95% CI, 22.1–32.7; p < 0.0001) (Fig. 1B, Table 2). We did not identify a change in the relationship of COVID-19 with hypercoagulable ROTEM measurements when adjusting for baseline nonmatched medical comorbidities (FIBTEM mean intergroup difference, 27.8 mm; 95% CI, 22.6–32.9; p < 0.0001) in separate sensitivity analyses.
TABLE 1.
Baseline Characteristics of Critically Ill COVID-19 Patients Compared with Matched Non-COVID-19 Patients
| COVID-19 Patients (n = 30) | Non-COVID-19 Patients (n = 30) | |
|---|---|---|
| Age, mean (SD), y | 63 (12) | 62 (12) |
| Male, n (%) | 15 (50) | 15 (50) |
| BMI, mean (SD) | 33.0 (8.1) | 32.8 (7.8) |
| Medical history, n (%) | ||
| Hypertension | 23 (77) | 17 (57) |
| Diabetes | 15 (50) | 12 (40) |
| Congestive heart failure | 2 (7) | 0 (0) |
| Coronary artery disease | 2 (7) | 2 (7) |
| Dyslipidemia | 9(30) | 8 (27) |
| Laboratory coagulation testing at time of ROTEM, mean (SD) | ||
| INR | 1.2 (0.2) | 1.1 (0.2) |
| PTT, s | 35.1 (7.1) | 30.9 (7.1) |
| PT, s | 15.4 (1.4) | Na |
| Platelet count, 103/μL | 255 (103) | 200 (66) |
| D-dimer, μg/mL* | 255 (103) | 200 (66) |
Available in 25 of 30 COVID-19 patients.
BMI, body mass index.
TABLE 2.
ROTEM Intergroup Differences Between COVID-19 and Non-COVID-19 Patients
| Reference Ranges | COVID-19 Patients (n = 30) | Non–COVID-19 Patients (n = 30) | Intergroup Mean Difference (95% CI; p Value) | |
|---|---|---|---|---|
| EXTEM | ||||
| CT, mean (SD), s | 43–82 | 108 (54) | 57 (31) | 51.3 (28.5–74.2; p <0.0001) |
| MCF, mean (SD), mm | 52–70 | 75 (5) | 65 (8) | 10.0 (6.4–13.7; p < 0.0001) |
| Hypercoagulability (MCF, >70 mm), n (%) | 24 (80) | 5 (17) | ||
| INTEM | ||||
| CT, mean (SD), s | 122–208 | 205 (65) | 169 (57) | 36.6(10.1–63.1; p = 0.01) |
| MCF, mean (SD), mm | 51–72 | 76 (5) | 65 (9) | 11.1 (7.2–15.0; p <0.0001) |
| Hypercoagulability (MCF, >72 mm), n (%) | 18 (60) | 3 (10) | ||
| FIBTEM | ||||
| MCF, mean (SD), mm | 7–24 | 47 (13) | 20 (7) | 27.4 (22.1–32.7; p <0.0001) |
| Hypercoagulability (MCF, >24 mm), n (%) | 29 (97) | 3 (10) |
In secondary analyses comparing ROTEM measurements of functional coagulation between COVID-19 and non-COVID-19 patients, we identified slower coagulation kinetics in COVID-19 in both the extrinsic (EXTEM CT) and intrinsic (INTEM CT) pathways (Table 2). When looking at analogous traditional plasma coagulation tests for these ROTEM coagulation kinetic assessments, we similarly identified prolonged INR and slower PTT times in COVID-19 patients; however, the clinical significance of these intergroup differences were marginal compared with the larger differences seen using ROTEM. We also identified higher platelet count in COVID-19 patients (Table 1).
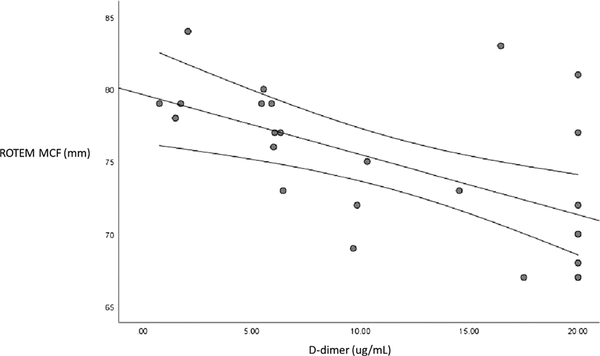
In our exploratory analyses investigating incident thrombotic complications in our COVID-19 cohort, we identified 10 patients (33%) who developed thrombotic complications. Of these patients, three had DVT, one had PE, one had DVT with PE, four had ischemic stroke, and one had DVT with ischemic stroke. No MI was identified. When comparing intergroup laboratory differences between COVID-19 patients with and without incident thrombotic complications, we identified higher D-dimer levels in those with thrombosis (17.5 ± 4.3 μg/mL vs. 8.0 ± 6.3 μg/mL; mean difference, 9.5 μg/mL; 95% CI, 13.9−5.1; p < 0.0001). Although 90% of COVID-19 patients with thrombosis had hypercoagulable FIBTEM MCF measurements, the FIBTEM MCF was lower in patients identified with thrombosis compared with those without thrombosis (39.7 ± 10.8 mm vs. 50.1 ± 12.0 mm; mean difference, −11.2 mm; 95% CI, −2.1 to −20.2; p = 0.02). Fibrinolysis (FIBTEM maximum lysis) was slightly increased in patients with thrombosis compared with those without; however, this was not statistically significant (median [interquartile range], 3.0% [0%−6%] vs. 0.5% [0%−5.5%]; p = 0.29). When comparing D-dimer and ROTEM measurements of hypercoagulability in a subgroup of COVID-19 patients with concurrent testing (n = 25), we identified a negative correlation between D-dimer levels and ROTEM MCF using Pearson bivariate correlation analyses (r = −0.61; p = 0.001) (Fig. 2).
Figure 2.
Correlation of D-dimer and ROTEM MCF in COVID-19 patients.
DISCUSSION
We identified marked hypercoagulability in critically ill COVID-19 patients driven by profound elevations in fibrinogen contribution to clot strength using ROTEM, which parallels European data.5 However, in addition to hypercoagulable clot strength characteristics, we also identified slower coagulation kinetics on ROTEM testing in COVID-19 patients compared with matched non-COVID-19 patients. While these findings were dissimilar to other reported cohorts of COVID-19 patients receiving ROTEM,5 the analogous plasma-based coagulation tests for EXTEM and INTEM CT (INR and PTT, respectively) similarly showed slower coagulation kinetics, which has been seen in other work.3,10
When exploring the clinical implications of our laboratory findings, we identified thrombotic complications in 33% of our COVID-19 cohort. Although these data were limited by diagnostic imaging acquisition bias (67% received thromboembolic diagnostic imaging), this reflects real-world clinical practice. In addition, our thrombotic complication prevalence appeared similar to what has been reported in other centers,11,12 further supporting the role that hypercoagulability can have on thrombosis in critically ill patients with COVID-19. Using traditional plasma coagulation tests and whole blood ROTEM testing, we additionally compared coagulation profiles of COVID-19 patients who did and did not encounter incident thrombotic complications. We expectedly identified higher D-dimer levels in those with thrombosis. However, while ROTEM measurements of clot strength (MCF) were elevated above established reference ranges demonstrating hypercoagulability in COVID-19 patients with thrombosis, the extent of hypercoagulability and fibrinogen contribution to clot strength (FIBTEM MCF) was paradoxically less than COVID-19 patients without thrombosis. Furthermore, there were negative correlations when comparing D-dimer and these ROTEM measurements of hypercoagulability. It is unclear whether these findings reflect observations from prior non–COVID-19 studies that similarly identified lower fibrinogen and elevated D-dimer levels in patients with documented venous thromboembolic complications.13 These prior studies have postulated that such findings could be driven by a consumptive process where fibrinogen becomes depleted with pathologic clot formation in conjunction with activated fibrinolytic processes, which drive elevations in D-dimer levels. While we did identify higher ROTEM measurements of fibrinolysis in COVID-19 patients with thrombosis compared with those without, these differences were clinically small and not statistically significant. More importantly is that these overall measurements of fibrinolysis were lower than what has been reported in reference populations. This may parallel prior work that has suggested lower overall levels of fibrinolysis in critically ill COVID-19 patients suggesting a “fibrinolysis shutdown” mechanism of hypercoagulability.14
Although the mechanism for this inverse relationship of D-dimer and fibrinogen in our data is unclear and requires further investigation, our data seem to suggest that D-dimer and fibrinogen are not interchangeable tests. While D-dimer and fibrinogen levels both conceptually test clot burden, their changes reflect different time points in the clotting process. It is notable that fibrinogen is a substrate of clot formation, and conversely, D-dimer reflects downstream fibrin clot degradation. Alterations of fibrinolytic processes between clot formation and breakdown could impact correlations of D-dimer and fibrinogen. Consequently, it is plausible that changes in fibrinogen (both increases and decreases) temporally precede changes in D-dimer. Although these explanations are speculative, our results appear to highlight limitations in using single time-point data or D-dimer results in isolation in identifying coagulopathy, which is temporally dynamic process. The mechanisms for our aforementioned hypercoagulable clot strength and prolonged coagulation kinetics in COVID-19 patients are also unclear. Further studies will be required to establish whether these findings are replicable on a larger scale and then to assess whether the mechanisms for these findings involve abnormal fibrinolytic (or antifibrinolytic) processes or dysregulated “propagation” of coagulation and fibrin formation.15–17 However, these collective findings using a more representative physiologic assessment of blood clotting via ROTEM appear to support the complex coagulopathy that has been suggested in prior studies using traditional plasma coagulation assessments.2,3,10
Although our study appears to support prior literature, our findings need to be interpreted with caution because of several limitations. In addition to our limitations of analyzing single time-point laboratory results, our intensive care unit biased cohort was prohibitive in assessing variations of functional coagulation in different stages of COVID-19 severity. Similarly, we did not have reliable symptom onset timing data to assess the impact of time from presentation on the coagulopathy seen in our cohort. Furthermore, the small sample size and small thrombotic outcome numbers prevented the ability to account for confounders in the analysis and prohibited an effective look at laboratory thresholds that could be used to predict thrombosis risk. We were also unable to match our COVID-19 patients to analogous non–COVID-19 controls with similar critical illness severity or medical comorbidities to identify whether COVID-19 influences hypercoagulability independent of these potential confounders. However, our work appears to replicate findings seen in other studies and the thromboinflammation that has been seen in COVID-19 compared with other viral illnesses, and we continued to identify this relationship after adjusting for medical comorbidities in additional sensitivity analyses. Finally, we did not have complete data on fibrinogen levels to compare this widely available test with our ROTEM results and D-dimer levels, limiting the generalizability of our findings.
Despite these limitations, our study appears to provide further support that fibrinogen plays a role in the coagulopathy and thrombotic complications that are seen in critically ill COVID-19 patients. The associations of fibrinogen on poor cardiovascular outcomes18 and causal mechanism with thrombosis19 are known in non–COVID-19 diseases. Subsequently, further work is required to study the relationship and changes of D-dimer and fibrinogen levels over time and their relationship with thrombotic outcomes in COVID-19 to best identify diagnostic and treatment strategies for these patients.
ACKNOWLEDGMENT
We thank all the frontline providers: nurses, ancillary staff, physicians, residents, and fellows who made this clinical and research effort possible.
DISCLOSURE
D.J.R. is supported by the National Blood Foundation Grant. He discloses consulting fees for Portola Pharmaceuticals. The authors declare no conflicts of interest.
Contributor Information
David J. Roh, Division of Critical Care and Hospitalist Neurology, Columbia University.
Katherine Eiseman, Department of Anesthesiology, Columbia University Irving Medical Center/NewYork-Presbyterian Hospital, New York, New York.
Hannah Kirsch, Division of Critical Care and Hospitalist Neurology, Columbia University.
Nina Yoh, Department ofNeurological Surgery, New York-Presbyterian Hospital and Vagelos College of Physicians and Surgeons, Columbia University.
Amelia Boehme, Division of Critical Care and Hospitalist Neurology, Columbia University; Department of Neurology, New York-Presbyterian Hospital and Vagelos College of Physicians and Surgeons, Columbia University.
Sachin Agarwal, Division of Critical Care and Hospitalist Neurology, Columbia University.
Soojin Park, Division of Critical Care and Hospitalist Neurology, Columbia University.
E. Sander Connolly, Department ofNeurological Surgery, New York-Presbyterian Hospital and Vagelos College of Physicians and Surgeons, Columbia University.
Jan Claassen, Division of Critical Care and Hospitalist Neurology, Columbia University.
Gebhard Wagener, Department of Anesthesiology, Columbia University Irving Medical Center/NewYork-Presbyterian Hospital, New York, New York.
REFERENCES
- 1.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11): 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel P, Braun C, Patel P, et al. Diagnosis of deep vein thrombosis of the upper extremity: a systematic review and meta-analysis of test accuracy. Blood Adv. 2020;4(11):2516–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, Navalesi P, Simioni P. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020; 120(6):998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramacciotti E, Macedo AS, Biagioni RB, et al. Evidence-based practical guidance for the antithrombotic management in patients with coronavirus disease (COVID-19) in 2020. Clin Appl Thromb Hemost. 2020;26:1076029620936350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore HB, Barrett CD, Moore EE, et al. Study of alteplase for respiratory failure in SARS-Cov2/COVID-19: study design of the phase IIa STARS trial. Res Pract Thromb Haemost. 2020;4(6):984–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hincker A, Feit J, Sladen RN, Wagener G. Rotational thromboelastometry predicts thromboembolic complications after major non-cardiac surgery. Crit Care. 2014;18(5):549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kucher N, Kohler H-P, Dornhöfer T, Wallmann D, Lämmle B. Accuracy of D-dimer/fibrinogen ratio to predict pulmonary embolism: a prospective diagnostic study. J Thromb Haemost. 2003;1:708–713. [DOI] [PubMed] [Google Scholar]
- 14.Wright F, Vogler TO, Moore EE, Moore HB, Wohlauer MV, Urban S, Nydam TL, Moore PK, McIntyre RC Jr. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231(2): 193–203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliver JA, Monroe DM, Church FC, Roberts HR, Hoffman M. Activated protein C cleaves factor Va more efficiently on endothelium than on platelet surfaces. Blood. 2002;100:539–546. [DOI] [PubMed] [Google Scholar]
- 16.Monroe DM, Hoffman M. What does it take to make the perfect clot? Arterioscler Thromb Vasc Biol. 2006;26:41–48. [DOI] [PubMed] [Google Scholar]
- 17.Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133:511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fibrinogen Studies Collaboration, Danesh J, Lewington S, Thompson SG, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294(14):1799–1809. [DOI] [PubMed] [Google Scholar]
- 19.Machlus KR, Cardenas JC, Church FC, Wolberg AS. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood. 2011;117:4953–4963. [DOI] [PMC free article] [PubMed] [Google Scholar]