Abstract
We compared the performance of computed tomography (CT) and magnetic resonance imaging (MRI) for preoperative clinical staging of mass‐forming intrahepatic cholangiocarcinoma (iCCA), using the eighth American Joint Committee on Cancer (AJCC) system. This retrospective, multicenter, cohort study consecutively identified patients who underwent partial hepatectomy for mass‐forming iCCA and had preoperative CT and MRI performed from January 2009 to December 2015. CT and MRI characteristics were used to determine clinical stage based on the eighth AJCC system. Performances of CT and MRI for clinical T and N staging were compared using generalized estimating equations. In 334 patients (median age, 63 years; 221 men), MRI sensitivities were significantly higher than CT sensitivities for detecting T1b or higher stages (91.0% vs. 80.5%, respectively, P < 0.001), T2 or higher stages (89.1% vs. 73.8%, respectively, P < 0.001), and T3 or T4 stage (77.8% vs. 58.0%, respectively, P < 0.001). MRI was also more sensitive at identifying multiple tumors than CT (66.7% vs. 50.0%, respectively, P = 0.026), without a significant difference in specificity (78.1% vs. 80.1%, respectively, P = 0.342). Sensitivities were comparable between CT and MRI for determination of size >5 cm (i.e., T1b for single tumor) and extrahepatic organ invasion (i.e., T4). Sensitivities of CT and MRI were not different for N stage (65.0% vs. 64.0%, respectively, P = 0.808), but the specificity of CT was significantly higher than that of MRI (80.7% vs. 72.9%, respectively, P = 0.001) when using a composite reference standard. Conclusion: MRI showed superior sensitivity to CT for diagnosing T2 and T3 stages, particularly multiple tumors. CT and MRI had comparable sensitivity for N staging, but CT provided higher specificity than MRI.
Abbreviations
- AJCC
American Joint Committee on Cancer
- CT
computed tomography
- ECA
extracellular agent
- HBA
hepatobiliary agent
- iCCA
intrahepatic cholangiocarcinoma
- IQR
interquartile range
- MRI
magnetic resonance imaging
Intrahepatic cholangiocarcinoma (iCCA) is the second most common primary liver cancer, accounting for approximately 15% of primary liver cancers.( 1 , 2 ) The incidence of iCCA is highest in Eastern countries, such as Thailand, South Korea, and China, but its incidence and mortality are increasing worldwide.( 1 , 2 , 3 ) iCCA arises from canals of Hering to second‐order bile ducts and is differentiated from perihilar or distal cholangiocarcinoma by anatomic location. iCCA most commonly presents as a mass‐forming type that can be morphologically distinguished from periductal infiltrating or intraductal growing types.( 1 , 4 )
The American Joint Committee on Cancer (AJCC) system is a standard system used for cancer prognostication and treatment guidance. In 2010, the seventh edition of AJCC designated a unique staging system for iCCA separate from that for hepatocellular carcinoma.( 5 ) The AJCC system considers tumor number, local extent, regional lymph node metastasis, and distant metastasis; in the latest edition (eighth edition), tumor size, number, and invasion of vascular structures, visceral peritoneum, and/or extrahepatic organs are taken into account for T staging (Supporting Table S1).( 6 )
Multiphasic contrast‐enhanced computed tomography (CT) and magnetic resonance imaging (MRI) are imaging modalities of choice for staging iCCA.( 7 ) As surgery is the only potentially curative treatment for iCCA, it is important to accurately determine clinical stage using imaging studies and identify surgical candidates.( 3 ) Although a multimodal imaging approach is frequently used for preoperative evaluation of iCCA,( 8 ) recognizing the pros and cons of using CT or MRI for staging may help choose the optimal imaging modality before surgery. The high spatial resolution provided by CT and high soft tissue contrast provided by MRI may have different impacts on clinical staging.( 9 ) Moreover, the choice of contrast media for MRI can affect clinical staging due to differences in the properties of hepatobiliary agents (HBAs) and extracellular agents (ECAs).( 10 ) However, few studies have investigated the performance of clinical staging according to imaging modality and contrast agent. The current AJCC system does not provide specific recommendations regarding the usage of CT and MRI. Therefore, our aims in this study were to compare the performance of preoperative clinical staging using the eighth AJCC system between CT and MRI for mass‐forming iCCAs that were surgically resected and to explore differences in clinical staging according to the choice of MRI contrast media.
Patients and Methods
Patients
This retrospective, multicenter, cohort study was approved by the institutional review boards of all participating institutions. The requirement for written informed consent was waived due to retrospective review of medical records and images. We consecutively identified patients who underwent partial hepatectomy for mass‐forming iCCA at six participating academic medical centers in South Korea from January 2009 to December 2015. Inclusion criteria were (a) histopathologic diagnosis of mass‐forming iCCA, (b) multiphasic contrast‐enhanced CT performed within 3 months before surgery, and (c) multiphasic contrast‐enhanced MRI performed within 3 months before surgery. Exclusion criteria were (a) radiologically invisible tumor, (b) the absence of preoperative CT images or preoperative CT images of suboptimal quality, (c) the absence of preoperative MRI images or suboptimal preoperative MRI images, (d) previous surgery for iCCA, and (e) mortality within 1 month after surgery.
Image Acquisition
Multiphasic contrast‐enhanced CT was routinely performed at 120 kilovoltage peak using 16 or higher detector rows. Images were acquired before and after intravenous injection of iodinated contrast of 120‐150 mL at a rate of 2‐5 mL/second. Arterial phase images were obtained using a bolus‐tracking technique with a 10‐25‐second delay after reaching the peak abdominal aortic enhancement to 100 Hounsfield units. Portal venous phase and delayed‐phase images were obtained at 70‐80 seconds and 3 minutes, respectively, after contrast injection.
Multiphasic contrast‐enhanced MRI was routinely performed using either a 1.5‐T or 3.0‐T scanner. Routine protocols included unenhanced T1‐weighted in‐phase and opposed‐phase imaging and respiratory‐triggered or breath‐hold T2‐weighted imaging with or without fat suppression. Three‐dimensional, T1‐weighted, gradient‐echo images were obtained before and after intravenous injection of recommended contrast dose and subsequent saline flush. Because patients were recruited from multiple institutions, various contrast media were used for MRI, including gadoxetic acid (Primovist; Bayer), gadobenate dimeglumine (MultiHance; Bracco Diagnostics Inc.), and gadoterate meglumine (Dotarem; Guerbet). Arterial phase images were acquired using either the test‐bolus or bolus‐tracking technique. Portal venous phase and delayed‐phase images were acquired at 50‐70 seconds and 120‐180 seconds after contrast injection, respectively. Hepatobiliary phase images were acquired for gadoxetic acid‐ and gadobenate dimeglumine‐enhanced MRI at 20 minutes and 3 hours after contrast injection, respectively. Diffusion‐weighted imaging was performed at b values of 0‐50, 400‐500, and 800‐900 second/mm2.
Image Analysis
In the first image analysis session, three board‐certified radiologists from different institutions independently evaluated de‐identified CTs. In the second session, which was held a month later to minimize recall bias, the same radiologists independently evaluated de‐identified MRIs. Readers were blinded to clinical and pathologic information during image analysis. All images were provided as anonymized Digital Imaging and Communications in Medicine (DICOM) files and analyzed using a commercial DICOM viewer (RadiAnt DICOM viewer; Medixant, Poznan, Poland).
Typical imaging features of iCCA are described in Supporting Table S2, and Supporting Fig. S1. The following characteristics were evaluated on both CT and MRI to determine clinical stage based on the eighth AJCC system for iCCA( 6 ): Largest tumor size was measured on the axial plane of portal venous phase images by each reader, and values were averaged.( 11 ) Mean tumor size was dichotomized using the cutoff of 5 cm for staging of T1a (≤5 cm) or T1b (>5 cm) tumors. The following other characteristics were evaluated by combined reading of all sequences of CT or MRI to reflect daily radiology practice: Tumor multiplicity was determined based on the number of tumor nodules in the liver, including multifocal disease, satellite nodules, and intrahepatic metastasis; in patients with liver cirrhosis, cirrhosis‐associated regenerative nodules were not counted as multiple tumors. Vascular invasion was defined as luminal irregularity, narrowing, or obliteration of segmental or larger intrahepatic vessels due to encasement by the tumor or as tumor thrombosis in the vessels (enhancing or expansile thrombosis in contiguity with the tumor).( 12 , 13 ) Visceral peritoneal invasion was defined as tumor infiltration through the liver capsule on axial or coronal images. Extrahepatic organ invasion was defined as tumor infiltration of the colon, duodenum, stomach, hepatoduodenal ligament, inferior vena cava, abdominal wall, or diaphragm. Regional lymph node metastasis was considered positive when regional lymph nodes showed suspicious features, such as a short‐axis diameter of larger than 1 cm, central necrosis, or abnormal spiculate or round shape.( 14 ) Distant metastasis was considered positive when nonregional lymph nodes with suspicious features, peritoneal seeding metastasis, or extrahepatic organ metastasis was suspected. After independent image analysis, consensus results reached by at least two radiologists were summarized for data analysis.
Reference Standards
Surgical pathologic reports were reviewed to collect data on tumor size, number, microvascular or macrovascular invasion, visceral peritoneal invasion, and extrahepatic organ invasion to determine the pathologic T stage based on the eighth AJCC system for iCCA. The status of lymph node metastasis and the number of harvested regional lymph nodes were also recorded. Positive lymph node metastasis was recorded as pathologic N1 stage, regardless of the number of harvested regional lymph nodes; negative lymph node metastasis in the case of complete sampling of at least six regional lymph nodes was recorded as pathologic N0 stage. Patients with negative lymph node metastasis in case of incomplete lymph node sampling or those who did not undergo lymphadenectomy were classified as having pathologic Nx stage. The following composite reference standards were used to determine the N stage for these patients: development of suspicious regional lymph nodes on follow‐up cross‐sectional imaging, such as CT, MRI, or positron emission tomography‐CT, within 3 months after surgery was classified as N1; absence of suspicious regional lymph nodes on follow‐up cross‐sectional imaging for at least 1 year after surgery was classified as N0; the remaining cases were classified as Nx.
Statistical Analysis
Patient demographics were summarized descriptively. Sensitivity, specificity, and accuracy of CT and MRI for clinical T staging and clinical N staging were compared using generalized estimating equations. Interreader agreements for imaging characteristics were summarized using intraclass correlation coefficients for tumor size and Fleiss’ κ statistics for categorical variables. The κ statistics were interpreted as follows: poor, κ ≤ 0.00; slight, κ = 0.01‐0.20; fair, κ = 0.21‐0.40; moderate, κ = 0.41‐0.60; substantial, κ = 0.61‐0.80; and almost perfect, κ = 0.81‐0.99.( 15 ) Subgroup analysis was performed between CT and HBA‐enhanced MRI (i.e., gadoxetic acid‐ or gadobenate dimeglumine‐enhanced MRI with hepatobiliary phase) and between CT and ECA‐enhanced MRI (i.e., other gadolinium‐based agent‐ or gadobenate dimeglumine‐enhanced MRI without hepatobiliary phase). Subgroup analysis was also performed according to pathologic tumor differentiation. Statistical Analysis Software version 9.4 (SAS Institute Inc., Cary, NC) and R package version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) were used for analyses. A two‐tailed P < 0.05 indicated statistical significance.
Results
Patient Characteristics
Of 418 patients who underwent partial hepatectomy for mass‐forming iCCA at six participating institutions from January 2009 to December 2015, 84 patients were excluded for reasons specified in Fig. 1; 334 patients (median age, 63 years; interquartile range [IQR], 55‐70; 221 men) were finally included (Table 1). Liver cirrhosis and viral hepatitis without cirrhosis were present in 47 (14.1%) and 63 (18.9%) patients, respectively. Median serum carbohydrate antigen 19‐9 and carcinoembryonic antigen values before surgery were 47.2 U/mL (IQR, 12.1‐394.6) and 2.9 ng/mL (IQR, 1.7‐6.1), respectively. HBA was used as the MRI contrast agent in 251 (75.1%) patients. Median intervals between CT and MRI, CT and surgery, and MRI and surgery were 6 days (IQR, 1‐16), 12 days (IQR, 6‐22), and 10 days (IQR, 6‐18), respectively. CT was performed earlier than MRI in 216 (64.7%), on the same date with MRI in 31 (9.3%), and later than MRI in 87 (26.0%) patients. Surgery was performed with a curative intent in 311 (93.1%) patients. Major hepatectomy was performed in 199 (59.6%) patients, and lymphadenectomy was performed in 162 (48.5%) patients. Surgical resection margins were negative in 286 (85.6%) patients. Results of pathologic T staging, pathologic N staging, and N staging based on the composite reference standard are summarized in Table 1. The frequency of pathologic tumor multiplicity did not differ according to the presence or absence of liver cirrhosis (8.5% [4/47] vs. 13.2% [38/287], P = 0.503).
FIG. 1.
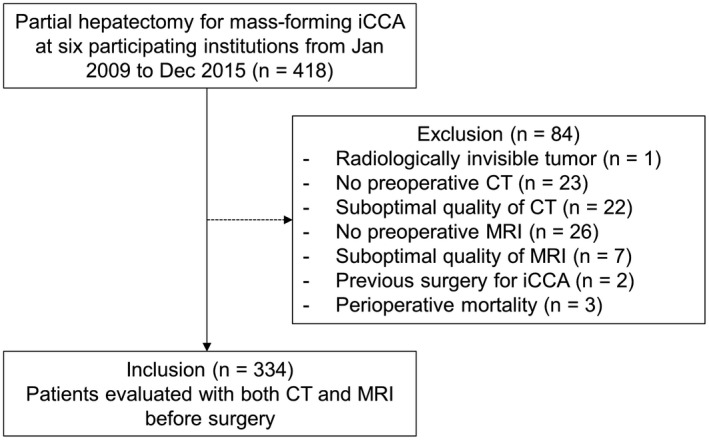
Flow diagram of patients.
Table 1.
Clinicopathologic characteristics
| Variable | Value (n = 334) |
|---|---|
| Age (years), median (IQR) | 63 (55, 70) |
| Men | 221 (66.2) |
| Liver disease | |
| Liver cirrhosis | 47 (14.1) |
| HBV infection/HCV infection/HBV and HCV coinfection | 23 (6.9)/2 (0.6)/1 (0.3) |
| Alcoholic/nonalcoholic fatty liver disease | 6 (1.8)/1 (0.3) |
| Unknown etiology | 14 (4.2) |
| Viral hepatitis without cirrhosis | 63 (18.9) |
| Hepatolithiasis without cirrhosis | 5 (1.5) |
| Clonorchiasis without cirrhosis | 2 (0.6) |
| Primary sclerosing cholangitis or autoimmune hepatitis | 2 (0.6) |
| Preoperative serum tumor markers | |
| Carbohydrate antigen 19‐9 (U/mL), median (IQR) (n = 305) (normal reference value, ≤34 U/mL) | 47.2 (12.1, 394.6) |
| Carcinoembryonic antigen (ng/mL), median (IQR) (n = 291) (normal reference value, ≤5 ng/mL) | 2.9 (1.7, 6.1) |
| MRI contrast media | |
| Hepatobiliary agent | 251 (75.1) |
| Extracellular agent | 83 (24.9) |
| Interval between | |
| CT and MRI (days), median (IQR) | 6 (1, 16) |
| CT and surgery (days), median (IQR) | 12 (6, 22) |
| MRI and surgery (days), median (IQR) | 10 (6, 18) |
| Acquisition order between CT and MRI | |
| CT earlier than MRI | 216 (64.7) |
| CT and MRI on the same date | 31 (9.3) |
| MRI earlier than CT | 87 (26.0) |
| CT/MRI T staging | |
| T1a | 102 (30.5)/65 (19.5) |
| T1b | 17 (5.1)/4 (1.2) |
| T2 | 75 (22.5)/64 (19.2) |
| T3 | 118 (35.3)/182 (54.5) |
| T4 | 22 (6.6)/19 (5.7) |
| CT/MRI N staging | |
| N0 | 227 (68.0)/209 (62.6) |
| N1 | 107 (32.0)/125 (37.4) |
| CT/MRI TNM staging | |
| IA | 96 (28.7)/59 (17.7) |
| IB | 12 (3.6)/3 (0.9) |
| II | 45 (13.5)/42 (12.6) |
| IIIA | 59 (17.7)/96 (28.7) |
| IIIB | 101 (30.2)/113 (33.8) |
| IV | 21 (6.3)/21 (6.3) |
| Type of surgery | |
| Major hepatectomy | 199 (59.6) |
| Minor hepatectomy | 133 (39.8) |
| Wedge resection | 2 (0.6) |
| Resection margin status | |
| R0 | 286 (85.6) |
| R1 | 34 (10.2) |
| R2 | 13 (3.9) |
| Not available | 1 (0.3) |
| Pathologic grade | |
| Well differentiated | 21 (6.3) |
| Moderately differentiated | 216 (64.7) |
| Poorly differentiated | 81 (24.3) |
| Undifferentiated | 3 (0.9) |
| Not available | 13 (3.9) |
| Lymphadenectomy status | |
| Not harvested | 172 (51.5) |
| Complete sampling | 40 (12.0) |
| Incomplete sampling | 53 (15.9) |
| Positive lymph node metastasis with unknown number of samples | 69 (20.7) |
| Pathologic T staging | |
| T1a | 78 (23.4) |
| T1b | 35 (10.5) |
| T2 | 140 (41.9) |
| T3 | 68 (20.4) |
| T4 | 13 (3.9) |
| Pathologic N staging | |
| N0 | 33 (9.9) |
| N1 | 78 (23.4) |
| Nx | 223 (66.8) |
| N staging using the composite reference standard | |
| N0 | 181 (54.2) |
| N1 | 100 (29.9) |
| Nx | 53 (15.9) |
| Adjuvant treatment | |
| No | 191 (57.2) |
| Yes | 143 (42.8) |
Data are number of patients with percentages in parentheses unless otherwise specified.
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; TNM, tumor‐node‐metastasis.
Diagnostic Performance of Clinical Staging
MRI sensitivities were significantly higher than those of CT for detecting T1b or higher stages (91.0% vs. 80.5%, respectively, P < 0.001), T2 or higher stages (89.1% vs. 73.8%, respectively, P < 0.001), and T3 or T4 stage (77.8% vs. 58.0%, respectively, P < 0.001) (Table 2). However, MRI specificities were significantly lower than CT specificities for identifying T1b or higher stages (53.9% vs. 69.2%, respectively, P = 0.001), T2 or higher stages (39.8% vs. 54.0%, respectively, P = 0.001), and T3 or T4 stage (45.5% vs. 63.2%, respectively, P < 0.001). The performance of the two modalities for identifying T4 stage was comparable.
Table 2.
Diagnostic performance of CT and MRI for T staging
| T staging | CT | MRI | P Value |
|---|---|---|---|
| Sensitivity | |||
| ≥T1b | 80.5 [206/256] (75.6, 85.3) | 91.0 [233/256] (87.5, 94.5) | <0.001 |
| ≥T2 | 73.8 [163/221] (68.0, 79.6) | 89.1 [197/221] (85.0, 93.2) | <0.001 |
| ≥T3 | 58.0 [47/81] (47.3, 68.8) | 77.8 [63/81] (68.7, 86.8) | <0.001 |
| T4 | 38.5 [5/13] (12.0, 64.9) | 23.1 [3/13] (17.3, 46.0) | 0.124 |
| Specificity | |||
| ≥T1b | 69.2 [54/78] (60.0, 79.5) | 53.9 [42/78] (42.8, 64.9) | 0.001 |
| ≥T2 | 54.0 [61/113] (44.8, 63.2) | 39.8 [45/113] (30.8, 48.9) | 0.001 |
| ≥T3 | 63.2 [160/253] (57.3, 69.2) | 45.5 [115/253] (39.3, 51.6) | <0.001 |
| T4 | 94.7 [304/321] (92.3, 97.2) | 95.3 [306/321] (93.0, 97.6) | 0.683 |
| Accuracy | |||
| ≥T1b | 77.8 [260/334] (73.4, 82.3) | 82.3 [275/334] (78.3, 86.4) | 0.031 |
| ≥T2 | 67.1 [224/334] (62.0, 72.1) | 72.5 [242/334] (67.7, 77.3) | 0.023 |
| ≥T3 | 62.0 [207/334] (56.8, 67.2) | 53.3 [178/334] (47.9, 58.6) | 0.001 |
| T4 | 92.5 [309/334] (89.7, 95.3) | 92.5 [309/334] (89.7, 95.3) | >0.999 |
Data are performance measures in percentages with numerators and denominators in square brackets and 95% confidence intervals in parentheses.
The diagnostic performances of CT and MRI for tumor characteristics and invasion are summarized in Table 3. MRI had a higher sensitivity for identifying multiple tumors than CT (66.7% vs. 50.0%, respectively, P = 0.026), without a significant difference in specificity (78.1% vs. 80.1%, respectively, P = 0.342) (Supporting Fig. S2). MRI had a significantly higher sensitivity for detecting vascular invasion and visceral peritoneal invasion than CT (73.8% vs. 56.8%, respectively, P < 0.001 for vascular invasion; 77.2% vs. 57.0%, respectively, P < 0.001 for visceral peritoneal invasion) but lower specificities than CT (47.0% vs. 60.3%, respectively, P < 0.001 for vascular invasion; 45.1% vs. 62.8%, respectively, P < 0.001 for visceral peritoneal invasion). CT and MRI showed similar accuracies with regard to tumor size (89.8% vs. 90.4%, respectively, P = 0.617) and extrahepatic organ invasion (92.5% for both CT and MRI).
Table 3.
Diagnostic performance of CT and MRI for tumor characteristics and invasion
| Performance | CT | MRI | P Value |
|---|---|---|---|
| Size >5 cm | |||
| Sensitivity | 83.4 [141/169] (77.8, 89.0) | 83.4 [141/169] (77.8, 89.0) | >0.999 |
| Specificity | 96.4 [159/165] (93.5, 99.2) | 97.6 [161/165] (95.2, 99.9) | 0.155 |
| Accuracy | 89.8 [300/334] (86.6, 93.1) | 90.4 [302/334] (87.3, 93.6) | 0.617 |
| Tumor multiplicity | |||
| Sensitivity | 50.0 [21/42] (34.9, 65.1) | 66.7 [28/42] (52.4, 80.9) | 0.026 |
| Specificity | 80.1 [234/292] (75.6, 84.7) | 78.1 [228/292] (73.3, 82.8) | 0.342 |
| Accuracy | 76.4 [255/334] (71.8, 80.9) | 76.7 [256/334] (72.1, 81.2) | 0.889 |
| Vascular invasion | |||
| Sensitivity | 56.8 [104/183] (49.7, 64.0) | 73.8 [135/183] (67.4, 80.1) | <0.001 |
| Specificity | 60.3 [91/151] (52.5, 68.1) | 47.0 [71/151] (39.1, 55.0) | <0.001 |
| Accuracy | 58.4 [195/334] (53.1, 63.7) | 61.7 [206/334] (56.5, 66.9) | 0.158 |
| Visceral peritoneal invasion | |||
| Sensitivity | 57.0 [45/79] (46.0, 67.9) | 77.2 [61/79] (68.0, 86.5) | <0.001 |
| Specificity | 62.8 [160/255] (56.8, 68.7) | 45.1 [115/255] (39.0, 51.2) | <0.001 |
| Accuracy | 61.4 [205/334] (56.2, 66.6) | 52.7 [176/334] (47.3, 58.1) | 0.001 |
| Extrahepatic organ invasion | |||
| Sensitivity | 38.5 [5/13] (12.0, 64.9) | 23.1 [3/13] (0.2, 46.0) | 0.124 |
| Specificity | 94.7 [304/321] (92.3, 97.2) | 95.3 [306/321] (93.0, 97.6) | 0.683 |
| Accuracy | 92.5 [309/334] (89.7, 95.3) | 92.5 [309/334] (89.7, 95.3) | >0.999 |
Data are performance measures in percentages with numerators and denominators in square brackets and 95% confidence intervals in parentheses.
Sensitivities of CT and MRI were similar for N staging using either a pathologic (65.4% vs. 62.8%, respectively, P = 0.617) or composite reference standard (65.0% vs. 64.0%, respectively, P = 0.808) (Table 4). The specificity of CT was significantly higher than that of MRI using either the pathologic (54.6% vs. 36.4%, respectively, P = 0.007) or composite (80.7% vs. 72.9%, respectively, P = 0.001) reference standard.
Table 4.
Diagnostic performance of CT and MRI for N staging
| Performance | CT | MRI | P Value |
|---|---|---|---|
| Pathologic reference standard | |||
| Sensitivity | 65.4 [51/78] (54.8, 75.9) | 62.8 [49/78] (52.1, 73.6) | 0.617 |
| Specificity | 54.6 [18/33] (37.6, 71.5) | 36.4 [12/33] (20.0, 56.8) | 0.007 |
| Accuracy | 62.2 [69/111] (53.1, 71.2) | 55.0 [61/111] (45.7, 64.2) | 0.084 |
| Composite reference standard | |||
| Sensitivity | 65.0 [65/100] (55.7, 74.4) | 64.0 [64/100] (54.6, 73.4) | 0.808 |
| Specificity | 80.7 [146/181] (74.9, 86.4) | 72.9 [132/181] (66.5, 79.4) | 0.001 |
| Accuracy | 75.1 [211/281] (70.0, 80.2) | 69.8 [196/281] (64.4, 75.1) | 0.010 |
Data are performance measures in percentages with numerators and denominators in square brackets and 95% confidence intervals in parentheses.
Interreader agreements for imaging characteristics are summarized in Supporting Table S3. Tumor size measurements showed almost perfect agreement, with intraclass correlation coefficients higher than 0.90 for both CT and MRI. Agreements were substantial for vascular invasion on CT, tumor multiplicity on MRI, and regional lymph node metastasis on both modalities (κ = 0.69‐0.71). Agreements were fair for visceral peritoneal invasion and extrahepatic organ invasion on MRI (κ = 0.27‐0.28) and moderate for all other characteristics (κ = 0.43‐0.60).
Subgroup Analysis
For T staging, accuracy of HBA‐enhanced MRI (n = 251) was significantly higher than that of CT for detecting T1b or higher stages (82.1% vs. 76.5%, P = 0.033) but lower for detecting T3 or higher stages (54.6% vs. 64.1%, P = 0.003). However, ECA‐enhanced MRI (n = 83) showed comparable accuracy to CT (Supporting Table S4).
For identifying multiple tumors, HBA‐enhanced MRI had higher sensitivity than CT (63.0% vs. 40.7%, P = 0.042) and no significant difference in specificity (76.3% vs. 78.1%, P = 0.492). However, ECA‐enhanced MRI and CT showed comparable sensitivity and specificity for detecting tumor multiplicity (Supporting Table S5).
For N staging, sensitivity of HBA‐enhanced MRI was not significantly different from that of CT but specificity was significantly lower than that of CT. However, the sensitivity and specificity of ECA‐enhanced MRI for N staging were comparable to those of CT (Supporting Table S6).
Diagnostic performances for T staging were overall similar between subgroups according to pathologic tumor differentiation (Supporting Table S7). Of note, MRI sensitivity for tumor multiplicity was significantly higher in poorly differentiated or undifferentiated tumors than in well or moderately differentiated tumors (81.8% vs. 45.5%, P = 0.012), with comparable specificities (80.8% vs. 79.5%, P = 0.763) (Supporting Table S8). Diagnostic performances for N staging were overall similar between subgroups according to pathologic tumor differentiation (Supporting Table S9).
Discussion
In this multicenter cohort study, 334 patients were evaluated with both CT and MRI before surgery for mass‐forming iCCA. When CT and MRI characteristics were analyzed based on AJCC eighth‐edition guidelines, significant discrepancies between CT and MRI were observed for clinical T staging. MRI showed better sensitivity for diagnosing T2 and T3 stages than CT. MRI was also more sensitive than CT at detecting multiple tumors, vascular invasion, and visceral peritoneal invasion, while MRI and CT showed comparable specificity for detecting tumor multiplicity. There was no difference in sensitivity between CT and MRI for N staging, but CT provided higher specificity than MRI.
Tumor multiplicity, one of the imaging characteristics used for T2 staging, was better appreciated with MRI, particularly HBA‐enhanced MRI, than with CT. These results are in accordance with studies that reported that MRI enabled detection of additional focal liver lesions to those detected by CT.( 16 , 17 , 18 , 19 ) Lesion conspicuity can be superior with MRI due to the high soft tissue contrast of MRI.( 20 ) Moreover, multiple tumors can be better detected in the hepatobiliary phase of HBA‐enhanced MRI. Hepatic tumors appear hypointense to the liver as liver‐specific contrast accumulates in the liver parenchyma; thus, the hepatobiliary phase provides excellent lesion conspicuity and lesion‐to‐liver contrast.( 21 ) In addition, diffusion‐weighted MRI can improve lesion detectability, particularly that of small metastases.( 19 , 22 , 23 ) Recent studies have demonstrated that intrahepatic metastasis is an important prognostic factor for iCCA, and T2 tumors with multiplicity actually have a worse prognosis than T3 tumors in the AJCC eighth edition.( 24 , 25 , 26 ) In our study, there were no significant differences in sensitivity and specificity between ECA‐enhanced MRI and CT in detecting tumor multiplicity. Therefore, we recommend the use of HBA‐enhanced MRI in addition to CT before surgery to detect additional intrahepatic metastases. Notably, the use of MRI may be especially helpful to identify multiple tumors in poorly differentiated or undifferentiated tumors.
MRI using either HBA or ECA was more sensitive but less specific for evaluating vascular and visceral peritoneal invasion than CT. Prior studies found that the performance of ECA‐enhanced MRI was similar to that of CT for evaluating vascular invasion in perihilar or distal cholangiocarcinoma, but evidence is limited regarding this comparison.( 27 , 28 ) Evaluation of vascular invasion may be easier on CT than on MRI due to higher spatial resolution,( 20 , 29 ) as reflected in lower interreader agreement of vascular invasion on MRI (κ = 0.46) than on CT (κ = 0.71) in this study. Moreover, as HBA not only diffuses in the extracellular space but is also transported into hepatocytes,( 10 ) vascular structures and extracellular connective tissue may enhance to a lesser degree with HBA‐enhanced MRI than CT, impairing specificity. Furthermore, tumor infiltration through the liver capsule or the vascular structure may be better depicted on CT than MRI due to the higher spatial resolution of CT.( 8 , 11 , 29 )
The sensitivities of CT and MRI were comparable for N staging using both pathologic and composite reference standards, at approximately 60% (62.8%‐65.4%). Studies have shown that MRI had a limited accuracy of 56.9% to 66% for predicting lymph node metastasis before surgery for cholangiocarcinoma, consistent with our results.( 30 , 31 ) CT has also been reported to have higher specificity for lymph node metastasis with perihilar or distal cholangiocarcinoma than MRI.( 32 ) Nevertheless, the sensitivity of both CT and MRI for positive lymph node metastasis was limited in our study, similar to the reported pooled sensitivity of CT (61%) in perihilar cholangiocarcinoma.( 33 ) Studies have shown that quantitative radiologic evaluation, including lymph node size measurement, may help predict lymph node metastasis in patients with iCCA.( 34 , 35 , 36 ) However, accurate prediction of lymph node metastasis on cross‐sectional imaging remains challenging as microscopic lymph node metastasis may not be apparent on qualitative radiologic evaluation and the size criterion inevitably impairs specificity for lymph node metastasis due to frequent reactive hyperplasia of lymph nodes.( 31 ) Although routine lymph node dissection in patients with iCCA is controversial, 162 of 334 (48.5%) patients in our study underwent lymphadenectomy, similar to previous studies.( 37 , 38 , 39 ) Only 40 (12.0%) patients received complete pathologic N staging by dissection of at least six regional lymph nodes per AJCC recommendations. Therefore, we also used the composite reference standard to evaluate the performance of N staging in patients who underwent incomplete lymph node sampling or did not undergo lymphadenectomy. However, this composite reference standard may be less accurate than the pathologic reference standard, and further investigation of survival outcomes according to radiologic N staging is warranted.
This study had several limitations. First, selection bias may have been introduced due to the retrospective design of the study. However, most patients underwent both CT and MRI before surgery in the participating institutions. Therefore, selection bias is unlikely to have affected our findings. Second, various scanners, techniques, and contrast agents were used for CT and MRI, which may have affected diagnostic performance. However, this could be a strength in terms of generalizability as it reflects actual clinical practice. Third, the number of patients who underwent ECA‐enhanced MRI was relatively small, and this decreased the statistical power of the subanalysis of this group. Nevertheless, our study showed interesting trends that ECA‐enhanced MRI had comparable diagnostic performance to CT in T‐staging accuracy, tumor multiplicity, and N staging. The reason could be that the contrast agents of ECA‐enhanced MRI and CT have common properties. Further studies using ECA‐enhanced MRI are needed. Fourth, only 40 (12.0%) patients received complete pathologic N staging per eighth AJCC recommendations; this decreased the statistical power of using the pathologic reference standard. However, the proportion (48.5%) of patients who underwent routine lymph node dissection was similar to that reported in previous studies, and the results showed a similar trend to those obtained using the composite reference standard. Fifth, there is a possibility that patients who underwent palliative surgery (n = 23, 6.9%) might have had an incomplete reference standard for tumor multiplicity using the pathology report. Despite these limitations, this is the first intra‐individual comparison study between CT and MRI for clinical staging of iCCA based on analyses of a relatively large number of patients from a multi‐institutional cohort and multiple experienced readers working at different hospitals.
In conclusion, MRI showed better sensitivity for diagnosing T2 and T3 stages than CT. MRI more sensitively detected multiple tumors than CT in patients with iCCA, without impaired specificity, and may help decide patient eligibility for curative resection. CT and MRI had similar sensitivities for N staging, but CT provided higher specificity than MRI. The use of MRI to better evaluate T2‐stage tumors, particularly multiple tumors, is therefore recommended.
Supporting information
Supplementary Material
Supported by the Research Supporting Program of The Korean Association for the Study of the Liver and The Korean Liver Foundation (KASLKLF 2019‐02 to M.S.P.).
Potential conflict of interest: Dr. Choi has received grants from Bayer Korea. The other authors have nothing to report.
Contributor Information
Seung Soo Lee, Email: seungsoolee@amc.seoul.kr.
Mi‐Suk Park, Email: radpms@yuhs.ac.
References
Author names in bold designate shared co‐first authorship.
- 1. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park J‐W, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268‐1289. [DOI] [PubMed] [Google Scholar]
- 2. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020;17:557‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS‐CCA). Nat Rev Gastroenterol Hepatol 2016;13:261‐280. [DOI] [PubMed] [Google Scholar]
- 4. Okabayashi T, Yamamoto J, Kosuge T, Shimada K, Yamasaki S, Takayama T, et al. A new staging system for mass‐forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer 2001;92:2374‐2383. [DOI] [PubMed] [Google Scholar]
- 5. Farges O, Fuks D, Le Treut YP, Azoulay D, Laurent A, Bachellier P, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: by the AFC‐IHCC‐2009 study group. Cancer 2011;117:2170‐2177. [DOI] [PubMed] [Google Scholar]
- 6. Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol 2018;7:52. [DOI] [PubMed] [Google Scholar]
- 7. Forner A, Vidili G, Rengo M, Bujanda L, Ponz‐Sarvise M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int 2019;39(Suppl. 1):98‐107. [DOI] [PubMed] [Google Scholar]
- 8. Joo I, Lee JM, Yoon JH. Imaging diagnosis of intrahepatic and perihilar cholangiocarcinoma: recent advances and challenges. Radiology 2018;288:7‐13. [DOI] [PubMed] [Google Scholar]
- 9. Jhaveri KS, Hosseini‐Nik H. MRI of cholangiocarcinoma. J Magn Reson Imaging 2015;42:1165‐1179. [DOI] [PubMed] [Google Scholar]
- 10. Frydrychowicz A, Lubner MG, Brown JJ, Merkle EM, Nagle SK, Rofsky NM, et al. Hepatobiliary MR imaging with gadolinium‐based contrast agents. J Magn Reson Imaging 2012;35:492‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olthof SC, Othman A, Clasen S, Schraml C, Nikolaou K, Bongers M. Imaging of cholangiocarcinoma. Visc Med 2016;32:402‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung YE, Kim M‐J, Park YN, Choi J‐Y, Pyo JY, Kim YC, et al. Varying appearances of cholangiocarcinoma: radiologic‐pathologic correlation. Radiographics 2009;29:683‐700. [DOI] [PubMed] [Google Scholar]
- 13. Fraum TJ, Tsai R, Rohe E, Ludwig DR, Salter A, Nalbantoglu I, et al. Differentiation of hepatocellular carcinoma from other hepatic malignancies in patients at risk: diagnostic performance of the Liver Imaging Reporting and Data System version 2014. Radiology 2018;286:158‐172. [DOI] [PubMed] [Google Scholar]
- 14. Yoh T, Cauchy F, Le Roy B, Seo S, Taura K, Hobeika C, et al. Prognostic value of lymphadenectomy for long‐term outcomes in node‐negative intrahepatic cholangiocarcinoma: a multicenter study. Surgery 2019;166:975‐982. [DOI] [PubMed] [Google Scholar]
- 15. Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull 1971;76:378‐382. [Google Scholar]
- 16. Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging‐a systematic review and meta‐analysis. Radiology 2015;275:97‐109. [DOI] [PubMed] [Google Scholar]
- 17. Kim H‐D, Lim Y‐S, Han S, An J, Kim G‐A, Kim SY, et al. Evaluation of early stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology 2015;148:1371‐1382. [DOI] [PubMed] [Google Scholar]
- 18. Corwin MT, Fananapazir G, Jin M, Lamba R, Bashir MR. Differences in liver imaging and reporting data system categorization between MRI and CT. AJR Am J Roentgenol 2016;206:307‐312. [DOI] [PubMed] [Google Scholar]
- 19. Marion‐Audibert A‐M, Vullierme M‐P, Ronot M, Mabrut J‐Y, Sauvanet A, Zins M, et al. Routine MRI with DWI sequences to detect liver metastases in patients with potentially resectable pancreatic ductal carcinoma and normal liver CT: a prospective multicenter study. AJR Am J Roentgenol 2018;211:W217‐W225. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Uchida M, Abe T, Nishimura H, Hayabuchi N, Nakashima Y. Intrahepatic peripheral cholangiocarcinoma: comparison of dynamic CT and dynamic MRI. J Comput Assist Tomogr 1999;23:670‐677. [DOI] [PubMed] [Google Scholar]
- 21. Peporte AR, Sommer WH, Nikolaou K, Reiser MF, Zech CJ. Imaging features of intrahepatic cholangiocarcinoma in Gd‐EOB‐DTPA‐enhanced MRI. Eur J Radiol 2013;82:e101‐e106. [DOI] [PubMed] [Google Scholar]
- 22. Taron J, Johannink J, Bitzer M, Nikolaou K, Notohamiprodjo M, Hoffmann R. Added value of diffusion‐weighted imaging in hepatic tumors and its impact on patient management. Cancer Imaging 2018;18:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Löwenthal D, Zeile M, Lim WY, Wybranski C, Fischbach F, Wieners G, et al. Detection and characterisation of focal liver lesions in colorectal carcinoma patients: comparison of diffusion‐weighted and Gd‐EOB‐DTPA enhanced MR imaging. Eur Radiol 2011;21:832‐840. [DOI] [PubMed] [Google Scholar]
- 24. Cheng Z, Lei Z, Si A, Yang P, Luo T, Guo G, et al. Modifications of the AJCC 8th edition staging system for intrahepatic cholangiocarcinoma and proposal for a new staging system by incorporating serum tumor markers. HPB (Oxford) 2019;21:1656‐1666. [DOI] [PubMed] [Google Scholar]
- 25. Yamamoto Y, Sugiura T, Okamura Y, Ito T, Ashida R, Ohgi K, et al. The evaluation of the eighth edition of the AJCC/UICC staging system for intrahepatic cholangiocarcinoma: a proposal of a modified new staging system. J Gastrointest Surg 2020;24:786‐795. [DOI] [PubMed] [Google Scholar]
- 26. Lamarca A, Santos‐Laso A, Utpatel K, La Casta A, Stock S, Forner A, et al.; European Network for the Study of Cholangiocarcinoma (ENS‐CCA) . Liver metastases of intrahepatic cholangiocarcinoma: implications for a potential new staging system. Hepatology 2020; 10.1002/hep.31598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park HS, Lee JM, Choi J‐Y, Lee MW, Kim HJ, Han JK, et al. Preoperative evaluation of bile duct cancer: MRI combined with MR cholangiopancreatography versus MDCT with direct cholangiography. AJR Am J Roentgenol 2008;190:396‐405. [DOI] [PubMed] [Google Scholar]
- 28. Engelbrecht MR, Katz SS, van Gulik TM, Lameris JS, van Delden OM. Imaging of perihilar cholangiocarcinoma. AJR Am J Roentgenol 2015;204:782‐791. [DOI] [PubMed] [Google Scholar]
- 29. Lin E, Alessio A. What are the basic concepts of temporal, contrast, and spatial resolution in cardiac CT? J Cardiovasc Comput Tomogr 2009;3:403‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Songthamwat M, Chamadol N, Khuntikeo N, Thinkhamrop J, Koonmee S, Chaichaya N, et al. Evaluating a preoperative protocol that includes magnetic resonance imaging for lymph node metastasis in the Cholangiocarcinoma Screening and Care Program (CASCAP) in Thailand. World J Surg Oncol 2017;15:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Promsorn J, Soontrapa W, Somsap K, Chamadol N, Limpawattana P, Harisinghani M. Evaluation of the diagnostic performance of apparent diffusion coefficient (ADC) values on diffusion‐weighted magnetic resonance imaging (DWI) in differentiating between benign and metastatic lymph nodes in cases of cholangiocarcinoma. Abdom Radiol (NY) 2019;44:473‐481. [DOI] [PubMed] [Google Scholar]
- 32. Kim NH, Lee SR, Kim YH, Kim HJ. Diagnostic performance and prognostic relevance of FDG positron emission tomography/computed tomography for patients with extrahepatic cholangiocarcinoma. Korean J Radiol 2020;21:1355‐1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ruys AT, van Beem BE, Engelbrecht MR, Bipat S, Stoker J, Van Gulik TM. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta‐analysis. Br J Radiol 2012;85:1255‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhu Y, Mao Y, Chen J, Qiu Y, Wang Z, He J. Preoperative computed tomography features of intrahepatic cholangiocarcinoma for predicting lymph node metastasis and overall survival. J Comput Assist Tomogr 2019;43:729‐735. [DOI] [PubMed] [Google Scholar]
- 35. Meng ZW, Lin XQ, Zhu JH, Han SH, Chen YL. A nomogram to predict lymph node metastasis before resection in intrahepatic cholangiocarcinoma. J Surg Res 2018;226:56‐63. [DOI] [PubMed] [Google Scholar]
- 36. Yoh T, Hatano E, Seo S, Terajima H, Uchida Y, Taura K, et al. Preoperative criterion identifying a low‐risk group for lymph node metastasis in intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2018;25:299‐307. [DOI] [PubMed] [Google Scholar]
- 37. Zhou R, Lu D, Li W, Tan W, Zhu S, Chen X, et al. Is lymph node dissection necessary for resectable intrahepatic cholangiocarcinoma? A systematic review and meta‐analysis. HPB (Oxford) 2019;21:784‐792. [DOI] [PubMed] [Google Scholar]
- 38. Jutric Z, Johnston WC, Hoen HM, Newell PH, Cassera MA, Hammill CW, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB (Oxford) 2016;18:79‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bagante F, Spolverato G, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, et al. Assessment of the lymph node status in patients undergoing liver resection for intrahepatic cholangiocarcinoma: the new eighth edition AJCC staging system. J Gastrointest Surg 2018;22:52‐59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


