Abstract
Hepatic steatosis (HS) is a growing problem in adults worldwide, with racial/ethnic disparity in the prevalence of the disease. The purpose of this study was to characterize the racial/ethnic prevalence of the stages (normal/mild [S0/S1], moderate [S2], and severe [S3]) of HS in Mexican Americans and other Hispanics compared to other racial/ethnic groups. We analyzed data for 5,492 individuals 12 years and older from the newly released National Health and Nutrition Examination Survey 2017‐2018, which is a representative sample of the US adult population. HS was diagnosed by FibroScan using controlled attenuation parameter values: S0, <238; S1, 238‐259; S2, 260‐290; S3, >290. We analyzed the data using the bivariate chi‐squared test and multinomial regression. The prevalence of HS overall was 46.9% (S2,16.6%; S3, 30.3%). The prevalence of S3 was highest among Mexican Americans (42.8%), lowest among Blacks (21.6%), 27.6% in other Hispanics, and 30.6% in Whites (P < 0.05). Mexican Americans were about 2 times more likely than Whites to have S2 and S3, while other Hispanics showed no difference from Whites. In an adjusted model, the common risk factors of S2 and S3 were male sex, older ages, high waist‐to‐hip ratio, body mass index ≥25, and high triglycerides (P < 0.05). Other risk factors for S3 were hemoglobin A1c ≥5.7 and highly sensitive C‐reactive protein ≥10 mg/dL (P < 0.05). Conclusion: Our study challenges the paradigm that HS is higher in Hispanics overall; rather, our data show that HS is higher in Mexican Americans and not non‐Mexican American Hispanics.
Hepatic steatosis is a growing global epidemic that it has long been thought that Hispanics are at higher risk for hepatic steatosis than other racial/ethnic groups.However, previous studies have often not taken the diverse ethnic origins of Hispanics into account.Our study shows that particularly Hispanics of Mexican descent are at increased risk for hepatic steatosis, while Hispanics of non‐Mexican descent are not.

Abbreviations
- ALT
alanine aminotransferase
- AOR
adjusted odds ratio
- AST
aspartate aminotransferase
- BMI
body mass index
- CAP
controlled attenuation parameter
- CI
confidence interval
- FPL
federal poverty level
- HbA1c
hemoglobin A1c
- HS
hepatic steatosis
- hsCRP
highly sensitive C‐reactive protein
- NAFLD
nonalcoholic fatty liver disease
- NHANES
National Health and Nutrition Examination Survey
- PNPLA3
patatin‐like phospholipase domain containing 3
Hepatic steatosis (HS), or fatty liver, is a common condition in the US population. It can be caused by excessive alcohol consumption, certain medications, and chronic liver diseases.( 1 , 2 ) In a large proportion of cases, falling under the term nonalcoholic fatty liver disease (NAFLD), the etiology is unknown. Recently, an international expert consensus statement recommended using the term metabolic dysfunction‐associated fatty liver disease.( 3 ) While HS can be benign, it is associated with the development of inflammation and progression to fibrosis and cirrhosis. Recent work showed that at least a quarter of patients with HS develop fibrosis within 6 years, and their mortality rates increase by ~10% within 20 years, primarily due to cancer and cirrhosis.( 4 , 5 ) Patients with HS may also have the inflammatory condition of nonalcoholic steatohepatitis, which has become the second‐leading reason for liver transplantation.( 6 ) HS is a growing health problem, with one model predicting some 100 million cases of NAFLD in the United States in the year 2030.( 7 ) Current estimates place the direct annual health care cost for NAFLD in the United States at $103 billion.( 8 )
The reported prevalence of HS in the United States varies widely as it depends on the specific population and methods of measurement. Liver biopsy is the reference method for detecting HS, but it is only available for patients in which the biopsy is done during abdominal surgery or percutaneously in patients with unexplained liver disease. A common noninvasive method for detecting HS is ultrasound transient elastography,( 9 ) which has a sensitivity and specificity of 84.4% and 93.6%, respectively.( 10 ) For example, the prevalence of HS as assessed by the controlled attenuation parameter (CAP) using ultrasound transient elastography was 31% in a cohort of patients with coinfection of human immunodeficiency virus/hepatitis C virus,( 11 ) while a study based on autopsies of aircrew in the United States found a prevalence of 16%.( 12 ) Furthermore, prevalence estimates are often reported for NAFLD alone, excluding patients that have HS from identified sources, such as alcohol or hepatotoxic drugs. A systematic review reported that the prevalence of NAFLD in the United States ranges from 10% to 35%.( 13 ) These estimates may underestimate the total prevalence of HS in the US population because they are restricted to HS resulting from NAFLD.
The prevalence of HS differs by ethnicity, with the majority of information derived from a database that is over 30 years old, the Third National Health and Nutrition Examination Survey (NHANES III), 1988‐1994. In this national sample of the general population, the prevalence of HS was found to be highest in Mexican Americans and lowest in Blacks. The prevalence in White subjects was intermediate.( 14 ) The only Hispanic group studied was Mexican Americans, and it has generally been assumed that the high prevalence of HS occurs in all Hispanics. The high prevalence in Mexican American subjects has been attributed to high rates of obesity and insulin resistance, but no similar associations were found for Black subjects.( 2 , 15 ) Several studies have found that variants of the gene patatin‐like phospholipase domain containing 3 (PNPLA3) that are associated with higher fat content are most common in Mexican Americans, with the lowest frequency of the allele occurring in African Americans.( 16 ) On the other hand, a variant of PNPLA3 associated with low liver‐fat content is common in African Americans but rarely seen in Mexican Americans or Whites.( 16 ) Among Hispanics, there is some variability in the prevalence of HS and NAFLD. A recent study found that the highest frequency of the PNPLA3 variant associated with HS was found in those of Mexican descent.( 17 ) Those of Mexican descent also show a high frequency of variants of other genes associated with development of NAFLD.( 18 )
Risk factors for HS include insulin resistance, hypertension, overweight or obesity, and a sedentary lifestyle.( 14 ) Several laboratory values have also been associated with HS, including triglycerides, gamma‐glutamyltransferase, alanine aminotransferase (ALT), and aspartate aminotransferase (AST).( 19 ) The recently released NHANES 2017‐2018 has the advantage over NHANES III in that it separates ethnicity into Mexican Americans and other Hispanics, while NHANES III only reported Mexican Americans. The current study identified the prevalence of HS by stage and race/ethnicity from NHANES data collected from 2017 to 2018 and identified risk factors associated with HS in the overall population. We hypothesized that Mexican Americans and other Hispanics would have a higher prevalence of HS than Whites and that Blacks would have the lowest prevalence.
Participants and Methods
Study Population
We analyzed data for 5,492 participants who were 12 years and older, using NHANES 2017‐2018. NHANES samples the US population using a complex multistage probability design and obtains informed consent from all participants. NHANES protocols were approved by the National Center for Health Statistics Research Ethics Review Board. Our analysis of these publicly available data was exempt from Charles R. Drew University Institutional Review Board review.
Dependent Variable
Liver fibrosis was measured by FibroScan, which uses ultrasound and vibration‐controlled transient elastography to derive liver stiffness. The device also simultaneously measures ultrasound attenuation related to the presence of HS and records the controlled attenuation parameter (CAP) as the indicator for liver fat. We categorized the steatosis status using the median CAP dB/m for steatosis grades, whereby S0, <238; S1, 238‐259; S2, 260‐290; and S3 >290, based on clinical guidelines.( 20 )
Independent Variables and Measures
The following variables were included in the analyses: demographics (age, sex, race/ethnicity, education, language spoken, and poverty), physical activity status, smoking status, body composition (waist‐to‐hip ratio and body mass index [BMI]), laboratory values (cholesterol, triglyceride, glucose, hemoglobin A1c [HbA1c], highly sensitive C‐reactive protein [hsCRP], AST, and ALT).
The physical activity variable was categorized into three categories (0, inactive; 1, does not meet guidelines, which specify moderate exercise <5 times/week or vigorous exercise <3 times/week; and 2, meets guidelines, which specify moderate exercise 5 or more times/week or vigorous exercise 3 times/week). Age was categorized as 12‐19 years, 20‐34 years, 35‐49 years, 50‐64 years, and ≥65. Education was categorized as less than high school (<12 grade), high school (12 grade), some college, and at least a college degree. Sex was categorized as male and female. Race/ethnicity was categorized as White, Black, Mexican American, other Hispanics, and other race, including multiracial. Language spoken at home was classified as English, Spanish, both, and other languages. Federal poverty level (FPL) was classified as <1, 1‐2, and >2 FPL. Smoking status was categorized as nonsmoker, former smoker, and current smoker. Participants were classified using BMI, with BMI <25 (normal), BMI 25‐29.9 (overweight), and BMI ≥30 (obese). Waist‐to‐hip ratio was classified as risk for women (≥0.85)/risk for men (≥1.0) versus healthy. Based on HbA1c, subjects were classified as normal (<5.7), with prediabetes (5.7‐6.4), and with diabetes (≥6.5). Total cholesterol was categorized as normal (<200 mg/dL), elevated (200‐239 mg/dL), and high (≥240 mg/dL). Triglyceride level was categorized as normal (<150 mg/dL), borderline (150‐199 mg/dL), and high (≥200 mg/dL). hsCRP was categorized as normal (0.1‐1.0 mg/dL), mild inflammation (1.0‐3 mg/dL), significant inflammation (3‐10 mg/dL), and highly significant inflammation (≥10 mg/dL). ALT was categorized as normal (<56 U/L) and elevated (≥56 U/L), and AST was categorized as normal (<40 U/L) and elevated (≥40 U/L).
Statistical Analyses
We used descriptive statistics, including using mean ± standard error for continuous variables and unweighted number and weighted percentage for categorical variables. Missing data were <10% for each variable. Bivariate analysis using the chi‐squared test for categorical variables was used to determine the statistical difference among racial/ethnic groups and the other independent variables in the prevalence of HS. We performed multinomial regression analysis with listwise deletion to determine racial/ethnic difference as well as the predictors of HS stages, comparing HS stages (S2 and S3) to the normal population (those with no or mild HS) and adjusting for confounding variables. Data are presented as adjusted odds ratio (AOR) and 95% confidence interval (CI). P < 0.05 was considered statistically significant. The data were analyzed using SAS (release V.9. 3, 2002; SAS, Inc). We used the sample weights provided by the National Center for Health Statistics to correct for differential selection probabilities and to adjust for noncoverage and nonresponse. All estimates were weighted as supplied by NHANES, and the complex multistage probability sampling design is being taken into consideration when estimating the variance.
Results
Population Characteristics
Of the 5,492 subjects in our sample from NHANES 2017‐2018, 24.8% were 50‐64 years of age and 16.8% were 65 years and older; 11.3% were Black, 9.9% were Mexican Americans, and 7% were other Hispanics. About half the population were male individuals (49.7%), 19.5% had less than high school education, and 13.8% were poor (<1 FPL). Most of the participants (80.2%) spoke English at home, 16.6% were current smokers, 19.7% were physically inactive (did no exercise), 50.3% had a high waist‐to‐hip ratio (≥0.85 for male sex, ≥0.9 for female sex), and 38% were obese by BMI. In addition, 9.5% had high total cholesterol (≥240 mg/dL), 15.7% had a high level of triglyceride (≥200 mg/dL), 25% had significant inflammation (as indicated by >3 mg/dL hsCRP), 4.3% had abnormal AST, 3.7% had abnormal ALT, 22.6% had prediabetes, and 8.5% had diabetes (Table 1).
Table 1.
Descriptive results comparing normal/mild, moderate, and severe groups using NHANES 2017‐2018
| Total | Normal/Mild (S0,S1) CAP <260 | Moderate (S2) CAP 260‐290 | Severe (S3) CAP >290 | P Value | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| Overall | 5,492 | 2,896 (53. 1%) | 900 (16.6%) | 1,696 (30.3%) | |
| Age, years | † | † | <0.0001 | ||
| 12‐19 | 983 (12.4%) | 760 (81.0%) | 97 (8.8%) | 126 (10.2%) | |
| 20‐34 | 1,033 (24.5%) | 650 (64.3%) | 149 (12.9%) | 234 (22.7%) | |
| 35‐49 | 1,008 (21.5%) | 474 (48.9%) | 183 (19.4%) | 351 (31.6%) | |
| 50‐64 | 1,349 (24.8%) | 537 (40.3%) | 254 (18.9%) | 558 (40.8%) | |
| 65+ | 1,119 (16.8%) | 475 (40.4%) | 217 (20.7%) | 427 (38.9%) | |
| Race/ethnicity | † | <0.0001 | |||
| White | 1,839 (61.2%) | 949 (53.1%) | 293 (16.3%) | 597 (30.6%) | |
| Black | 1,254 (11.3%) | 755 (61.4%) | 209 (17.1%) | 290 (21.6%) | |
| Hispanic | 502 (7.0%) | 260 (56.7%) | 83 (15.8%) | 159 (27.6%) | |
| Mexican American | 798 (9.9%) | 330 (41.4%) | 127 (15.9%) | 341 (42.8%) | |
| Other | 1,099 (10.6%) | 602 (52.9%) | 188 (18.8%) | 309 (28.3%) | |
| Sex | † | <0.0001 | |||
| Male | 2,744 (49.7%) | 1,333 (48.0%) | 434 (16.5%) | 977 (35.4%) | |
| Female | 2,748 (503%) | 1,563 (58.1%) | 466 (16.7%) | 719 (25.2%) | |
| Education | † | <0.0001 | |||
| Less than high school | 1,675 (19.5%) | 1,015 (63.5%) | 239 (14.6%) | 421 (22.0%) | |
| High school | 1,182 (25.2%) | 585 (48.8%) | 181 (14.6%) | 416 (36.6%) | |
| Some college | 1,519 (27.8%) | 722 (48.4%) | 262 (16.8%) | 535 (34.8%) | |
| At least college degree | 1,108 (27.6%) | 572 (54.5%) | 217 (19.7%) | 319 (25.9%) | |
| Language spoken at home | * | 0.0013 | |||
| English | 3,775 (80.2%) | 2,091 (54.4%) | 602 (16.5%) | 1,082 (29.0%) | |
| Spanish | 404 (4.4%) | 161 (40.8%) | 73 (16.7%) | 170 (42.4%) | |
| Both | 632 (8.5%) | 297 (50.0%) | 95 (14.9%) | 240 (35.0%) | |
| Other | 663 (6.9%) | 337 (49.1%) | 129 (19.5%) | 197 (31.4%) | |
| Federal income ratio | 0.5164 | ||||
| <1 | 975 (13.8%) | 548 (56.6%) | 130 (13.6%) | 297 (29.8%) | |
| 1‐2 | 1,342 (20.8%) | 692 (52.6%) | 225 (17.4%) | 425 (30.0%) | |
| >2 | 2,498 (65.4%) | 1,318 (53.2%) | 421 (16.4%) | 759 (30.4%) | |
| Waist‐to‐hip ratio | † | † | <0.0001 | ||
| Healthy | 2,574 (49.7%) | 1,767 (70.2%) | 354 (13.2%) | 453 (16.6%) | |
| Risk for women (≥0.85)/risk for men (≥1.0) | 2,724 (50.3%) | 1,038 (36.5%) | 505 (19.6%) | 1,181 (43.9%) | |
| BMI | † | † | <0.0001 | ||
| Normal or healthy | 1,802 (32.0%) | 1,541 (87.8%) | 155 (7.9%) | 106 (4.4%) | |
| Overweight | 1,657 (30.0%) | 847 (52.8%) | 340 (22.1%) | 470 (25.2%) | |
| Obese | 1,988 (38.0%) | 486 (24.2%) | 397 (19.7%) | 1,105 (56.1%) | |
| Smoking status | † | 0.0003 | |||
| Current | 826 (16.6%) | 444 (52.6%) | 118 (16.0%) | 264 (31.4%) | |
| Former | 1,081 (24.1%) | 427 (41.5%) | 200 (19.0%) | 454 (39.4%) | |
| Nonsmoker | 2,838 (59.3%) | 1,442 (53.0%) | 504 (17.0%) | 892 (29.9%) | |
| Physical activity | † | <0.0001 | |||
| Inactive | 1,145 (19.7%) | 497 (43.6%) | 202 (18.0%) | 446 (38.4%) | |
| Does not meet guidelines | 764 (15.5%) | 337 (40.2%) | 133 (18.7%) | 284 (41.1%) | |
| Meets guidelines | 2,846 (64.8%) | 1,479 (54.6%) | 487 (16.8%) | 880 (28.6%) | |
| Serum cholesterol | * | † | <0.0001 | ||
| Good (<200 mg/dL) | 3,550 (66.0%) | 1,971 (56.2%) | 553 (15.8%) | 1,026 (28.0%) | |
| Elevated (200‐239 mg/dL) | 1,144 (24.5%) | 516 (45.9%) | 217 (18.6%) | 411 (35.5%) | |
| High (≥240 mg/dL) | 468 (9.5%) | 192 (41.5%) | 91 (20.1%) | 185 (38.3%) | |
| Serum triglycerides | † | † | <0.0001 | ||
| Normal (<150 mg/dL) | 3,622 (69.4%) | 2,228 (62.9%) | 564 (15.5%) | 830 (21.6%) | |
| Borderline (150‐199 mg/dL) | 728 (14.9%) | 244 (33.8%) | 157 (23.8%) | 327 (42.3%) | |
| High (≥200 mg/dL) | 781 (15.7%) | 186 (22.5%) | 137 (16.5%) | 458 (61.0%) | |
| hsCRP | † | † | <0.0001 | ||
| Normal (0.1 to <1 mg/dL) | 1,772 (33.8%) | 1,272 (72.5%) | 230 (12.5%) | 270 (14.9%) | |
| Mild inflammation (1‐3 mg/dL) | 1,725 (34.7%) | 816 (47.9%) | 334 (20.0%) | 575 (32.1%) | |
| Significant inflammation (3‐10 mg/dL) | 1,285 (25.0%) | 452 (35.6%) | 236 (19.1%) | 597 (45.3%) | |
| Highly significant inflammation (≥10 mg/dL) | 349 (6.4%) | 119 (33.9%) | 56 (14.1%) | 174 (52.0%) | |
| AST | † | <0.0001 | |||
| Normal (≤40 U/L) | 4,899 (95.7%) | 2,579 (53.3%) | 821 (16.9%) | 1,499 (29.8%) | |
| Elevated (>40 U/L) | 215 (4.3%) | 71 (30.4%) | 35 (17.7%) | 109 (51.9%) | |
| ALT | † | † | <0.0001 | ||
| Normal (≤56 U/L) | 4,955 (96.3%) | 2,618 (53.3%) | 831 (16.9%) | 1,506 (29.8%) | |
| Elevated (>56 U/L) | 177 (3.7%) | 42 (26.0%) | 26 (16.9%) | 109 (57.1%) | |
| HbA1c | † | † | <0.0001 | ||
| Healthy (<5.7%) | 3,232 (68.9%) | 2,057 (62.6%) | 496 (16.6%) | 679 (20.7%) | |
| Prediabetes (5.7%‐6.4%) | 1,405 (22.6%) | 555 (35.5%) | 275 (18.8%) | 575 (45.6%) | |
| Diabetes (>6.5%) | 625 (8.5%) | 135 (15.9%) | 103 (13.8%) | 387 (70.2%) | |
| Variable names | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | |
| Age | 43.91 (0.57) | 39.16 (0.62) | 48.05 (1.04)† | 49.97 (0.71)† | <0.0001 |
| Waist‐to‐hip ratio | 0.92 (0.01) | 0.88 (0.01) | 0.94 (0.01)† | 0.98 (0.01)† | <0.0001 |
| BMI | 28.84 (0.25) | 25.40 (0.27) | 30.06 (0.30)† | 34.21 (0.42)† | <0.0001 |
| Serum cholesterol | 185.65 (1.63) | 180.46 (1.62) | 192.36 (2.55)† | 190.78 (2.17)† | <0.0001 |
| Serum triglycerides | 137.21 (3.25) | 105.28 (2.63) | 145.99 (5.18)† | 186.50 (4.87)† | <0.0001 |
| hsCRP | 3.54 (0.14) | 2.68 (0.20) | 3.50 (0.23)* | 5.02 (0.26)† | <0.0001 |
| AST | 22.12 (0.26) | 20.96 (0.43) | 22.39 (0.65) | 23.94 (0.55)† | <0.0001 |
| ALT | 22.65 (0.37) | 18.79 (0.46) | 22.77 (0.62)† | 29.13 (0.86)† | <0.0001 |
| HbA1c | 5.61 (0.01) | 5.38 (0.02) | 5.58 (0.03)† | 6.02 (0.04)† | <0.0001 |
Post hoc tests: *P < 0.05 compared to normal/mild (S0,S1); † P < 0.01 compared to normal/mild (S0,S1).
Abbreviation: SE, standard error.
Prevalence of HS Stages
For HS, 53.1% had normal/mild HS, 16.6% had moderate HS, and 30.3% had severe HS. Participants with moderate and severe HS were older, had a higher average waist‐to‐hip ratio, BMI, and levels of cholesterol, triglyceride, hsCRP, AST, ALT, and HbA1c, relative to those without HS (P < 0.05) (Table 1; Fig. 1).
FIG. 1.
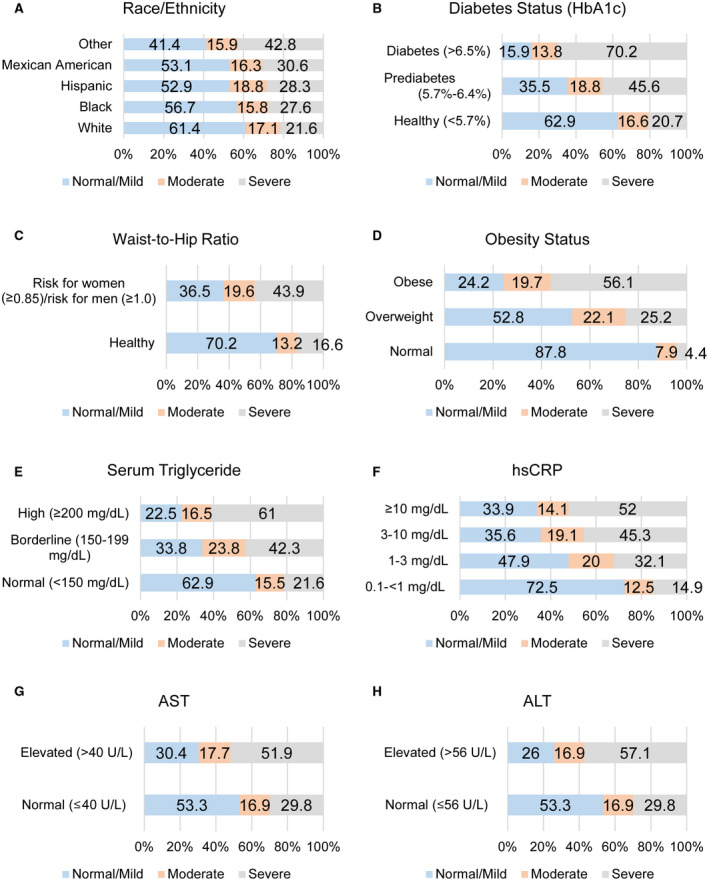
Prevalence of HS by risk factor. The prevalence of normal/mild (S0,S1), moderate (S2), and severe (S3) HS are shown for (A) race/ethnicity, (B) level of HbA1c, (C) waist‐to‐hip ratio, (D) level of BMI, (E) level of triglycerides, (F) level of hsCRP, (G) normal and elevated levels of AST, and (H) normal and elevated levels of ALT. The numbers on the bars indicate the prevalence.
The prevalence of HS varied significantly by all independent variables (P < 0.05) except FPL (P > 0.05). The highest prevalence of moderate HS was among subjects 65 years and older (20.7%), while the highest severe HS was among the 50‐64‐year age group (40.8%) (P < 0.05). More than one third of the male subjects (35.4%) had severe HS compared to 25.2% of the female subjects (P < 0.05). The highest prevalence of moderate HS was among the other racial/ethnic group (18.8%). The highest prevalence of severe HS was among Mexican Americans (43.8%), and the lowest prevalence was among the Black population (21.6%) (P < 0.05). The highest prevalence of moderate HS was among participants with at least a college degree, and the highest prevalence of severe HS was among those with a high school education (36.6%) (P < 0.05). The prevalence of severe HS was highest among Spanish speakers (42.4%), followed by those who spoke both English and Spanish (35.0%) (P < 0.05). HS prevalence did not vary by federal income ratio (P > 0.05). About 44% of subjects with a high waist‐to‐hip ratio had severe HS (P < 0.05). The highest prevalence of moderate HS was among the overweight group (21.1%), and the highest prevalence of severe HS was among the obese group (56.1%) (P < 0.05). Former smokers had the highest prevalence of moderate HS (19.0%) and severe HS (39.4%) (P < 0.05). About 19% of the physically inactive subjects had moderate HS and 38.4% has severe HS (P < 0.05). Of the subjects with a high cholesterol level, 20.1% had moderate HS and 38.3% had severe HS (P < 0.05). Of those with a high triglyceride level, 16.5% had moderate HS and 61% had severe HS (P < 0.05). The highest prevalence of moderate HS was among those with mild inflammation (20.0%), and the highest prevalence of severe HS was among those with an hsCRP level ≥10 mg/dL (52.0%) (P < 0.05). The highest prevalence of moderate and severe HS was among those with elevated AST (17.7% and 51.9%, respectively) and elevated ALT (16.9% and 57.1%, respectively) (P < 0.05). The highest prevalence of moderate HS was among the prediabetes group (18.8%), while the highest prevalence of severe HS was among the diabetes group (70.2%) (P < 0.05).
Factors Associated With HS Stages (Multinomial Regression Analysis)
In the multinomial adjusted model (Table 2), race/ethnicity was significantly associated with HS stages; Mexican Americans had 77% higher odds of moderate HS (AOR, 1.77; 95% CI, 1.13‐2.80; P < 0.05) and were more than twice as likely to have severe HS (AOR, 2.11; 95% CI, 1.44‐3.09; P < 0.05) relative to the White population. There was a trend for the other Hispanic population to have lower odds of moderate or severe HS relative to the White population, but this was not statistically significant (P > 0.05). In addition, the Black population was significantly less likely to have severe HS relative to the White population (AOR, 0.47; 95% CI, 0.36‐0.63; P < 0.05). Relative to the 20‐34‐year‐old age group, persons 12‐19 years old were less likely to have moderate HS (AOR, 0.48; 95% CI, 0.24‐0.94; P < 0.05); those 50‐64 years old were more likely to have severe HS (AOR, 1.81; 95% CI, 1.27‐2.57; P < 0.05), and the 35‐49‐year‐old group had higher odds of moderate HS (P < 0.05).
Table 2.
Multinomial regression (unadjusted and adjusted) for HS and factors associated with HS (reference population of normal/mild HS) using NHANES 2017‐2018
| Unadjusted OR (95% CI) | AOR (95% CI) | |||
|---|---|---|---|---|
| Moderate (S2) | Severe (S3) | Moderate (S2) | Severe (S3) | |
| Age, years | ||||
| 12‐19 versus 20‐34 | 0.53 (0.37‐0.77)* | 0.36 (0.28‐0.47)* | 0.48 (0.24‐0.94)* | 0.70 (0.40‐1.23) |
| 35‐49 versus 20‐34 | 1.96 (1.34‐2.84)* | 1.82 (1.36‐2.45)* | 1.63 (1.12‐2.39)* | 1.27 (0.89‐1.79) |
| 50‐64 versus 20‐34 | 2.33 (1.53‐3.54)* | 2.88 (2.08‐3.98)* | 2.01 (1.14‐3.54)* | 1.81 (1.27‐2.57)* |
| 65+ versus 20‐34 | 2.53 (1.73‐3.69)* | 2.72 (2.02‐3.66)* | 1.85 (1.05‐3.25)* | 1.47 (0.97‐2.22) |
| Race/ethnicity | ||||
| Black versus White | 0.91 (0.72‐1.15) | 0.61 (0.49‐0.77)* | 0.80 (0.56‐1.16) | 0.47 (0.36‐0.63)* |
| Mexican‐American versus White | 1.22 (1.03‐1.45)* | 1.78 (1.41‐2.24)* | 1.77 (1.13‐2.80)* | 2.11 (1.44‐3.09)* |
| Other Hispanic versus White | 0.90 (0.75‐1.08) | 0.85 (0.67‐1.08) | 0.76 (0.47‐1.24) | 0.67 (0.43‐1.04) |
| Other versus White | 1.16 (0.93‐1.45) | 0.93 (0.64‐1.34) | 1.35 (0.82‐2.22) | 0.99 (0.55‐1.78) |
| Sex | ||||
| Female versus male | 0.83 (0.62‐1.11) | 0.59 (0.50‐0.70)* | 0.65 (0.44‐0.95)* | 0.41 (0.31‐0.54)* |
| Education | ||||
| Less than high school versus high school | 0.77 (0.64‐0.92)* | 0.46 (0.37‐0.57)* | 1.17 (0.90‐1.53) | 0.73 (0.43‐1.24) |
| Some college versus high school | 1.16 (0.87‐1.56) | 0.96 (0.73‐1.27) | 1.24 (0.84‐1.84) | 1.08 (0.71‐1.64) |
| At least college degree versus high school | 1.20 (0.85‐1.69) | 0.63 (0.47‐0.85)* | 1.22 (0.73‐2.05) | 0.77 (0.49‐1.22) |
| Language spoken at home | ||||
| Spanish versus English | 1.36 (1.07‐1.72)* | 1.96 (1.37‐2.79)* | 0.86 (0.49‐1.50) | 0.90 (0.55‐1.47) |
| Both versus English | 0.96 (0.78‐1.17) | 1.30 (0.97‐1.73) | 0.74 (0.49‐1.13) | 1.13 (0.72‐1.77) |
| Other versus English | 1.30 (0.96‐1.76) | 1.17 (0.77‐1.80) | 1.37 (0.76‐2.47) | 1.74 (0.93‐3.25) |
| Federal income ratio | ||||
| <1 versus >2 | 0.82 (0.61‐1.10) | 0.93 (0.73‐1.18) | 1.03 (0.73‐1.46) | 0.93 (0.68‐1.27) |
| 1‐2 versus >2 | 1.06 (0.83‐1.35) | 0.98 (0.80‐1.21) | 1.23 (0.84‐1.80) | 1.00 (0.74‐1.36) |
| Waist‐to‐hip ratio | ||||
| Risk for women (≥0.85)/risk for men (≥1.0) versus healthy | 2.84 (2.10‐3.86)* | 5.09 (4.15‐6.24)* | 1.72 (1.00‐2.96)* | 2.18 (1.42‐3.32)* |
| BMI | ||||
| Overweight versus normal | 4.67 (3.49‐6.25)* | 9.57 (7.05‐12.99)* | 3.02 (2.06‐4.41)* | 6.11 (3.92‐9.51)* |
| Obese versus normal | 9.10 (5.84‐14.18)* | 46.59 (29.05‐74.72)* | 5.04 (2.98‐8.53)* | 21.92 (12.08‐39.77)* |
| Smoking status | ||||
| Current versus never | 0.95 (0.70‐1.29) | 1.06 (0.84‐1.34) | 0.89 (0.59‐1.34) | 0.91 (0.65‐1.28) |
| Former versus never | 1.42 (1.07‐1.90)* | 1.68 (1.30‐2.16)* | 1.24 (0.86‐1.78) | 1.14 (0.79‐1.64) |
| Physical activity | ||||
| Inactive versus meets guidelines | 1.33 (1.08‐1.63)* | 1.67 (1.29‐2.17)* | 0.97 (0.76‐1.24) | 1.25 (0.90‐1.74) |
| Does not meet guidelines versus meets guidelines | 1.50 (1.04‐2.15)* | 1.95 (1.46‐2.60)* | 0.92 (0.61‐1.38) | 1.45 (0.97‐2.15) |
| Serum cholesterol | ||||
| Elevated (200‐239 mg/dL) versus good (<200 mg/dL) | 1.45 (1.11‐1.88)* | 1.55 (1.24‐1.94)* | 0.83 (0.59‐1.17) | 0.92 (0.66‐1.28) |
| High (≥240 mg/dL) versus good (<200 mg/dL) | 1.72 (1.16‐2.55)* | 1.85 (1.46‐2.34)* | 0.90 (0.68‐1.19) | 0.85 (0.63‐1.14) |
| Serum triglycerides | ||||
| Borderline (150‐199 mg/dL) versus normal (≤150 mg/dL) | 2.86 (2.07‐3.95)* | 3.66 (2.64‐5.08)* | 1.85 (1.38‐2.49)* | 1.88 (1.24‐2.85)* |
| High (≥200 mg/dL) versus normal (≤150 mg/dL) | 2.99 (2.09‐4.29)* | 7.93 (6.26‐10.05)* | 1.64 (1.17‐2.29)* | 3.29 (2.36‐4.58)* |
| hsCRP | ||||
| Mild inflammation (1‐3 mg/dL) versus normal (<1 mg/dL) | 2.40 (1.85‐3.12)* | 3.26 (2.44‐4.35)* | 1.37 (0.94‐2.02) | 1.12 (0.78‐1.62) |
| Significant inflammation (3‐10 mg/dL) versus normal (<1 mg/dL) | 3.12 (2.21‐4.40)* | 6.24 (4.63‐8.41)* | 1.28 (0.85‐1.94) | 1.55 (0.92‐2.59) |
| Highly significant inflammation (≥10 mg/dL) versus normal (<1 mg/dL) | 2.43 (1.52‐3.88)* | 7.58 (4.43‐12.97)* | 1.45 (0.96‐2.19) | 2.08 (1.10‐3.91)* |
| AST | ||||
| Elevated (>40 U/L) versus normal (≤40 U/L) | 1.85 (0.93‐3.68) | 3.05 (1.80‐5.16)* | 1.83 (0.48‐6.94) | 2.21 (0.87‐5.58) |
| ALT | ||||
| Elevated (>56 U/L) versus normal (≤56 U/L) | 2.07 (1.34‐3.22)* | 3.96 (2.10‐7.45)* | 1.47 (0.51‐4.20) | 1.65 (0.53‐5.12) |
| HbA1c | ||||
| Prediabetic versus healthy (<5.7%) | 1.99 (1.51‐2.63)* | 3.85 (2.99‐4.95)* | 1.28 (0.87‐1.89) | 2.29 (1.65‐3.18)* |
| Diabetic (≥6.5) versus healthy (<5.7%) | 3.13 (2.12‐4.62)* | 13.35 (8.90‐20.03)* | 1.38 (0.76‐2.50) | 4.85 (2.61‐9.03)* |
Statistically significant at P < 0.05.
Female subjects had lower adjusted odds of moderate HS (AOR, 0.65; 95% CI, 0.44‐0.95; P < 0.05) and severe HS (AOR, 0.41; 95% CI, 0.31‐0.54; P < 0.05) compared to male subjects. Participants in the high‐risk group of the waist‐to‐hip ratio were more likely to have moderate HS (AOR, 1.72; 95% CI, 1.001‐2.96; P < 0.05) and severe HS (AOR, 2.18; 95% CI, 1.42‐3.32; P < 0.05) relative to those in the normal waist‐to‐hip group. Relative to the group with normal BMI, overweight participants were 3 times more likely to have moderate HS (AOR, 3.02; 95% CI, 2.06‐4.41; P < 0.05) and >6 times more likely to have severe HS (AOR, 6.11; 95% CI, 3.92‐9.51; P < 0.05). This relationship was stronger among the population with obesity for both moderate HS (AOR, 5.04; 95% CI, 2.98‐8.53; P < 0.05) and severe HS (AOR, 21.92; 95% CI, 12.08‐39.77; P < 0.05).
Participants with borderline and high levels of triglycerides had higher odds than the normal group of having moderate and severe HS (P < 0.05). Participants with borderline values of triglycerides were more likely to have moderate (AOR, 1.85; 95% CI, 1.38‐2.49; P < 0.05) and severe HS (AOR, 1.88; 95% CI, 1.24‐2.85; P < 0.05) relative to those with normal values of triglycerides. Participants with high levels of triglycerides were more likely to have moderate (AOR, 1.88; 95% CI, 1.24‐2.85; P < 0.05) and severe HS (AOR, 3.29; 95% CI, 2.36‐4.58; P < 0.05) relative to those with normal values of triglycerides. Participants with an hsCRP level of 10 mg/dL or higher were twice as likely to have severe HS relative to those with a normal level (<1 mg/dL) (AOR, 2.08; 95% CI, 1.10‐3.91; P < 0.05). Those with HbA1c in the prediabetes and diabetes range had higher odds of severe HS relative to the normal group (P < 0.05). Participants with prediabetes had twice the odds of severe HS compared to the normal group (AOR, 2.29; 95% CI, 1.65‐3.18; P < 0.05). Participants with diabetes were almost 5 times more likely to have severe HS relative to the normal group (AOR, 4.85; 95% CI, 2.61‐9.03; P < 0.05) (Table 2).
Discussion
We analyzed data from the NHANES 2017‐2018 database to examine racial/ethnic differences and the associated risk factors of HS in the representative sample of the noninstitutionalized US population. The data indicated that slightly less than half the US population has moderate or severe HS. Our data are consistent with the finding that the prevalence of HS is rising globally, including in the United States.( 21 ) Studies based on NHANES III data collected between 1988 and 1994 using ultrasound reported the prevalence of moderate or severe HS as 21%.( 14 ) In another study of NHANES data between 1988 and 2012 that used biomarkers to identify subjects with NAFLD, the authors calculated an increasing prevalence of NAFLD from 18% in 1988‐1991 to 29% in 1999‐2000 to 31% in 2011‐2012.( 22 ) Similarly, a study of subjects in Dallas in 2000‐2002 estimated the prevalence of HS to be 34%.( 2 ) This time‐dependent increase in the prevalence of HS is consistent with increases observed in its risk factors, such as obesity, insulin resistance, and metabolic syndrome.( 23 ) Previous non‐US studies were also consistent with our results of a higher prevalence of HS. A 2018 study in Italy( 24 ) reported the prevalence of NAFLD at 48% among 890 participants.
While we observed a small difference in the prevalence of moderate HS among racial/ethnic groups, there were larger racial/ethnic differences in the prevalence of severe HS. Mexican Americans had the highest prevalence of severe HS (43.2%), while other Hispanics had a slightly lower prevalence than the White population (28.3% vs. 30.9%; P < 0.05). Mexican Americans also had higher odds of both moderate and severe HS compared to the White population, while the odds of HS (moderate or severe) in other Hispanics was not significantly different from Whites. Among the racial/ethnic groups, the Black population had the lowest prevalence of severe HS and showed a significantly lower adjusted odds in having severe HS relative to the White population.
Previous studies, mostly based on data from NHANES III, had similarly found the highest prevalence of HS and NAFLD in Mexican Americans and the lowest in non‐Hispanic Blacks.( 14 , 25 ) Although the high prevalence seen in Mexican Americans has often been generalized with other Hispanics,( 9 ) our results indicate a clear difference between the Mexican American group and other Hispanic groups. Our findings are consistent with recent work showing that among Hispanics the prevalence of NAFLD is higher in those of Mexican descent compared to those of other backgrounds, such as Cuban, Dominican, or Puerto Rican,( 26 , 27 ) although our report is the first to describe this disparity for HS based on Mexican Americans versus other Hispanic lineages. Browning et al.( 2 ) found a higher prevalence of HS in Hispanics than Whites and Blacks due to the higher prevalence of obesity and insulin resistance. Fleischman et al.( 27 ) found that Hispanics of Mexican origin had a significantly higher prevalence of NAFLD compared to Hispanics of Dominican or Puerto Rican origin.
Our study extends these previous findings in important ways. First, the previous studies were of multisite cohorts predominantly based in urban locations, while our study is based on a nationally representative sample of the noninstitutionalized US population. Furthermore, while previous studies used computed tomography (CT) scans or elevated AST or ALT levels to detect HS, the NHANES 2017‐2018 data used FibroScan, which has a higher sensitivity than CT or methods based on hepatic enzyme levels.( 28 )
Genetic factors are a strong candidate for the mechanism underlying the racial/ethnic disparities in HS. The prevalence of the PNPLA3 G allele, which is associated with greater severity of NAFLD, parallels the prevalence of NAFLD, with the highest frequency among Hispanics( 29 , 30 ) that were not divided into Mexican Americans versus other Hispanics and low frequencies found in African Americans.( 16 ) Variation in the PNPLA3 gene may also contribute to the difference between Mexican Americans and other Hispanic groups. The highest frequencies of the G allele have been found in populations in Mexico and other Central and South American countries, while similar to the prevalence of NAFLD, lower frequencies are seen in other Latino populations, such as those in Puerto Rico and Cuba.( 31 , 32 ) Future studies should examine the genetic and environmental factors among different ethnic backgrounds to determine their contribution to the high prevalence of HS among Mexican Americans.
We identified several other risk factors for moderate and severe HS, including older age (50 years and older), male sex, high BMI (overweight/obese), high waist‐to‐hip ratio, very high level of hsCRP, and elevated triglycerides, diabetes, and prediabetes.
Although severe HS was more likely among the 50‐64‐year‐old age group, the highest prevalence of moderate HS was among subjects 65 years and older. Interestingly, in the adjusted model, only those in the 50‐64‐year‐old group were more likely to have severe HS relative to the 20‐34‐year‐old group. Male subjects had a higher prevalence of moderate and severe HS relative to female subjects. Participants in the high‐risk group of the waist‐to‐hip ratio had a higher prevalence of HS and were more likely to have HS relative to those in the healthy group. Additionally, an overweight status was associated with the likelihood of moderate HS, and the obese group was associated with severe HS. Participants with borderline and high levels of triglycerides had a high prevalence of both moderate and severe HS compared to those without HS. While hsCRP level was not associated with moderate HS, the higher level of 10 mg/dL was associated with a higher odds of severe HS.
Because most researchers choose to focus on NAFLD or other specific forms of liver disease, few studies have identified risk factors for HS generally. Consistent with our results, Lazo et al.( 14 ) identified male sex, high BMI, high waist‐to‐hip ratio, former smoking, low levels of physical activity, and elevated cholesterol and triglycerides as risk factors for HS, based on NHANES III data. In our study, smoking, low level of physical activity, and elevated cholesterol level were not associated with HS stages. This difference could be attributed to different methods of HS diagnosis and variation in the methodology of data collection. Others have also found significant associations between age,( 14 , 33 ) high waist‐to‐hip ratio,( 29 , 30 ) and elevated CRP levels( 34 ) with NAFLD, consistent with the risk factors that we herein identified for HS, indicating the persistence of these risk factors in the population.
Our results using HbA1c indicated that individuals with prediabetes as well as those with diabetes are more likely to develop severe HS relative to the group with normoglycemia. Consistent with our findings, other investigators have identified diabetes as a risk factor, although their definition was based on self‐reporting and serum glucose( 14 ) while ours was based on HbA1c level.
The current study uses data from the most recently released cycle of NHANES, a database that has several strengths. First, the data are far more recent compared to prior NHANES releases that contained imaging data; this makes the data more relevant for determining current prevalence and risk factors. Second, the data are a large national representative sample of the noninstitutionalized population of the United States. Third, FibroScan, the method used for detecting HS in the 2017‐2018 cycle, is more sensitive than the ultrasound method used in the NHANES III cycle. Fourth, the new data categorize Mexican Americans separately from Americans of other Hispanic background, revealing an important dissociation of the prevalence in Mexican Americans compared to the other Hispanic population. The main strength of our present study is that it challenges the previous paradigm that HS is higher in Hispanics overall. It specifically differentiates that HS is only higher in Mexican Americans but not in non‐Mexican American Hispanics.
One limitation of our study is that the NHANES data are cross‐sectional, so we cannot determine the causal direction between HS and its associated factors. The FibroScan method used to identify HS in this study is not 100% accurate when compared to liver biopsy. However, because HS is considered a relatively benign condition, biopsy is not typically implemented for its diagnosis. Because FibroScan data are only available in the latest release of NHANES (2017‐2018), we could only use data from this cycle for the analysis. Additionally, these data were not collected from all participants in the cycle. Both these factors limited the size of the sample available. Additional potential predictors, such as C‐peptide and low‐density lipoprotein, could not be included because data were not available for the former variable and data were only collected from a small number of participants for the latter variable. Additionally, some variables, such as smoking and physical activity, were collected by self‐reporting, so these estimates are prone to recall bias. Although we controlled for major confounders and robust associations, it is possible that other unknown confounders could account for the associations found.
Using a large nationally representative sample of a noninstitutionalized population in the United States, our study showed persistence of the high prevalence of HS in Mexican Americans. Overall, the NHANES 2017‐2018 data set challenges the previous paradigm that HS is higher in Hispanics. Rather, our data show that HS is only higher in Mexican Americans and not in non‐Mexican American Hispanics. This is an important factor for clinicians to keep in mind when considering risk factors of HS and other liver conditions and when considering liver biopsy. Our results also serve as a reminder that the Hispanic population is genetically and culturally diverse and that care should be taken in generalizing across groups of different backgrounds. Further studies are needed to explain the mechanisms and genetics of the differentially high prevalence of HS among Mexican Americans compared to other Hispanics.
Supported by the National Institutes of Health (NIH) National Institute on Minority Health and Health Disparities (grants R01MD012579 to S.M.N., T.C.F, U54MD007598 to T.C.F., S21MD000103 to M.S.), NIH National Institute on Drug Abuse (R24DA017298 to T.C.F.), and NIH National Center for Advancing Translational Sciences (UL1TR000124 to M.S.).
Potential conflict of interest: Nothing to report.
References
- 1. Day CP, James OF. Hepatic steatosis: innocent bystander or guilty party? Hepatology 1998;27:1463‐1466. [DOI] [PubMed] [Google Scholar]
- 2. Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387‐1395. [DOI] [PubMed] [Google Scholar]
- 3. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero‐Gomez M, et al. A new definition for metabolic dysfunction‐associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73:202‐209. [DOI] [PubMed] [Google Scholar]
- 4. Adams LA, Ratziu V. Non‐alcoholic fatty liver ‐ perhaps not so benign. J Hepatol 2015;62:1002‐1004. [DOI] [PubMed] [Google Scholar]
- 5. Simon TG, Roelstraete B, Khalili H, Hagström H, Ludvigsson JF. Mortality in biopsy‐confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut 2021;70:1375‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547‐555. [DOI] [PubMed] [Google Scholar]
- 7. Estes C, Anstee QM, Arias‐Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden In China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016‐2030. J Hepatol 2018;69:896‐904. [DOI] [PubMed] [Google Scholar]
- 8. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577‐1586. [DOI] [PubMed] [Google Scholar]
- 9. Chinchilla‐López P, Ramírez‐Pérez O, Cruz‐Ramón V, Canizales‐Quinteros S, Domínguez‐López A, Ponciano‐Rodríguez G, et al. More evidence for the genetic susceptibility of Mexican population to nonalcoholic fatty liver disease through PNPLA3. Ann Hepatol 2018;17:250‐255. [DOI] [PubMed] [Google Scholar]
- 10. Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta‐analysis. Hepatology 2011;54:1082‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chromy D, Mandorfer M, Bucsics T, Schwabl P, Bauer D, Scheiner B, et al. Prevalence and predictors of hepatic steatosis in patients with HIV/HCV coinfection and the impact of HCV eradication. AIDS Patient Care STDS 2019;33:197‐206. [DOI] [PubMed] [Google Scholar]
- 12. Ground KE. Liver pathology in aircrew. Aviat Space Environ Med 1982;53:14‐18. [PubMed] [Google Scholar]
- 13. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274‐285. [DOI] [PubMed] [Google Scholar]
- 14. Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988‐1994. Am J Epidemiol 2013;178:38‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 2004;114:147‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461‐1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kallwitz ER, Tayo BO, Kuniholm MH, Cai J, Daviglus M, Cooper RS, et al. American ancestry is a risk factor for suspected nonalcoholic fatty liver disease in Hispanic/Latino adults. Clin Gastroenterol Hepatol 2019;17:2301‐2309. [DOI] [PubMed] [Google Scholar]
- 18. Larrieta‐Carrasco E, Flores YN, Macías‐Kauffer LR, Ramírez‐Palacios P, Quiterio M, Ramírez‐Salazar EG, et al. Genetic variants in COL13A1, ADIPOQ and SAMM50, in addition to the PNPLA3 gene, confer susceptibility to elevated transaminase levels in an admixed Mexican population. Exp Mol Pathol 2018;104:50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Memorial Sloan Kettering Cancer Center . Understanding your FibroScan® results. https://www.mskcc.org/cancer‐care/patient‐education/understanding‐your‐fibroscan‐results. Published February 27, 2018. Accessed April 2020.
- 21. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 22. Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther 2015;41:65‐76. [DOI] [PubMed] [Google Scholar]
- 23. Liu A, Galoosian A, Kaswala D, Li AA, Gadiparthi C, Cholankeril G, et al. Nonalcoholic fatty liver disease: epidemiology, liver transplantation trends and outcomes, and risk of recurrent disease in the graft. J Clin Transl Hepatol 2018;6:420‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petta S, Di Marco V, Pipitone RM, Grimaudo S, Buscemi C, Craxì A, et al. Prevalence and severity of nonalcoholic fatty liver disease by transient elastography: genetic and metabolic risk factors in a general population. Liver Int 2018;38:2060‐2068. [DOI] [PubMed] [Google Scholar]
- 25. Schneider ALC, Lazo M, Selvin E, Clark JM. Racial differences in nonalcoholic fatty liver disease in the U.S. population. Obesity (Silver Spring) 2014;22:292‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kallwitz ER, Daviglus ML, Allison MA, Emory KT, Zhao L, Kuniholm MH, et al. Prevalence of suspected nonalcoholic fatty liver disease in Hispanic/Latino individuals differs by heritage. Clin Gastroenterol Hepatol 2015;13:569‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fleischman MW, Budoff M, Zeb I, Li D, Foster T. NAFLD prevalence differs among hispanic subgroups: the multi‐ethnic study of atherosclerosis. World J Gastroenterol: WJG 2014;20:4987‐4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non‐invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int 2012;32:911‐918. [DOI] [PubMed] [Google Scholar]
- 29. Zheng R‐D, Chen Z‐R, Chen J‐N, Lu Y‐H, Chen J. Role of body mass index, waist‐to‐height and waist‐to‐hip ratio in prediction of nonalcoholic fatty liver disease. Gastroenterol Res Practice 2012;2012:362147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kral JG, Schaffner F, Pierson RN Jr, Wang J. Body fat topography as an independent predictor of fatty liver. Metabolism 1993;42:548‐551. [DOI] [PubMed] [Google Scholar]
- 31. Walker RW, Belbin GM, Sorokin EP, Van Vleck T, Wojcik GL, Moscati A, et al. A common variant in PNPLA3 is associated with age at diagnosis of NAFLD in patients from a multi‐ethnic biobank. J Hepatol 2020;72:1070‐1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lazo M, Bilal U, Perez‐Escamilla R. Epidemiology of NAFLD and type 2 diabetes: health disparities among persons of Hispanic origin. Curr Diab Rep 2015;15:116. [DOI] [PubMed] [Google Scholar]
- 33. Fan J‐G, Zhu J, Li X‐J, Chen L, Li L, Dai F, et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol 2005;43:508‐514. [DOI] [PubMed] [Google Scholar]
- 34. Ndumele CE, Nasir K, Conceiçao RD, Carvalho JA, Blumenthal RS, Santos RD. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol 2011;31:1927‐1932. [DOI] [PMC free article] [PubMed] [Google Scholar]


