Abstract
Objectives:
Radiomics is the conversion of medical images into quantitative high-dimensional data. Laryngeal cancer, one of the most common head and neck cancers, has risen globally by 58.7%. CT, MRI and PET are acquired during the diagnostic process providing potential data for radiomic analysis and correlation with outcomes.
This review aims to examine the applications of this technique to laryngeal cancer and the future considerations for translation into clinical practice.
Methods:
A comprehensive systematic review-informed search of the MEDLINE and EMBASE databases was undertaken. Keywords “laryngeal cancer” OR “larynx“ OR “larynx cancer” OR “head and neck cancer” were combined with “radiomic” OR “signature” OR “machine learning” OR “artificial intelligence”. Additional articles were obtained from bibliographies using the “snowball method”.
Results:
The included studies (n = 15) demonstrated that radiomic features are significantly associated with various clinical outcomes (including stage, overall survival, treatment response, progression-free survival) and that predictive models incorporating radiomic features are superior to those that do not. Two studies demonstrated radiomics could improve laryngeal cancer staging whilst 12 studies affirmed its predictive capability for clinical outcomes.
Conclusions:
Radiomics has potential for improving multiple aspects of laryngeal cancer care; however, the heterogeneous cohorts and lack of data on laryngeal cancer exclusively inhibits firm conclusions. Large prospective well-designed studies in laryngeal cancer are required to progress this field. Furthermore, to implement radiomics into clinical practice, a unified research effort is required to standardise radiomics practice.
Advances in knowledge:
This review has highlighted the value of radiomics in enhancing laryngeal cancer care (including staging, prognosis and predicting treatment response).
Introduction
Since the 1990s, head and neck cancer (HNC) incidence rates have risen by 33% and it is now the sixth most common cancer worldwide.1 A large proportion of these cases occur in the larynx.2,3 Laryngeal cancers are staged according to the American Joint Committee on Cancer (AJCC) Tumour Node Metastases (TNM) system.4 Early-stage cancers incorporate T-stage 1 and 2 tumours whilst T-stage 3 and 4 are deemed advanced-stage cancers.4 Early-stage disease has a 3-year disease-specific survival rate of 89%; however, advanced stage disease has a significantly worse survival rate of around 50%.5 The uncertainty regarding the optimal treatment modality (surgery or chemoradiotherapy) for advanced laryngeal cancer is an important issue and to improve outcomes, personalised (tumour-specific) treatment plans are required.6–9 With the currently available methods, it is a substantial challenge to predict which patients are likely to respond to a specific treatment modality. Therefore, creating robust clinical decision-making models incorporating all available data sources (including medical imaging) are crucial.
During the diagnostic and staging process for laryngeal cancer contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) are typically acquired.10 These images hold valuable information on the tumour and its surrounding microenvironment. Although historically, medical imaging had been solely interpreted subjectively by a clinician, with advances in computational algorithms it is now possible to extract quantitative (objective) information such as tumour shape, size, texture and intensity from such images in a reproducible and robust manner. These data can be analysed and correlated with clinical outcomes.11,12 This process of image conversion into mineable high-dimensional data is known as radiomics. The radiomics workflow involves four key steps: image acquisition, tumour segmentation, feature extraction and subsequent analysis resulting in a radiomic-based model (Figure 1). Furthermore, biopsies for laryngeal provide restricted data since samples are obtained from one anatomical site of the tumour at a single time point whilst the patient is under general anaesthetic. However, radiomics provides a non-invasive method of evaluating the whole tumour over time without the need for anaesthesia.13
Figure 1.
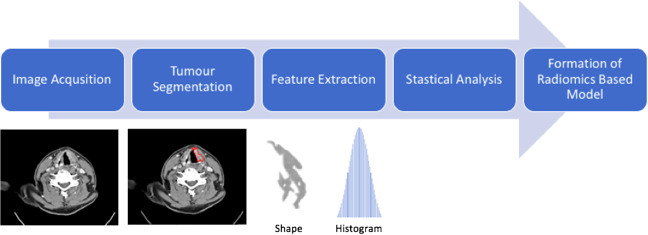
Radiomics Workflow.
To date, radiomics has shown great promise in various specialties and diseases.14 In lung cancer, radiomic techniques can15,16 accurately predict the cancer status (malignant or benign) of pulmonary nodules,15 the histological subtype of lung cancers16 and predict both survival and response to (chemo)radiotherapy.14 In oesophageal cancer, radiomics-based models can identify the stage of oesophageal squamous cell carcinomas,17 predict treatment response18 and prognosis19. The correlation with gene expression profiles have been demonstrated in multiple cancer sites.20,21
Despite this growing body of evidence, to our knowledge, no study has critically reviewed the value of radiomics in laryngeal cancer. Thus, this narrative review aims to evaluate the applications of radiomics in laryngeal cancer to date, highlight the limitations and the potential future uses.
Methods
Search strategy and selection criteria
A comprehensive systematic review informed search of the Ovid MEDLINE and EMBASE online databases (no interval/period stipulated) was undertaken in November 2020. Keywords “laryngeal cancer” OR “larynx” OR “larynx cancer” OR “head and neck cancer” were combined with “radiomic” OR “signature” OR “machine learning” OR “artificial intelligence”. This resulted in 889 publications. Subsequently duplicates were removed, abstracts were screened for relevance and only full peer-reviewed articles in the English language were included. Additional articles were obtained from bibliographies using the “snowball method.” Two independent reviewers conducted the search and screened the quality of the articles for inclusion. Discrepancies were resolved by consensus. All peer-reviewed articles incorporating radiomic analysis of patients with laryngeal cancer were included. This resulted in 15 articles (Figure 2). We synthesised study findings narratively.
Figure 2.
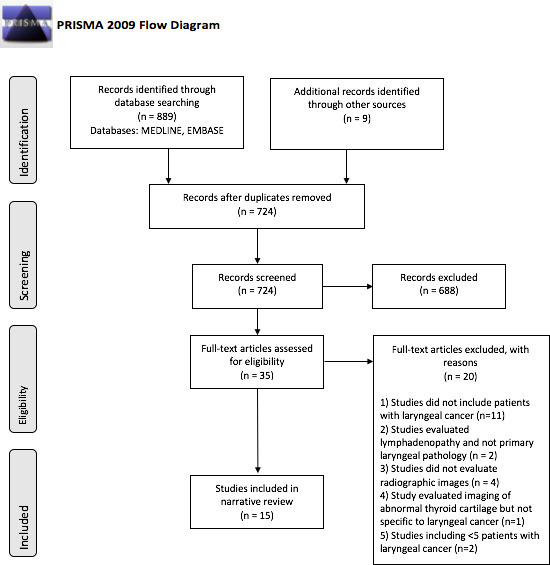
PRISMA flow diagram of the literature review process for studies on the application of radiomics in laryngeal cancer
Results
Through our structured search we identified 997 records, of which 15 studies met the inclusion criteria. Findings from the included studies are detailed below in three sections; the use of radiomics in laryngeal cancer staging (Table 1), the predictive value of CT-based radiomics (Table 2) and the predictive value of PET-based radiomics in laryngeal cancer (Table 3).
Table 1.
Summary of literature on the value of radiomics in the accuracy of staging for laryngeal cancer
| Author | Radiomics Software Used | Image Modality | Study Objective | Total Number of Laryngeal Cancer Patients (n) | Primary Treatment | Model Evaluation | Significant Radiomic Features | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Wang et al22 a | Pyradiomics | CT | Determine whether CT radiomics could enhance the accuracy of T-staging in advanced laryngeal cancer | 211 (TC: 150) (VC: 61) |
Surgery | Single Institute Cohort divided into training and validation cohorts |
Associated with T-Stage:
|
Developed a nomogram (combining the radiomic signature and T-stage reported by radiologists) with good accuracy (AUC: 0.892, 95% CI: 0.811–0.974) for T-staging. |
| Guo et al23 | Radcloud Platform & Anaconda3 platform | CT | Determine whether CT radiomics could aid in the prediction of thyroid cartilage invasion from laryngeal and hypopharyngeal cancer | 236 | Surgery | Single Institute 5-fold Cross-Validation |
four shape features seven first order features 5 GLRLM associated features 4 GLCM features 3 GLSZM features |
The Radiomics-based models (AUC 0.905, 95% CI 0.863–0.937) were more accurate than a clinical radiologist alone (0.721, 95% CI: 0.663–0.774) in predicting thyroid cartilage invasion |
AUC, area under curve; CT, Computed tomography, GLCM, Gray level co-occurrence matrix; GLRLM, gray level run-length matrix; GLSZM, Gray level size zone matrix; IDN, Inverse difference normalized; IMC, Informational measure of correlation; TR, Training cohort; VC, Validation cohort.
Study evaluates laryngeal cancer patients exclusively.
For wavelets where L and H are low- and high-frequency signals, respectively.
Table 2.
Summary of literature on the prognostic and predictive value of CT-based radiomics in laryngeal cancer
| Author | Radiomics Software Used | Image Modality | Study Objective | Total Number of Laryngeal Cancer Patients (n) | Primary Treatment | Model Evaluation | Significant Radiomic Features | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Chen et al24 a | LIFEx | CT | Evaluate the prognostic value of CT-based radiomics in patients with laryngeal SCC following surgical resection. | 136 (TC: 96) (VC: 40) |
Surgery ± adjuvant therapy | Cohort from single institute divided into training and validation cohorts | HGRE, LRHGE, ZLNU | A CT-based radiomics nomogram incorporating radiomic and clinicopathological features has significant value in predicting overall survival |
| Agarwal et al25 | TexRad | CT | Evaluate the predictive value of pre-treatment CT-radiomics in locally advanced laryngo-pharyngeal SCC with regards to local control and laryngectomy-free-survival. | 31 | Chemoradiotherapy | Nil | Radiomic Features predictive of:
Medium texture entropy Skewness |
Radiomic feature, medium texture entropy predicts inferior local control and laryngectomy-free survival in advanced laryngopharyngeal cancer |
| Ou et al26 | Oncoradiomics | CT | Evaluate the value of radiomics in patients with locally advanced HNSCC treated with chemoradiotherapy or bio-radiotherapy | 18 | Chemoradiotherapy Bio-radiotherapy | 10-fold cross-validation in single institute | Intensity features: 3 Shape features: 6 Texture features: 1 Wavelet features: 15 |
|
| Zhang et al27 | TexRad | CT | Evaluate whether CT-radiomics is associated with overall survival in patients with locally advanced HNSCC previously treated with induction chemotherapy | 21 | Induction Chemotherapy (cisplatin/5-FU/docetaxel) | Nil | Primary mass entropy Skewness |
Independent of size, N-stage, and other clinical variables, primary tumour mass texture analysis features mass entropy & skewness are associated with overall survival in patients treated with induction chemotherapy |
| Kuno et al28 | In-house developed radiomics software (MATLAB) | CT | Assess the value of radiomic textural features obtained from pre-treatment CT imaging of patients with HNSCC treated with chemoradiotherapy | 19 | Radiotherapy Chemoradiotherapy | Nil | three histogram features: Geometric mean, Harmonic mean, Fourth moment. 4 GLRLM features: SRE. GLNU, RLNU, and SRLGE |
Following adjustment for clinical variables, 3 histogram and 4 GLRL were associated with local failure in patients with HNSCC. |
| Cozzi et al29 | LIFEx | CT | Evaluate the ability of a CT-based radiomics signature to predict clinical outcome following chemoradiotherapy in stage III-IV HNSCC | 8 | Chemoradiotherapy | Nil | Radiomic Features predictive of: 1)Overall Survival: GLRLM RLNU, GLZLM GLNU, NGLDM Coarseness
Shape Volume, NGLDM Coarseness |
CT-based radiomic features correlate well with overall survival, progression-free survival and local tumour control in head and neck cancer patients |
| Meneghetti et al30 | Medical Imaging Radiomics Processor (MIRP) Python package | CT | To develop and validate a CT-based radiomics signature for the prognosis of loco-regional tumour control in patients with HNSCC treated by primary chemoradiotherapy | 8 | Chemoradiotherapy | 3-fold cross- validation across six partner sites | 10th percentile of intensity histogram High-dependence high-emphasis of the NGLM |
The final signature combined the tumour volume with two independent radiomics features and achieved moderately good discriminatory performance in determining locoregional control in a validation cohort. |
| Vallieres et al31 | In-house developed radiomics software (MATLAB) | 18F-FDG PET & CT | Investigate the value of PET and CT imaging based radiomics in combination with clinical variables to formulate a prediction model for risk of recurrence in HNCs. | 45 | Radiotherapy Chemoradiotherapy | Multi centre cohort divided into training and validation cohorts | Radiomic feature with the highest association with: 1)Locoregional recurrence: LZHGE [GLSZM] (from CT imaging) 2)Distant metastases: ZSN [GLSZM] (from CT imaging) 3)Overall survival: GLV [GLRLM] (from CT imaging). |
Radiomics provide important prognostic information regarding locoregional recurrence and distant metastases in HNC. |
| Keek et al32 | Oncoradiomics | CT | Investigate the use of CT-radiomics for prediction of overall survival, locoregional recurrence and distant metastases in advanced HNSCC peri-tumoural tissue treated with chemoradiotherapy. | 57 | Chemoradiotherapy | Multi centre cohort divided into training and validation cohorts | - | CT-based radiomic features obtained from peri-tumoural regions do not predict overall survival, locoregional recurrence and distant metastases |
5-FU, Fluorouracil; GLNU, Gray-level non-uniformity; GLCM, Gray-level co-occurrence matrix; GLSZM, Gray level size zone matrix; GLRLM, Gray-level run-length matrix; GLV, Gray-level variance; HGRE, High gray-level run emphasis; HNSCC, head and neck squamous cell carcinoma; LRHGE, Long-run high gray-level emphasis, LZHGE, Long-zone high gray-level emphasis; NGLDM, Neighbourhood gray-level different matrix; RLNU, Run-length non-uniformity, SCC, Squamous cell carcinoma; SRLGE, Short-run low gray-level emphasis; SRLGE, Short-run low gray-level emphasis; SRE, Short run emphasis; ZLNU, Zone length non-uniformity; ZSN, Zone size non-uniformity.
Study evaluates laryngeal cancer patients exclusively.
Table 3.
Summary of literature on the prognostic and predictive value of PET-based radiomics in laryngeal cancer
| Author | Radiomics Software Used | Image Modality | Primary Study Objective | Total number of Laryngeal Cancer Patients (n) | Primary Treatment | Model Evaluation | Significant Radiomic Features | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Guezennec et al33 | LIFEx | 18F-FDG PET-CT | Evaluate the prognostic value of radiomic features extracted from pre-treatment 18F-FDG PET/CT images in HNSCC | 32 | Surgery Chemoradiotherapy Palliative Treatment Radiotherapy alone Chemotherapy alone |
30-fold cross- validation in single institute | MTV Correlation |
MTV and one textural index extracted from pretherapeutic 18 F FDG‐PET/CT (Correlation) were independent prognostic factors of overall survival in patients with HNSCC |
| Feliciani et al34 | CGITA v1.3 | 18F-FDG PET-CT | Evaluate the value of pre-treatment 18F-FDG PET radiomics for the prediction of treatment failure in primary HNSCC treated with concurrent chemoradiotherapy | 14 | Chemoradiotherapy | 10-fold cross-validation in single Institute | LILRE | Lower LILRE, is associated with higher local failure in patients with HNSCC treated with chemoradiotherapy |
| Bogowicz et al35 | In house developed radiomics software:
|
18F-FDG PET-CT | Evaluate the association of post-chemoradiotherapy PET radiomics and local tumour control in HNSCC | 11 | Chemoradiotherapy | Cohort from single institute divided into training and validation cohorts | Histogram Range GLCM Difference Entropy. |
Higher histogram range and higher GLCM difference entropy corresponds to a greater risk of tumour recurrence. Both post-treatment PET-CT radiomic models were prognostic for local tumour control and performed equally well. |
| Bogowicz et al36 | In-house developed radiomics software (Python) | 18F-FDG PET, CT | Investigate the value of pre-treatment 18F-FDG PET radiomics for determining local tumour control in HNSCC patients. | 10 | Chemoradiotherapy | 5-fold cross-validation in single Institute | CT: GLSZM entropy, HLHa intensity energy. PET: spherical disproportion, SZLGE [GLSZM] |
Tumours more homogenous in CT density and with a focused region of high FDG uptake suggested a better prognosis. Both CT and PET radiomics showed equally good discriminative power for local tumour control in HNSCC. |
GLCM, Gray-level co-occurrence matrix; GLSZM, Gray-level size zone matrix; GLNU, Gray-level non-uniformity, GLRLM, Gray-level run length matrix; LILRE, Low-intensity long-run emphasis; MTV, Metabolic tumour volume; SZLGE, Short-zone low gray-level emphasis.
For wavelets where L and H are low- and high-frequency signals, respectively.
Radiomics in laryngeal cancer staging
Two studies were identified from the indexed literature (summarised in Table 1). Guo et al23 evaluated the use of CT-based radiomics to predict thyroid cartilage invasion from squamous cell carcinomas (SCC) of the larynx and hypopharynx. In this patient cohort (n = 236), 80 had evidence of thyroid cartilage invasion (confirmed by histopathological assessment). The authors demonstrated that a radiomics-based model had greater capability than a radiologist alone in determining thyroid cartilage invasion from CT imaging. The model performance was assessed by the area under the receiver operator curve (AUC). The AUC is a measure of the accuracy of a model. A value <0.5 is deemed as no better accuracy than chance, whilst a value with perfect accuracy is 1. The AUCs for predicting thyroid cartilage invasion were 0.905 (95%CI, 0.863–0.937) and 0.876 (95%CI 0.830–0.913) for the two radiomics-based models and 0.721 (95%CI, 0.663–0.774) for the radiologist alone. This study was limited due to its use of three different scanners (with different scanning parameters) and tumour demarcation was conducted by two junior radiologists, which may result in inaccurate segmentation.
Wang et al22 assessed the value of CT-radiomics in distinguishing between T3 and T4 tumours, since surgical decision-making is often influenced by this distinction. 211 patients with locally advanced laryngeal cancer who underwent a total laryngectomy were included. Eight radiomic features were associated with the T-stage. Three models were subsequently formed and assessed for their predictive capability of T-stage (as determined by histopathological assessment following total laryngectomy); a radiologist alone, a radiomics signature and a nomogram (a pictorial representation of a complex mathematical model that generates a probability of an event37) combining both. The AUCs were 0.775 (95%CI, 0.667–0.883), 0.862 (95% CI: 0.772–0.952) and 0.892 (95% CI: 0.811–0.974) for the radiologist alone, the radiomics signature and a combination of radiology reporting and the radiomics signature, respectively. The main limitations of this study are it was a single-centre retrospective study and the CT imaging analysed may have been taken some time before the total laryngectomy thus the histopathological assessment may not be a true reflection of the CT findings.
Predictive value of radiomics in laryngeal cancer
Overall, there is limited literature on the value of predictive radiomics exclusively in laryngeal cancer. However, studies have been conducted in HNCs, which incorporate a subcohort of laryngeal cancer patients. Detailed below are studies evaluating the predictive capability of CT- and PET-based radiomics.
Predictive value of computed tomography–based radiomics
Nine studies were included that evaluated the predictive value of CT-based radiomics (summarised in Table 2). Chen et al24 used LIFEx radiomics software to analyse CT imaging of 96 laryngeal cancer patients. They subsequently generated a radiomics nomogram incorporating three radiomic textural features (high gray-level run emphasis, long-run high gray-level emphasis and zone length non-uniformity) and clinicopathological outcomes. The predictive capability of the model was assessed by the concordance index (C-index). The C-index provides a goodness-of-fit statistic for a predictive model whilst accounting for censored data. Therefore, it is often used to evaluate predictive models for survival. Similar to AUC, a value <0.5 suggests a poor model whilst a value closer to one suggests a strong model. The radiomics nomogram had better prognostic capability (overall survival – OS) than cancer staging alone (C-index, 0.817 vs 0.682, p = 0.009) and demonstrated good agreement between actual and predicted survival. However, this retrospective study conducted in a single centre included a relatively small population, with potential selection bias. Although the training and validation cohorts were similar, the treatment received, and complications of treatment could also be potential confounders.
Agarwal et al25, analysed pre-treatment imaging of advanced laryngopharyngeal SCC (n = 31) treated with chemoradiotherapy and demonstrated that medium texture entropy was an independent predictor of local control (p < 0.001) and laryngectomy-free-survival (p < 0.001). This study had numerous strengths; firstly, the patient cohort was uniformly treated with chemoradiotherapy unlike other studies which have an array of different treatment approaches. Furthermore, all images were obtained using a uniform protocol from the same CT scanner. However, the tumour was contoured by a single operator thus cannot account for interoperator variability. Also, the tumour was delineated at the point of maximal cross-sectional area, meaning that the entire tumour was not analysed; although this may be less time-consuming if implemented into clinical practice.
In the indexed literature, eight further studies evaluating CT-radiomics were identified that incorporated a subcohort of laryngeal cancer patients. Ou et al26 analysed pre-treatment images of patients with locally advanced HNCs treated with chemoradiotherapy (cisplatin with radiotherapy) or bio-radiotherapy (cetuximab with radiotherapy). 18 of 120 patients included in the study had laryngeal cancer. Multivariate analysis showed a radiomic signature (incorporating 24 radiomic features) significantly predicted OS (HR 0.3, p = 0.02) and progression-free survival (PFS) (HR 0.3, p = 0.02). Further combination with the molecular phenotype (p16 status) created an enhanced predictive model (AUC = 0.78 vs AUC = 0.67, p = 0.01) for overall survival. This study highlights the utility of a multitude of data sources such as clinical staging, risk factors for disease, radiomic features and molecular phenotypes to form robust predictive models. However, this retrospective study included a small heterogenous population (primary tumours of distinct anatomical subsites) and during the study period the standard of care for CT acquisition changed, which could impact the validity of the results. Furthermore, the implementation of the p16 status into the model has limited value in laryngeal cancer since this is of prognostic importance in oropharyngeal cancers.
Zhang et al27, incorporating 21 patients with laryngeal cancer, evaluated the association of CT radiomics with survival in HNC patients treated with induction chemotherapy (cisplatin, 5-flurouracil, docetaxel). The authors identified primary mass entropy (HR 2.10, p = 0.036) and skewness measurements (HR 3.67, p = 0.009) as independent predictors of overall survival. There were multiple limitations in this retrospective study including a small heterogeneous patient population, lack of validation in an external cohort, exclusion of non-contrast CT imaging which excludes patients with renal impairment and only a single user performed the segmentation. Kuno et al28 also demonstrated that numerous textural features including three histogram features (geometric mean [HR 4.68, p = 0.026], harmonic mean [HR 8.61, p = 0.004], and fourth moment [HR 4.56, p = 0.048]) and four gray-level run-length features (short-run emphasis [HR 3.75, p = .044], gray-level nonuniformity [HR 5.72, p = 0.004], run-length nonuniformity (HR 4.15, p = 0.043), and short-run low gray-level emphasis [HR 5.94, p = 0.035]) were associated with local treatment failure in HNSCC (19/62 patients with laryngeal cancer). However, the study included a heterogeneous population, with differing CT protocols, slice thickness and exclusion of regions demonstrating necrosis and ulceration. Cozzi et al29 also confirmed that CT-based radiomic features correlated well with overall survival (Run length Non-Uniformity, Gray-Level Non-Uniformity, Neighbourhood Gray-Level Different Matrix (NGLDM) Coarseness), PFS (Shape Compacity, Gray-Level Co-occurrence Matrix (GLCM) Correlation) and local control (Shape Volume, NGLDM Coarseness) in HNC patients (8/110 with laryngeal cancer) treated with chemoradiotherapy. Although CT acquisition parameters were uniform, limitations include the study’s retrospective nature, inclusion of a heterogeneous population from a single centre, lack of external validation and segmentation by one radiation oncologist.
Meneghetti et al30 both, developed and validated a radiomics signature incorporating two significant radiomic features (high-dependence high-emphasis of the NGLDM and 10th percentile of histogram intensity) locoregional control in advanced HNSCC. This model achieved good discriminatory performance when performed on an external validation cohort (C-Index 0.66, 95% CI 0.55–0.75). However as with a lot of these studies there were a limited sub cohort of laryngeal cancer patients, only 8 of 233, to draw conclusions regarding laryngeal cancer.
Vallières et al31 conducted one of the largest studies to date, and extracted 1615 radiomic features from pre-treatment CT and PET images of 300 patients (45 with laryngeal cancer) to correlate with the risk of locoregional recurrence and distant metastases in HNCs. The prediction models formulated (combining both clinical and radiomic features) were good predictors of locoregional control (AUC 0.69, C-Index 0.67) and distant metastases (AUC 0.86, C-Index 0.88). This study had numerous strengths, in particular that patient recruitment was undertaken at multiple sites and the combination of PET- and CT-based radiomics.
Finally, Keek et al32 identified that CT-based radiomic features extracted from peri-tumoural tissue (surrounding microenvironment) from HNSCCs were not useful in the prediction of locoregional recurrence or distant metastasis. This study incorporated 57 (of 444) patients with laryngeal cancer. Although Keek et al32 used an external validation dataset, this study was limited due to its retrospective nature, leading to several clinical features (such as weight loss) not being comparable between the training and validation cohorts and the omission of valuable semantic imaging.
Predictive value of positron emission tomography–based radiomics
Alongside CT, PET imaging with fluorodeoxyglucose (18F-FDG) provides useful functional and metabolic information on a tumour38. As such, PETCT has been targeted for radiomic analysis. Although to date, there are no studies that evaluate PET radiomics exclusively in laryngeal cancer, four studies were identified from the literature that include a subcohort of laryngeal cancer patients (summarised in Table 3).
Guezennec et al33 identified metabolic tumour volume (p = 0.008) and the textural feature correlation (p = 0.028) obtained from 18F-FDG PET in HNCs were prognostic factors for overall survival. Of the 284 patients included, 32 patients had laryngeal cancer. There are several limitations in this study including analysis of a heterogeneous cohort of patients with tumours of differing anatomical subsites and staging and in 99 patients, due to very small regions of interest, they were unable to extract radiomic features. Feliciani et al34 used PET radiomics to predict treatment failure in HNCs treated with chemoradiotherapy. 14 (of 90) patients had laryngeal cancer and retrospective radiomic analysis identified a lower Low-intensity Long-run Emphasis (LILRE) was associated with greater risk of treatment failure. Additionally, the model based on radiomics markers was superior in predicting local failure compared to the model based on clinical variables alone (C-index 0.76 vs 0.65).
Bogowicz et al also conducted two studies to determine the benefits of 18F-FDG PET radiomics in head and neck cancer.35,36 In one study they extracted radiomic features using two different “in-house” radiomics software packages from images three months post-radiotherapy.35 The aim was to predict tumour control and determine whether the radiomics software used had a substantial impact. 11 of 128 patients had laryngeal cancer, and the authors demonstrated that radiomic features histogram range and GLCM difference entropy were associated with local control and both models (from differing software) performed equally well (with C-indices > 0.7 in all models). This emphasises that different software implementations can be equally valuable. Their second study compared the use of 18F-FDG PET to CT radiomics for patients with HNSCC.36 This study included 10 (of 121) patients with laryngeal cancer. The model demonstrated that more homogeneous tumours (Gray-Level Size Zone Matrix (GLSZM) entropy) with a focused region of high FDG uptake (GLSZM Short-Zone Low Gray-level Emphasis) suggested a better prognosis. Both PET, CT and PET/CT (combined)-based models performed equally well in predicting local tumour control with C-indices of 0.72, 0.74, 0.77, respectively. This study has certain limitations including its retrospective nature, the lack of external validation and patients were recruited from a single centre potentially resulting in selection bias. Furthermore, it is well known that not the entire tumour exhibits increased metabolic activity; therefore, areas of the tumour may have been missed through the auto-segmentation process.
Discussion
This review has demonstrated that radiomic features extracted from CT and PET imaging can help in predicting treatment response, molecular phenotypes, prognosis and assist in the staging process for laryngeal cancer. 14 of the 15 studies showed that radiomic features are significantly associated with various outcomes (including OS, treatment response, PFS) and that predictive models incorporating radiomic features are superior to models that exclude radiomics. Despite these promising findings, the lack of studies evaluating laryngeal cancer exclusively means drawing valid conclusions is challenging. Further large collaborative multicentre prospective studies exclusive to laryngeal cancer are required to progress this field.
Laryngeal cancer staging
Accurate pre-therapeutic staging of laryngeal carcinomas is an important factor in guiding treatment. For patients undergoing surgery studies have often demonstrated, there are often discrepancies between the clinical stage (clinical examination with imaging) and the pathological stage (based on the pathology specimen), which can result in inadequate or excessive treatment.39
There are two key challenges regarding laryngeal cancer staging that need to be addressed. Firstly, the distinction between T2 and T3 tumours because current guidelines advises different treatment options. Due to its excellent soft tissue resolution, MRI imaging is often used to aid in this distinction as it can delineate cartilage involvement and deep tumour extension.40 T2 tumours are advised microsurgery or radiotherapy, whilst T3 tumours are recommended chemoradiotherapy or surgery with adjuvant (chemo)radiotherapy.41 The second challenge is the distinction between T3 and T4 tumours, which is predominantly based on the extent of extra laryngeal spread and/or destruction of the thyroid cartilage.42 This distinction is crucial in deciding between chemoradiotherapy or a total laryngectomy. Thus, it is important to identify additional methods to stage the cancer correctly as this could have a significant impact on a patient’s outcome.
There are two studies detailed in the results (Guo et al23, Wang et al22), which focus on advanced laryngeal cancer and show the benefits of radiomics in predicting thyroid cartilage invasion and distinguishing between T3 and T4 tumours. Clinically, accurate assessment of cartilage invasion is essential as this will influence the treatment strategy. Firstly, invasion of the inner cortex of the thyroid cartilage suggests T3 disease, which has a differing treatment approach relative to T2 tumours.4 Furthermore, invasion through the thyroid cartilage would suggest T4 disease and would thus meet criteria for a potential laryngectomy.42
To date, no studies have sought to use radiomics to tackle the challenge of early stage disease (i.e. the distinction between T2 and T3). Further large-scale studies addressing the challenges of staging are required.
Predictive value of radiomics in laryngeal cancer
Of the 13 studies evaluating, the predictive value of radiomics only one study (Chen et al24) focussed exclusively on laryngeal cancer. The authors demonstrated that a radiomics-based model incorporating three radiomic features and clinicopathological features (tumour stage, anatomical subsite, laryngectomy) had good predictive capability for OS. In fact, 12 of the 13 studies demonstrated that including radiomic features significantly improves prediction models for various outcomes. However, this may be due to publication bias, where negative results have not been published. Only one study, conducted by Keek et al32, highlighted that radiomic features were not useful, although this study did evaluate peri-tumoural tissue rather than the entire tumour. Although these results show promise it is important to highlight that the majority of studies include heterogeneous cohorts of patients with an array of different HNCs of distinct anatomical subsites at various stages. Thus, further studies are required before extrapolating these findings to laryngeal cancer.
A key focus in laryngeal cancer should be the formation of robust prediction models to aid in stratifying patients more likely to respond to specific treatments. Radiomics alone is not a solution for these models but if combined with other key clinicopathological features (such as demographics, risk factors for disease, histopathological features and genomic structure)43 can form robust clinical decision-making models.
Limitations of literature
There are several limitations that resonate throughout the literature and should be addressed in future studies. Firstly, the majority of these studies have been conducted in single centres and have failed to validate their findings externally. They also include heterogeneous cohorts with an array of different HNCs with laryngeal cancer generally poorly represented. Secondly, the use of different scanners with different scanning parameters and different protocols also limits the literature. The radiomic software implementations ranged from in-house developed software to readily available free software (e.g. LIFEx) (as seen in Table 2). The reliability and prognostic value of radiomic features can be highly dependent on the platform used, thus making this process coherent and unified is a significant challenge44. Although the image biomarker standardisation initiative (an international collaboration) is making strides in standardising this process.31 In addition, radiomic contouring was often conducted by a single operator and thus could not account for interobserver variability and has greater potential for human error.
Finally, the lack of biological and genomic data to allow correlation with radiomic features. The correlation will improve our understanding of specific radiomic features and how they reflect tumour biology.
Future considerations
Radiomics has significant promise but further developments are required before translation from bench to bedside. Firstly, the level of evidence regarding laryngeal cancer is insufficient and large multicentre prospective studies exclusively in laryngeal cancer are required. A consensus also needs to be reached regarding scanning protocols, image segmentation and feature extraction software. In the future, deep learning techniques could also be implemented to perform its own implicit feature extraction.45,46 Finally, to improve reproducibility, publications should allow access to raw data, and methods used to extract features.
The strengths of this review include the comprehensive search strategy and the screening process using two independent reviewers. Furthermore, the pitfalls that resonate throughout the literature have been highlighted. These can act as a guide for researchers formulating new studies to ensure these are addressed. However, there are some limitations. The lack of studies exclusively on laryngeal cancer, the use of different software implementations and the use of different scanning parameters means drawing meaningful conclusions is difficult. Finally, our review was limited to studies in the English language.
Conclusion
This review highlights that radiomics has great potential for improving multiple aspects of laryngeal cancer care from staging, prognosis to predicting treatment response. By evaluating the current literature, this review provides a stepping stone for the future integration of radiomics in laryngeal cancer care.
However, the lack of data on laryngeal cancer exclusively means drawing conclusions is challenging. Further large prospective studies exclusively in laryngeal cancer, utilising genomic and biological data are required to progress this field. In order to implement radiomics into clinical practice, a unified research effort is required to standardise radiomic practice.
Footnotes
Additional Information: AR is a National Institute for Health Research Academic Clinical Fellow. There was no funding source for this review.
Contributors: AR, DH, EA, BO developed the concept of this review. Titles and abstracts were screened by two researchers (AR and SP). Full text screening for eligibility was done by two reviewers (AR and SP). AR quality appraised studies and produced the tables and the figure. AR wrote the first draft of the manuscript with input from all authors. All authors contributed to drafting and editing the manuscript.
Contributor Information
Amarkumar Dhirajlal Rajgor, Email: amar.rajgor@nhs.net.
Shreena Patel, Email: shreenapatel93@gmail.com.
David McCulloch, Email: david.mcculloch@nhs.net.
Boguslaw Obara, Email: boguslaw.obara@newcastle.ac.uk.
Jaume Bacardit, Email: Jaume.Bacardit@newcastle.ac.uk.
Andrew McQueen, Email: andrew.mcqueen1@nhs.net.
Eric Aboagye, Email: eric.aboagye@imperial.ac.uk.
Tamir Ali, Email: t.ali@nhs.net.
James O’Hara, Email: j.ohara@nhs.net.
David Winston Hamilton, Email: david.hamilton3@nhs.net.
REFERENCES
- 1.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, regional, and National cancer incidence, mortality, years of life lost, years lived with disability, and Disability-Adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 2017; 3: 524–48. doi: 10.1001/jamaoncol.2016.5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Research UK. Head and neck cancers incidence statistics. 2020. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/head-and-neck-cancers/incidence#heading-Four.
- 3.Nocini R, Molteni G, Mattiuzzi C, Lippi G. Updates on larynx cancer epidemiology. Chin J Cancer Res 2020; 32: 18–25. doi: 10.21147/j.issn.1000-9604.2020.01.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin MB, Edge S, Greene F. et al.AJCC Cancer staging manual. 8th edition. New York: Springer International Publishing: American Joint Commission on Cancer; 2017. [Google Scholar]
- 5. National Cancer Intelligence Network . Head and neck cancers in England relative survival by age and stage. Oxford: Oxford Cancer Intelligence Unit;. 2011. [Google Scholar]
- 6.Olsen KD. Reexamining the treatment of advanced laryngeal cancer. Head Neck 2010; 32: 1–7. doi: 10.1002/hed.21294 [DOI] [PubMed] [Google Scholar]
- 7.Hoffman HT, Porter K, Karnell LH, Cooper JS, Weber RS, Langer CJ, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope 2006; 116: 1–13. doi: 10.1097/01.mlg.0000236095.97947.26 [DOI] [PubMed] [Google Scholar]
- 8.Department of Veterans Affairs Laryngeal Cancer Study Group, Wolf GT, Fisher SG, Hong WK, Hillman R, Spaulding M, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med 1991; 324: 1685–90. doi: 10.1056/NEJM199106133242402 [DOI] [PubMed] [Google Scholar]
- 9.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003; 349: 2091–8. doi: 10.1056/NEJMoa031317 [DOI] [PubMed] [Google Scholar]
- 10.Joshi VM, Wadhwa V, Mukherji SK. Imaging in laryngeal cancers. Indian J Radiol Imaging 2012; 22: 209–26. doi: 10.4103/0971-3026.107183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017; 14: 749–62. doi: 10.1038/nrclinonc.2017.141 [DOI] [PubMed] [Google Scholar]
- 12.Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RGPM, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012; 48: 441–6. doi: 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sollini M, Antunovic L, Chiti A, Kirienko M. Towards clinical application of image mining: a systematic review on artificial intelligence and radiomics. Eur J Nucl Med Mol Imaging 2019; 46: 2656–72. doi: 10.1007/s00259-019-04372-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arshad MA, Thornton A, Lu H, Tam H, Wallitt K, Rodgers N, et al. Discovery of pre-therapy 2-deoxy-2-18F-fluoro-D-glucose positron emission tomography-based radiomics classifiers of survival outcome in non-small-cell lung cancer patients. Eur J Nucl Med Mol Imaging 2019; 46: 455–66. doi: 10.1007/s00259-018-4139-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Balagurunathan Y, Atwater T, Antic S, Li Q, Walker RC, et al. Radiological image traits predictive of cancer status in pulmonary nodules. Clin Cancer Res 2017; 23: 1442–9. doi: 10.1158/1078-0432.CCR-15-3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkins S, Wang H, Liu Y, Garcia A, Stringfield O, Krewer H, et al. Predicting malignant nodules from screening CT scans. J Thorac Oncol 2016; 11: 2120–8. doi: 10.1016/j.jtho.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, Wang C, Tan X, Cheng Z, Zhao K, Yan L, et al. Radiomics approach for preoperative identification of stages I-II and III-IV of esophageal cancer. Chin J Cancer Res 2018; 30: 396–405. doi: 10.21147/j.issn.1000-9604.2018.04.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aerts HJWL, Grossmann P, Tan Y, Oxnard GR, Rizvi N, Schwartz LH, et al. Defining a radiomic response phenotype: a pilot study using targeted therapy in NSCLC. Sci Rep 2016; 6: 33860. doi: 10.1038/srep33860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coroller TP, Grossmann P, Hou Y, Rios Velazquez E, Leijenaar RTH, Hermann G, et al. Ct-Based radiomic signature predicts distant metastasis in lung adenocarcinoma. Radiother Oncol 2015; 114: 345–50. doi: 10.1016/j.radonc.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Kim J, Qu F, Liu S, Wang H, Balagurunathan Y, et al. Ct features associated with epidermal growth factor receptor mutation status in patients with lung adenocarcinoma. Radiology 2016; 280: 271–80. doi: 10.1148/radiol.2016151455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gevaert O, Mitchell LA, Achrol AS, Xu J, Echegaray S, Steinberg GK, et al. Glioblastoma multiforme: exploratory radiogenomic analysis by using quantitative image features. Radiology 2014; 273: 168–74. doi: 10.1148/radiol.14131731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, Zhang B, Wu X, Liu L, Fang J, Chen Q, et al. Radiomic nomogram improves preoperative T category accuracy in locally advanced laryngeal carcinoma. Front Oncol 2019; 9: 1064. doi: 10.3389/fonc.2019.01064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo R, Guo J, Zhang L, Qu X, Dai S, Peng R, et al. CT-based radiomics features in the prediction of thyroid cartilage invasion from laryngeal and hypopharyngeal squamous cell carcinoma. Cancer Imaging 2020; 20: 81. doi: 10.1186/s40644-020-00359-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Wang H, Zeng H, Zhang Y, Ma X. Evaluation of CT-based radiomics signature and nomogram as prognostic markers in patients with laryngeal squamous cell carcinoma. Cancer Imaging 2020; 20: 28. doi: 10.1186/s40644-020-00310-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal JP, Sinha S, Goda JS, Joshi K, Mhatre R, Kannan S, et al. Tumor radiomic features complement clinico-radiological factors in predicting long-term local control and laryngectomy free survival in locally advanced laryngo-pharyngeal cancers. Br J Radiol 2020; 93: 20190857. doi: 10.1259/bjr.20190857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ou D, Blanchard P, Rosellini S, Levy A, Nguyen F, Leijenaar RTH, et al. Predictive and prognostic value of CT based radiomics signature in locally advanced head and neck cancers patients treated with concurrent chemoradiotherapy or bioradiotherapy and its added value to human papillomavirus status. Oral Oncol 2017; 71: 150–5. doi: 10.1016/j.oraloncology.2017.06.015 [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Graham CM, Elci O, Griswold ME, Zhang X, Khan MA, et al. Locally advanced squamous cell carcinoma of the head and neck: CT texture and histogram analysis allow independent prediction of overall survival in patients treated with induction chemotherapy. Radiology 2013; 269: 801–9. doi: 10.1148/radiol.13130110 [DOI] [PubMed] [Google Scholar]
- 28.Kuno H, Qureshi MM, Chapman MN, Li B, Andreu-Arasa VC, Onoue K, et al. Ct texture analysis potentially predicts local failure in head and neck squamous cell carcinoma treated with chemoradiotherapy. AJNR Am J Neuroradiol 2017; 38: 2334–40. doi: 10.3174/ajnr.A5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cozzi L, Franzese C, Fogliata A, Franceschini D, Navarria P, Tomatis S, et al. Predicting survival and local control after radiochemotherapy in locally advanced head and neck cancer by means of computed tomography based radiomics. Strahlenther Onkol 2019; 195: 805–18. doi: 10.1007/s00066-019-01483-0 [DOI] [PubMed] [Google Scholar]
- 30.Rabasco Meneghetti A, Zwanenburg A, Leger S, Leger K, Troost EGC, Linge A, et al. Definition and validation of a radiomics signature for loco-regional tumour control in patients with locally advanced head and neck squamous cell carcinoma. Clinical and Translational Radiation Oncology 2021; 26: 62–70. doi: 10.1016/j.ctro.2020.11.011 [DOI] [Google Scholar]
- 31.Zwanenburg A, Vallières M, Abdalah MA, Aerts HJWL, Andrearczyk V, Apte A, et al. The image biomarker standardization initiative: standardized quantitative Radiomics for high-throughput image-based phenotyping. Radiology 2020; 295: 328–38. doi: 10.1148/radiol.2020191145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keek S, Sanduleanu S, Wesseling F, de Roest R, van den Brekel M, van der Heijden M, et al. Computed tomography-derived radiomic signature of head and neck squamous cell carcinoma (peri)tumoral tissue for the prediction of locoregional recurrence and distant metastasis after concurrent chemo-radiotherapy. PLoS One 2020; 15: e0232639. doi: 10.1371/journal.pone.0232639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guezennec C, Robin P, Orlhac F, Bourhis D, Delcroix O, Gobel Y, et al. Prognostic value of textural indices extracted from pretherapeutic 18-F FDG-PET/CT in head and neck squamous cell carcinoma. Head Neck 2019; 41: 495–502. doi: 10.1002/hed.25433 [DOI] [PubMed] [Google Scholar]
- 34.Feliciani G, Fioroni F, Grassi E, Bertolini M, Rosca A, Timon G, et al. Radiomic Profiling of Head and Neck Cancer: 18 F-FDG PET Texture Analysis as Predictor of Patient Survival. Contrast Media Mol Imaging 2018; 2018: 1–8. doi: 10.1155/2018/3574310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bogowicz M, Leijenaar RTH, Tanadini-Lang S, Riesterer O, Pruschy M, Studer G, et al. Post-radiochemotherapy PET radiomics in head and neck cancer - The influence of radiomics implementation on the reproducibility of local control tumor models. Radiother Oncol 2017; 125: 385–91. doi: 10.1016/j.radonc.2017.10.023 [DOI] [PubMed] [Google Scholar]
- 36.Bogowicz M, Riesterer O, Stark LS, Studer G, Unkelbach J, Guckenberger M, et al. Comparison of PET and CT radiomics for prediction of local tumor control in head and neck squamous cell carcinoma. Acta Oncol 2017; 56: 1531–6. doi: 10.1080/0284186X.2017.1346382 [DOI] [PubMed] [Google Scholar]
- 37.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015; 16: e173–80. doi: 10.1016/S1470-2045(14)71116-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomasi G, Aboagye EO. Introduction to the analysis of PET data in oncology. J Pharmacokinet Pharmacodyn 2013; 40: 419–36. doi: 10.1007/s10928-013-9307-3 [DOI] [PubMed] [Google Scholar]
- 39.Contrera KJ, Hair BB, Prendes B, Reddy CA, Zimmer DI, Burkey BB, et al. Clinical versus pathologic laryngeal cancer staging and the impact of stage change on outcomes. Laryngoscope 2021; 131: 559–65. doi: 10.1002/lary.28924 [DOI] [PubMed] [Google Scholar]
- 40.Agnello F, Cupido F, Sparacia G, et al. Computerised tomography and magnetic resonance imaging of laryngeal squamous cell carcinoma: a practical approach. computerised tomography and magnetic resonance imaging of laryngeal squamous cell carcinoma: a practical approach. Neuroradiol J 2017; 30: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Institute for Health and Care Excellence. Treatment of upper aerodigestive tract cancer – Larynx. 2021. Available from: https://pathways.nice.org.uk/pathways/upper-aerodigestive-tract-cancer#path=view%3A/pathways/upper-aerodigestive-tract-cancer/treatment-of-upper-aerodigestive-tract-cancer.xml&content=view-node%3Anodes-larynx.
- 42.Timmermans AJ, Lange CAH, de Bois JA, van Werkhoven E, Hamming-Vrieze O, Hilgers FJM, et al. Tumor volume as a prognostic factor for local control and overall survival in advanced larynx cancer. Laryngoscope 2016; 126: E60–7. doi: 10.1002/lary.25567 [DOI] [PubMed] [Google Scholar]
- 43.Gatenby RA, Grove O, Gillies RJ. Quantitative imaging in cancer evolution and ecology. Radiology 2013; 269: 8–14. doi: 10.1148/radiol.13122697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fornacon-Wood I, Mistry H, Ackermann CJ, Blackhall F, McPartlin A, Faivre-Finn C, et al. Reliability and prognostic value of radiomic features are highly dependent on choice of feature extraction platform. Eur Radiol 2020; 30: 6241–50. doi: 10.1007/s00330-020-06957-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosny A, Aerts HJ, Mak RH. Handcrafted versus deep learning radiomics for prediction of cancer therapy response [published correction appears in Lancet Digit Health. 2019 Aug;1(4):e160. Lancet Digit Health 2019; 1: e106–7. [DOI] [PubMed] [Google Scholar]
- 46.Bibault J-E, Giraud P, Housset M, Durdux C, Taieb J, Berger A, et al. Author correction: deep learning and Radiomics predict complete response after neo-adjuvant chemoradiation for locally advanced rectal cancer. Sci Rep 2018; 8: 16914. doi: 10.1038/s41598-018-35359-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


