Abstract
We previously demonstrated, for the first time, the abscopal effect of Boron Neutron Capture Therapy (BNCT) in an ectopic model of syngeneic colon cancer in BDIX rats.
Objective:
The aim of the present study was to evaluate the local and regional therapeutic efficacy and abscopal effect of BNCT mediated by boronophenyl-alanine, combined with Bacillus Calmette-Guerin (BCG) as an immunotherapy agent in this model.
Methods:
The local effect of treatment was evaluated in terms of tumor response in the irradiated tumor-bearing right hind flank. Metastatic spread to tumor-draining lymph nodes was analyzed as an indicator of regional effect. The abscopal effect of treatment was assessed as tumor growth inhibition in the contralateral (non-irradiated) left hind flank inoculated with tumor cells 2 weeks post-irradiation. The experimental groups BNCT, BNCT + BCG, BCG, Beam only (BO), BO +BCG, SHAM (tumor-bearing, no treatment, same manipulation) were studied.
Results:
BNCT and BNCT + BCG induced a highly significant local anti-tumor response, whereas BCG alone induced a weak local effect. BCG and BNCT + BCG induced a significant abscopal effect in the contralateral non-irradiated leg. The BNCT + BCG group showed significantly less metastatic spread to tumor-draining lymph nodes vs SHAM and vs BO.
Conclusion:
This study suggests that BNCT + BCG-immunotherapy would induce local, regional and abscopal effects in tumor-bearing animals. BNCT would be the main effector of the local anti-tumor effect whereas BCG would be the main effector of the abscopal effect.
Advances in knowledge:
Although the local effect of BNCT has been widely evidenced, this is the first study to show the local, regional and abscopal effects of BNCT combined with immunotherapy, contributing to comprehensive cancer treatment with combined therapies.
Introduction
Boron Neutron Capture Therapy (BNCT) combines selective tumor uptake of 10B compounds and neutron irradiation. The capture reaction between 10B and a thermal neutron gives rise to the formation of short-range (5–9 µm), high Linear Energy Transfer (LET) α and lithium-7 particles. BNCT protocols are designed to maximize the tumor-specific boron dose component, proportional to the concentration of boron and produced in the above-mentioned reaction, and minimize background dose that affects tumor and normal tissue alike.1 Background dose in the facility used to perform the irradiations results from the protons produced in the interaction of thermal neutrons with the 14N present in the tissue and from the γ field, both structural and generated in the interaction of thermal neutrons with the hydrogen also present in the tissue.
Clinical trials of BNCT for different tumor types and localizations such as glioblastoma multiforme, melanoma and head and neck tumors have shown therapeutic efficacy with room for improvement.2–6 The optimization of BNCT for different pathologies continues to be a field of much needed research. In this sense, our group has performed translational and pre-clinical research in different animal models to improve the safety and efficacy of BNCT protocols for different pathologies.7–15
We described, for the first time, the abscopal effect of BNCT mediated by borono-phenylalanine (BPA) in an ectopic model of colon cancer in BDIX rats.16 The abscopal effect was originally described by Mole (1953)17 as the “out-of-field” inhibitory effect of standard radiotherapy on tumor growth.18,19 Demaria et al20 suggested that the abscopal effect would be mediated by radiation-induced immune responses. Tumors create an immunosuppressive environment that would allow them to escape destruction by the immune system. Radiotherapy would have the capacity to induce immunogenic cell death, leading to cross-priming of tumor-specific T cells that would induce an in-situ tumor vaccine effect.21 More recently, Khan et al22 described immunomodulatory effects of BNCT that contribute to tumor growth inhibition.
The immune system can be stimulated by the administration of attenuated or genetically modified microorganisms, in turn causing tumor regression.23 Within this context, Bacillus Calmette-Guerin (BCG) would act as an immune stimulator and has been shown to effectively treat certain malignancies.24,25 Treatment with BCG does not entail major complications or compromise survival, being the gold-standard treatment for non-muscle invasive bladder cancer.26 In addition, BCG alone is not an effective therapy in more aggressive pathologies like invasive bladder tumors. However, studies in preclinical models demonstrated that it improves tumor response to γ irradiation through the enhancement of local and systemic immune anti tumor effects.25
Having provided proof of principle of the abscopal effect of BNCT alone employing an ectopic model of colon cancer in BDIX rats,16 the aim of the present study was to evaluate, for the first time, the local and regional therapeutic efficacy and the abscopal effect of BNCT combined with BCG as an immunotherapy agent in the same model. The knowledge of these effects would conceivably contribute to the comprehensive treatment of cancer with combined therapies.
Methods and materials
Experimental model
A total of 100 male or female adult BDIX rats (Charles River Lab., MA, USA), 170–250 g body mass (bm) were used throughout. The animals were housed as described previously.11 All rats were injected subcutaneously (sc) in the right hind flank, under ketamine (36.5 mg/kg bm)-xylazine (5.4 mg/kg bm) anesthesia, with 1 × 106 DHD/K12/TRb syngeneic colon cancer cells (ECACC, UK) in 100 µl of F-10-DMEM culture medium (GIBCO) using a syringe with a 27-gauge needle. Based on previous studies,16 all experiments were performed 3–4 weeks post-inoculation. At this time, the animals have developed vascularized, measurable subcutaneous tumor nodules. This time point was considered T0.
Animal experiments were carried out in accordance with the guidelines of the National Institute of Health in the USA regarding the care and use of animals for experimental procedures and with protocols approved by the National Atomic Energy Commission Animal Care and Use Committee (CICUAL-CNEA #10/2018).
Experimental groups
3–4 weeks post-inoculation of DHD/K12/TRb colon carcinoma cells, a total of 100 tumor-bearing rats were assigned at random to each of the following experimental groups:
BNCT-group: tumor-bearing rats injected with BPA (46.5 mg 10B/kg bm) intravenously (i.v.) and irradiated at the RA-3 Nuclear Reactor (Argentina) as described below.
BNCT + BCG-group: tumor-bearing rats treated similarly to the BNCT-group plus three intratumoral applications (3 days pre-BNCT, 1 and 7 days post-BNCT) of BCG (strain Pasteur 1173P2; 0.2 mg/0.1 ml per injection, viability 6 × 105 colony forming units [CFU]).
BCG-group: tumor-bearing rats treated with BCG only as described above.
Beam only-group (BO-group): tumor-bearing rats exposed to the same neutron fluence as the BNCT-group, without boron compound administration.
(BO + BCG)-group: combines the two previous protocols.
SHAM-group: untreated tumor-bearing animals exposed to the same manipulation.
In-vivo BNCT
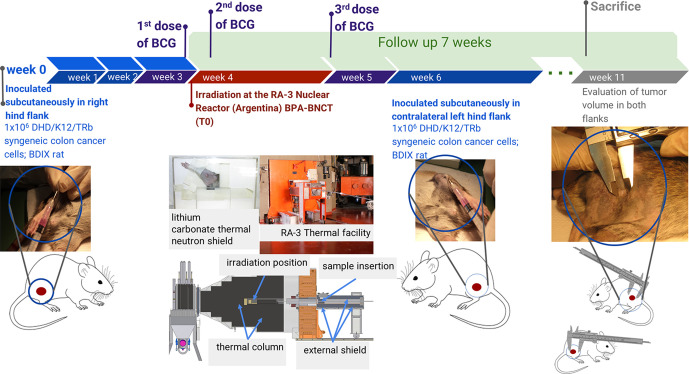
3–4 weeks (depending on Reactor logistics) post-inoculation of DHD/K12/TRb colon carcinoma cells, the rats in the corresponding groups (BNCT, BNCT + BCG, BO, BO + BCG) were irradiated at the thermal neutron facility constructed by the National Atomic Energy Commission at the RA-3 research and production reactor in Buenos Aires.11,27 Irradiations were performed under ketamine (36.5 mg/kg bm)-xylazine (5.4 mg/kg bm) anesthesia. The animals were inserted into a near-isotropic neutron field while the reactor was in normal operation. A self-powered neutron detector (SPND),28 was used to perform neutron flux measurements at a monitor position during each irradiation and calculate exposure time to reach the prescribed dose. Dosimetric calculations were performed employing dosimetry data for the RA-3 facility reported by Farías.29 In the case of the groups treated with BNCT, BPA was administered iv at a dose of 46.5 mg 10B/kg bm in the jugular vein under ketamine (36.5 mg/kg bm)-xylazine (5.4 mg/kg bm) anesthesia as previously described.30 The right leg bearing the tumor nodule was locally irradiated 3 h post-administration of BPA. A lithium carbonate thermal neutron shield (enriched to 95% in lithium-6), developed and fabricated by our group, was used to protect the body of the animals from thermal neutrons while exposing the tumor-bearing leg through a collimated aperture. The schematic representation of the experimental protocol is shown in Figure 1. An absorbed dose of 7.6 Gy was administered to exposed skin as the “organ-at-risk” based on skin radiotolerance data.31In Table 1, we show the prescribed absorbed doses from the different radiation components, the total absorbed BNCT dose and the gross boron concentration data employed for dose calculations.16 Irradiation time to reach the prescribed dose was approximately 13–16 min. The thermal neutron fluence at the irradiation position was 4.2 × 1012 n cm−2, while the thermal neutron fluence at all locations within the shield container was at least a factor of 20 lower than the fluence on the exposed leg.
Figure 1.
Schematic representation of the experimental protocol.
Table 1.
Boron concentration in tissue16 used for boron dose calculation and absorbed dose (Gy) expressed as mean [min;max] as indicated
| Tissue | ppm 10B mean ± SD | Induced protons | Total γ ray dose | Boron dose | Total BNCT dose |
|---|---|---|---|---|---|
| Leg (Skin) | 18.2 ± 2.4 | 1.0 [0.9;1.0] | 1.1 [0.9;1.3] | 5.5 [5.0;5.7] | 7.6 [6.8;8.1] |
| Tumor | 20.1 ± 4.5 | 1.0 [0.9;1.0] | 1.1 [0.9;1.3] | 6.0 [5.6;6.3] | 8.1 [7.3;8.7] |
BNCT, Boron Neutron Capture Therapy; SD, standard deviation.
Induced protons result from the interaction of thermal neutrons with the nitrogen present in the tissue, total γ ray dose includes both structural γ rays and those generated in the interaction of thermal neutrons with the hydrogen present in the tissue, and boron dose includes the α and Li-7 particles generated in the capture reaction between 10B and a thermal neutron.
In the case of the groups that include the BCG protocol, three intratumoral applications of BCG (strain Pasteur 1173P2; 0.2 mg/0.1 ml per injection, viability 6 × 105 CFU) were given 3 days pre-BNCT, 1 and 7 days post- BNCT, or at matched time points in the BCG only group.
Two weeks post-irradiation (or at the matched time point in the non-irradiated SHAM and BCG only groups), 1 × 106 DHD/K12/TRb cells were injected sc in the contralateral left hind flank in all BDIX rats. The choice of this time interval was based on experimental studies of abscopal effect of standard radiotherapy by Zenkoh et al32 which suggest that the immune responses that are responsible for the abscopal effect in certain cases set in 2 weeks after treatment. A two-stage tumor cell inoculation protocol was used to avoid a potential influence of γ irradiation (not shielded by the lithium carbonate device) on tumor growth inhibition.33 The response of the tumor in the right leg (irradiated in the corresponding groups) was used to evaluate the local effect of treatment whereas the inhibitory effect on tumor growth in the left leg (not exposed to irradiation in any of the groups) was used to evaluate the abscopal effect of treatment. The metastatic spread to tumor-draining lymph nodes was used as an end-point to monitor the regional effect of treatment.
Follow-up
Tumor volume was determined by external caliper measurement of the three largest orthogonal diameters (d) and calculated as d1 x d2 x d3 as previously described16 in the right leg pre-irradiation and once a week post-irradiation until sacrifice at 7 weeks (or at matched time points in the non-irradiated groups). Throughout follow-up and at the end of the treatment, the post/pre-treatment tumor volume ratio was calculated to evaluate the local response. Similarly, tumor volume was measured weekly in the contralateral left flank to assess a potential influence of treatment in the right leg on tumor growth in the left leg. The potential inhibitory effect on tumor development in the left leg induced by treatment in the right leg was taken as an indicator of abscopal effect. The end point to evaluate tumor growth in the left leg for each group was incidence of animals with a tumor volume ≤50 mm3.
The percentage of animals with metastatic spread to the tumor-draining lymph nodes in the right irradiated leg was analyzed at the time of sacrifice as an indicator of regional effect. Macroscopic evaluation and confirmatory histological analysis were performed in each case. This study was carried out in the last rounds of treatment once the detection and dissection technique of the draining lymph node were optimized.
Clinical signs and local toxicity were monitored throughout. Local toxicity was assessed employing a radiation dermatitis scale adapted from the National Cancer Institute Common Terminology Criteria for Adverse Events and the Radiation Therapy Oncology Group.34
Statistical analysis
All statistical analyses were performed using a 5% level of significance. For Normality tests we used Shapiro–Wilk and Kolmogorov–Smirnov tests, whereas for homoscedasticity (to compare treatment group variances), we used Hartley (Maximum variance) test.
We used Kruskal–Wallis non-parametric test with post hoc Dunn´s test to compare right or left tumor volume for each treatment group (baseline and over time for each week evaluated), because this variable does not fit a Normal distribution. For relative measurements such as post-/pre-tumor volume (compared to baseline at time point T0), that fit a Normal distribution, we used ANOVA and post hoc Tukey´s test.
For categorized variables such as left leg tumor volume (≤or>than 50 mm3) and lymph node macroscopic and histological features (positive or negative in terms of metastatic spread), we compared treatment group proportions with Chi-Squared or z tests for proportion differences.
Results
The initial (pre-treatment) conditions (tumor volume and body mass) of all the experimental groups were compared using a Kruskal–Wallis test (since they did not fit a normal distribution p > 0.05) and no statistically significant differences were found (p = 0.3), rendering them comparable.
No clinical systemic signs of toxicity were identified in any of the experimental groups. Local toxicity was observed only in the exposed skin of the BNCT and BNCT + BCG groups. It involved reversible Grade 3 dermatitis in the thigh (moist desquamation and bleeding induced by minor trauma) and reversible Grade 4 dermatitis in the more radiosensitive soles of the feet (moist desquamation, ulceration and spontaneous bleeding). The peak of dermatitis was observed approximately 10 days post-BNCT and lasted 5–7 days, followed by healing. When necessary, the animals were medicated with analgesics and antibiotics to mitigate the symptoms of dermatitis.
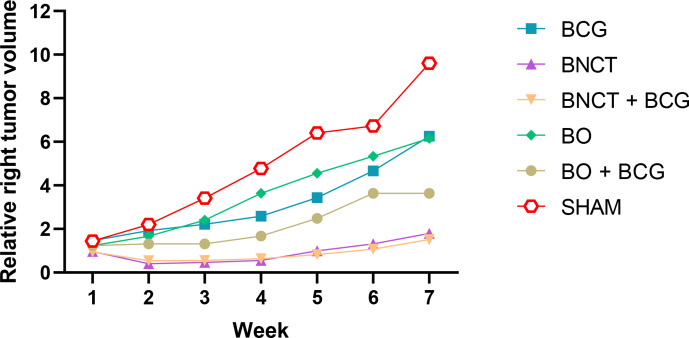
The relative right leg tumor volume (relative to pre-treatment tumor volume) was compared between the experimental groups at each time point, as shown in Figure 2. At each time point, the ratios were calculated individually for each tumor and then used to determine the mean ratio for each group. Fixed effects (Type III) two-way ANOVA showed a significant interaction between group and week (p < 0.0001) and a significant effect for group (p = 0.008) and for week (p < 0.0001). Simple effects for treatment group were evaluated within each time point (weeks 1–7) through Tukey´s multiple comparison test, where significant differences between at least some of the groups became apparent 3 weeks after treatment. In particular, significant differences were observed between the BNCT groups (BNCT and BNCT + BCG) and the remaining groups (SHAM, BO, BO +BCG and BCG). The greatest difference between BNCT and BNCT + BCG vs SHAM and BO (p < 0.05 in all cases) was observed at weeks 6 and 7.
Figure 2.
Temporal evolution of post-/pre-treatment tumor volume ratio in the right leg from 0 to 7 weeks. Error bars indicating the SD were omitted for image clarity.
The curves (Figure 2) show the evolution in time of relative tumor volume in the right leg for each group, revealing the trend in each case and the differences between some of the experimental groups at each time point (week) evaluated. The values of relative tumor volume over time showed that both the BNCT and BNCT + BCG values were very small and significantly different from SHAM at weeks 6 and 7, revealing a robust local response to treatment for both groups. While BCG alone did exert a certain degree of local effect on tumor (albeit not statistically significant) vs SHAM, a statistically significant inhibitory effect on tumor growth was achieved when BNCT and BCG were combined. However, BCG did not seem to contribute to local tumor control beyond the local effect achieved by BNCT alone (local tumor control was almost identical for BNCT and BNCT + BCG). Conversely, BNCT did contribute to local tumor control when combined with BCG compared to BCG alone. However, this difference between BNCT + BCG and BCG did not reach statistical significance. Within this context, BNCT would provide the main contribution to local tumor control when combined with BCG. BO and BCG exhibited a slight and similar inhibitory effect on tumor growth vs SHAM that did not reach statistical significance. The combination of BO and BCG seemed to favor tumor control compared to each treatment alone, albeit not significantly. The Sham mean tumor volume ratio progressed from over 1 at 1 week to almost 10 at 7 weeks. This implies that, left untreated, the tumor volume increased almost 10 times on average in 7 weeks. The volume ratio for BNCT and BNCT + BCG was always close to 1, i.e. the tumors remained stable over time. At 7 weeks, the tumor volume ratio of the SHAM group was six times greater than that of the groups treated with BNCT (BNCT and BNCT + BCG). Likewise, between the second and fourth weeks the post-/pre-treatment ratios for the BNCT and BNCT + BCG groups were below 1, showing a reduction in tumor volume from its initial value. At weeks 5, 6 and 7, these values recovered their pre-treatment value or increased a little. This finding suggests that a second treatment around the fourth week could prevent or delay tumor recurrence or even achieve complete tumor remission.
Table 2 shows absolute tumor volume values pre-treatment and at 7 weeks post-treatment. These values are presented only as a reference because in contrast with Figure 2, they fail to represent the behavior of individual tumors with respect to their pre-treatment volume. If we evaluate absolute tumor volume at 7 weeks, as an indicator of local effect of the treatment, we see significant differences between both BNCT and BNCT + BCG groups vs both the SHAM control group and the BO group (p < 0.05 in all cases). Furthermore, the BNCT + BCG group also exhibited differences with the BCG alone group that reached statistical significance in this analysis (p < 0.05), providing additional evidence of the main role of BNCT in local tumor control. Follow-up ended at 7 weeks because the SHAM group exceeded admissible tumor growth after that.
Table 2.
Absolute tumor volume values in the right leg for each of the experimental groups pre-treatment and at 7 weeks
|
Groups
(N) |
Tumor volume (mm3)
Pre-treatment Mean ± SD Median[Min;Max] |
Tumor volume (mm3)
7 weeks post-treatment Mean ± SD Median[Min;Max] |
|---|---|---|
|
SHAM (20) |
178 ± 123
137 [19;441] |
1178 ± 754
945 [307;3591] |
|
BO (10) |
258 ± 128
233 [123;586] |
1545 ± 1258
1248 [551;4708] |
|
BO + BCG (8) |
219 ± 113
248 [54;372] |
938 ± 864
890 [0;2697] |
|
BCG (21) |
157 ± 125
128 [14;381] |
1018 ± 1317
598 [0;5321] |
|
BNCT (20) |
207 ± 174
154 [31;722] |
394 ± 468
368 [0;2017] |
|
BNCT + BCG (21) |
156 ± 99
119 [39;405] |
229 ± 439
12 [0;1932] |
BCG, Bacillus Calmette-Guerin; BNCT, Boron Neutron Capture Therapy; BO, beam only.
Tumor volume in the right leg expressed as the mean ± SD and Median [Min;Max]. N = number of animals.
Table 3 shows the percentage of animals with a tumor volume ≤50 mm3 in the left leg that was assessed for each of the experimental groups at the end of follow-up. This time-point corresponds to 11 weeks post-inoculation and 7 weeks post-treatment for the right leg, and 5 weeks post-inoculation for the left leg (Figure 1 above).
Table 3.
Number of animals with tumor volume ≤50 mm3 for the left (non-irradiated) leg and percentage for each group
| Groups (N) | # of animals with tumor volume ≤50 mm3(%) |
|---|---|
| SHAM (20) | 1 (5%) |
| BO (10) | 1 (10%) |
| BO + BCG (8) | 3 (37%) |
| BCG (21) | 12 (57%) |
| BNCT (20) | 3 (15%) |
| BNCT + BCG (21) | 9 (43%) |
BCG, Bacillus Calmette-Guerin; BNCT, Boron Neutron Capture Therapy; BO, beam only.
N = number of animals.
The abscopal effect was identified as an inhibitory effect on tumor growth in the left contralateral leg (untreated). The percentage of animals with a tumor volume ≤50 mm3 was evaluated with a Chi-Squared analysis and significant differences were observed between BCG and BNCT + BCG vs SHAM (p = 0.0005 and p = 0.02 respectively). Likewise, BCG showed significant differences with BO (p = 0.02). BO, BO + BCG and BNCT exhibited some degree of abscopal effect vs SHAM. However, this effect did not reach statistical significance. BNCT + BCG did not differ significantly from BCG alone, revealing that BCG would be mainly responsible for inducing an abscopal effect in the BNCT + BCG protocol. Although BNCT alone would generate some degree of abscopal response, it would be less powerful than that induced by either BCG alone or BNCT + BCG. These data reveal that BCG and BNCT + BCG induced a statistically significant abscopal effect in the contralateral non-irradiated leg and that BCG would be the principal effector of the abscopal response.
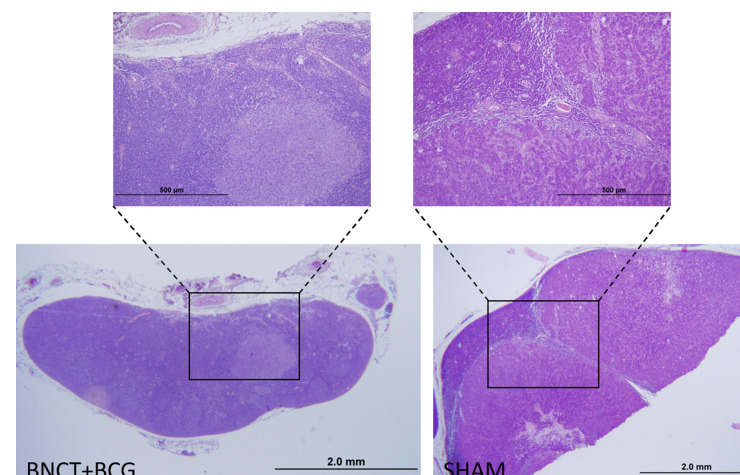
Table 4 shows the regional effect of treatment expressed as the incidence of animals without metastatic tumor-draining lymph nodes in terms of macroscopic and histological evaluation. This regional effect would combine the abscopal effect that results from local tumor treatment and the local effect of radiation in the exposed area. Metastatic nodes were enlarged and indurated and were considered macroscopically positive when visual inspection revealed pale, high density, nodular areas. All the lymph nodes that were considered macroscopically positive also proved to be positive on histological analysis. Conversely, some of the lymph nodes that were considered negative on macroscopic assessment exhibited a varying degree of metastatic invasion on histological analysis. Most of the lymph nodes that were positive on histological analysis, exhibited only small areas of metastasis in the BNCT and BNCT + BCG groups whereas in the case of the BO and SHAM groups, the lymph nodes were completely replaced by tumor tissue. In the latter case, only the border of the lymph node was identifiable as shown in Figure 3. Metastatic spread to tumor-draining lymph nodes was significantly inhibited by BNCT + BCG vs the SHAM group as revealed by macroscopic (p < 0.05) and histological (p = 0.01) analysis. Similarly, metastatic spread was significantly inhibited by BNCT + BCG vs BO both macroscopically and histologically (p = 0.02 and p = 0.001 respectively). BNCT, BCG and BO + BCG exhibited some degree of regional effect vs SHAM and BO. However, this effect did not reach statistical significance. Although we did not detect statistically significant differences between BNCT + BCG and BCG, a trend is evident and suggests that BNCT + BCG would exert a more potent regional effect than BCG alone. In fact, the combination of BNCT and BCG would be more effective than either of the treatments alone. Both BNCT and BCG seemed to contribute importantly to the statistically significant regional effect of BNCT + BCG. These data are reported as preliminary but contributory and indicative that future studies in this sense are warranted.
Table 4.
Number of animals with negative lymph nodes (-) in terms of macroscopic and histological evaluation, total number of animals (N) and % of animals with negative lymph nodes for each of the experimental groups
| Groups | Macroscopic analysis of lymph nodes -/N (%) | Histological analysis of lymph nodes -/N (%) |
|---|---|---|
| SHAM | 4/9 (44%) | 1/9 (11%) |
| BO | 3/9 (33%) | 0/9 (0%) |
| BO + BCG | 3/6 (50%) | 2/6 (33%) |
| BCG | 5/9 (56%) | 4/8 (50%) |
| BNCT | 6/9 (67%) | 3/9 (33%) |
| BNCT + BCG | 10/11 (91%) | 8/11 (73%) |
BCG, Bacillus Calmette-Guerin; BNCT, Boron Neutron Capture Therapy; BO, beam only.
Figure 3.
Microphotograph of a lymph node corresponding to an animal in the BNCT + BCG group. A small area of metastasis can be observed in the follicular area of the lymph node. Microphotograph of a lymph node corresponding to an animal in the SHAM group. The lymphatic tissue has almost completely been replaced by tumor tissue with the typical features of colon adenocarcinoma: atypical glandular structures with scarce stroma. There is only a small portion of remaining lymphatic tissue in the subcapsular area. H&E, scale bar is shown in the images. BCG, Bacillus Calmette-Guerin; BNCT, Boron Neutron Capture Therapy
Discussion
It is known that when ionizing radiation is applied to a primary tumor, it can induce immunogenic cell death which may in turn trigger a cytotoxic immune response against the primary tumor and its metastasis.25,35,36 This phenomenon, known as abscopal effect, has been described in association with localized standard radiotherapy.37 Proof of principle of the abscopal effect of BNCT was provided for the first time by our group.16 More recently, Kahn et al22 also demonstrated an immunomodulatory effect of BNCT, as it induced an anti-tumor phenotype in peripheral blood mononuclear cells.
BCG has successfully been used as an immunotherapy agent or as an immunological adjuvant against human neoplasms.38,39 It is an immune stimulator that was shown to improve the anti tumor immune response in combination with ionizing radiation, inducing a systemic immune response to both the primary tumor and metastases.25
In the present study we examined, for the first time, the local, regional and abscopal effects of BNCT combined with BCG as an anti-tumor immune stimulator. The use of an experimental model that employs the inoculation of syngeneic colon cancer cells in immunocompetent rats was essential to examine abscopal effect that it is dependent on a functional immune system.20,33,40 The fact that BPA-BNCT is approved for use in patients4 and that BCG is approved as an immunological adjuvant in the development of anti-tumor vaccines,38 contributes to bridge the gap between translational research and a clinical scenario.
We showed that BNCT alone and BNCT + BCG induced a highly statistically significant local tumor response. BNCT has been widely shown to induce local tumor control,11 mainly as a result of cell death induced by the direct action of the high LET α and Li particles on DNA, that in turn leads to complex, clustered DNA damage that is difficult to repair. While BCG alone exhibited weak local tumor control, it failed to significantly enhance the local effect of BNCT when BNCT and BCG were combined, rendering BNCT mainly responsible for local tumor control. The combination of BCG and BNCT might act as an “anti-tumor vaccine” where BNCT would trigger the process of tumor antigen generation and BCG would promote antigen presentation in an inflammatory microenvironment. Within this context, Antonelli et al. (2020)41 demonstrated that BCG therapy enhanced an anti-tumor effect induced by tumor-specific T cells in the bladder tumor microenvironment. Robust regional and abscopal effects of BNCT + BCG were observed. BCG would be the main effector of the abscopal effect since BCG alone exhibited a powerful abscopal effect that was not enhanced by the combination of BNCT + BCG. In the case of the regional effect, both BNCT and BCG would contribute importantly, given that the regional effect of BNCT + BCG was more pronounced than for either treatment alone.
BCG alone has been used as a local sensitizer to overcome hypoxia-associated radioresistance, inducing the local production of the free radical Nitric Oxide42,43 and improving the local and abscopal response to ionizing radiation in the context of low LET radiation.25 Furthermore, BCG has been used for local immunomodulation. The combined treatment with immunomodulation and radiofrequency ablation (RFA), resulted in a complete cure of local and distant colorectal carcinoma in an experimental model. The lack of an effective distant immune response in patients treated with RFA alone supports this new combined treatment strategy.44 In the case of BNCT, the main radiation dose component is high LET and as such, induces direct damage to DNA rather than indirect damage associated to the production of free radicals.1 In a high-LET radiation scenario, the local effect of BCG (albeit weak) would be mainly related to its immune-stimulator effect. In addition, pre-clinical studies showed that double strand DNA damage activates innate immune signaling pathways,45 suggesting that BNCT would contribute to an abscopal effect.
In the experimental conditions employed herein, the immune-stimulator effect of BCG alone would not, on average, be enough to induce a statistically significant local tumor response. This weak local effect of BCG alone might be related to the high tumor burden at the time of treatment as previously described. It is known that close contact between the Mycobacterium and cancer cells is required for successful therapy.25,46 It has been described that with a high tumor burden, immune cells alone are not effective in controlling disease and complementary therapies are necessary.47
Regarding the similar and statistically significant abscopal effect of BCG and BNCT + BCG groups vs SHAM the reduction of tumor bulk in the right, treated leg could contribute to reduce the suppressive immune response developed by tumor cells, in turn favoring abscopal response.16 It is known that cell death leads to the release of endogenous damage-associated molecular patterns (DAMPs) that contribute to the priming of the immune system by triggering dendritic cells, in turn improving antigen presentation to T cells that are present in the lymph nodes. This presentation stimulates an adaptive immune response against tumor cells, that impacts locally and systemically affecting the same tumor in unirradiated sites.48–50 Other authors reported that immunogenic cell death induced by ionizing radiation alone or combined with immunotherapy would elicit local and abscopal effects.47 The systemic immune response induced by BCG alone would suffice to induce a robust abscopal effect, inhibiting or reducing the development of experimental “out-of-field” tumors. BCG primary tumor inoculation was the most powerful strategy to induce an abscopal effect, alone or in combination with BNCT. These observations are supported by clinical studies from other authors that conclude that the use of BCG as tumor vaccine in combination with ionizing radiation therapy help to control the distant disease in locally advanced recurrent hepatocellular carcinoma and in advanced breast cancer.51
To analyze the regional effect (a combination of abscopal and local effects) induced by our treatment protocols, we studied the metastatic spread to tumor-draining lymph nodes. The regional therapeutic efficacy in terms of a statistically significant reduction in metastatic spread was achieved only with the combined BNCT + BCG treatment, suggesting that both BNCT and BCG play an important role in triggering a regional effect.
As reported by Hatanaka et al,52 BNCT would be ideally suited to integrate a multimodal tumor treatment. It is capable of inhibiting tumor growth, has low local and systemic toxicity compared to conventional chemo- and radiotherapy and it is not immunosuppressive. Extensive studies are necessary to elucidate the best combination of radiotherapy and immune therapies to induce effective and long-lasting tumor response and abscopal effect.47 Within this context, a reduction in local toxicity would allow for dose-escalation to tumor and an increase in therapeutic advantage without exceeding radiotolerance.
BNCT mediated by BPA clinical trials are currently mainly devoted to treating glioblastoma and head and neck cancer.6,53,54 BPA-BNCT in dog patients with spontaneous head and neck cancer with no other therapeutic option showed the potential value of BNCT in veterinary medicine.55 Clinical studies also demonstrated that BNCT would be useful to treat melanoma.56,57 Experimental studies have shown that BNCT could be expanded to treat prostate and breast cancer.58,59 None of these targets would require complex ex-vivo irradiations as described for liver metastases and have been proved clinically feasible.60 Experimental and clinical studies in veterinary and human patients showed that BCG would also be useful to treat the above mentioned illnesses.61–64 In this sense, and based on the results of this study, the combination of BNCT + BCG for the treatment of these tumors in a clinical scenario could be a promising approach.
While the efficacy of reactor based BNCT has been confirmed for certain malignancies, the difficulty to install a nuclear reactor in a hospital environment encouraged the BNCT community worldwide to work on the development of accelerator-based neutron sources for BNCT. Several accelerators destined for hospital placement have been introduced and have already afforded encouraging results, conceivably favoring widespread BNCT clinical trials.65
Ongoing studies by our group are addressing the issue of minimizing dermatitis and/or enhancing local therapeutic efficacy of BPA-BNCT using the seaweed extract Oligo-Fucoidan (Hi-Q Marine Biotech International Ltd). Given that multimodal tumor treatments would be the best suited to optimize local and systemic response, translational studies in this area are pivotal to optimize outcome. Tailored studies are necessary to determine the radiation doses and sequence that will maximize immune stimulation and to establish the best combination of immunostimulatory molecules and radiation that will neutralize radioinduced immunosuppression.49
Conclusions
The present results show that the best option for local control of disease is BNCT (alone or combined with BCG). On the other hand, BCG is the best strategy to control the distant disease by immunological activation and the induction of a powerful abscopal response. Regarding the regional spread of the disease to the lymph nodes, it is the combined treatment of BNCT + BCG that provides the most promising results. The findings on regional spread, albeit preliminary, are contributory and warrant further studies. Multimodal therapies would be the best option for the comprehensive treatment of this type of disease.
Contributor Information
Verónica A. Trivillin, Email: verotrivillin@gmail.com.
Yanina V. Langle, Email: yaninalangle@yahoo.com.ar.
Mónica A. Palmieri, Email: monica.ale.palmieri@gmail.com.
Emiliano C.C. Pozzi, Email: eccpozzi@gmail.com.
Silvia I. Thorp, Email: thorp@cae.cnea.gov.ar.
Debora N. Benitez Frydryk, Email: debora_nbh@hotmail.com.
Marcela A. Garabalino, Email: marcegarabalino@gmail.com.
Andrea Monti Hughes, Email: andre.mh@gmail.com.
Paula M. Curotto, Email: paulacurotto@gmail.com.
Lucas L. Colombo, Email: lucascol2003@yahoo.com.ar.
Iara S. Santa Cruz, Email: iarasofiasantacruz@gmail.com.
Paula S. Ramos, Email: paula.s.ramos@outlook.com.
María E. Itoiz, Email: marielitoiz35@gmail.com.
Claudia Argüelles, Email: claudia.arguelles@gmail.com.
Ana M. Eiján, Email: anamariaeijan@gmail.com.
Amanda E. Schwint, Email: mandyschwint@gmail.com.
REFERENCES
- 1.Coderre JA, Morris GM. The radiation biology of boron neutron capture therapy. Radiat Res 1999; 151: 1–18Review. [PubMed] [Google Scholar]
- 2.González SJ, Bonomi MR, Santa Cruz GA, Blaumann HR, Calzetta Larrieu OA, Menéndez P, et al. First BNCT treatment of a skin melanoma in Argentina: dosimetric analysis and clinical outcome. Appl Radiat Isot 2004; 61: 1101–5. doi: 10.1016/j.apradiso.2004.05.060 [DOI] [PubMed] [Google Scholar]
- 3.Kankaanranta L, Seppälä T, Koivunoro H, Välimäki P, Beule A, Collan J, et al. Serèn T Paetau a, Saarilahti K, Savolainen S, Joensuu H. L-boronophenylalanine-mediated boron neutron capture therapy for malignant glioma progressing after external beam radiation therapy: a phase I study. Int J Radiat Oncol Biol Phys 2011; 80: 369–76. [DOI] [PubMed] [Google Scholar]
- 4.Kankaanranta L, Seppälä T, Koivunoro H, Saarilahti K, Atula T, Collan J, et al. Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: final analysis of a phase I/II trial. Int J Radiat Oncol Biol Phys 2012; 82: e67–75. doi: 10.1016/j.ijrobp.2010.09.057 [DOI] [PubMed] [Google Scholar]
- 5.Miyatake S-I, Kawabata S, Hiramatsu R, Furuse M, Kuroiwa T, Suzuki M. Boron neutron capture therapy with bevacizumab may prolong the survival of recurrent malignant glioma patients: four cases. Radiat Oncol 2014; 9: 6. doi: 10.1186/1748-717X-9-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L-W, Liu Y-WH, Chou F-I, Jiang S-H. Clinical trials for treating recurrent head and neck cancer with boron neutron capture therapy using the Tsing-Hua open pool reactor. Cancer Commun 2018; 38: 37. doi: 10.1186/s40880-018-0295-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreimann EL, Itoiz ME, Longhino J, Blaumann H, Calzetta O, Schwint AE. Boron neutron capture therapy for the treatment of oral cancer in the hamster cheek pouch model. Cancer Res 2001; 61: 8638–42. [PubMed] [Google Scholar]
- 8.Trivillin VA, Heber EM, Nigg DW, Itoiz ME, Calzetta O, Blaumann H, et al. Therapeutic success of boron neutron capture therapy (BNCT) mediated by a chemically non-selective boron agent in an experimental model of oral cancer: a new paradigm in BNCT radiobiology. Radiat Res 2006; 166: 387–96. doi: 10.1667/RR3592.1 [DOI] [PubMed] [Google Scholar]
- 9. Molinari AJ, Pozzi ECC, Monti Hughes A, Heber EM, Garabalino MA, Thorp SI, Miller M, Itoiz ME, Aromando RF, Nigg DW, Quintana J, Santa Cruz GA, Trivillin VA, Schwint AE. "Sequential" boron neutron capture therapy (BNCT): a novel approach to BNCT for the treatment of oral cancer in the hamster cheek pouch model. Radiat Res 2011; 175: 463–72. [DOI] [PubMed] [Google Scholar]
- 10.Molinari AJ, Pozzi ECC, Monti Hughes A, Heber EM, Garabalino MA, Thorp SI, et al. Tumor blood vessel "normalization" improves the therapeutic efficacy of boron neutron capture therapy (BNCT) in experimental oral cancer. Radiat Res 2012; 177: 59–68. doi: 10.1667/rr2729.1 [DOI] [PubMed] [Google Scholar]
- 11.Pozzi ECC, Trivillin VA, Colombo LL, Monti Hughes A, Thorp SI, Cardoso JE, et al. Boron neutron capture therapy (BNCT) for liver metastasis in an experimental model: dose–response at five-week follow-up based on retrospective dose assessment in individual rats. Radiat Environ Biophys 2013; 52: 481–91. doi: 10.1007/s00411-013-0490-9 [DOI] [PubMed] [Google Scholar]
- 12.Monti Hughes A, Pozzi ECC, Thorp S, Garabalino MA, Farías RO, González SJ, et al. Boron neutron capture therapy for oral precancer: proof of principle in an experimental animal model. Oral Dis 2013; 19: 789–95. doi: 10.1111/odi.12077 [DOI] [PubMed] [Google Scholar]
- 13.Schwint AE, Trivillin VA. 'Close-to-ideal' tumor boron targeting for boron neutron capture therapy is possible with 'less-than-ideal' boron carriers approved for use in humans. Ther Deliv 2015; 6: 269–72. doi: 10.4155/tde.14.108 [DOI] [PubMed] [Google Scholar]
- 14.Trivillin VA, Serrano A, Garabalino MA, Colombo LL, Pozzi EC, Hughes AM, Monti Hughes A, et al. Translational boron neutron capture therapy (BNCT) studies for the treatment of tumors in lung. Int J Radiat Biol 2019; 95: 646–54. doi: 10.1080/09553002.2019.1564080 [DOI] [PubMed] [Google Scholar]
- 15.Garabalino MA, Olaiz N, Portu A, Saint Martin G, Thorp SI, Pozzi ECC, et al. Electroporation optimizes the uptake of boron-10 by tumor for boron neutron capture therapy (BNCT) mediated by GB-10: a boron biodistribution study in the hamster cheek pouch oral cancer model. Radiat Environ Biophys 2019; 58: 455–67. doi: 10.1007/s00411-019-00796-z [DOI] [PubMed] [Google Scholar]
- 16.Trivillin VA, Pozzi ECC, Colombo LL, Thorp SI, Garabalino MA, Monti Hughes A, et al. Abscopal effect of boron neutron capture therapy (BNCT): proof of principle in an experimental model of colon cancer. Radiat Environ Biophys 2017; 56: 365–75. doi: 10.1007/s00411-017-0704-7 [DOI] [PubMed] [Google Scholar]
- 17.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953; 26: 234–41. doi: 10.1259/0007-1285-26-305-234 [DOI] [PubMed] [Google Scholar]
- 18.Okuma K, Yamashita H, Niibe Y, Hayakawa K, Nakagawa K. Abscopal effect of radiation on lung metastases of hepatocellular carcinoma: a case report. J Med Case Rep 2011; 5: 111. doi: 10.1186/1752-1947-5-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013; 1: 365–72. doi: 10.1158/2326-6066.CIR-13-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004; 58: 862–70. doi: 10.1016/j.ijrobp.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 21.Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol 2014; 4: 325. doi: 10.3389/fonc.2014.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan AA, Maitz C, Quanyu C, Hawthorne F. Bnct induced immunomodulatory effects contribute to mammary tumor inhibition. PLoS One 2019; 14: e0222022. doi: 10.1371/journal.pone.0222022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kucerova P, Cervinkova M. Spontaneous regression of tumour and the role of microbial infection--possibilities for cancer treatment. Anticancer Drugs 2016; 27: 269–77. doi: 10.1097/CAD.0000000000000337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rumi LS, García MS, Barañao RI, Schwint AE. Growth of sarcoma 180 in normal and splenectomized BALB/c mice immunized with BCG. Acta Physiol Lat Am 1982; 32: 123–30. [PubMed] [Google Scholar]
- 25.Prack Mc Cormick B, Belgorosky D, Langle YV, Balarino N, Sandes E, Eiján AM. Bacillus Calmette-Guerin improves local and systemic response to radiotherapy in invasive bladder cancer. Nitric Oxide 2017; 64: 22–30. Epub 2017 Jan 23. doi: 10.1016/j.niox.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 26.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol 1976; 116: 180. doi: 10.1016/s0022-5347(17)58737-6 [DOI] [PubMed] [Google Scholar]
- 27.Miller M, Quintana J, Ojeda J, Langan S, Thorp S, Pozzi E, et al. New irradiation facility for biomedical applications at the RA-3 reactor thermal column. Appl Radiat Isot 2009; 67(7-8 Suppl): S226–9. doi: 10.1016/j.apradiso.2009.03.107 [DOI] [PubMed] [Google Scholar]
- 28.Miller ME, Mariani LE, Gonçalves-Carralves MLS, Skumanic M, Thorp SI. Implantable self-powered detector for on-line determination of neutron flux in patients during NCT treatment. Appl Radiat Isot 2004; 61: 1033–7. doi: 10.1016/j.apradiso.2004.05.041 [DOI] [PubMed] [Google Scholar]
- 29.Farías R. "Dosimetry and computational modeling for extracorporeal irradiations in humans within boron neutron capture therapy". Ph. D. Thesis UNSAM, Buenos Aires, Argentina 2015;. [Google Scholar]
- 30.Garabalino MA, Monti Hughes A, Molinari AJ, Heber EM, Pozzi ECC, Cardoso JE, et al. Boron neutron capture therapy (BNCT) for the treatment of liver metastases: biodistribution studies of boron compounds in an experimental model. Radiat Environ Biophys 2011; 50: 199–207. doi: 10.1007/s00411-010-0345-6 [DOI] [PubMed] [Google Scholar]
- 31.González SJ, Casal M, Pereira MD, Santa Cruz GA, Carando DG, Blaumann H, et al. Tumor control and normal tissue complications in BNCT treatment of nodular melanoma: a search for predictive quantities. Appl Radiat Isot 2009; 67(7-8 Suppl): S153–6. doi: 10.1016/j.apradiso.2009.03.038 [DOI] [PubMed] [Google Scholar]
- 32.Zenkoh J, Gerelchuluun A, Suzuki K, Wang X, Ito A, Ohno T, et al. Radiation Induced Tumor Cell Death Bring About the “Abscopal Effect” in the Brain. 15th. International Congress of Radiation Research ICRR2015 Kyoto May 2015. [Google Scholar]
- 33.Camphausen K, Moses MA, Ménard C, Sproull M, Beecken W-D, Folkman J, et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res 2003;; ; 63: 1990–315. [PubMed] [Google Scholar]
- 34.Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology 2017; 31):, : 894–9885-7. [PubMed] [Google Scholar]
- 35.Ludgate CM. Optimizing cancer treatments to induce an acute immune response: radiation Abscopal effects, PAMPs, and DAMPs. Clin Cancer Res 2012; 18: 4522–5. doi: 10.1158/1078-0432.CCR-12-1175 [DOI] [PubMed] [Google Scholar]
- 36.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys 2013; 85: 293–5. doi: 10.1016/j.ijrobp.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366: 925–31. doi: 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel SG, Cohen A, Weiner AB, Steinberg GD. Intravesical therapy for bladder cancer. Expert Opin Pharmacother 2015; 16: 889–901. doi: 10.1517/14656566.2015.1024656 [DOI] [PubMed] [Google Scholar]
- 39.Morales A. Treatment of superficial bladder cancer. Can Med Assoc J 1980; 122: 1133–8. [PMC free article] [PubMed] [Google Scholar]
- 40.Chakravarty PK, Alfieri A, Thomas EK, Beri V, Tanaka KE, Vikram B, et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res 1999; 59: 6028–32. [PubMed] [Google Scholar]
- 41.Antonelli AC, Binyamin A, Hohl TM, Glickman MS, Redelman-Sidi G. Bacterial immunotherapy for cancer induces CD4-dependent tumor-specific immunity through tumor-intrinsic interferon-γ signaling. Proc Natl Acad Sci U S A 2020; 117: 18627–37. doi: 10.1073/pnas.2004421117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Ridder M, Van Esch G, Engels B, Verovski V, Storme G. Hypoxic tumor cell radiosensitization: role of the iNOS/NO pathway. Bull Cancer 2008; 95: 282–91. doi: 10.1684/bdc.2008.0592 [DOI] [PubMed] [Google Scholar]
- 43.Scicinski J, Oronsky B, Ning S, Knox S, Peehl D, Kim MM, et al. No to cancer: the complex and multifaceted role of nitric oxide and the epigenetic nitric oxide donor, RRx-001. Redox Biol 2015; 6: 1–8. doi: 10.1016/j.redox.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemdani K, Mignet N, Boudy V, Seguin J, Oujagir E, Bawa O, et al. Local immunomodulation combined to radiofrequency ablation results in a complete cure of local and distant colorectal carcinoma. Oncoimmunology 2019;; ; 8: 155034210. doi: 10.1080/2162402X.2018.1550342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dutt S, Ahmed MM, Loo BW, Strober S. Novel radiation therapy paradigms and immunomodulation: heresies and hope. Semin Radiat Oncol 2020; 30: 194–200. doi: 10.1016/j.semradonc.2019.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rapp HJ, Zbar B. Bcg and cancer. J Natl Cancer Inst 1981; 67: 991. [PubMed] [Google Scholar]
- 47.Rubner Y, Wunderlich R, Rühle P-F, Kulzer L, Werthmöller N, Frey B, et al. How does ionizing irradiation contribute to the induction of anti-tumor immunity? Front Oncol 2012; 2: 75. doi: 10.3389/fonc.2012.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beyer C, Stearns NA. Giessl a Distler JH, Schett G, Pisetsky Ds. the extracellular release of DNA and HMGB1 from Jurkat T cells during in vitro necrotic cell death. Innate Immun 2012; 18: 727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu ZI, McArthur HL, Ho AY, ZI H, AY H. The Abscopal effect of radiation therapy: what is it and how can we use it in breast cancer? Curr Breast Cancer Rep 2017; 9: 45–51. doi: 10.1007/s12609-017-0234-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoopes PJ, Wagner RJ, Duval K, Kang K, Gladstone DJ, Moodie KL, et al. Treatment of canine oral melanoma with nanotechnology-based immunotherapy and radiation. Mol Pharm 2018; 15: 3717–22. doi: 10.1021/acs.molpharmaceut.8b00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abei M, Okumura T, Fukuda K, Hashimoto T, Araki M, Ishige K, et al. Koji Tsuboi K. a phase I study on combined therapy with proton-beam radiotherapy and in situ tumor vaccination for locally advanced recurrent hepatocellular carcinoma, Radiat. Oncol 2013; 8: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hatanaka H, Amano K, Kamano S, Fankhauser H, Hanamura T, Sano K. Boron-neutron capture therapy in relation to immunotherapy. Acta Neurochir 1978; 42(1-2): 57–72. doi: 10.1007/BF01406631 [DOI] [PubMed] [Google Scholar]
- 53.Suzuki M. Boron neutron capture therapy (BNCT): a unique role in radiotherapy with a view to entering the accelerator-based BNCT era. Int J Clin Oncol 2020; 25: 43–50. doi: 10.1007/s10147-019-01480-4 [DOI] [PubMed] [Google Scholar]
- 54.Lan T-L, Chou F-I, Lin K-H, Pan P-S, Lee J-C, Huang W-S, et al. Using salvage boron neutron capture therapy (BNCT) for recurrent malignant brain tumors in Taiwan. Appl Radiat Isot 2020; 160: 109105. doi: 10.1016/j.apradiso.2020.109105 [DOI] [PubMed] [Google Scholar]
- 55.Schwint AE, Monti Hughes A, Garabalino MA, Santa Cruz GA, González SJ, Longhino J. 1 Provenzano L, Oña P, Rao M, , Cantarelli MA, Leiras A, Olivera MS, Trivillin VA, Alessandrini P, Brollo F, Boggio E, Costa H, Ventimiglia R, Binia S, Pozzi ECC, Nievas SI, Santa Cruz IS. Clinical Veterinary Boron Neutron Capture Therapy (BNCT) Studies in Dogs with Head and Neck Cancer: Bridging the Gap between Translational and Clinical Studies. Biology 2020; 9: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Menéndez PR, Roth BMC, Pereira MD, Casal MR, González SJ, Feld DB, et al. BNCT for skin melanoma in extremities: updated Argentine clinical results. Appl Radiat Isot 2009; 67(7-8 Suppl): S50–3. doi: 10.1016/j.apradiso.2009.03.020 [DOI] [PubMed] [Google Scholar]
- 57.Yong Z, Song Z, Zhou Y, Liu T, Zhang Z, Zhao Y, et al. Boron neutron capture therapy for malignant melanoma: first clinical case report in China. Chin J Cancer Res 2016; 28: 634–40. doi: 10.21147/j.issn.1000-9604.2016.06.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahara K, Inamoto T, Minami K, Yoshikawa Y, Takai T, Ibuki N, et al. The anti-proliferative effect of boron neutron capture therapy in a prostate cancer xenograft model. PLoS One 2015; 10: e0136981. doi: 10.1371/journal.pone.0136981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alberti D, Michelotti A, Lanfranco A, Protti N, Altieri S, Deagostino A, et al. In vitro and in vivo BNCT investigations using a carborane containing sulfonamide targeting CAIX epitopes on malignant pleural mesothelioma and breast cancer cells. Sci Rep 2020; 10: 19274. doi: 10.1038/s41598-020-76370-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zonta A, Pinelli T, Prati U, Roveda L, Ferrari C, Clerici AM, et al. Extra-Corporeal liver BNCT for the treatment of diffuse metastases: what was learned and what is still to be learned. Appl Radiat Isot 2009; 67(7-8 Suppl): S67–75. doi: 10.1016/j.apradiso.2009.03.087 [DOI] [PubMed] [Google Scholar]
- 61.Henry CJ, Downing S, Rosenthal RC, Klein MK, Meleo K, Villamil JA, et al. Evaluation of a novel immunomodulator composed of human chorionic gonadotropin and Bacillus Calmette-Guerin for treatment of canine mast cell tumors in clinically affected dogs. Am J Vet Res 2007; 68: 1246–51. doi: 10.2460/ajvr.68.11.1246 [DOI] [PubMed] [Google Scholar]
- 62.Sánchez-Rodríguez C, Cruces KP, Riestra Ayora J, Martín-Sanz E, Sanz-Fernández R. Bcg immune activation reduces growth and angiogenesis in an in vitro model of head and neck squamous cell carcinoma. Vaccine 2017; 35: 6395–403. doi: 10.1016/j.vaccine.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 63.Benitez MLR, Bender CB, Oliveira TL, Schachtschneider KM, Collares T, Seixas FK, Ruiz Benitez ML, Bonnemann Bender C, Larré Oliveira T, Kömmling Seixas F. Mycobacterium bovis BCG in metastatic melanoma therapy. Appl Microbiol Biotechnol 2019; 103: 7903–16. doi: 10.1007/s00253-019-10057-0 [DOI] [PubMed] [Google Scholar]
- 64.Kremenovic M, Schenk M, Lee DJ. Clinical and molecular insights into BCG immunotherapy for melanoma. J Intern Med 2020; 288: 625–40. doi: 10.1111/joim.13037 [DOI] [PubMed] [Google Scholar]
- 65.Hirose K, Konno A, Yoshimoto S, Ono K, Otsuki N, Hatazawa J, et al. Updated results of a phase II study evaluating accelerator-based boron neutron capture therapy (AB-BNCT) with borofalan(10B) (SPM-011) in recurrent squamous cell carcinoma (R-SCC-HN) and recurrent and locally advanced non-SCC (R/LA-nSCC-HN) of the head and neck. Ann. Oncol 2019; 30. [Google Scholar]