Abstract
Introduction
The rate of hospital-acquired coronavirus disease 2019 has reduced from 14.3% to 4.2% over the last year, but substantial differences still exist between English National Health Service (NHS) hospital trusts.
Methods
This study assessed rates of hospital-acquired infection (HAI), comparing NHS hospital trusts using airborne respiratory protection (e.g. FFP3 masks) for all staff, as a marker of measures to reduce airborne spread, with NHS hospital trusts using mainly droplet precautions (e.g. surgical masks).
Results/discussion
The use of respiratory protective equipment was associated with a 33% reduction in the odds of HAI in the Delta wave, and a 21% reduction in the odds of HAI in the Alpha wave (P<0.00001). It is recommended that all hospitals should prioritize airborne mitigation.
Keywords: COVID-19, Infection control, Nosocomial infections, Personal protective equipment, Respiratory protective devices
Introduction
Hospital-acquired infections (HAIs) are associated with poorer outcomes for both the individual and the wider healthcare system, and hospital-acquired coronavirus disease 2019 (COVID-19) in the UK National Health Service (NHS) has been a significant driver of the pandemic [1]. National reporting and investigation of other nosocomial infections have led to a significant reduction in rates [2], but there has been little central work to understand the large variation in nosocomial COVID-19 rates between NHS hospital trusts. Over the last 18 months, there has been substantial development in the understanding of airborne transmission, asymptomatic spread, use of personal protective equipment (PPE), and regular testing of patients and staff [[3], [4], [5]]. UK NHS hospital trusts have common guidance as to COVID-19 pathways and isolation [6], but resources and implementation vary between trusts. The improvements and variation still seen suggest that nosocomial spread of COVID-19 is not inevitable, but the question remains as to how best to reduce rates and keep them low.
The 2020 guidance stated that severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was spread by the droplet route except for a list of aerosol-generating procedures (AGPs); changes in 2021 acknowledged airborne transmission but stated that droplet precautions alone are required for healthcare staff unless a local risk assessment suggests otherwise [6]. However, some NHS hospital trusts have chosen to protect staff against airborne transmission by allowing the use of respiratory protective equipment (RPE, e.g. FFP3 masks) for all staff caring for patients with COVID-19. Early in 2020, shortages of PPE may have limited the opportunity to use higher levels of protection, but by summer 2020, PPE supplies had improved [7]. It is hypothesized that this acceptance of airborne transmission, and the mitigation measures it entails, is associated with a reduction in the nosocomial transmission of SARS-CoV-2.
Methods
Hospital-acquired COVID-19 was calculated as ‘probable’ or ‘definite’ HAI from NHS England weekly COVID-19 statistics, defined as COVID-19 diagnoses made ≥8 days after hospital admission, and before hospital discharge. The first available data were from 1st August 2020; data were separated into waves, with the ‘Alpha’ wave from 1st August 2020 to 30th April 2021, and the ‘Delta’ wave from 1st May 2021 onwards. Data were downloaded from NHS England on 16th September 2021, and the last data point was 12th September 2021. The HAI rate for COVID-19 (‘HAI rate’) was calculated as a percentage of the total COVID-19 cases for each NHS hospital trust. Hospital size was calculated from NHS England overnight bed data, as the average number of acute hospital beds open overnight from July to September 2020. Hospital COVID-19 pressure was calculated as the total number of patients with COVID-19 treated per acute hospital bed.
RPE use was determined from a public dataset maintained by FreshAirNHS, compiled from news reports, public statements and private communication. All NHS hospital trusts recorded as using RPE were contacted on 20th September 2021 to confirm their current practice, and an open call was made to other trusts who may have been missing from the dataset to come forward. For each wave in the data, an NHS hospital trust was marked as using RPE if, at any point during the wave, it was recorded as allowing staff to use RPE when caring for patients with COVID-19 outside the setting of AGPs.
Data were analysed using R 4.0.4 (R Core Team). Significance testing used the exact 2x2 test for patient-level analysis, and Wilcoxon rank-sum test for NHS hospital trust-level analysis. Correlations were calculated as Spearman's rank correlation coefficient. Data used in this analysis are publicly available and accompany this article (see online supplementary material).
Results
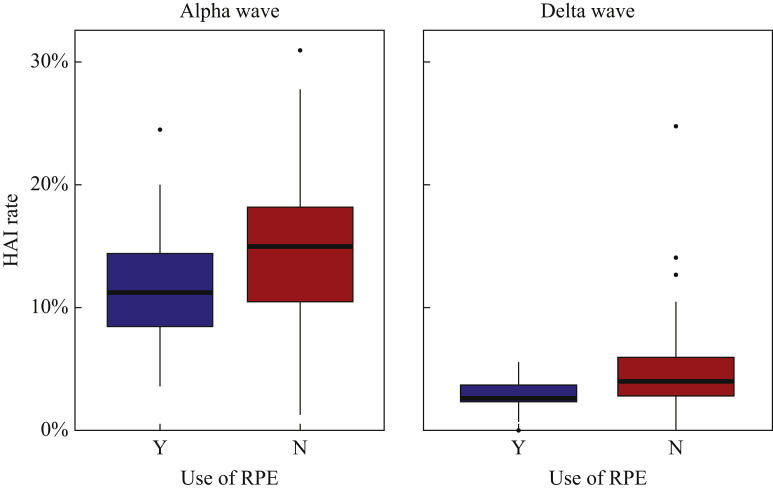
Results for NHS hospital trust-level and patient-level analyses are given in Table I , and overall NHS hospital trust-level rates are shown in Figure 1 . During the Alpha wave, it is estimated that 6000 additional patients (95%CI 5150–6800) at non-RPE NHS hospital trusts caught COVID-19 in hospital than if rates at NHS hospital trusts using RPE had been replicated across England.
Table I.
Hospital-acquired infection (HAI) rates during the Alpha and Delta waves
| Alpha wave (Aug 2020–Apr 2021) HAI rates | Delta wave (May 2021–Sep 2021) HAI rates |
|---|---|
| Trusts using RPE (N=14) | Trusts using RPE (N=16) |
| Mean 11.9% | Mean 2.8% |
| Median 11.2% (IQR 8.4–14.4%) | Median 2.6% (IQR 2.2–3.7%) |
| Trusts not using RPE (N=109) | Trusts not using RPE (N=107) |
| Mean 14.7% | Mean 4.7% |
| Median 15.0% (IQR 10.4–18.2%) | Median 4.0% (IQR 2.7–6.0%) |
| Reduction in HAI rates | Reduction in HAI rates |
| Absolute: 3.0% (-0.3–6.3%) | Absolute: 1.4% (0.3–2.7%) |
| P=0.0713 | P=0.0088 |
| Patient level | Patient level |
| Overall proportion 14.3% | Overall proportion 4.2% |
| At trusts using RPE (N=31,163) | At trusts using RPE (N=8746) |
| Proportion 11.8% | Proportion 3.0% |
| At trusts not using RPE (N=224,156) | At trusts not using RPE (N=45,542) |
| Proportion 14.6% | Proportion 4.4% |
| Reduction in HAI proportion | Reduction in HAI proportion |
| Absolute: 2.7% (2.4–3.1%) | Absolute: 1.4% (1.0–1.8%) |
| Relative odds: 21.3% (18–24%) | Relative odds: 33.1% (24–42%) |
| P<0.00001 | P<0.00001 |
RPE, respiratory protective equipment.
Figure 1.
Boxplot of hospital-acquired infection (HAI) rates by National Health Service trust during the Alpha and Delta waves. RPE, respiratory protective equipment.
No significant correlation was found between HAI rate and hospital size (Alpha: P=0.25; Delta: P=0.16). A significant correlation was found between HAI rate and hospital COVID-19 pressure (Alpha: P=0.03; Delta: P=0.002); however, this had limited predictive value (linear regression – Alpha: R 2=0.05; Delta: R 2=0.05). The direction of the correlation indicated that hospitals with more cases of COVID-19 had lower HAI rates, probably because there were fewer patients without COVID-19 available to contract it. RPE use was not associated with hospital COVID-19 pressure (Alpha: P=0.74; Delta: P=0.29). HAI rate was not correlated with the total number of COVID-19 cases (Alpha: P=0.76; Delta: P=0.21).
A time series analysis of HAI rates across all acute NHS hospital trusts shows a large peak in December 2020 with average HAI rates >20%, followed by improvement to June 2021, corresponding with the rollout of vaccines in the UK. However, in this present wave up to September 2021, rates are increasing again.
Discussion
RPE use for all COVID-19-facing staff in English NHS hospital trusts is associated with a significant reduction in hospital-acquired COVID-19. Whilst RPE use outside AGPs was used as a marker in this study, it is considered that the results are likely due to a multi-modal series of interventions directed against airborne transmission in NHS hospital trusts which have prioritized action in this way. The reduction in HAI rates demonstrated is greater than would be expected directly from RPE use (i.e. by preventing asymptomatically infected healthcare workers from infecting patients, reported as 9%) [8]. The data presented demonstrate that the measures associated with RPE implementation, likely involving improvements to ventilation and air filtration, were associated with a 21% and 33% relative reduction in the odds of nosocomial COVID-19 for the Alpha and Delta waves, respectively.
The authors made efforts to obtain accurate data on RPE use; however, it is possible that some NHS hospital trusts using RPE officially or unofficially may have been missed in this study. This would tend to reduce the effect size, so the results may underestimate the true impact. These findings are in keeping with modelling suggesting that screening and effective PPE use are effective interventions to reduce nosocomial COVID-19 transmission [9]. The finding that high NHS hospital trust COVID-19 pressure was associated with a lower HAI rate is novel, but the effect size is relatively small and is potentially because high COVID-19 occupancy means there are few patients without COVID-19 who are available to catch it. It also suggests that community rates are not the main driver of hospital-acquired COVID-19 once considered as a percentage of cases. The combined patient-level and NHS hospital trust-level analyses ensure that small hospitals with large outbreaks do not skew the analysis, but care must be taken in interpretation – the patient-level numbers do not represent risk for a COVID-19-negative patient subsequently catching it in hospital, but the proportion of COVID-19-positive patients in hospital who probably caught it there.
The greater relative reduction in HAI rates seen with the Delta wave in NHS hospital trusts implementing RPE likely reflects the underlying hierarchy of controls being more effective from April 2021, coinciding with updates to national infection prevention and control guidance which emphasized their use [6]. In the later wave, NHS hospital trusts were seemingly better able to isolate cases through enhanced staff and patient testing, achieving reductions in HAI rates in all trusts irrespective of airborne mitigation. Once all NHS hospital trusts are implementing the hierarchy appropriately, this increased homogeneity may cause differences such as airborne mitigation to have a larger relative effect on reducing nosocomial transmission.
At the peak in December 2020, high HAI rates could have been driven by a variety of factors including overcrowding as England's COVID-19 hospitalizations rose rapidly, leading to a breakdown in the ability to cohort patients. The drop in HAI at the January peak of hospitalizations may have represented the early vaccine rollout, other mitigations, or simply there being fewer patients without COVID-19 in the hospitals to catch it. Whilst vaccines and other mitigations have reduced the rates of hospital-acquired COVID-19, there is room for improvement as further mitigations directed against airborne transmission are rolled out. Whilst it is relatively quick to deploy FFP3 respirators outside of AGP areas, it is much slower to audit ventilation and deploy air filtration units, and even slower to upgrade hospital estates to include more side rooms including negative pressure isolation rooms. However, it is important to recognize that even hospitals with predominantly side room provision have seen nosocomial spread (personal communication, C. Peters). The potential role of ventilation and air flows in these types of wards is in keeping with detailed investigations of quarantine hotel outbreaks in Australia.
Recent data highlight that >4% of hospital COVID-19 cases are still nosocomial, and by only including patients who became positive after >7 days, this is likely to be an underestimate, particularly for Delta where the peak of infectivity is earlier than previous lineages [10]. Also, patients who have acquired infection but are not yet shedding the virus at their last test before discharge will likewise not be counted; this is a limitation of the underlying data. Equally, healthcare workers themselves have been at risk of contracting COVID-19 in hospitals, and this is not covered by these data; other work has shown that airborne mitigation can reduce the risk to staff [4]. The time series data show that COVID-19 HAI rates have been worsening since July 2021, lending an urgency to finding ways to improve further. The Scientific Advisory Group for Emergencies advice from early 2021 suggests a range of ways to mitigate airborne transmission of COVID-19 in hospitals, and more general advice has existed for longer [11]. It is recommended that all hospitals should adopt appropriate airborne aerosol mitigation to protect staff and patients.
Acknowledgements
Data provided by NHS England under Open Government Licence v3.0.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2021.11.018.
Conflict of interest statement
TL and MB are clinicians at NHS hospital trusts which use RPE for all patients with COVID-19.
Funding
No funding was sought or provided for this study.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Scientific Advisory Group for Emergencies . 2021. PHE and LSHTM: the contribution of nosocomial infections to the first wave, 28 January. Report No.: S1056. London: SAGE; 2021. [Google Scholar]
- 2.National Audit Office . TSO; London: 2009. Reducing healthcare associated infections in hospitals in England: report. [Google Scholar]
- 3.Greenhalgh T., Jimenez J.L., Prather K.A., Tufekci Z., Fisman D., Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. 2021;397:1603–1605. doi: 10.1016/S0140-6736(21)00869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferris M., Ferris R., Workman C., O’Connor E., Enoch D.A., Goldesgeyme E., et al. Efficacy of FFP3 respirators for prevention of SARS-CoV-2 infection in healthcare workers. ELife. 2021;10 doi: 10.7554/eLife.71131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivett L., Sridhar S., Sparkes D., Routledge M., Jones N.K., Forrest S., et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. ELife. 2020;9 doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Public Health England . PHE; London: 2021. COVID-19 infection prevention and control guidance – version 1.2. Report No.: GOV-8505. [Google Scholar]
- 7.National Audit Office . National Audit Office; London: 2020. The supply of personal protective equipment (PPE) during the COVID-19 pandemic. Report No.: HC 961. [Google Scholar]
- 8.Illingworth C.J., Hamilton W.L., Warne B., Routledge M., Popay A., Jackson C., et al. Superspreaders drive the largest outbreaks of hospital onset COVID-19 infections. ELife. 2021;10 doi: 10.7554/eLife.67308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pham T.M., Tahir H., van de Wijgert J.H.H.M., Van der Roest B.R., Ellerbroek P., Bonten M.J.M., et al. Interventions to control nosocomial transmission of SARS-CoV-2: a modelling study. BMC Med. 2021;19:211. doi: 10.1186/s12916-021-02060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J., Li B., Deng A., Li K., Hu Y., Li Z., et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 Delta variant. MedRxiv. 2021 doi: 10.1101/2021.07.07.21260122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morawska L., Tang J.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., et al. How can airborne transmission of COVID-19 indoors be minimised? Environ Int. 2020;142:105832. doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.