Abstract
High dietary protein may increase susceptibility of weaned pigs to enteric pathogens. Dietary supplementation with functional amino acids (FAA) may improve growth performance of pigs during disease challenge. The objective of this study was to evaluate the interactive effects of dietary protein content and FAA supplementation above requirements for growth on performance and immune response of weaned pigs challenged with Salmonella. Sixty-four mixed-sex weanling pigs (13.9 ± 0.82 kg) were randomly assigned to dietary treatments in a 2 × 2 factorial arrangement with low (LP) or high protein (HP) content and basal (AA–) or FAA profile (AA+; Thr, Met, and Trp at 120% of requirements) as factors. After a 7-d adaptation period, pigs were inoculated with either a sterile saline solution (CT) or saline solution containing Salmonella Typhimurium (ST; 3.3 × 109 CFU/mL). Growth performance, body temperature, fecal score, acute-phase proteins, oxidant/antioxidant balance, ST shedding score in feces and intestinal colonization, fecal and digesta myeloperoxidase (MPO), and plasma urea nitrogen (PUN) were measured pre- and postinoculation. There were no dietary effects on any measures pre-inoculation or post-CT inoculation (P > 0.05). Inoculation with ST increased body temperature and fecal score (P < 0.05), serum haptoglobin, plasma superoxide dismutase (SOD), malondialdehyde (MDA), PUN, and fecal MPO, and decreased serum albumin and plasma reduced glutathione (GSH):oxidized glutathione (GSSG) compared with CT pigs (P < 0.05). ST-inoculation reduced average daily gain (ADG) and feed intake (ADFI) vs. CT pigs (P < 0.05) but was increased by AA+ vs. AA– in ST pigs (P < 0.05). Serum albumin and GSH:GSSG were increased while haptoglobin and SOD were decreased in ST-inoculated pigs fed AA+ vs. AA– (P < 0.05). PUN was higher in HP vs. LP-fed pigs postinoculation (P < 0.05). Fecal ST score was increased in ST-inoculated pigs on days 1 and 2 postinoculation and declined by day 6 (P < 0.05) in all pigs while the overall score was reduced in AA+ vs. AA– pigs (P < 0.05). Cecal digesta ST score was higher in HP vs. LP-fed pigs and were lower in AA+ compared with AA– fed pigs in the colon (P < 0.05). Fecal and digesta MPO were reduced in ST pigs fed AA+ vs. AA– (P < 0.05). These results demonstrate a positive effect of FAA supplementation, with minimal effects of dietary protein, on performance and immune status in weaned pigs challenged with Salmonella.
Keywords: crude protein, functional amino acids, growth performance, pigs, Salmonella Typhimurium
Introduction
The difference between growth potential and achieved performance in commercial herds is due to a number of factors, including heat stress (Pearce et al., 2013), stocking density (Fu et al., 2016), feed characteristics (Boumans et al., 2015), and disease challenge (Pastorelli et al., 2012). The weaned piglet is particularly susceptible to a variety of challenges arising from an abrupt change from a milk-based to a cereal-based diet, potentially resulting in gastrointestinal health issues and exposure to immune and other stressors (Jha and Berrocoso, 2016). In particular, exposure to pathogens, such as Salmonella and Escherichia coli, may potentiate the negative effects of weaning on growth performance and inflammatory response in the gastrointestinal tract (Heo et al., 2009; Boyer et al., 2015). In addition, high protein (HP) content in nursery diets may exacerbate these negative effects on piglet gastrointestinal health due to the potential for increased production of protein fermentation metabolites (e.g., sulfur compounds, N-nitroso compounds, ammonia, heterocyclic amines, and organic acids) and increased proliferation of pathogenic bacteria (Htoo et al., 2007; Heo et al., 2009). As a result, current recommendations are to feed reduced protein diets with sufficient supplementation of essential amino acids (AA) to meet requirements for growth (van Milgen and Dourmad, 2015). This has been shown to improve gastrointestinal health (Fan et al., 2017) and reduce predisposition to enteric pathogens (Kim et al., 2011) as well as the incidence of postweaning diarrhea in challenged (Heo et al., 2009) and unchallenged (Heo et al., 2008) pigs.
It is well documented that during periods of immune stimulation (e.g., enteric disease challenge), there is a redistribution of nutrients from growth to immune system support (Reeds et al., 1994; Reeds and Jahoor, 2001). Under such situations, studies have shown that there is an increase in requirements of individual AA for growth, including Thr (Wellington et al., 2018), Met (Litvak et al., 2013), and Trp (de Ridder et al., 2012). More recently, “functional” roles of AA in supporting the immune system, maintaining the intestinal mucosal barrier, regulating the antioxidant defense, and synthesizing immune molecules have received greater attention (Le Floc’h et al., 2018). It has been suggested, therefore, that supplementation with key functional AA (FAA) may mitigate the negative effects of stressors. It has previously been reported that both the dietary protein content and the supplementation of Met, Thr, and Trp modulate the immune status of grower pigs under poor sanitary conditions (van der Meer et al., 2016). It is not known whether supplementation with FAA will improve growth performance, gut health, and immune status during the postweaning period.
The objective of the present study was to determine whether supplementation of FAA will improve growth performance and immune status of Salmonella-challenged weaned pigs and whether the effect of FAA is dependent on dietary protein content. It was hypothesized that supplementation with FAA would improve performance and immune status in pigs inoculated with Salmonella and/or fed an HP diet.
Materials and Methods
The experimental protocol was approved by the University of Saskatchewan’s Animal Research Ethics Board (AUP #20190003) and followed the Canadian Council on Animal Care guidelines (CCAC, 2009).
Animals, housing, and diets
A total of 64 mixed-sex weanling pigs (Camborough Plus × C3378; PIC Canada Ltd.) of 13.9 ± 0.82 kg initial body weight (BW) were obtained from the Prairie Swine Centre, Inc. (Saskatoon, SK) and transported to the Animal Care Unit of the Western College of Veterinary Medicine (Saskatoon, SK). The pigs were placed on trial in 2 blocks using 2 experimental rooms for each block. In each experimental room (25 °C ambient temperature), pigs were housed individually on solid floors lined with rubber mats. Pigs were randomly assigned to 1 of 8 treatments in a 2 × 2 × 2 factorial arrangement in a randomized complete block design (RCBD; n = 8 pigs/treatment) for 14 d, which consisted of a 7-d adaptation period (no inoculation) and 7 d postinoculation period. Dietary treatments consisted of a low protein [LP; 16% crude protein (CP)] or HP (20% CP) diet with either a basal (AA–) or functional (AA+) AA profile. Diets were corn-, wheat-, barley-, and soybean meal-based and were formulated using the reported nutrient content and analyzed AA content of ingredients to meet or exceed nutrient requirements for 11 to 25 kg pigs according to NRC (2012) and AMINODat 5.0 (Evonik, 2016; Tables 1 and 2). The HP diets were formulated by partly replacing corn in the LP diet with soybean meal. The AA– profile met the standardized ileal digestible (SID) AA requirements according to NRC (2012) and the AA+ profile contained Thr, Met, and Trp at 120% of requirements. Pigs were fed ad libitum and had unrestricted access to water.
Table 1.
Ingredient and nutrient composition of experimental diets1 (as-fed basis)
| LP | HP | |||
|---|---|---|---|---|
| Ingredients, % | AA– | AA+ | AA– | AA+ |
| Corn | 54.53 | 54.18 | 36.41 | 35.91 |
| Wheat | 13.00 | 13.00 | 13.00 | 13.00 |
| Barley | 17.50 | 17.50 | 17.50 | 17.50 |
| Soybean meal, 46% CP | 7.50 | 7.50 | 26.50 | 26.50 |
| Canola oil | — | — | 3.00 | 3.00 |
| l-Lys HCl2 | 1.13 | 1.13 | 0.55 | 0.55 |
| dl-Met2 | 0.33 | 0.48 | 0.17 | 0.31 |
| l-Trp2 | 0.10 | 0.15 | — | 0.05 |
| l-Thr2 | 0.42 | 0.57 | — | 0.31 |
| l-Arg2 | 0.55 | 0.55 | — | — |
| l-Leu2 | 0.43 | 0.43 | — | — |
| l-Iso2 | 0.32 | 0.32 | — | — |
| l-Val2 | 0.33 | 0.33 | 0.02 | 0.02 |
| l-His2 | 0.18 | 0.18 | — | — |
| l-Phe2 | 0.57 | 0.57 | - | - |
| Salt | 0.35 | 0.35 | 0.35 | 0.35 |
| Vitamin/mineral premix3 | 0.20 | 0.20 | 0.20 | 0.20 |
| Limestone | 1.38 | 1.38 | 1.32 | 1.32 |
| Monocalcium phosphate | 1.18 | 1.18 | 0.98 | 0.98 |
| Calculated nutrient content 4 | ||||
| DM, % | 87.07 | 87.12 | 84.52 | 84.57 |
| CP, % | 16.36 | 16.57 | 20.26 | 20.48 |
| ME, kcal/kg | 3259 | 3264 | 3343 | 3347 |
| NE, kcal/kg | 2510 | 2514 | 2507 | 2510 |
| SID Lys:CP, %:% | 0.08 | 0.08 | 0.06 | 0.06 |
| AA, % SID | ||||
| Arg | 1.16 | 1.16 | 1.16 | 1.16 |
| His | 0.45 | 0.45 | 0.45 | 0.45 |
| Ile | 0.72 | 0.72 | 0.72 | 0.72 |
| Leu | 1.40 | 1.40 | 1.40 | 1.40 |
| Lys | 1.28 | 1.28 | 1.28 | 1.28 |
| Met+Cys | 0.70 | 0.85 | 0.70 | 0.85 |
| Phe+Tyr | 0.86 | 0.86 | 1.08 | 1.08 |
| Thr | 0.76 | 0.91 | 0.76 | 0.91 |
| Trp | 0.20 | 0.25 | 0.20 | 0.25 |
| Val | 0.81 | 0.81 | 0.81 | 0.81 |
1LP, low protein; HP, high protein; AA–, basal AA profile; AA+, functional AA profile (Thr, Met, and Trp at 120% of requirements for growth); SID, standardized ileal digestible.
2l-Lys HCl, Archer Daniels Midland Company (Decatur, IL); dl-Met, Evonik Operations GmbH (Hanau-Wolfgang, Germany); l-Trp, l-Thr, and l-Val, Jefo Nutrition Inc. (Saint-Hyacinthe, QC, Canada); all other AA, ACP Chemicals, Inc. (St. Leonard, QC, Canada).
3Supplied per kilogram of complete diet: vitamin A, 6,000 IU; vitamin D, 9.3 mg; vitamin E, 35 IU; menadione, 2.5 mg; vitamin B12, 0.02 mg; thiamine, 1.00 mg; biotin, 0.10 mg; niacin, 20 mg; riboflavin, 4 mg; pantothenate, 12 mg; folic acid, 0.50 mg; pyridoxine, 5.0 mg; Fe,75 mg; Zn, 75 mg; Mg, 20 mg; Cu, 10 mg; Se, 0.15 mg, and I, 0.50 mg.
4Nutrient content of diets based on estimated nutrient contents of ingredients according to NRC (2012) and analyzed AA content according to Evonik Operations GmbH.
Table 2.
Analyzed nutrient content of experimental diets1 (as-fed basis)
| LP | HP | |||
|---|---|---|---|---|
| Item, % | AA– | AA+ | AA– | AA+ |
| DM | 88.5 | 89.1 | 88.8 | 88.9 |
| CP | 16.6 | 16.6 | 19.3 | 19.8 |
| Total AA2 | ||||
| Arg | 1.18 (1.21) | 1.22 (1.21) | 1.08 (1.24) | 1.17 (1.24) |
| His | 0.49 (0.49) | 0.49 (0.49) | 0.45 (0.49) | 0.48 (0.49) |
| Ile | 0.77 (0.77) | 0.83 (0.77) | 0.74 (0.81) | 0.78 (0.81) |
| Leu | 1.53 (1.51) | 1.44 (1.51) | 1.40 (1.57) | 1.47 (1.57) |
| Lys | 1.38 (1.36) | 1.48 (1.36) | 1.36 (1.41) | 1.47 (1.41) |
| Met+Cys | 0.74 (0.76) | 0.88 (0.90) | 0.72 (0.79) | 0.87 (0.93) |
| Phe+Tyr | 1.14 (1.51) | 0.98 (1.51) | 0.91 (1.57) | 1.17 (1.57) |
| Thr | 0.87 (0.83) | 0.98 (0.97) | 0.83 (0.85) | 0.99 (1.01) |
| Trp | 0.22 (0.22) | 0.29 (0.27) | 0.23 (0.23) | 0.26 (0.28) |
| Val | 0.85 (0.88) | 0.84 (0.88) | 0.85 (0.92) | 0.87 (0.92) |
1LP, low protein; HP, high protein; AA–, basal AA profile; AA+, functional AA profile (Thr, Met, and Trp at 120% of requirements for growth).
2Analyzed values of total AA with calculated values in parenthesis.
Inoculation, rectal swab protocol, and fecal sampling
On day 0 of the inoculation period (day 8 of the experiment), after being confirmed negative for the inoculated pathogen, half of the pigs (n = 32) were orally inoculated twice within 4 hr, each time with 1 mL of sterile saline solution (n = 32; CT) or a solution containing 3.3 × 109 CFU/mL of Salmonella enterica subsp. enterica (S.) serovar Typhimurium var. Copenhagen (n = 32; ST) selected for antibiotic resistance to Nalidixic acid and Novobiocin (Nal+/ Nov+) (Pieper et al., 2009; Wellington et al., 2019). In each block, all ST pigs and all CT pigs were housed in separate rooms and treatment/room was switched between blocks. On day 2 pre-inoculation and days 1, 2, 4, and 6 postinoculation, rectal swabs were obtained from individual pigs, diluted 1:10 in buffered peptone water (BPW), and cultured on brilliant green agar (BG agar) plates containing 30 μg/mL Nalidixic acid and 50 μg/mL Novobiocin (Nal+/ Nov+). Further, for pigs inoculated with ST, 1 mL of the dilution was enriched in 4 mL of selenite-cysteine broth (30 μg/mL Nal+/50 μg/mL Nov+) and incubated overnight at 37 °C and later 200 μL was cultured on BG agar plates (30 μg/mL Nal+/50 μg/mL Nov+). Colony counts were recorded on all plates after incubation for 24 hr at 37 °C. A scoring system was used for each plate to assign fecal shedding scores (Wellington et al., 2019). Plates prepared from swabs with colony counts >30 were given a score of 3. Plates positive for antibiotic-resistant ST but with colony counts <30 were given a score of 2. A score of 1 was assigned to swabs that were negative for antibiotic resistant ST after direct plating but positive after enrichment. Swabs negative for antibiotic resistant ST after direct plating and on enrichment were given a score of zero. On day 2 pre-inoculation and day 4 postinoculation, fecal samples were obtained from individual pigs for analysis of myeloperoxidase (MPO) activity as a pro-inflammatory biomarker according to adapted methodology by Bloomer (2018). Briefly, fecal samples were stored at –80 °C, allowed to thaw and diluted (1:1) before centrifugation twice at 1,150 × g for 10 min at 4 °C. For each sample, 750 μL of the resulting supernatant was transferred to 2 mL polypropylene microcentrifuge tubes and centrifuged again at 7,000 × g for 10 min at room temperature. The samples were then assayed for determination of MPO activity using a colorimetric assay kit according to the manufacturer’s instruction (ab105136; Abcam, Cambridge, MA).
Growth performance
Individual pig BW and feed intake was obtained on days –7, 0, and 7 of the study for calculation of pre- and postinoculation average daily gain (ADG), average daily feed intake (ADFI), and gain:feed (G:F).
Rectal temperature and fecal score
Rectal temperature and fecal score were obtained from all pigs over a period of 7 d (day –1 pre-inoculation and daily postinoculation until day 6). Rectal temperatures were obtained using a digital thermometer (Life Brand, ON, Canada). A scoring system was used to assign fecal scores, with normal consistency feces given a score of 0, semisolid feces without blood given a score of 1, watery feces without blood given a score of 2, and blood-tinged feces given a score of 3.
Blood sampling and analysis
Blood samples were obtained at 0900 hours from all pigs before inoculation (day 0) and on days 4 and 7 postinoculation via jugular vein puncture into 10 mL heparin coated vacutainer tubes (BD, Mississauga, ON, Canada) or tubes containing no additive. Blood samples collected into additive-free tubes were allowed to clot. Serum and plasma were obtained by centrifugation at 2,500 × g at 4 °C for 15 min and stored at −20 °C for subsequent analysis. Serum albumin was analyzed by bromocresol green method using a Cobas C 311 (Roche Diagnostics, Laval, QC, Canada) according to Doumas et al. (1971). Serum haptoglobin was analyzed in the Animal Health Laboratory at the University of Guelph (Guelph, ON) on a Roche Cobas 6000 c501 analyzer according to the method described by Makimura and Suzuki (1982). Plasma content of superoxide dismutase (SOD, ab65354), malondialdehyde (MDA, ab118970), reduced glutathione:oxidized glutathione (GSH:GSSG, ab138881), and urea nitrogen (PUN, ab83362) were determined according to the manufacturer’s instructions (Abcam, Cambridge, MA) of the respective kits.
Digesta and tissue collection and analysis
On day 7 postinoculation, ST pigs (n = 32) were euthanized by penetrating captive bolt followed by exsanguination. Subsequently, mesenteric lymph nodes (MLN) and spleen were sampled under aseptic conditions into sterile tubes containing 20 mL BPW, weighed, and homogenized followed by plating of 200 μL of the dilution on Nal+/ Nov+ BG agar plates. Further, 1 mL was diluted in 4 mL selenite-cysteine broth (Nal+/Nov+) for enrichment overnight at 37 °C with shaking, after which 200 μL was plated and incubated. Intestinal digesta samples (~1 g) were obtained from the ileum, cecum, and colon and each diluted in 4 mL BPW and kept at 4 °C. The digesta samples were serially diluted to 10–7, and 200 μL of each dilution was plated on BG agar (Nal+/Nov+) and cultured at 37 °C for 24 hr after which colonies were counted on each plate, with colony counts of 30 to 300 used in the calculation of colony forming units per gram digesta (CFU/g). Digesta (ileum, cecum, and colon) MPO activity was measured using a colorimetric kit (ab105136, Abcam; Cambridge, MA) according to the same methodology described above for fecal samples.
Statistical analysis
Data were tested for normality using the UNIVARIATE procedure of SAS (version 9.4, SAS Institute Inc., Cary, NC) and the Shapiro-Wilk test and the studentized residual was used to identify outliers (>3 SD from the mean). Outliers were identified and removed from the dataset of growth performance (n = 2), ST quantification in intestinal contents (n = 3), SOD (n = 3), MDA (n = 3), fecal (n = 4), digesta (n = 2) MPO, and PUN (n = 1) for final statistical analysis with removed data considered unrelated to experimental treatment. Data were analyzed using the MIXED procedure of SAS as an RCBD with a 2 × 2 × 2 factorial arrangement of treatments. The factors were (a) dietary CP content (low vs. high), (b) FAA supplementation (AA– vs. AA+), and (c) challenge (CH) (CT vs. ST). Data including only ST pigs were analyzed as an RCBD with a 2 × 2 factorial arrangement with factors of dietary CP content and FAA supplementation. These factors and their interactions were included as fixed effects and block as a random effect variable. For data collected over time, day (pre- and postinoculation) was included in the analysis as a repeated effect [i.e., acute-phase response, oxidant/antioxidant balance, and PUN (days 4 and 7 postinoculation); Salmonella shedding score in feces (days 1, 2, 4, and 6 postinoculation); and fecal MPO (day 4 postinoculation)]. For digesta Salmonella quantification and MPO data, section of the intestine (ileum, cecum, and colon) was also included as a fixed effect. Differences between means were determined using the Tukey post hoc test and considered significant at P ≤ 0.05. A trend toward significance was considered at 0.05 < P < 0.10.
Results
Rectal temperature and fecal score
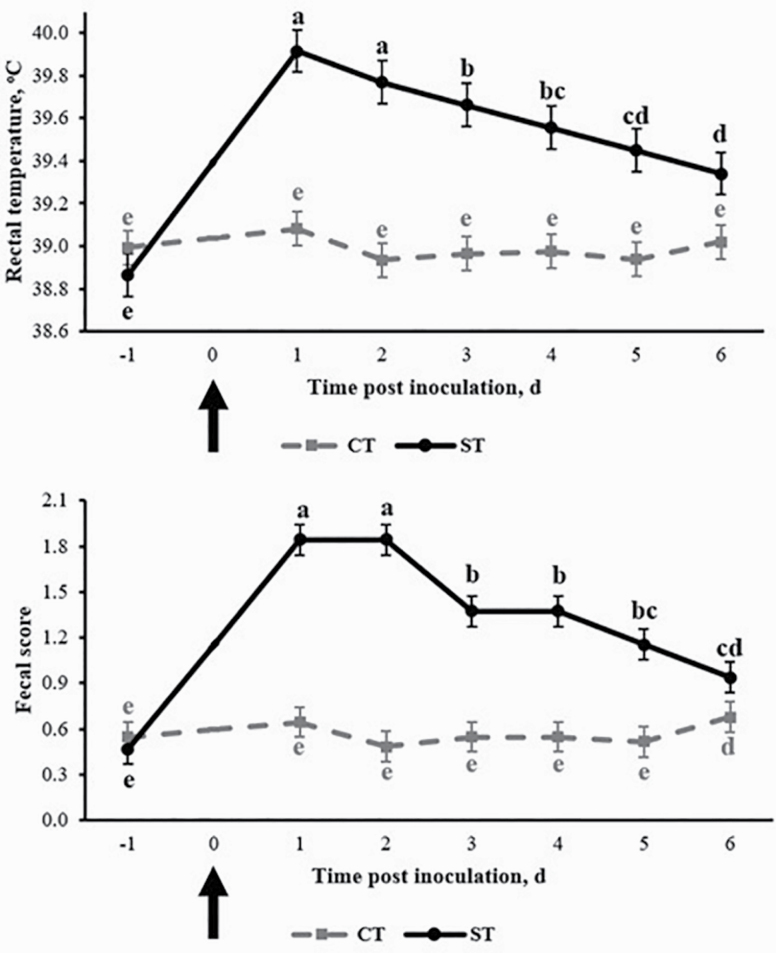
Rectal temperature and fecal score of CT and ST pigs are shown in Figure 1. Inoculation with ST increased rectal temperature within 24 hr, which remained elevated for the duration of the study compared with the pre-inoculation measurement and temperature of CT pigs throughout the study (P < 0.05). There was no effect of inoculation on rectal temperature of CT pigs (P > 0.10). Fecal score was negatively affected in ST pigs during the first 5 d postinoculation (P < 0.05). There was no effect of inoculation in CT pigs (P > 0.10). There was no effect of dietary treatment on rectal temperature or fecal score in either CT or ST pigs (P > 0.10).
Figure 1.
Rectal temperature and fecal score of pigs inoculated with saline (CT) or Salmonella Typhimurium var. Copenhagen (ST) (indicated by arrow). Normal consistency feces were given a score of 0, semisolid feces without blood were given a score of 1, watery feces without blood were given a score of 2 and blood-tinged feces were given a score of 3. Within days, points with different superscripts differ (P < 0.05). No significant (P > 0.10) effects of dietary treatments on rectal temperature and fecal score were observed. Values are least squares means; n = 32 pigs/treatment.
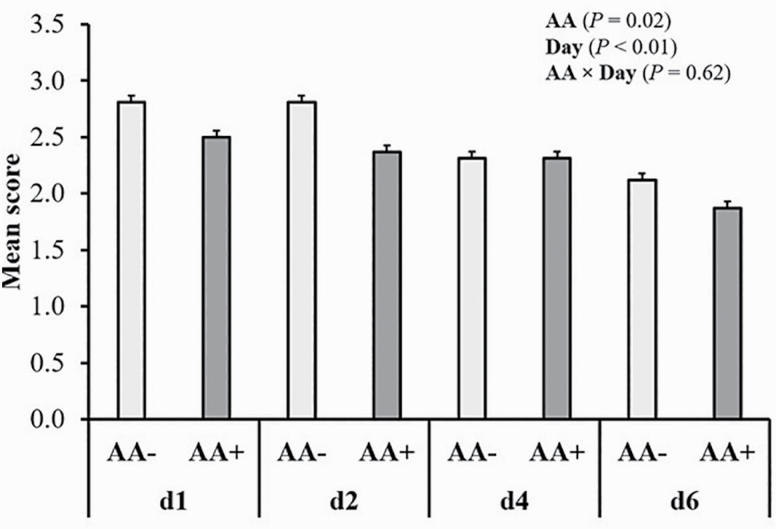
Salmonella scoring for shedding in feces and colonization
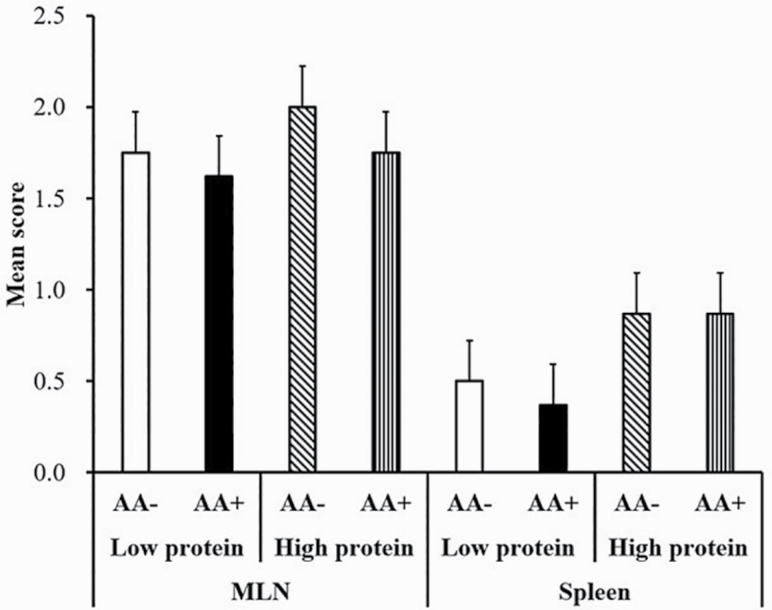
Pigs inoculated with saline (CT) remained negative for Salmonella throughout the study and ST pigs were negative prior to inoculation. Salmonella shedding score in feces in inoculated pigs was quantified on days 1, 2, 4, and 6 postinoculation (Figure 2). Fecal ST shedding score was increased on days 1 and 2 postinoculation and declined by day 6 regardless of dietary treatment (P < 0.05). Pigs fed AA+ diets showed decreased overall ST shedding score in feces compared with AA– (P < 0.05). There was no effect of CP content on fecal ST score (P > 0.10). Table 3 shows ST quantification in ileum, cecum, and colon digesta of inoculated pigs. The ST counts in cecal digesta were increased in HP-fed pigs compared with LP-fed pigs and ST counts in colon were reduced in AA+ pigs compared to AA– pigs (P < 0.05). Ileal counts of ST were not affected by dietary treatment and there was no effect of section on quantification (P > 0.10). Figure 3 shows the presence of the inoculated ST in the MLN and spleen of inoculated pigs. There was no effect of dietary treatment on the presence of the inoculated ST in the spleen and MLN (P > 0.10). Although not significant (P > 0.05), in MLN, 100% (8/8) of pigs fed AA– while 87.5% (7/8) of pigs fed AA+ had a positive ST score. Likewise, in spleen, only 25% (2/8) pigs fed LP and 50% (4/8) fed HP had positive ST score.
Figure 2.
Postinoculation scoring for shedding in feces of Salmonella Typhimurium var. Copenhagen in pigs fed a basal (AA–) or functional amino acid profile (AA+; Thr, Met and Trp 120% of requirements for growth). A score for shedding in feces of 3 was assigned to plates positive for the inoculated ST with counts >30 and plates positive but with counts <30 were given a score for shedding in feces of 2. A score for shedding in feces of 1 was assigned to plates that were only positive after enrichment and plates negative after enrichment were scored zero. Probability values corresponding to main effects of, and interactions among, dietary CP content, AA profile, and day not shown in graph were not statistically significant (P > 0.10). Values are least squares means; n = 16 pigs/treatment.
Table 3.
Salmonella Typhimurium var. Copenhagen quantification in intestinal contents (log 10 CFU/g; day 7 postinoculation) of Salmonella-inoculated pigs fed diets differing in functional amino acid and protein content1
| LP | HP | P-value1 | |||||
|---|---|---|---|---|---|---|---|
| Item | AA– | AA+ | AA– | AA+ | SEM | CP | AA |
| Ileum | 2.54 | 2.32 | 2.69 | 2.42 | 0.37 | NS | NS |
| Cecum | 2.30 | 2.17 | 2.75 | 2.81 | 0.24 | 0.03 | NS |
| Colon | 2.87 | 2.01 | 2.36 | 2.15 | 0.24 | NS | 0.03 |
1LP, low protein; HP, high protein; AA–, basal AA profile; AA+, functional AA profile (Thr, Met, and Trp at 120% of requirements for growth); SEM, standard error of the mean. Values are least squares means; n = 8 pigs/treatment.
1CP, effect of CP content; AA, effect of AA profile. The interaction CP × AA was not statistically significant (NS) and there was no effect of section (ileum, cecum, and colon; P > 0.10).
Figure 3.
Salmonella Typhimurium var. Copenhagen translocation to the MLN and spleen in pigs fed HP or LP diets with basal (AA–) or functional amino acid profile (AA+; Thr, Met and Trp at 120% of requirements for growth). A score of 3 was assigned to plates positive for the inoculated ST with counts >30 and plates positive but with counts <30 were given a score of 2. A score of 1 was assigned to plates that were only positive after enrichment and plates negative after enrichment were scored zero. No significant (P > 0.10) effects of dietary treatments on bacteria translocation in either MLN or spleen were observed. Values are least squares means; n = 8 pigs/treatment.
Growth performance
Growth performance data for pre- and postinoculation is presented in Table 4. There were no dietary treatment effects on growth performance during the pre-inoculation period (P > 0.10). There was no effect of FAA supplementation or CP content on postinoculation performance of CT pigs (P > 0.10). Both ADG and ADFI were reduced postinoculation in ST compared with CT pigs (P < 0.05). Salmonella-inoculated pigs fed AA+ diets had greater ADG (P < 0.05) and tended to have increased G:F compared with ST pigs fed AA– diets (P < 0.10). There was no effect of CP content and no significant interactive effect among dietary treatments on postinoculation growth performance of ST pigs (P > 0.10).
Table 4.
Pre- and postinoculation growth performance of control (CT) and Salmonella-inoculated (ST) pigs fed diets differing in functional AA and protein content1
| CT | ST | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LP | HP | LP | HP | ||||||
| Item | AA– | AA+ | AA– | AA+ | AA– | AA+ | AA– | AA+ | SEM |
| Initial BW (day –7), kg | 13.96 | 13.93 | 13.93 | 13.94 | 13.98 | 13.96 | 13.95 | 13.90 | 0.31 |
| Inoculation BW (day 0), kg | 16.92 | 17.33 | 17.08 | 17.18 | 17.14 | 17.15 | 17.18 | 17.22 | 0.97 |
| Final BW (day 7), kg | 20.92av | 21.36ax | 21.18av | 21.24ax | 19.22bv | 20.36bx | 19.28bv | 20.40bx | 1.31 |
| Pre-inoculation period (days –7 to 0) | |||||||||
| ADG, kg | 0.423 | 0.486 | 0.450 | 0.463 | 0.451 | 0.456 | 0.461 | 0.474 | 0.05 |
| ADFI, kg | 0.580 | 0.602 | 0.563 | 0.636 | 0.614 | 0.646 | 0.632 | 0.648 | 0.05 |
| Gain:feed, kg/kg | 0.72 | 0.80 | 0.79 | 0.72 | 0.73 | 0.70 | 0.73 | 0.73 | 0.10 |
| Postinoculation period (days 0 to 7) | |||||||||
| ADG, kg | 0.571a | 0.576a | 0.586a | 0.580a | 0.297bv | 0.459bx | 0.300bv | 0.456bx | 0.06 |
| ADFI, kg | 0.880a | 0.906a | 0.916a | 0.936a | 0.738b | 0.673b | 0.744b | 0.686b | 0.08 |
| Gain:feed, kg/kg | 0.64 | 0.63 | 0.63 | 0.62 | 0.40y | 0.68z | 0.40y | 0.66z | 0.13 |
1LP, low protein; HP, high protein; AA–, basal AA profile; AA+, functional AA profile (Thr, Met, and Trp at 120% of requirements for growth); SEM, standard error of the mean. Values are least squares means; n = 8 pigs/treatment. Main or interactive effects not presented were not statistically significant for any of the parameters measured.
a,bMeans within a row with different superscripts differ (CT vs. ST) (P < 0.05).
v,xMeans within a row with different superscripts differ (AA– vs. AA+) (P < 0.05).
y,zMeans within a row with different superscripts tend to differ (AA– vs. AA+) (P < 0.10).
Blood parameters
Serum indicators of acute-phase response (albumin and haptoglobin), plasma indicators of oxidant/antioxidant balance (SOD and MDA, GSH, GSSG, GSH:GSSG), and PUN are shown in Table 5 with probabilities corresponding to treatments effects shown in Table 6.
Table 5.
Pre- and postinoculation blood parameters of control (CT) and Salmonella-inoculated (ST) pigs fed diets differing in functional AA and protein content1
| CT | ST | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LP | HP | LP | HP | ||||||
| Item | AA– | AA+ | AA– | AA+ | AA– | AA+ | AA– | AA+ | SEM |
| Serum albumin, g/L | |||||||||
| Preinoculation (day 0) | 39.12 | 35.13 | 37.12 | 39.87 | 33.95 | 36.12 | 35.62 | 36.12 | 2.31 |
| Postinoculation (day 4) | 37.12 | 37.75 | 36.12 | 39.75 | 37.52 | 37.00 | 33.12 | 37.62 | |
| Postinoculation (day 7) | 34.50 | 39.62 | 38.12 | 33.87 | 35.66 | 30.12 | 29.25 | 37.12 | |
| Serum haptoglobin, g/L | |||||||||
| Pre-inoculation (day 0) | 1.95 | 1.83 | 1.95 | 1.75 | 1.71 | 1.58 | 1.64 | 1.78 | 0.33 |
| Postinoculation (day 4) | 1.47 | 1.24 | 1.28 | 1.00 | 2.10 | 2.10 | 2.31 | 2.24 | |
| Postinoculation (day 7) | 1.25 | 0.73 | 1.23 | 0.88 | 1.57 | 0.89 | 1.55 | 1.22 | |
| Plasma SOD, mU/mL | |||||||||
| Preinoculation (day 0) | 53.9 | 52.6 | 52.0 | 53.5 | 49.8 | 49.2 | 51.4 | 53.8 | 2.51 |
| Postinoculation (d 4) | 51.9 | 52.5 | 48.5 | 48.5 | 63.5 | 51.4 | 63.0 | 54.3 | |
| Postinoculation (day 7) | 54.0 | 53.0 | 53.1 | 51.6 | 60.7 | 50.5 | 60.5 | 50.4 | |
| Plasma malondialdehyde, nmol/mL | |||||||||
| Preinoculation (day 0) | 0.67 | 0.54 | 0.51 | 0.48 | 0.51 | 0.63 | 0.55 | 0.65 | 0.20 |
| Postinoculation (day 4) | 0.59 | 0.59 | 0.76 | 0.60 | 1.68 | 1.05 | 1.27 | 1.04 | |
| Postinoculation (day 7) | 0.52 | 0.40 | 0.62 | 0.63 | 1.13 | 0.87 | 1.13 | 0.98 | |
| Reduced glutathione (GSH), μM | |||||||||
| Preinoculation (day 0) | 3.78 | 3.63 | 3.35 | 3.67 | 3.17 | 3.61 | 3.95 | 3.35 | 0.51 |
| Postinoculation (day 4) | 3.09 | 4.75 | 2.54 | 4.22 | 1.95 | 2.35 | 2.18 | 2.58 | |
| Postinoculation (day 7) | 3.33 | 3.96 | 3.35 | 3.29 | 1.73 | 2.40 | 1.96 | 2.19 | |
| GSSG, μM | |||||||||
| Pre-inoculation (day 0) | 1.34 | 1.62 | 1.90 | 1.73 | 1.99 | 1.63 | 1.25 | 1.84 | 0.34 |
| Postinoculation (day 4) | 2.21 | 1.46 | 2.47 | 1.69 | 5.57 | 5.24 | 5.56 | 4.98 | |
| Postinoculation (day 7) | 1.71 | 1.52 | 1.75 | 1.82 | 4.85 | 4.32 | 4.82 | 4.64 | |
| GSH:GSSG | |||||||||
| Pre-inoculation (day 0) | 2.82 | 2.24 | 1.76 | 2.12 | 1.59 | 2.21 | 3.16 | 1.82 | 0.81 |
| Postinoculation (day 4) | 1.40 | 3.25 | 1.03 | 2.50 | 0.35 | 0.45 | 0.39 | 0.52 | |
| Postinoculation (day 7) | 1.95 | 2.61 | 1.91 | 1.81 | 0.36 | 0.56 | 0.41 | 0.47 | |
| PUN, mmol/L | |||||||||
| Pre-inoculation (day 0) | 3.66 | 3.49 | 2.94 | 3.67 | 4.07 | 3.66 | 3.60 | 4.15 | 0.44 |
| Postinoculation (day 4) | 3.70 | 3.33 | 3.51 | 3.28 | 3.58 | 3.63 | 4.79 | 5.41 | |
| Postinoculation (day 7) | 3.37 | 2.94 | 2.75 | 3.91 | 3.28 | 4.38 | 4.26 | 5.07 | |
1LP, low protein; HP, high protein; AA–, basal AA profile; AA+, functional AA profile (Thr, Met, and Trp at 120% of requirements for growth); SEM, standard error of the mean. Values are least squares means; n = 8 pigs/treatment. Probability values are presented in Table 6.
Table 6.
Significance of main and interactive effects of challenge (CH), dietary CP content, AA profile, and day for pre- and postinoculation blood parameters1
| Item | CH | AA | CP | Day | CH × AA | CH × CP | AA × CP | CH × day | AA × day |
|---|---|---|---|---|---|---|---|---|---|
| Serum albumin | <0.01 | NS | NS | NS | 0.03 | 0.06 | NS | 0.03 | NS |
| Serum haptoglobin | <0.01 | 0.04 | NS | <0.01 | <0.01 | NS | NS | <0.01 | NS |
| Plasma SOD | <0.01 | <0.01 | NS | NS | <0.01 | NS | NS | <0.01 | 0.06 |
| Plasma malondialdehyde | <0.01 | NS | NS | <0.01 | NS | NS | NS | 0.01 | NS |
| PUN | <0.01 | 0.04 | 0.09 | NS | NS | 0.01 | 0.06 | NS | NS |
| GSH | <0.01 | 0.02 | NS | <0.01 | NS | NS | NS | 0.04 | NS |
| GSSG | <0.01 | 0.09 | NS | <0.01 | NS | NS | NS | <0.01 | NS |
| GSH:GSSG | 0.02 | 0.04 | NS | <0.01 | NS | NS | NS | 0.05 | NS |
1Interactive effects not presented were not statistically significant for any of the parameters measured.
Inoculation with ST reduced albumin (P < 0.05), which was increased by feeding the AA+ profile (P < 0.05). There was no effect of day on albumin for CT pigs (P > 0.10), while it was decreased in ST pigs on day 7 (P < 0.05). Inoculation with ST increased haptoglobin, which was reduced in pigs fed AA+ diets (P < 0.05). The ST pigs fed AA– diets showed the highest haptoglobin, CT pigs fed AA+ diets the lowest, and ST pigs fed AA+ and CT pigs fed AA– diets were intermediate (P < 0.05). Additionally, haptoglobin in ST pigs peaked at day 4 and returned to baseline at day 7 while CT pigs showed progressively decreasing haptoglobin from days 0 to 7 (P < 0.05). No significant 3- or 4-way interactions for serum albumin and haptoglobin were observed (P > 0.10).
The ST pigs showed increased overall SOD, which were decreased in pigs receiving the AA+ diet (P < 0.05). The ST pigs had higher SOD at day 4, returning to baseline at day 7 (P < 0.05). Moreover, ST pigs fed AA+ diets had SOD comparable to CT pigs, regardless of FAA supplementation (P > 0.10), and these concentrations were all lower than in ST pigs fed AA– diets (P < 0.05). The ST pigs had increased overall MDA compared with CT pigs (P < 0.05) and had higher MDA at day 4 which had not decreased by day 7 (P < 0.05). Plasma GSH:GSSG was reduced by day 4 in ST compared with CT pigs, which remained lower at day 7, as a result of reduced plasma GSH and increased plasma GSSG (P < 0.05). Overall plasma GSH:GSSG was increased in pigs fed AA+ compared with AA– diets, mainly due to greater GSH (P < 0.05). There was an interaction between ST inoculation and CP content, such that feeding the HP diet increased PUN in the ST inoculated pigs only (P < 0.05).
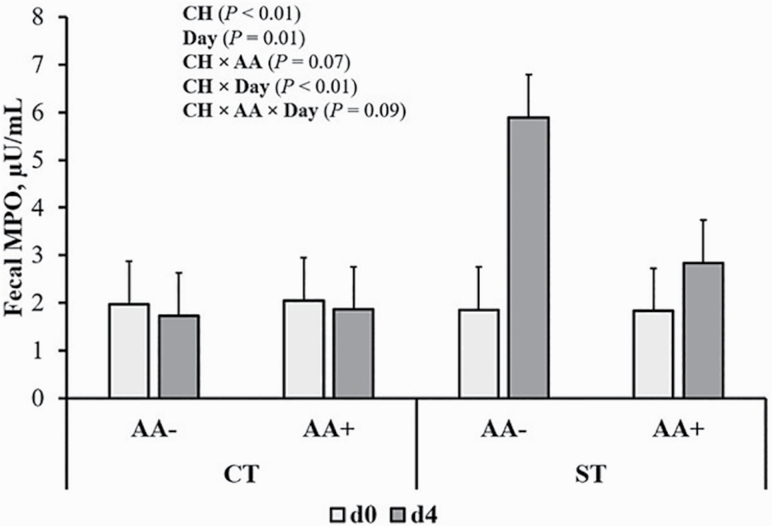
Fecal and digesta MPO
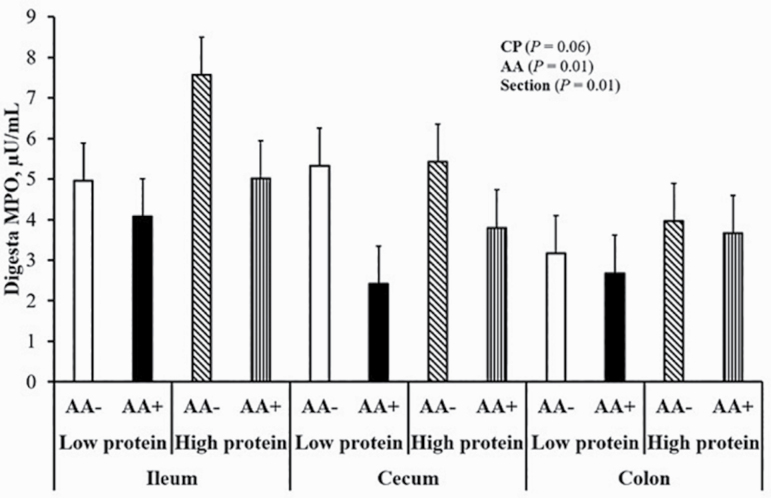
Fecal MPO of pigs is shown in Figure 4. The ST inoculated pigs had greater fecal MPO (P < 0.05) compared with CT pigs. ST pigs had higher fecal MPO at day 4, returning to pre-inoculation at day 7 (P < 0.05). Myeloperoxidase of pigs inoculated with ST was also analyzed in digesta samples (Figure 5). Myeloperoxidase was highest in ileal, lowest in colonic, and intermediate in cecal digesta, regardless of dietary treatment (P < 0.05). Pigs fed AA+ diets showed reduced MPO in digesta compared with AA– regardless of section (P < 0.05). There was a trend for increased MPO in digesta samples of HP pigs compared with LP pigs, regardless of section (P < 0.10).
Figure 4.
Pre- (day 0) and postinoculation (day 4) fecal MPO in control (CT) and Salmonella-inoculated pigs (ST) fed a basal (AA–) or functional amino acid (AA) profile (AA+; Thr, Met and Trp at 120% of requirements for growth). Probability values corresponding to main effects of, and interactions among, challenge (CH), dietary CP content, and AA profile not shown in graph were not statistically significant (P > 0.10). Values are least squares means; n = 16 pigs/treatment.
Figure 5.
Postinoculation (day 7) ileal (a), cecal (b), and colonic (c) digesta MPO in Salmonella-inoculated pigs fed HP or LP diets with basal (AA–) or functional AA profile (AA+; Thr, Met, and Trp at 120% of requirements for growth). Probability values corresponding to main effects of, and interactions among, dietary CP content, AA profile, and section (ileum, cecum, and colon) not shown in graph were not statistically significant (P > 0.10). Values are least squares means; n = 8 pigs/treatment.
Discussion
The objective of the present study was to determine whether growth performance and immune status of weanling pigs would be improved by supplementation of specific FAA (i.e., Thr, Met, and Trp) at 120% of requirements for growth (NRC, 2012) when inoculated with an enteric pathogen or fed an HP diet. High protein content in nursery diets may increase the production of protein fermentation metabolites and potentiate the proliferation of pathogenic bacteria (Htoo et al., 2007; Heo et al., 2009). Furthermore, immune stimulation increases requirements of Thr (Wellington et al., 2018), Met (Litvak et al., 2013), and Trp (de Ridder et al., 2012) for growth. Recently, both the dietary protein content and the supplementation of Met, Thr, and Trp have been shown to modulate the immune status of grower pigs under poor sanitary conditions (van der Meer et al., 2016).
Response of pigs to Salmonella inoculation
Prior to ST inoculation, there was no effect of dietary CP or FAA content on pig growth performance or any indicators of health. Moreover, there was no effect of dietary treatment on postinoculation performance or indicators of health in pigs inoculated with saline. This confirms that diets were properly formulated to meet or exceed nutrient requirements for this age and weight range of pigs.
After inoculation, ST pigs had increased rectal temperature and deterioration in fecal score compared with CT pigs. In addition, ST pigs showed decreased serum albumin and plasma GSH:GSSG, while showing increased concentrations of serum haptoglobin, and plasma SOD and MDA. Furthermore, fecal MPO was increased in ST compared with CT pigs. Rectal swabs collected throughout the postinoculation period revealed a progressively lower, but still present, bacterial shedding in feces following inoculation, which correlates with serum and plasma indicators. Our fecal score results are in agreement with previous studies using the same scoring system and similar ST inoculation dose in pigs. Burkey et al. (2004) orally inoculated 6.8 kg piglets with 1.33 × 109 CFU/mL and reported scoring for shedding ranging from 1 to 3 until day 7 postinoculation. Likewise, our recent study showed fecal scores ranging from 2 to 3 until day 6 post-ST inoculation (2.30 × 109 CFU/mL) in 22.6 kg pigs (Wellington et al., 2019). Moreover, the presence of ST in digesta samples (ileum, cecum, and colon) and translocation to lymphoid tissues (MLN and spleen) were detected on day 7 postinoculation. Collectively, these results confirm a successful and uniform stimulation of immune system of pigs by ST, which is mainly characterized by diarrhea (Correa-Matos et al., 2003) and fever (Gebru et al., 2010), activation of acute-phase response (Wellington et al., 2019), disturbance to the antioxidant balance (Lv et al., 2020), and poor gut health (Barba-Vidal et al., 2017).
Inoculation with ST resulted in a 35% reduction in growth and 22% reduction in ADFI as well as increased PUN. These findings are in agreement with Price et al. (2010) who inoculated postweaned piglets with 109 CFU of ST and observed a reduction of 42% and 26% in ADG and ADFI, respectively, 7 d postinoculation compared with a control group. Conversely, Bruno et al. (2013) inoculated 5 kg piglets with a lower concentration (1 × 105 CFU of ST) and reported no difference in growth (210 g/d vs. 210 g/d) or ADFI (320 g/d vs. 320 g/d) after 7 d postinoculation when compared with a control group, which indicates that growth and feed intake impairment due to ST inoculation is dose dependent. Overall, our findings corroborate the anorectic and reduced growth response to disease challenge (Wichterman et al., 1980; Fink and Heard, 1990; Balaji et al., 2000; Burkey et al., 2004) which can be related to both a reduction in feed intake and reduced nutrient utilization efficiency (Coma et al., 1995; Pastorelli et al., 2012). Taken together, these results demonstrate successful disease challenge in the current study.
Effects of dietary protein content on parameters of growth performance, immune system stimulation, and intestinal inflammation in Salmonella-challenged pigs
No interactive effects were observed between dietary treatment factors, therefore, the effects of dietary CP content and FAA profile are discussed independently. Dietary components, such as dietary protein, can have an impact on immune status and gut health. In the postweaning period, high dietary protein can predispose pigs to postweaning diarrhea and proliferation of enteric pathogens (Rist et al., 2013). The lack of effect of dietary protein content on growth performance in the postinoculation period was unexpected, as it was hypothesized that this would increase proliferation of pathogenic bacteria and production of harmful metabolites, having an overall negative impact on gut health (Wellock et al., 2007, 2008; Opapeju et al., 2009) and nutrient utilization (Heo et al., 2010b).
The cecum is the primary site of microbial fermentation of undigested protein in pigs (Htoo et al., 2007), and lowering the dietary CP content reduces the availability of fermentable substrates to intestinal microbes, mitigating growth of pathogenic bacteria (Rist et al., 2013). Toxic compounds of protein fermentation could impair mucosal development (Visek, 1984; Lin and Visek, 1991), leading to villus atrophy (Nousiainen, 1991) and consequently diarrhea (Heo et al., 2008). In the present study, the observed increase in ST count in cecal digesta when pigs were fed the HP diet is in agreement with the concept that high dietary CP content can contribute to proliferation of pathogenic bacteria (Heo et al., 2009). This is further supported by the increased PUN and tendency for greater digesta MPO observed in the present study in HP vs. LP-fed pigs, indicating that higher dietary CP content may have reduced gut barrier function likely mediated by production of harmful microbial metabolites. Interestingly, feeding HP diets did not result in greater ST shedding score in feces and did not further increase the severity of diarrhea, which suggests that the negative effects of dietary CP may have been limited in the current study. The limited effect of CP is further supported by the lack of effect on indicators of pig health, specifically plasma acute-phase protein and antioxidant balance.
These results are contrary to previous studies, indicating that HP diets impaired overall health of pigs. For example, after E. coli challenge, weaned piglets fed 25.1% CP showed looser faces compared with piglets fed 19.2% CP (Heo et al., 2010a). Likewise, pigs fed 22.5% CP had shorter villi and reduced villus height:crypt depth 3 d after an enteric challenge compared to pigs fed 17.6% CP (Opapeju et al., 2009). It should be noted that the abovementioned studies investigated higher CP contents than in the present study. Moreover, recent findings suggest that ileal microbiota diversity in pigs is dramatically impacted by higher dietary protein levels and E. coli exposure, and the effects are exacerbated when both factors are combined (e.g., E. coli-challenged pigs fed HP diets; Pollock et al. (2019)). It has been suggested that feeding HP diets increase proliferation of saccharo-proteolytic microbes, which preferentially gain energy from carbohydrate fermentation but are able switch to protein fermentation when the protein:carbohydrate ratio increases in digesta (Roy, 1969; Abe et al., 1995; Nollet et al., 1999). Indeed, there is evidence of Salmonella being highly competitive for carbohydrates as a carbon source (Martín-Peláez et al., 2008), which is supported by increased severity of infection by Salmonella in mice fed different carbohydrate sources (Petersen et al., 2009). Also, this is also consistent with recent findings from our research group where growing pigs showed reduced ADG after but not before Salmonella challenge when fed a high vs. low fiber diet (Wellington et al., 2019). Thus, it is possible that the response to dietary CP content may be related to the type of enteric pathogen present and may have less impact with Salmonella challenge. Moreover, it should be noted that the negative effects of HP diets in previous studies were observed in younger pigs postweaning (Heo et al., 2009; Opapeju et al., 2009; Song et al., 2010). Indeed, Heo et al. (2008) suggested that the immediate postweaning period (e.g., 5 to 7 d after weaning) is the critical window where dietary CP should be reduced to minimize diarrhea incidence, which is an earlier period than investigated in the present study. Finally, despite not being evaluated here, plant, nondigestible, presumably fermentable protein sources, as used in the present study, are known to impair growth performance, nutrient digestibility, and gut structure when compared with animal protein sources (Makkink et al., 1994; Yun et al., 2005; Jones et al., 2010) which could be another factor contributing to the variation among studies.
Effects of FAA supplementation on parameters of growth performance, immune system stimulation, and intestinal inflammation in Salmonella-challenged pigs
It has been shown that immune stimulation increases requirements for some AA for growth, including Met and Cys (Litvak et al., 2013; Rakhshandeh et al., 2014), Thr (Jayaraman et al., 2015; Wellington et al., 2018), and Trp (Le Floc’h et al., 2009; de Ridder et al., 2012), suggesting that supplementation with these AA may improve performance in disease-challenged pigs. Indeed, using the same inoculation model, we reported previously that supplementing dietary Thr at 20% above requirements for growth improved growth performance of 22 kg pigs (Wellington et al., 2019). Therefore, in the current study, we supplied Met, Thr, and Trp at 120% of NRC (2012) requirements and examined the impact on performance measures and key indicators of immune status and gut health in pigs fed LP or HP diets and inoculated with an enteric pathogen.
The increased ADG and tendency for improved feed efficiency in AA+ fed pigs after inoculation with ST compared with those fed AA– diets confirms our hypothesis that supplementation with key FAA supports improved growth and nutrient utilization in pigs exposed to an enteric pathogen challenge. This is in line with findings from Capozzalo et al. (2017) and Wellington et al. (2020) in which it was shown that supplementation with Trp/Met or Thr attenuated gut inflammation and improved protein utilization and performance in E. coli and Salmonella-challenged pigs, respectively. Moreover, van der Meer et al. (2016) reported improved performance of growing pigs housed in low sanitary conditions when fed Met, Thr, and Trp at 120% of requirements. Further support for an altered AA profile during disease challenge is provided by the observation that the positive effects of AA+ diets on ADG and feed efficiency of ST-inoculated pigs were achieved without a concurrent increase in ADFI. This is in line with results of a meta-analysis performed by Pastorelli et al. (2012) in which it was shown that 74% of the reduction in growth due to enteric pathogen challenge was due to feed efficiency (i.e., nutrient utilization) and not due to the decrease in feed intake. Serum haptoglobin and albumin are a positive and negative acute-phase proteins, respectively, generally regarded as key biomarkers for health status of pigs (Le Floc’h et al., 2009; Kampman-van de Hoek et al., 2016). Under situations of poor health, altered nutrient utilization and metabolism redirect dietary and body reserves nutrients to support the immune system and excessive demands for AA may be expected for the synthesis of acute-phase proteins (Reeds et al., 1994). In the current study, haptoglobin was increased while albumin was decreased after the enteric infection, which agrees with previous findings (Dritz et al., 1996; Turner et al., 2000; Wellington et al., 2019). The overall reduction in haptoglobin and increase in albumin content in AA+ fed compared with AA– fed pigs suggests that supplementation of FAA may have attenuated the inflammatory response in these pigs. Indeed, further evidence for this is provided by the reduced colonization of the inoculated Salmonella in the distal gut (i.e., colon) as well as reduced overall score for ST shedding in AA+ compared with AA– pigs. Moreover, the reduced MPO content in digesta and tendency for reduced MPO content in fecal samples of pigs fed AA+ indicates reduced intestinal inflammation (Kansagra et al., 2003; Young et al., 2012) with FAA supplementation. Indeed, our previous work showed improved intestinal barrier function in Salmonella-challenged pigs fed diets with supplemental Thr (Wellington et al., 2020). Likewise, Chen et al. (2018) reported an attenuation of the inflammatory response and improved intestinal barrier function in broiler chickens injected with E. coli lipopolysaccharide when supplemented with Thr, suggesting that attenuation in the immune response may be due to improved intestinal health. Interestingly, a reduction in serum haptoglobin has not been observed in previous studies which provided Thr, Met+Cys, or Trp individually (Rakhshandeh et al., 2014; Jayaraman et al., 2017; Wellington et al., 2019), suggesting that attenuation of acute-phase response in enteric challenged pigs might be dependent on provision of a combination of these AA. Translocation of ST to lymphoid tissues (MLN and spleen) was detected on day 7 postinoculation; however, there was no effect of FAA supplementation. This could be associated with ST remaining in a latent state in surrounding lymphoid tissues even after gut barrier was re-established and some degree of recovery of the disease was observed (Bellido-Carreras et al., 2019).
Disruption of the intestinal barrier and overall immune system stimulation may also trigger oxidative stress (Circu and Aw, 2012), with reactive oxygen species released as a consequence of the inflammatory reaction (Oz et al., 2007). It is well known that the antioxidant capacity of enterocytes is compromised in the presence of Salmonella, which makes them more susceptible to oxidative damage (Mehta et al., 1998). We measured SOD and MDA as enzymatic and GSH and GSSG as nonenzymatic antioxidants. Increased plasma SOD and MDA in ST compared with CT pigs confirms disruption of antioxidant balance, as plasma SOD is a systemic indicator of an activated intestinal mucosa to oxidative stress (Dincer et al., 2007), while higher plasma MDA has been associated with intestinal neutrophil activity, atrophy, and metaplasia (Siregar et al., 2018). Reduced plasma SOD in Salmonella-inoculated pigs fed AA+ vs. AA– supports an attenuation of ST damage to the gastrointestinal tract and is consistent with reduced score for ST shedding in feces, lower ST colonization in colon, and attenuated acute-phase response in pigs fed AA+ compared with AA– diets. Indeed, increasing dietary Trp content attenuated the alterations in plasma and hepatic SOD and MDA in piglets intraperitoneally injected with diquat (Mao et al., 2014). Likewise, cisplatin-induced intestinal damage was suppressed in rats supplemented with D-Met mainly through antioxidative effects (Wu et al., 2019). Glutathione (GSH) is an important cellular antioxidant which eliminates peroxides, being converted to its oxidized (disulfide) form (GSSG). Under normal conditions, there is an increased proportion in GSH relative to GSSG, while under oxidative stress the proportion of prooxidants exceeds the proportion of antioxidants and the GSH:GSSG ratio is reduced (Jones, 2002). In the present study, Salmonella inoculation significantly decreased plasma GSH and increased GSSG, resulting in a reduced GSH:GSSG, indicating increased use of GSH to mitigate the effects of pathogen challenge. Production of GSH has been largely associated with the increase in estimated sulfur amino acid (SAA) requirements in pigs under immune stimulation (Rakhshandeh et al., 2010; Rakhshandeh and de Lange, 2010). Furthermore, Riedijk et al. (2007) and Shoveller et al. (2003) demonstrated the importance of SAA as key precursors for maintenance of cell redox status via GSH synthesis, which is pivotal for epithelial cell proliferation. Previous evidence indicated that immune system stimulation increases the utilization of Cys for the production of GSH (Malmezat et al., 2000), with an increase in Cys requirement being met, in part, with Met supplementation (Lu, 2009). In this sense, AA+ fed pigs showing increased GSH, which elevated GSH:GSSG, regardless of CH and CP, suggests an improved antioxidant system compared with AA– pigs. This indicates that the increased availability of Met in AA+ diets supported GSH synthesis. Indeed, Chen et al. (2014) reported higher GSH and lower GSSG concentration in plasma, duodenum, and jejunum of postweaning piglets fed diets supplemented with Met. Overall, these results support the hypothesis that supplementation of key FAA is necessary to support the immune response of pigs to pathogen challenge, and specifically result in increased antioxidant defense mechanisms (Stipanuk et al., 2002; Stipanuk, 2004).
Conclusions
Taken together, our results clearly show that diet supplementation with key FAA, specifically Thr, Met, and Trp, above estimated requirements for growth improves growth performance and immune status of pigs, regardless of dietary protein content. Our data further suggest that the positive effects of these FAA are due to beneficial effects on intestinal health and antioxidant defense systems, attenuating the overt immune response, even with no impact on Salmonella presence in lymphoid tissues.
Acknowledgment
The authors would like to thank the staff at the Animal Care Unit of the Western College of Veterinary Medicine, Canadian Feed Research Centre, and the Prairie Swine Centre, Inc. for their assistance.
Glossary
Abbreviations
- AA
amino acid
- ADFI
average daily feed intake
- ADG
average daily gain
- BW
body weight
- CP
crude protein
- DM
dry matter
- G:F
gain:feed
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- MDA
malondialdehyde
- ME
metabolizable energy
- MLN
mesenteric lymph nodes
- MPO
myeloperoxidase
- NE
net energy
- PUN
plasma urea nitrogen
- SID
standardized ileal digestible
- SOD
superoxide dismutase
- ST
Salmonella Typhimurium
Conflict of interest statement
J.C.G.-V. and J.K.H. are employees of Evonik Operations GmbH. All other authors declare no real or perceived conflicts of interest.
Authors’ Contributions
L.A.R., J.C.G.V., J.K.H., A.G.V.K., and D.A.C. designed the study; L.A.R. and M.O.W. conducted the study; L.A.R. and M.O.W. performed lab and data analysis; L.A.R., M.O.W., and D.A.C. wrote the manuscript. All the authors contributed to the interpretation of the results throughout the study and have read and approved the manuscript. D.A.C. was responsible for content of the final manuscript.
Funding
Funding for this project was provided by Swine Innovation Porc (1794), Evonik Operations GmbH, and Mitacs (IT12203). General program funding for the Prairie Swine Centre, Inc. is provided by the Government of Saskatchewan, Saskatchewan Pork Development Board, Manitoba Pork, Alberta Pork, and Ontario Pork.
Data Availability
Data available upon reasonable request to the corresponding author.
Literature Cited
- Abe, F., Ishibashi N., and Shimamura S.. . 1995. Effect of administration of bifidobacteria and lactic acid bacteria to newborn calves and piglets. J. Dairy Sci. 78:2838–2846. doi: 10.3168/jds.S0022-0302(95)76914-4. [DOI] [PubMed] [Google Scholar]
- Balaji, R., Wright K. J., Hill C. M., Dritz S. S., Knoppel E. L., and Minton J. E.. . 2000. Acute phase responses of pigs challenged orally with Salmonella Typhimurium. J. Anim. Sci. 78:1885–1891. doi: 10.2527/2000.7871885x. [DOI] [PubMed] [Google Scholar]
- Barba-Vidal, E., Castillejos L., Roll V. F. B., Cifuentes-Orjuela G., Moreno Muñoz J. A., and Martín-Orúe S. M.. . 2017. The probiotic combination of Bifidobacterium longum subsp. infantis CECT 7210 and Bifidobacterium animalis subsp. lactis BPL6 reduces pathogen loads and improves gut health of weaned piglets orally challenged with Salmonella Typhimurium. Front. Microbiol. 8:1570. doi: 10.3389/fmicb.2017.01570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido-Carreras, N., Argüello H., Zaldívar-López S., Jiménez-Marín Á., Martins R. P., Arce C., Morera L., Carvajal A., and Garrido J. J.. . 2019. Salmonella Typhimurium infection along the porcine gastrointestinal tract and associated lymphoid tissues. Vet. Pathol. 56:681–690. doi: 10.1177/0300985819843682. [DOI] [PubMed] [Google Scholar]
- Bloomer, S. A. 2018. Combinational use of Na-butyrate and phytobiotics on growth performance and intestinal health of nursery pigs [MSc Diss.]. Raleigh: North Caroline State University. [Google Scholar]
- Boumans, I. J. M. M., Bokkers E. A. M., Hofstede G. J., and de Boer I. J. M.. . 2015. Understanding feeding patterns in growing pigs by modelling growth and motivation. Appl. Anim. Behav. Sci. 171:69–80. 10.1016/j.applanim.2015.08.013. [DOI] [Google Scholar]
- Boyer, P. E., D’Costa S., Edwards L. L., Milloway M., Susick E., Borst L. B., Thakur S., Campbell J. M., Crenshaw J. D., Polo J., . et al. 2015. Early-life dietary spray-dried plasma influences immunological and intestinal injury responses to later-life Salmonella Typhimurium challenge. Br. J. Nutr. 113:783–793. doi: 10.1017/S000711451400422X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno, D. G., Martins S. M. M. K., Parazzi L. J., Afonso E. R., Del Santo T. A., Teixeira S. M. N., Moreno A. M., and Moretti A. S.. . 2013. Phytogenic feed additives in piglets challenged with Salmonella Typhimurium. R. Bras. Zootec. Sci. 42:137–43. 10.1590/S1516-35982013000200009. [DOI] [Google Scholar]
- Burkey, T. E., Dritz S. S., Nietfeld J. C., Johnson B. J., and Minton J. E.. . 2004. Effect of dietary mannanoligosaccharide and sodium chlorate on the growth performance, acute-phase response, and bacterial shedding of weaned pigs challenged with Salmonella enterica serotype Typhimurium. J. Anim. Sci. 82:397–404. doi: 10.2527/2004.822397x. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care (CCAC) . 2009. Guidelines on the care and use of farm animals in research, teaching and testing. Ottawa, ON: CCAC. [Google Scholar]
- Capozzalo, M. M., Kim J. C., Htoo J. K., de Lange C. F. M., Mullan B. P., Hansen C. F., Resink J. W., and Pluske J. R.. . 2017. Pigs experimentally infected with an enterotoxigenic strain of Escherichia coli have improved feed efficiency and indicators of inflammation with dietary supplementation of tryptophan and methionine in the immediate post-weaning period. Anim. Prod. Sci. 57:935–947. 10.1071/AN15289. [DOI] [Google Scholar]
- Chen, Y., Li D., Dai Z., Piao X., Wu Z., Wang B., Zhu Y., and Zeng Z.. . 2014. L-methionine supplementation maintains the integrity and barrier function of the small-intestinal mucosa in post-weaning piglets. Amino Acids 46:1131–1142. doi: 10.1007/s00726-014-1675-5. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Zhang H., Cheng Y., Li Y., Wen C., and Zhou Y.. . 2018. Dietary l-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br. J. Nutr. 119:1254–1262. 10.1017/S0007114518000740. [DOI] [PubMed] [Google Scholar]
- Circu, M. L., and Aw T. Y.. . 2012. Intestinal redox biology and oxidative stress. Semin. Cell Dev. Biol. 23:729–737. doi: 10.1016/j.semcdb.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coma, J., Zimmerman D. R., and Carrion D.. . 1995. Relationship of rate of lean tissue growth and other factors to concentration of urea in plasma of pigs. J. Anim. Sci. 73:3649–3656. doi: 10.2527/1995.73123649x. [DOI] [PubMed] [Google Scholar]
- Correa-Matos, N. J., Donovan S. M., Isaacson R. E., Gaskins H. R., White B. A., and Tappenden K. A.. . 2003. Fermentable fiber reduces recovery time and improves intestinal function in piglets following Salmonella Typhimurium infection. J. Nutr. 133:1845–1852. doi: 10.1093/jn/133.6.1845. [DOI] [PubMed] [Google Scholar]
- Dincer, Y., Erzin Y., Himmetoglu S., Gunes K. N., Bal K., and Akcay T.. . 2007. Oxidative DNA damage and antioxidant activity in patients with inflammatory bowel disease. Dig. Dis. Sci. 52:1636–1641. doi: 10.1007/s10620-006-9386-8. [DOI] [PubMed] [Google Scholar]
- Doumas, B. T., Watson W. A., and Biggs H. G.. . 1971. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta. 31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- Dritz, S. S., Owen K. Q., Goodband R. D., Nelssen J. L., Tokach M. D., Chengappa M. M., and Blecha F.. . 1996. Influence of lipopolysaccharide-induced immune challenge and diet complexity on growth performance and acute-phase protein production in segregated early-weaned pigs. J. Anim. Sci. 74:1620–1628. doi: 10.2527/1996.7471620x. [DOI] [PubMed] [Google Scholar]
- Evonik . 2016. AMINODat®5.0 Platinum. Germany: Evonik Degussa GmbH, Hanau-Wolfgang. [Google Scholar]
- Fan, P., Liu P., Song P., Chen X., and Ma X.. . 2017. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 7:43412. doi: 10.1038/srep43412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink, M. P., and Heard S. O.. . 1990. Laboratory models of sepsis and septic shock. J. Surg. Res. 49:186–196. doi: 10.1016/0022-4804(90)90260-9. [DOI] [PubMed] [Google Scholar]
- Fu, L., Li H., Liang T., Zhou B., Chu Q., Schinckel A. P., Yang X., Zhao R., Li P., and Huang R.. . 2016. Stocking density affects welfare indicators of growing pigs of different group sizes after regrouping. Appl. Anim. Behav. Sci. 174:42–50. 10.1016/j.applanim.2015.10.002. [DOI] [Google Scholar]
- Gebru, E., Lee J. S., Son J. C., Yang S. Y., Shin S. A., Kim B., Kim M. K., and Park S. C.. . 2010. Effect of probiotic-, bacteriophage-, or organic acid-supplemented feeds or fermented soybean meal on the growth performance, acute-phase response, and bacterial shedding of grower pigs challenged with Salmonella enterica serotype Typhimurium. J. Anim. Sci. 88:3880–3886. doi: 10.2527/jas.2010-2939. [DOI] [PubMed] [Google Scholar]
- Heo, J. M., Kim J. C., Hansen C. F., Mullan B. P., Hampson D. J., and Pluske J. R.. . 2008. Effects of feeding low protein diets to piglets on plasma urea nitrogen, faecal ammonia nitrogen, the incidence of diarrhoea and performance after weaning. Arch. Anim. Nutr. 62:343–358. doi: 10.1080/17450390802327811. [DOI] [PubMed] [Google Scholar]
- Heo, J. M., Kim J. C., Hansen C. F., Mullan B. P., Hampson D. J., and Pluske J. R.. . 2009. Feeding a diet with decreased protein content reduces indices of protein fermentation and the incidence of postweaning diarrhea in weaned pigs challenged with an enterotoxigenic strain of Escherichia coli. J. Anim. Sci. 87:2833–2843. doi: 10.2527/jas.2008-1274. [DOI] [PubMed] [Google Scholar]
- Heo, J. M., Kim J. C., Hansen C. F., Mullan B. P., Hampson D. J., Maribo H., Kjeldsen N., and Pluske J. R.. . 2010a. Effects of dietary protein level and zinc oxide supplementation on the incidence of post-weaning diarrhoea in weaner pigs challenged with an enterotoxigenic strain of Escherichia coli. Livest. Sci. 133:210–213. 10.1016/j.livsci.2010.06.066. [DOI] [Google Scholar]
- Heo, J. M., Kim J. C., Hansen C. F., Mullan B. P., Hampson D. J., and Pluske J. R.. . 2010b. Feeding a diet with a decreased protein content reduces both nitrogen content in the gastrointestinal tract and post-weaning diarrhoea, but does not affect apparent nitrogen digestibility in weaner pigs challenged with an enterotoxigenic strain of Escherichia coli. Anim. Feed Sci. Technol. 160:148–159. 10.1016/j.anifeedsci.2010.07.005. [DOI] [Google Scholar]
- Htoo, J. K., Araiza B. A., Sauer W. C., Rademacher M., Zhang Y., Cervantes M., and Zijlstra R. T.. . 2007. Effect of dietary protein content on ileal amino acid digestibility, growth performance, and formation of microbial metabolites in ileal and cecal digesta of early-weaned pigs,. J. Anim. Sci. 85:3303–3312. 10.2527/jas.2007-0105. [DOI] [PubMed] [Google Scholar]
- Jayaraman, B., Htoo J., and Nyachoti C. M.. . 2015. Effects of dietary threonine:lysine ratioes and sanitary conditions on performance, plasma urea nitrogen, plasma-free threonine and lysine of weaned pigs. Anim. Nutr. 1:283–288. doi: 10.1016/j.aninu.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman, B., Regassa A., Htoo J. K., and Nyachoti C. M.. . 2017. Effects of dietary standardized ileal digestible tryptophan:lysine ratio on performance, plasma urea nitrogen, ileal histomorphology and immune responses in weaned pigs challenged with Escherichia coli K88. Livest. Sci. 203:114–119. 10.1016/j.livsci.2017.07.014. [DOI] [Google Scholar]
- Jha, R., and Berrocoso J. F. D.. . 2016. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Anim. Feed Sci. Technol. 212:18–26. 10.1016/j.anifeedsci.2015.12.002. [DOI] [Google Scholar]
- Jones, D. P. 2002. [11] Redox potential of GSH/GSSG couple: assay and biological significance. In: Sies H., and Packer L., editors. Methods in enzymology. San Diego, CA: Academic Press; p. 93–112. [DOI] [PubMed] [Google Scholar]
- Jones, C. K., DeRouchey J. M., Nelssen J. L., Tokach M. D., Dritz S. S., and Goodband R. D.. . 2010. Effects of fermented soybean meal and specialty animal protein sources on nursery pig performance. J. Anim. Sci. 88:1725–1732. 10.2527/jas.2009-2110. [DOI] [PubMed] [Google Scholar]
- Kampman-van de Hoek, E., Jansman A. J., van den Borne J. J., van der Peet-Schwering C. M., van Beers-Schreurs H., and Gerrits W. J.. . 2016. Dietary amino acid deficiency reduces the utilization of amino acids for growth in growing pigs after a period of poor health. J. Nutr. 146:51–58. 10.3945/jn.115.216044. [DOI] [PubMed] [Google Scholar]
- Kansagra, K., Stoll B., Rognerud C., Niinikoski H., Ou C. N., Harvey R., and Burrin D.. . 2003. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 285:G1162–G1170. doi: 10.1152/ajpgi.00243.2003. [DOI] [PubMed] [Google Scholar]
- Kim, J. C., Heo J. M., Mullan B. P., and Pluske J. R.. . 2011. Efficacy of a reduced protein diet on clinical expression of post-weaning diarrhoea and life-time performance after experimental challenge with an enterotoxigenic strain of Escherichia coli. Anim. Feed Sci. Technol. 170:222–230. 10.1016/j.anifeedsci.2011.08.012. [DOI] [Google Scholar]
- Le Floc’h, N., Lebellego L., Matte J. J., Melchior D., and Sève B.. . 2009. The effect of sanitary status degradation and dietary tryptophan content on growth rate and tryptophan metabolism in weaning pigs. J. Anim. Sci. 87:1686–1694. doi: 10.2527/jas.2008-1348. [DOI] [PubMed] [Google Scholar]
- Le Floc’h, N., Wessels A., Corrent E., Wu G., and Bosi P.. . 2018. The relevance of functional amino acids to support the health of growing pigs. Anim. Feed Sci. Technol. 245:104–116. 10.1016/j.anifeedsci.2018.09.007. [DOI] [Google Scholar]
- Lin, H. C., and Visek W. J.. . 1991. Colon mucosal cell damage by ammonia in rats. J. Nutr. 121:887–893. doi: 10.1093/jn/121.6.887. [DOI] [PubMed] [Google Scholar]
- Litvak, N., Rakhshandeh A., Htoo J. K., and de Lange C. F.. . 2013. Immune system stimulation increases the optimal dietary methionine to methionine plus cysteine ratio in growing pigs. J. Anim. Sci. 91:4188–4196. doi: 10.2527/jas.2012-6160. [DOI] [PubMed] [Google Scholar]
- Lu, S. C. 2009. Regulation of glutathione synthesis. Mol. Aspects Med. 30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, L., Zhang H., Liu Z., Lei L., Feng Z., Zhang D., Ren Y., and Zhao S.. . 2020. Comparative study of yeast selenium vs. sodium selenite on growth performance, nutrient digestibility, anti-inflammatory and anti-oxidative activity in weaned piglets challenged by Salmonella Typhimurium. Innate Immun. 26:248–258. doi: 10.1177/1753425919888566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makimura, S., and Suzuki N.. . 1982. Quantitative determination of bovine serum haptoglobin and its elevation in some inflammatory diseases. Nihon Juigaku Zasshi. 44:15–21. 10.1292/jvms1939.44.15. [DOI] [PubMed] [Google Scholar]
- Makkink, C. A., Negulescu G. P., Qin G., and Verstegen M. W.. . 1994. Effect of dietary protein source on feed intake, growth, pancreatic enzyme activities and jejunal morphology in newly-weaned piglets. Br. J. Nutr. 72:353–368. doi: 10.1079/bjn19940039. [DOI] [PubMed] [Google Scholar]
- Malmezat, T., Breuillé D., Capitan P., Mirand P. P., and Obled C.. . 2000. Glutathione turnover is increased during the acute phase of sepsis in rats. J. Nutr. 130:1239–1246. doi: 10.1093/jn/130.5.1239. [DOI] [PubMed] [Google Scholar]
- Mao, X., Lv M., Yu B., He J., Zheng P., Yu J., Wang Q., and Chen D.. . 2014. The effect of dietary tryptophan levels on oxidative stress of liver induced by diquat in weaned piglets. J. Anim. Sci. Biotechnol. 5:49. doi: 10.1186/2049-1891-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Peláez, S., Gibson G. R., Martín-Orúe S. M., Klinder A., Rastall R. A., La Ragione R. M., Woodward M. J., and Costabile A.. . 2008. In vitro fermentation of carbohydrates by porcine faecal inocula and their influence on Salmonella Typhimurium growth in batch culture systems. FEMS Microbiol. Ecol. 66:608–619. doi: 10.1111/j.1574-6941.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- van der Meer, Y., Lammers A., Jansman A. J., Rijnen M. M., Hendriks W. H., and Gerrits W. J.. . 2016. Performance of pigs kept under different sanitary conditions affected by protein intake and amino acid supplementation. J. Anim. Sci. 94:4704–4719. doi: 10.2527/jas.2016-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, A., Singh S., and Ganguly N. K.. . 1998. Impairment of intestinal mucosal antioxidant defense system during Salmonella Typhimurium infection. Dig. Dis. Sci. 43:646–651. doi: 10.1023/a:1018887813713. [DOI] [PubMed] [Google Scholar]
- van Milgen, J., and Dourmad J. Y.. . 2015. Concept and application of ideal protein for pigs. J. Anim. Sci. Biotechnol. 6:15. doi: 10.1186/s40104-015-0016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (NRC) . 2012. Nutrient requirements of swine. 11th rev. ed. Washington, DC: National Academies Press. [Google Scholar]
- Nollet, H., Deprez P., Van Driessche E., and Muylle E.. . 1999. Protection of just weaned pigs against infection with F18+ Escherichia coli by non-immune plasma powder. Vet. Microbiol. 65:37–45. doi: 10.1016/s0378-1135(98)00282-x. [DOI] [PubMed] [Google Scholar]
- Nousiainen, J. 1991. Comparative observations on selected probiotics and olaquindox as feed additives for piglets around weaning. J. Anim. Physiol. Anim. Nutr. 66:224–230. 10.1111/j.1439-0396.1991.tb00290.x. [DOI] [Google Scholar]
- Opapeju, F. O., Krause D. O., Payne R. L., Rademacher M., and Nyachoti C. M.. . 2009. Effect of dietary protein level on growth performance, indicators of enteric health, and gastrointestinal microbial ecology of weaned pigs induced with postweaning colibacillosis. J. Anim. Sci. 87:2635–2643. doi: 10.2527/jas.2008-1310. [DOI] [PubMed] [Google Scholar]
- Oz, H. S., Chen T. S., and Nagasawa H.. . 2007. Comparative efficacies of 2 cysteine prodrugs and a glutathione delivery agent in a colitis model. Transl. Res. 150:122–129. doi: 10.1016/j.trsl.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorelli, H., van Milgen J., Lovatto P., and Montagne L.. . 2012. Meta-analysis of feed intake and growth responses of growing pigs after a sanitary challenge. Animal 6:952–961. doi: 10.1017/S175173111100228X. [DOI] [PubMed] [Google Scholar]
- Pearce, S. C., Mani V., Boddicker R. L., Johnson J. S., Weber T. E., Ross J. W., Rhoads R. P., Baumgard L. H., and Gabler N. K.. . 2013. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One 8:e70215. doi: 10.1371/journal.pone.0070215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, A., Heegaard P. M., Pedersen A. L., Andersen J. B., Sørensen R. B., Frøkiaer H., Lahtinen S. J., Ouwehand A. C., Poulsen M., and Licht T. R.. . 2009. Some putative prebiotics increase the severity of Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol. 9:245. doi: 10.1186/1471-2180-9-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper, R., Bindelle J., Rossnagel B., Van Kessel A., and Leterme P.. . 2009. Effect of carbohydrate composition in barley and oat cultivars on microbial ecophysiology and proliferation of Salmonella enterica in an in vitro model of the porcine gastrointestinal tract. Appl. Environ. Microbiol. 75:7006–7016. doi: 10.1128/AEM.01343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock, J., Hutchings M. R., Hutchings K. E. K., Gally D. L., and Houdijk J. G. M.. . 2019. Changes in the Ileal, but not fecal, microbiome in response to increased dietary protein level and enterotoxigenic Escherichia coli exposure in pigs. Appl. Environ. Microbiol. 85:e01252-19. 10.1128/AEM.01252-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, K. L., Totty H. R., Lee H. B., Utt M. D., Fitzner G. E., Yoon I., Ponder M. A., and Escobar J.. . 2010. Use of Saccharomyces cerevisiae fermentation product on growth performance and microbiota of weaned pigs during Salmonella infection. J. Anim. Sci. 88:3896–3908. doi: 10.2527/jas.2009-2728. [DOI] [PubMed] [Google Scholar]
- Rakhshandeh, A., and De Lange C. F. M.. . 2010. Immune system stimulation increases reduced glutathione synthesis rate in growing pigs. In: Crovetto G. M., editor. Energy and protein metabolism and nutrition. Wageningen, Netherlands: Wageningen Academic Publisher; p. 501–502. [Google Scholar]
- Rakhshandeh, A., Htoo J. K., and de Lange C. F. M.. . 2010. Immune system stimulation of growing pigs does not alter apparent ileal amino acid digestibility but reduces the ratio between whole body nitrogen and sulfur retention. Livest. Sci. 134:21–23. 10.1016/j.livsci.2010.06.085. [DOI] [Google Scholar]
- Rakhshandeh, A., Htoo J. K., Karrow N., Miller S. P., and de Lange C. F.. . 2014. Impact of immune system stimulation on the ileal nutrient digestibility and utilisation of methionine plus cysteine intake for whole-body protein deposition in growing pigs. Br. J. Nutr. 111:101–110. doi: 10.1017/S0007114513001955. [DOI] [PubMed] [Google Scholar]
- Reeds, P. J., Fjeld C. R., and Jahoor F.. . 1994. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J. Nutr. 124:906–910. doi: 10.1093/jn/124.6.906. [DOI] [PubMed] [Google Scholar]
- Reeds, P. J., and Jahoor F.. . 2001. The amino acid requirements of disease. Clin. Nutr. 20:15–22. 10.1054/clnu.2001.0402. [DOI] [Google Scholar]
- Riedijk, M. A., Stoll B., Chacko S., Schierbeek H., Sunehag A. L., van Goudoever J. B., and Burrin D. G.. . 2007. Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract. Proc. Natl. Acad. Sci. USA. 104:3408–3413. doi: 10.1073/pnas.0607965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ridder, K., Levesque C. L., Htoo J. K., and de Lange C. F.. . 2012. Immune system stimulation reduces the efficiency of tryptophan utilization for body protein deposition in growing pigs. J. Anim. Sci. 90:3485–3491. doi: 10.2527/jas.2011-4830. [DOI] [PubMed] [Google Scholar]
- Rist, V. T., Weiss E., Eklund M., and Mosenthin R.. . 2013. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: a review. Animal 7:1067–1078. doi: 10.1017/S1751731113000062. [DOI] [PubMed] [Google Scholar]
- Roy, J. H. 1969. Diarrhoea of nutritional origin. Proc. Nutr. Soc. 28:160–170. doi: 10.1079/pns19690027. [DOI] [PubMed] [Google Scholar]
- Shoveller, A. K., Brunton J. A., Pencharz P. B., and Ball R. O.. . 2003. The methionine requirement is lower in neonatal piglets fed parenterally than in those fed enterally. J. Nutr. 133:1390–1397. doi: 10.1093/jn/133.5.1390. [DOI] [PubMed] [Google Scholar]
- Siregar, G. A., Sari D. K., and Sungkar T.. . 2018. Degree of neutrophil, atrophy, and metaplasia intestinal were associate with malondialdehyde level in gastritis patients. IOP Conf. Ser. Earth Environ. Sci. 125:012213. 10.1088/1755-1315/125/1/012213. [DOI] [Google Scholar]
- Song, Y. S., Pérez V. G., Pettigrew J. E., Martinez-Villaluenga C., and de Mejia E. G.. . 2010. Fermentation of soybean meal and its inclusion in diets for newly weaned pigs reduced diarrhea and measures of immunoreactivity in the plasma. Anim. Feed Sci. Technol. 159:41–49. 10.1016/j.anifeedsci.2010.04.011. [DOI] [Google Scholar]
- Stipanuk, M. H. 2004. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu. Rev. Nutr. 24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- Stipanuk, M. H., Londono M., Lee J. I., Hu M., and Yu A. F.. . 2002. Enzymes and metabolites of cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels. J. Nutr. 132:3369–3378. doi: 10.1093/jn/132.11.3369. [DOI] [PubMed] [Google Scholar]
- Turner, J. L., Dritz S. S., Werner J. R., Hill C. M., Skjolaas K., Hogge S., Herkleman K., and Minton J. E.. . 2000. Effects of a Quillaja saponaria extract on weanling pig growth performance and immune function during an acute enteric disease challenge. Kans. State Univ. Swine Day 2000 Rep. Prog. 858:37–40. [Google Scholar]
- Visek, W. J. 1984. Ammonia: its effects on biological systems, metabolic hormones, and reproduction. J. Dairy Sci. 67:481–498. doi: 10.3168/jds.S0022-0302(84)81331-4. [DOI] [PubMed] [Google Scholar]
- Wellington, M. O., Agyekum A. K., Hamonic K., Htoo J. K., Van Kessel A. G., and Columbus D. A.. . 2019. Effect of supplemental threonine above requirement on growth performance of Salmonella Typhimurium challenged pigs fed high-fiber diets. J. Anim. Sci. 97:3636–3647. 10.1093/jas/skz225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington, M. O., Hamonic K., Krone J. E. C., Htoo J. K., Van Kessel A. G., and Columbus D. A.. . 2020. Effect of dietary fiber and threonine content on intestinal barrier function in pigs challenged with either systemic E. coli lipopolysaccharide or enteric Salmonella Typhimurium. J. Anim. Sci. Biotechnol. 11:38. doi: 10.1186/s40104-020-00444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington, M. O., Htoo J. K., Van Kessel A. G., and Columbus D. A.. . 2018. Impact of dietary fiber and immune system stimulation on threonine requirement for protein deposition in growing pigs. J. Anim. Sci. 96:5222–5232. doi: 10.1093/jas/sky381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellock, I. J., Fortomaris P. D., Houdijk J. G. M., and Kyriazakis I.. . 2007. Effect of weaning age, protein nutrition and enterotoxigenic Escherichia coli challenge on the health of newly weaned piglets. Livest. Sci. 108:102–105. 10.1016/j.livsci.2007.01.004. [DOI] [Google Scholar]
- Wellock, I. J., Fortomaris P. D., Houdijk J. G., and Kyriazakis I.. . 2008. Effects of dietary protein supply, weaning age and experimental enterotoxigenic Escherichia coli infection on newly weaned pigs: health. Animal 2:834–842. doi: 10.1017/S1751731108002048. [DOI] [PubMed] [Google Scholar]
- Wichterman, K. A., Baue A. E., and Chaudry I. H.. . 1980. Sepsis and septic shock—a review of laboratory models and a proposal. J. Surg. Res. 29:189–201. 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- Wu, C. H., Ko J. L., Liao J. M., Huang S. S., Lin M. Y., Lee L. H., Chang L. Y., and Ou C. C.. . 2019. D-methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation. Ther. Adv. Med. Oncol. 11:1758835918821021. doi: 10.1177/1758835918821021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, D., Ibuki M., Nakamori T., Fan M., and Mine Y.. . 2012. Soy-derived di- and tripeptides alleviate colon and ileum inflammation in pigs with dextran sodium sulfate-induced colitis. J. Nutr. 142:363–368. doi: 10.3945/jn.111.149104. [DOI] [PubMed] [Google Scholar]
- Yun, J. H., Kwon I. K., Lohakare J. D., Choi J. Y., Yong J. S., Zheng J., Cho W. T., and Chae B. J.. . 2005. Comparative efficacy of plant and animal protein sources on the growth performance, nutrient digestibility, morphology and caecal microbiology of early-weaned pigs. Asian-Australas. J. Anim. Sci. 18:1285–1293. doi:2005.18.9.1285. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available upon reasonable request to the corresponding author.