Recently, Whitaker et al. reported changes in SARS-CoV-2 seroprevalence in adults after introduction of a vaccination programme. Here we describe the impact of the delta wave and initiation of vaccination on seroprevalence in children.1 Sero-epidemiological surveys are important to monitor temporal and geographical distribution of SARS-CoV-2 and provide information on asymptomatic infections. Age-stratified surveys enable monitoring of prevalence estimates in different age groups and their contribution to transmission.
The UK Health Security Agency (UKHSA, formerly PHE) along with NHS partners and academic collaborators implemented a range of national sero-surveillance programmes to monitor antibody prevalence to COVID-19 in children and young adults, which included expansion of existing collections. Here we present results from residual serum samples collected from children aged one to 17 years in England from September 2020 to October 2021.
The UKHSA Sero-epidemiology Unit (SEU) coordinates a national collection across seven NHS regions of residual serum samples from routine microbiological testing which was enhanced at the start of the pandemic to increase sample numbers and geographic representation. Overall, a total of 26 hospital trusts have participated in the main SEU collection since the start of the pandemic with an average of 200 samples from children aged one to 17 years tested each month. In addition, a targeted paediatric collection from 18 hospitals across England providing paediatric services was established, with approximately 500 paediatric samples collected each month. Demographical data collected include age, sex and geographical region. The SEU has ethical approval for collection of anonymised samples for serosurveys of diseases for which a vaccine exists or is in active development (05/Q0505/45).
Samples were tested using two serological assays. The Roche Elecsys assay was used for detection of high avidity total antibody to SARS-CoV-2 nucleocapsid (N) protein, which informs on previous exposure to SARS-CoV-2. Sensitivity and specificity are 83.9% (95% CI 74.8–90.7) and 100% (95% CI 99.1–100), respectively in samples collected within 12 weeks of onset.2 The Roche Elecsys assay was used to detect antibodies to SARS-CoV-2 spike (S) protein receptor binding domain with a sensitivity of 95.5% (95%CI 93.2–97.1) and specificity of 100% (95% CI 99.1–100).3 This assay detects previous infection as well as vaccine induced immune response. As waning with assays based on S antibody detection are less pronounced than for N-based assays, analyses are focused on results from the S assay.4
Bayesian multilevel regression and poststratification (MRP) models5 were used to estimate seropositivity, with poststratification by age group and NHS region based on Office for National Statistics population estimates. Analyses were carried using RStan within R.6
From 1st September 2020 to 31st October 2021, 5209 paediatric sera (age groups 1–4 years, n = 945; 5–11 years, n = 1525; 12–15 years, n = 2033; 16–17 years, n = 706) were obtained from the SEU and targeted paediatric collections.
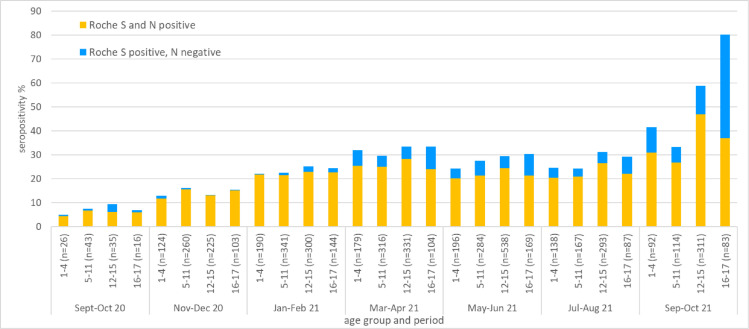
The overall national prevalence estimate of seropositivity, weighted by age group and NHS region based on results from the Roche S assay, increased from 7.6% (95% CrI 3.2–18.2%) for the period September to October 2020 to 31.5% (25.3%–39.1%) in March and April 2021, and, after remaining stable over summer, increased to 46.1% (38.3–53.6%) in October 2021 (Fig. 1 and Table 1 ).
Fig. 1.
Population weighted seropositivity estimates (posterior median) of residual samples from the SEU and paediatric collections by period and age group, obtained from September 2020 to October 2021 using the Roche S and N assay. Stacked columns represent the proportion of samples testing positive with both assays (yellow) and the proportion testing positive with the Roche S, but negative with Roche N (blue).
Table 1.
Population weighted seropositivity estimates (posterior median with 95% credible interval) of residual SEU and paediatric collections samples collected September 2020 to October 2021 using the Roche S and N assays.
| Roche S | Roche N | ||||||
|---|---|---|---|---|---|---|---|
| Period | Age, region | Pos | Total | Population weighted% pos (95% CI) | Pos | Total | Population weighted% pos (95% CI) |
| Sept-Oct 2020 | All | 8 | 119 | 7.6% (3.2% - 18.2%) | 6 | 120 | 6.3% (2.2% - 17%) |
| 1–4 | 0 | 26 | 5.1% (0.4% - 16.4%) | 0 | 26 | 4.5% (0.4% - 15.7%) | |
| 5–11 | 3 | 42 | 7.5% (2.4% - 20.5%) | 3 | 43 | 6.7% (2% - 20.3%) | |
| 12–15 | 4 | 35 | 9.5% (3.3% - 25.5%) | 2 | 35 | 6.1% (1.6% - 19.2%) | |
| 16–17 | 1 | 16 | 7% (1.4% - 21.8%) | 1 | 16 | 6% (1.3% - 20.4%) | |
| Lon | 3 | 14 | 10.7% (3.3% - 32.4%) | 3 | 14 | 10.5% (2.5% - 33.2%) | |
| NE | 2 | 63 | 4.3% (1% - 10.2%) | 1 | 64 | 2.7% (0.4% - 8%) | |
| NW | 3 | 27 | 8.1% (2.7% - 20%) | 2 | 27 | 5.6% (1.4% - 15.9%) | |
| SW | 0 | 13 | 4.6% (0.3% - 14.6%) | 0 | 13 | 3.3% (0.2% - 12.9%) | |
| Nov-Dec 2020 | All | 94 | 711 | 14.8% (11.2% - 19.8%) | 90 | 712 | 14.1% (10.6% - 19%) |
| 1–4 | 13 | 124 | 13% (7.6% - 19.2%) | 11 | 124 | 11.7% (6.3% - 18%) | |
| 5–11 | 42 | 259 | 16.2% (11.7% - 22.8%) | 40 | 260 | 15.6% (11.2% - 22.1%) | |
| 12–15 | 25 | 225 | 13.4% (9% - 19.1%) | 25 | 225 | 13.1% (8.8% - 18.8%) | |
| 16–17 | 14 | 103 | 15.5% (10.2% - 24.4%) | 14 | 103 | 15.3% (9.9% - 24.4%) | |
| Lon | 20 | 61 | 30.3% (19.8% - 42.6%) | 20 | 61 | 30.3% (19.8% - 42.5%) | |
| Mid | 3 | 20 | 13.3% (4.6% - 28.9%) | 3 | 20 | 12.9% (4.3% - 28.6%) | |
| NE | 30 | 206 | 14.5% (10.2% - 19.6%) | 28 | 206 | 13.5% (9.4% - 18.5%) | |
| NW | 32 | 199 | 15.6% (11.2% - 21%) | 31 | 200 | 15% (10.6% - 20.2%) | |
| SW | 3 | 35 | 9.2% (3.2% - 19.6%) | 2 | 35 | 7% (1.9% - 16.5%) | |
| Jan-Feb 2021 | All | 201 | 970 | 23.3% (18.4% - 29.5%) | 182 | 975 | 22.1% (17.3% - 28.3%) |
| 1–4 | 32 | 190 | 22.2% (15.5% - 29.3%) | 31 | 190 | 21.8% (15.6% - 28.7%) | |
| 5–11 | 68 | 340 | 22.6% (17.1% - 29.4%) | 62 | 341 | 21.6% (16.3% - 28.3%) | |
| 12–15 | 70 | 298 | 25.2% (19.4% - 32.6%) | 61 | 300 | 23% (17.6% - 30.2%) | |
| 16–17 | 31 | 142 | 24.5% (18.3% - 32.6%) | 28 | 144 | 22.8% (17% - 30.5%) | |
| Lon | 32 | 88 | 34% (24.7% - 44.3%) | 31 | 90 | 32.6% (23.6% - 42.7%) | |
| Mid | 10 | 22 | 39.4% (21.8% - 59.6%) | 10 | 22 | 39.5% (21.9% - 59.7%) | |
| NE | 74 | 412 | 17.7% (14.3% - 21.6%) | 67 | 413 | 16.1% (12.8% - 19.8%) | |
| NW | 75 | 288 | 25.6% (20.9% - 30.8%) | 66 | 288 | 22.6% (18.1% - 27.7%) | |
| SW | 2 | 66 | 4.8% (1.3% - 11.3%) | 2 | 68 | 4.5% (1.2% - 10.8%) | |
| Mar-Apr 2021 | All | 288 | 925 | 31.5% (25.3% - 39.1%) | 244 | 930 | 25.7% (20.1% - 33%) |
| 1–4 | 53 | 178 | 31.9% (24.3% - 40.6%) | 43 | 179 | 25.4% (18.5% - 33.8%) | |
| 5–11 | 87 | 315 | 29.7% (22.6% - 37.9%) | 78 | 316 | 25% (18.7% - 32.8%) | |
| 12–15 | 107 | 329 | 33.6% (26.4% - 42.3%) | 97 | 331 | 28.3% (21.4% - 37.1%) | |
| 16–17 | 41 | 103 | 33.5% (25.5% - 43.8%) | 26 | 104 | 24.1% (16.4% - 32.7%) | |
| Lon | 41 | 102 | 38% (29% - 47.7%) | 35 | 102 | 33% (24.5% - 42.6%) | |
| Mid | 9 | 20 | 39.4% (22.9% - 59.5%) | 8 | 21 | 32.9% (17.8% - 52.6%) | |
| NE | 114 | 489 | 23.1% (19.4% - 27%) | 103 | 492 | 20.3% (16.9% - 24.1%) | |
| NW | 118 | 267 | 43.3% (37.4% - 49.2%) | 95 | 267 | 34.9% (29.4% - 40.7%) | |
| SW | 4 | 35 | 15.9% (6.5% - 29%) | 2 | 36 | 10.1% (2.8% - 22.2%) | |
| May-Jun 2021 | All | 328 | 1186 | 27.5% (20.7% - 37.2%) | 281 | 1187 | 21.8% (16.1% - 31.1%) |
| 1–4 | 43 | 196 | 24.3% (16.3% - 34.9%) | 39 | 196 | 20.3% (13.6% - 30.1%) | |
| 5–11 | 76 | 284 | 27.5% (19.9% - 37.9%) | 63 | 284 | 21.4% (15.1% - 31.3%) | |
| 12–15 | 152 | 537 | 29.4% (22% - 39.7%) | 141 | 538 | 24.4% (17.7% - 34.7%) | |
| 16–17 | 57 | 169 | 30.5% (22.1% - 42.2%) | 38 | 169 | 21.3% (14.6% - 31.5%) | |
| Lon | 96 | 211 | 42.6% (35.6% - 49.9%) | 80 | 211 | 36.1% (29.6% - 43.1%) | |
| Mid | 5 | 16 | 27.5% (12.4% - 48.7%) | 3 | 16 | 17.4% (6% - 36.2%) | |
| NE | 125 | 511 | 23.8% (20% - 27.9%) | 120 | 512 | 22% (18.3% - 26.1%) | |
| NW | 87 | 276 | 31.2% (25.9% - 36.9%) | 69 | 276 | 24.7% (19.8% - 30.1%) | |
| SW | 15 | 171 | 9% (5.4% - 13.9%) | 9 | 171 | 5.8% (3% - 10%) | |
| Jul-Aug 2021 | All | 202 | 685 | 26.4% (20.1% - 35.3%) | 176 | 685 | 22.2% (16.5% - 31.4%) |
| 1–4 | 29 | 138 | 24.7% (16.8% - 34.9%) | 24 | 138 | 20.5% (13.3% - 30.6%) | |
| 5–11 | 39 | 167 | 24.2% (16.7% - 34.1%) | 36 | 167 | 21% (14.3% - 30.9%) | |
| 12–15 | 98 | 293 | 31.2% (23.1% - 41.8%) | 89 | 293 | 26.6% (19% - 37.5%) | |
| 16–17 | 36 | 87 | 29.2% (20.4% - 41.2%) | 27 | 87 | 22.1% (14.6% - 33%) | |
| Lon | 81 | 161 | 45.7% (37.1% - 54.4%) | 72 | 161 | 41.4% (33.2% - 49.9%) | |
| Mid | 35 | 111 | 30% (22.1% - 38.8%) | 24 | 111 | 20.8% (14.1% - 28.9%) | |
| NE | 80 | 354 | 21.3% (17.1% - 26%) | 76 | 354 | 20% (15.9% - 24.6%) | |
| NW | 3 | 12 | 24.3% (8.7% - 47.6%) | 3 | 12 | 22.5% (7.5% - 46.6%) | |
| SW | 3 | 45 | 8.6% (2.8% - 18.6%) | 1 | 45 | 4.7% (0.9% - 13.3%) | |
| Sep-Oct 2021 | All | 327 | 600 | 46.1% (38.3% - 53.6%) | 239 | 600 | 33.5% (26.8% - 40.9%) |
| 1–4 | 42 | 92 | 41.5% (30.2% - 53.6%) | 30 | 92 | 31.1% (21.7% - 41.9%) | |
| 5–11 | 37 | 114 | 33.3% (24% - 44.1%) | 29 | 114 | 26.7% (18.3% - 36.8%) | |
| 12–15 | 182 | 311 | 59% (48.9% - 67.5%) | 152 | 311 | 46.9% (37.5% - 56%) | |
| 16–17 | 66 | 83 | 80.3% (69.2% - 88.4%) | 28 | 83 | 36.9% (26.5% - 48.9%) | |
| Lon | 39 | 63 | 43.3% (31.8% - 55.4%) | 31 | 63 | 40.2% (29.3% - 52.8%) | |
| Mid | 52 | 87 | 55% (45.3% - 65.1%) | 34 | 87 | 36.6% (27.7% - 46.5%) | |
| NE | 159 | 316 | 44.1% (38.2% - 50.2%) | 135 | 316 | 34.4% (28.6% - 40.8%) | |
| NW | 37 | 55 | 59.5% (47% - 72.1%) | 25 | 55 | 39.4% (28.5% - 52%) | |
| SW | 39 | 76 | 34.9% (24.7% - 46.9%) | 14 | 76 | 18.2% (10.4% - 29%) | |
Roche S seropositivity varies by age with higher seropositivity persisting in children aged above 12 years at 59% (48.9–67.5%) for 12 to 15 year olds and 80.3% (69.2–88.4%) in those aged 16 to 17 in October.
Estimates based on the Roche N assay were largely comparable to results from the S assay from September to February 2021. However thereafter, N-based estimates were overall lower, with more pronounced increases of S-based estimates, particularly in those aged 16 to 17-years in recent months sowing an increase from 36.9% in August to 80.3% in October.
Seropositivity also varies by geographical region with the higher seropositivity in London and Northern regions compared to the South West throughout the surveillance period (see Table 1).
Our findings show large recent increases in seropositivity in children from September to October 2021 after a plateau which had persisted since the beginning of a phased exit out of national lockdown. Whilst increases of estimates in all age groups based on the Roche N assay indicate an increase in transmission following the start of the school academic year and is consistent with other surveillance data,7 the more pronounced increases seen in S-based estimates during this time period in older children reflect the deployment of a vaccine programme for 16–17 year olds. Over 80% of this age group had detectable antibodies through infection and /or vaccination by October. The initial moderately higher estimates through S-based assays during the summer months is likely to reflect early waning of antibodies in the Roche N-assay. In comparison, in those aged 12 to 15 years for whom a vaccine has been made available at the end of the reported period there was a significant increase in N-based estimates (26.6–46.9%) in October.
Seroprevalence studies are required to understand transmission dynamics and inform on the amount of asymptomatic infection; in children, almost half of all COVID−19 infection have been shown to be asymptomatic.8
This study has limitations, residual samples are not collected at random but obtained from individuals undergoing diagnostic and screening tests. Individuals having to provide regular blood samples may be more vulnerable, using more precautions and thus are unlikely to be representative of the general population. However, these provide valuable information on trends over time and enable comparison with other surveillance data which show trends consistent with our findings; school based studies report large increases in the beginning of the year with a third of students seropositive by Ladhani9 which then stabilized over summer.10
These findings highlight the importance of ongoing surveillance of paediatric seroprevalence to assess the extent of transmission in the paediatric population during the third wave and inform plans for future interventions, including the offer of a second dose to adolescents and expanding the paediatric programme with potential future availability of vaccines approved for use in children from 5 years of age. Acknowledgement: We would like to thank all hospital trusts that made this surveillance possible by providing samples throughout the pandemic.
Acknowledgement
We would like to thank all hospital trusts that made this surveillance possible by providing samples throughout the pandemic.
References
- 1.Whitaker H.J., et al. Impact of COVID-19 vaccination program on seroprevalence in blood donors in England, 2021. J Infect. 2021;83:237–279. doi: 10.1016/j.jinf.2021.04.037. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duggan J et al Public Health England Evaluation of Roche elecsys antiSARS-CoV-2 serology assay for the detection of anti-SARS-CoV-2 antibodies. 18th March 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/891598/Evaluation_of_Roche_Elecsys_anti_SARS_CoV_2_PHE_200610_v8.1_FINAL.pdf, accessed 25th November 2021.
- 3.Duggan J et al Public Health England Evaluation of Roche elecsys antiSARS-CoV-2 serology assay for the detection of anti-SARS-CoV-2 antibodies. 11th March 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/989460/Evaluation_of_Roche_Elecsys_anti_SARS_CoV_2_S_assay_PHE.pdf, accessed 25th November 2021.
- 4.Harris R.J., Whitaker H.J., Andrews N.J., Aiano F., Amin-Chowdhury Z., Flood J., et al. Serological surveillance of SARS-CoV-2: six-month trends and antibody response in a cohort of public health workers. J Infect. 2021;82:162–169. doi: 10.1016/j.jinf.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park D.K., Gelman A., Bafumi J. Bayesian multilevel estimation with poststratification: state level estimates from national polls. Polit Anal. 2004;12:375–385. [Google Scholar]
- 6.Stan Development Team (2020). “RStan: the R interface to stan.” R package version 2.21.2, http://mc-stan.org/, accessed 25th November 2021.
- 7.UKHSA Weekly National Influenza and COVID-19 Surveillance Report: Week 42 report 21 October 2021 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1027644/Weekly_Flu_and_COVID-19_report_w42_v2.pdf, accessed 25th November 2021.
- 8.Pratha S., et al. Asymptomatic SARS-CoV-2 infection: a systematic review and meta-analysis. Proc Natl Acad Sci. 2021;118(34) doi: 10.1073/pnas.2109229118. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladhani S.N., et al. Emergence of SARS-CoV-2 alpha (B.1.1.7) variant, infection rates, antibody seroconversion and seroprevalence rates in secondary school students and staff: active prospective surveillance, December 2020 to March 2021, England. J Infect. 2021;S0163-4453(21) doi: 10.1016/j.jinf.2021.08.019. Aug 1300401-1Epub ahead of print. PMID: 34400220; PMCID: PMC8361003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.COVID-19 Schools Infection Survey, England: round 6, pupil antibody data, June 2021 (21st October 2021) https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/covid19schoolsinfectionsurveyengland/round6pupilantibodydatajune2021, accessed 15th November 2021.