Abstract
Rationale & Objective:
Past studies show that, on average, predialysis BP runs higher than BP measured at home in a dialysis population. We hypothesized that in a subset of patients, BP was higher at home than in the dialysis unit and that the prevalence of left ventricular hypertrophy, a surrogate for cardiovascular events and death, was increased.
Study Design:
Prospective cohort
Settings and Participants:
97 hypertensive hemodialysis patients enrolled in the Blood Pressure in Dialysis Study (BID), a randomized trial of treatment to predialysis BP ≤140/90 vs. 155–165 /90 mm Hg, with ≥6 pairs home and predialysis BP readings.
Exposure:
Differences between predialysis and next day home systolic BP measured over one year
Outcome:
Left ventricular mass index (LVMI) by cardiac magnetic resonance imaging
Analytic Approach:
A hierarchical clustering analysis divided patients into 3 clusters based on the average and variability of differences in systolic BP predialysis and at home. We compared clinical factors and LVMI across clusters.
Results:
The predialysis-to-home systolic BP differences were least square (LS) means (95% CIs) 19.1 (17.0, 21.1) for Cluster 1 (‘home lower’), 3.7 (1.6, 5.8) for Cluster 2 (‘home and predialysis similar’), and −9.7 (−12.0, −7.4) mm Hg for Cluster 3 (‘home higher’). Systolic BP declined during dialysis in Clusters 1 and 2 but increased in Cluster 3. Interdialytic weight gains did not differ. After adjusting for sex and treatment arm, LVMI was higher in Cluster 3 versus Clusters 1 and 2 (differences in LS means of 10.6 (SE 4.96, p=0.04) and 12.0 (SE 5.08, p=0.02) gm/m2, respectively).
Limitations:
Limited statistical power
Conclusions:
In Cluster 3, which accounted for 31% of participants, home BP was higher than predialysis BP. Patients in Cluster 3 had a higher LVMI than those in Clusters 1 and 2 indicating that their BP may have been undertreated.
Keywords: masked hypertension, cardiovascular risk, left ventricular mass
INTRODUCTION
Hypertension is common among hemodialysis (HD) patients.1 However, the optimal blood pressure (BP) target, as well as the timing and location for BP measurements, remain controversial. Thrice weekly HD results in significant BP variability throughout the week. BP usually decreases significantly during dialysis and increases slowly during the interdialytic interval.2,3
The KDOQI and the Canadian Society of Nephrology recommend using pre- and post-dialysis BP measurements to guide hypertension management.4,5 Results from previous studies indicate that home BP is lower than predialysis BP.6,7 However, these observations were based on population averages from a two week period of intermittent home readings and a single 44-h ambulatory blood pressure monitoring (ABPM) session. Since BP is highly variable there may be significant variability in the observed differences between predialysis and home BP within individuals over repeated measurements and, across individuals in a population. Assessing population averages over a short time period may miss the detection of a pattern in which home BP is consistently higher or lower than dialysis unit readings within certain individuals. To the extent that home and dialysis unit BP measurements differ, excessive reliance on dialysis unit BP measurements may lead to over or under treatment of BP.
The present study was designed to explore the hypothesis that there is a subset of HD patients in whom BP is often higher at home than in the dialysis unit. As treatment decisions are based on dialysis unit BP these patients maybe undertreated, leading to left ventricular hypertrophy (LVH). We explored this hypothesis using data obtained in the Blood Pressure in Dialysis (BID) Pilot Study, a randomized trial of treating hypertensive HD patients to a predialysis systolic BP (SBP) of 110–140 mm Hg versus 155–165 mm Hg.8 BP was measured immediately before each dialysis and at home the day after the midweek HD over one year. In the present study we classified individuals into three clusters based on the mean and variability of the predialysis minus home BP differences over one year. We then assessed the differences in demographic and clinical factors, including left ventricular mass index (LVMI) across the clusters.
METHODS
Study Design
This is a prospective cohort study involving individuals who participated in the BID Study. Examining differences in home and standardized dialysis unit BP measurements was a pre-specified secondary outcome.
Study Population and Setting
We previously described the BID methods.9 Briefly, patients were recruited from 18 dialysis units in Albuquerque NM, Boston MA, Charleston SC, Pittsburgh PA, and Cleveland OH. The study was approved by the university affiliated Institutional Review Boards at each site and patients provided informed consent. Inclusion criteria for the BID Study were (1) age ≥ 18 years; (2) treated with HD for ≥ 90 days; and (3) 2-week averaged standardized predialysis systolic BP ≥ 155 mm Hg. Exclusion criteria were (1) fluid overload evident from clinical exam; (2) frequent intradialytic hypotension; and (3) life expectancy ≤ 1 year. Patients had to have ≥6 weekly pairs of midweek predialysis and next day home BP readings to be included in the present study.
BP Measurements
Standardized predialysis BP was measured each treatment after five minutes of rest in a sitting position with back supported, legs uncrossed, and in accord with other aspects of the American Heart Association guidelines for the duration of the study.10 Three readings were taken and the latter two were averaged. Patients and dialysis unit staff were trained to obtain standardized predialysis and home measurements, respectively, using the Lifesource UA-767 oscillometric device (A&D Medical, San Jose, CA). Home measurements were taken twice daily the day after the midweek dialysis. We requested readings on only one day per week to increase patient adherence over the one year study. The day after the midweek treatment was chosen because a prior study had demonstrated that BP taken the morning after the midweek HD matched most closely with the average of twice daily home and pre- and postdialysis readings over 7 days.7 Quarterly 44-hour ambulatory blood pressure monitoring (ABPM), beginning immediately after a midweek HD, was obtained in all sites except Boston. We collected intradialysis BP readings (taken every 30 minutes) from one dialysis session every other week. These were averaged starting at time 0 and excluding the standardized pre- and postdialysis readings. Intra- and postdialysis BPs were measured via the sphygmomanometer attached to the dialysis machine.
Outcome Variables
Cardiac magnetic resonance imaging was done the day after the midweek treatment at both baseline and 12 months. Left ventricular mass, excluding papillary muscle, was estimated by manual tracing. LVH was defined as LVMI (LVM/ body surface area) > 84.1 g/m2 in males and > 66.8 g/m2 in females.11,12
Statistical Analyses
Data were described as boxplots of the mean of observed values per individual for each BP type (pre-, post-, intradialytic, home, ABPM). Linear mixed models were constructed with patients as random effects to obtain least squares (LS) means with 95% confidence intervals (95% CIs) for differences between home and other BP measures after accounting for non-independence of observations and adjusting for time since randomization, treatment arm, and an interaction between time and treatment arm. Pre-, post-, and intradialysis BP were time matched to the closest home sitting the following day. The daytime (8 AM to 10 PM) average from the ABPM was matched to the average of the AM and PM home BP the following day. Dipping was defined as a decrease in average nocturnal SBP of 10% or more as compared to the average daytime SBP.
Cluster Analysis
Using a hierarchical clustering analysis ( R version 3.6.0) we constructed a dissimilarity matrix and calculated Euclidian distances13 based on the average and variability of differences between matched predialysis and home SBP per patient. We chose Ward’s minimum variance method,13,14 a clustering structure based on an agglomerative coefficient.
We compared characteristics across clusters using generalized linear models adjusted for treatment arm. Continuous data were log transformed if not normally distributed. Continuous data were expressed as LS means with 95% CIs. Count (IDH rate, cramps rate) and categorical data were modeled with generalized linear mixed effects modeling. To examine potential differences in LVMI and the change in LVMI over 12 months by cluster, we constructed a linear regression model with LVMI (or change in LVMI, respectively) as the dependent variable with adjustment for sex and treatment arm.
Reported p values are two-sided, and were considered statistically significant at p<0.05. Analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC) and R version 3.6.0.
RESULTS
Characteristics of the 97 participants with ≥6 matched pairs of home and predialysis readings are shown (Table 1). The median number (IQR) of weekly BP pairs per individual was 24 (15, 38). These participants were not significantly different from the original 126 BID participants (Table S1). The mean age of the study population was 56.7, while 48.5% were African American, 54% had diabetes as the cause of ESKD, 4.1% had a history of myocardial infarction, 8.3% of peripheral vascular disease, and 12.4% of congestive heart failure. The median time on dialysis prior to study start was 3.0 years, the median (IQR) duration of a dialysis treatment was 3.8 (3.5–4.0) hours. The median (IQR) number of antihypertensive medications prescribed was 3 (2, 3) and 21% held at least one antihypertensive prior to starting dialysis.
Table 1.
Baseline characteristics (N = 97).
| Randomized to Intensive study arm a | 46 (47.4) |
| Male a | 56 (57.7) |
| Race a | |
| White | 35 (36.1) |
| Black | 47 (48.5) |
| Others | 15 (15.5) |
| Age (years) b | 56.7 (13.4) |
| Vintage (years) c | 3.0 (1.1, 4.7) |
| Cause of end stage kidney disease a | |
| Diabetes mellitus | 52 (53.6) |
| Hypertension | 28 (28.9) |
| Glomerulonephritis | 8 (8.3) |
| Other | 9 (9.3) |
| Comorbid conditions a | |
| Myocardial infarction | 4 (4.1) |
| Congestive heart failure | 12 (12.4) |
| Peripheral vascular disease | 8 (8.3) |
| Current or Former Smoking history | 45 (46.4) |
| Body mass index (kg/m 2 ) c | 26.2 (22.9, 30.2) |
| Interdialytic weight gain (kg) d | 2.7 (1.2) |
| Interdialytic weight gain (% of estimated dry weight) d | 3.5 (1.3) |
| Prescribed treatment time (hours) e | 3.8 (3.5, 4.0) |
| Ultrafiltration volume (L) e | 2.9 (2.3, 3.5) |
| Predialysis systolic blood pressure (mm Hg) d | 159.4 (9.2) |
| Predialysis diastolic blood pressure (mm Hg) d | 80.0 (11.6) |
| Number of antihypertensive medications taken d | 2.6 (1.3) |
| Patients who had antihypertensive medication held predialysis a | |
| 1 medication held | 14 (14.4) |
| 2 medications held | 2 (2.1) |
| Percent of patients receiving specific classes of antihypertensive medications | |
| Angiotensin converting enzyme inhibitor | 42 |
| Angiotensin receptor blocker | 18 |
| Beta-adrenergic blocking agent | 75 |
| Calcium channel blocker | 60 |
| Central alpha agonist | 19 |
n(%)
mean (standard deviation)
median (interquartile range)
mean (standard deviation) for the month prior to randomization
median (interquartile range) for the month prior to randomization
Differences between Blood Pressure Measurements
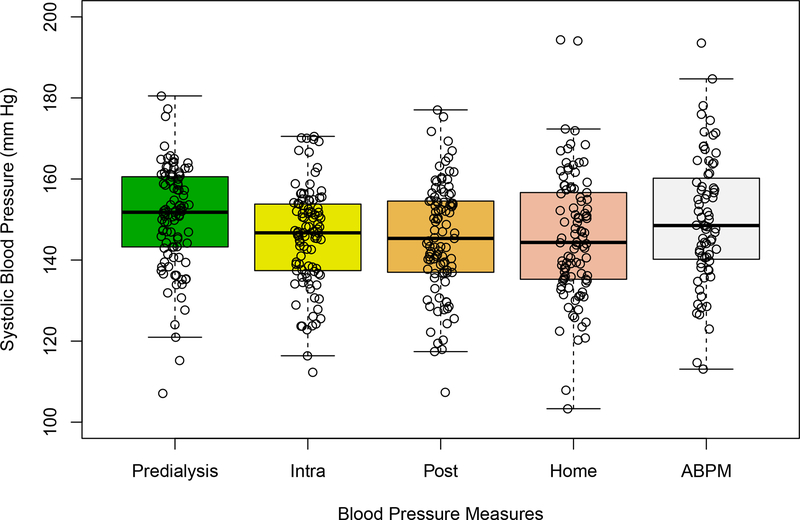
The distributions of pre-, post-, intradialytic and home SBP measurements in the 97 patients who had ≥ 6 pairs of matched midweek standardized dialysis unit and next day home readings and in the 72 patients who had all of the former readings as well as ABPM are shown (Figure 1, Table S2). Predialysis SBP was 5.91 (4.50, 7.33) mm Hg higher than home SBP (Table 2). Intradialytic and ABPM were similar to home SBP, postdialysis was lower than home SBP. Home, intradialytic, pre- and postdialysis diastolic BP (DBP) readings were similar.
Figure 1. Distributions of Standardized Predialysis, Home, Postdialysis, and Average Intradialysis Systolic Blood Pressures.
Figure 1. Boxplots with jitter show the distributions of the midweek pre-(standardized), post-, and intradialysis blood pressure, next day home blood pressure readings averaged over all dialysis sessions over the course of the year in each of 97 patients, and all possible ABPM over the year in each of 72 patients. Each data point represents one participant.
Table 2.
LS Means Differences between Home and Other Blood Pressure Measurements
| BP Difference | No. of patients | No. of BP pairs per patient | Systolic BP (mm Hg) | P value | Diastolic BP (mm Hg) | P value |
|---|---|---|---|---|---|---|
|
| ||||||
| Predialysis – Home | 97 | 24(15, 38) | 5.91 (4.50, 7.33) | <0.001 | 0.16 (−0.54, 0.85) | 0.9 |
| Intradialysis – Home | 24(16,33) | −0.74 (−2.18, 0.69) | 0.9 | 0.56 (−0.15, 1.27) | 0.2 | |
| Postdialysis – Home | 24(13,38) | −1.25 (−2.62, 0.13) | 0.1 | 0.54 (−0.14, 1.22) | 0.2 | |
|
| ||||||
| ABPM – Home | 72 | 2 (2,3) | 0.57 (−2.09, 3.23) | 0.7 | 3.99 (2.77, 5.21) | < 0.001 |
Predialysis, average intradialytic BP, and postdialysis BP were time matched to the closest home BP the following day. The daytime average from the ABPM was matched to the average of the AM and PM home BP. LS mean differences were estimated in linear mixed effects models adjusted for treatment arm, time between BP measurements and randomization.
. BP, blood pressure; ABPM, ambulatory blood pressure monitoring.
Pattern of the Home-to-Predialysis Difference over Repeated Measures within Individuals
Ninety-seven participants were divided into three clusters based on the within-patient differences between predialysis and home SBP and the variability in these differences over one year. There were 31 (32%), 36 (37%), and 30 (31%) patients allocated to clusters 1 (‘home lower’), 2 (‘home and predialysis similar’), and 3 (‘home higher’), respectively. The LS mean (95% CI) predialysis minus home SBP differences were 19.1 (17.0, 21.1) vs. 3.7 (1.6, 5.8) vs. −9.7 (−12.0, −7.4) mm Hg in clusters 1, 2 and 3, respectively (p<0.001). Home SBP was lower than predialysis by ≥10 mm Hg for 69%, 34%, and 17% of readings per patient in Clusters 1, 2 and 3, respectively, while, home was higher than predialysis SBP by ≥10 mm Hg for 9%, 24% and 48% in Clusters 1, 2 and 3, respectively. Patients in cluster 1 were older than those in clusters 2 and 3, however, these differences did not attain statistical significance (Table 3a). There were no significant differences in sex, race, vintage, cause of ESKD, body mass index, smoking status, the number of antihypertensive drugs taken or the number held prior to dialysis at baseline or in quarter 4, respectively, by cluster. There was no difference in predialysis systolic BP across clusters (Table 3b). BP declined during dialysis in Clusters 1 and 2 (LS means pre-post systolic difference (95% CI) 10.0 (5.4, 14.6), and 9.3 (5.0, 13.6) mm Hg, respectively) while there was a nonsignificant increase in Cluster 3 (−3.3 (−8.0, 1.4) mm Hg). Postdialysis BP was higher in Cluster 3 than Clusters 1 and 2 (LS means systolic BP: 151.7 vs. 142.4 vs. 142.9 mm Hg, and diastolic BP 80.4 vs. 71.7 vs. 77.9 mm Hg, respectively). The daytime ABPM (n=56) was higher for systolic in Cluster 3 vs. Clusters 1 and 2 (LS means 149.0 vs. 135.6 and 146.6 mm Hg, respectively, p=0.05) and for diastolic BP (86.0 vs. 72.8 and 81.1 mm Hg, respectively, p<0.01). Non-dipping status was highly prevalent and did not differ by cluster (100%, 85%, and 87% in Clusters 1, 2 and 3, respectively). Interdialytic weight gain (IDWG) did not differ by cluster. Intradialytic hypotension (1 or more occurrences of intradialytic SBP ≤90 mm Hg during a treatment) was less frequent in Cluster 3 vs. Clusters 1 and 2 (incidence rates per 100 patient-treatments (95% CI) 1.8 (1.0, 3.1) vs. 4.9 (2.9, 8.1) vs. 3.2 (2.3, 4.3), respectively p=0.04).
Table 3a:
Patient Characteristics Across Clusters
| Total | Cluster 1 “Home Lower” | Cluster 2 “Home and Predialysis Similar” | Cluster 3 “Home Higher” | ||
|---|---|---|---|---|---|
| No. of patients | 97 | 31 | 36 | 30 | p valuee |
|
| |||||
| Age (years) a | 56.7 (13.4) | 61.0 (12.9) | 54.3 (13.4) | 55.1 (13.2) | 0.09 |
| Male b | 56 (57.7) | 16 (51.6) | 19 (52.8) | 21 (70.0) | 0.2 |
| Vintage (years) c,d | 3.0 (1.1, 4.7) | 3.3 (0.9, 5.3) | 2.6 (0.7, 3.8) | 2.9 (1.4, 4.6) | 0.6 |
| Race b | |||||
| White | 35 (36.1) | 11 (35.5) | 14 (38.9) | 10 (33.3) | 0.9 |
| Black | 47 (48.5) | 14 (45.2) | 17 (47.2) | 16 (53.3) | |
| Other | 15 (15.5) | 6 (19.4) | 5 (13.9) | 4 (13.3) | |
| Body mass index (kg/m 2 ) c,d | 26.2 (22.9, 30.2) | 25.7 (22.6, 31.6) | 26.8 (24.3, 31.9) | 26.0 (22.5, 29.6) | 0.7 |
| Cause of ESKD b | 0.9 | ||||
| Diabetes | 52 (53.6) | 17 (54.8) | 17 (47.2) | 18 (60.0) | |
| Hypertension | 28 (28.9) | 9 (29.0) | 13 (36.1) | 6 (20.0) | |
| Glomerulonephritis | 8 (8.3) | 2 (6.5) | 3 (8.3) | 3 (10.0) | |
| Other | 9 (9.3) | 3 (9.7) | 3 (8.3) | 3 (10.0) | |
| Smoking status b | 0.2 | ||||
| Never | 52 (53.6) | 15 (48.4) | 24 (66.7) | 13 (43.3) | |
| Current | 12 (12.4) | 4 (12.9) | 5 (13.9) | 3 (10.0) | |
| Former | 33 (34.0) | 12 (38.7) | 7 (19.4) | 14 (46.7) | |
| Number of antihypertensive medications c | |||||
| Baseline | 3 (2, 3) | 2 (2, 3) | 2 (1, 3) | 3 (2, 4) | 0.2 |
| Quarter 4 | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 3 (3, 4) | 0.3 |
| Percent of patients who had 1 or more antihypertensive medications held predialysis b | |||||
| Baseline | 20 (20.8) | 6 (19.4) | 8 (22.9) | 6 (20.0) | 0.8 |
| Quarter 4 | 26 (27.1) | 6 (19.4) | 13 (37.1) | 7 (23.3) | 0.3 |
| BID randomized arm b | |||||
| Intensive Arm | 46 (47.4) | 15 (48.4) | 18 (50.0) | 13 (43.3) | 0.8 |
| Left ventricular hypertrophy b | |||||
| Baseline | 40 (48.2) | 14 (46.7) | 11 (40.7) | 15 (57.7) | 0.4 |
| Quarter 4 | 37 (44.6) | 11 (36.7) | 10 (37.0) | 16 (61.5) | 0.1 |
mean (standard deviation)
n(%)
median (interquartile range)
Log transformed for statistical testing
The p-value represents results of comparing factor by cluster using generalized linear regressions adjusted for treatment arm
Abbreviations: BID, Blood Pressure in Dialysis Pilot Study.
Table 3b:
Blood Pressure Measurements and Dialysis Treatment CharacteristicsAcross Clusters
| Total | Cluster 1 “Home Lower” | Cluster 2 “Home and Predialysis Similar” | Cluster 3 “Home Higher” | ||
|---|---|---|---|---|---|
| No. of patients | 97 | 31 | 36 | 30 | |
| Total No. of BP pairs per cluster | 2479 | 951 | 852 | 676 | p valuec |
|
| |||||
| Cluster Definition | |||||
| Predialysis minus home systolic BP (mm Hg)a | 4.8 (2.2, 7.4) | 19.1 (17.0, 21.1) | 3.7 (1.6, 5.8) | −9.7 (−12.0, −7.4) | <.001 |
|
| |||||
| Blood Pressure (BP) Measurements | |||||
| Percent of observations per patient in which predialysis minus home systolic BP b | |||||
| ≥ 10 mm Hg | 36.1 (21.4, 60.0) | 68.8 (57.1, 75.0) | 33.7 (27.4, 43.2) | 17.2 (9.1, 23.5) | <.001 |
| ± <10 mm Hg | 29.6 (21.9, 44.4) | 21.9 (16.7, 31.6) | 42.4 (28.6, 50.0) | 29.6 (24.0, 42.9) | <.001 |
| ≤ −10 mm Hg | 23.5 (12.5, 41.9) | 8.7 (2.6, 12.5) | 23.5 (21.1, 30.0) | 48.3 (41.9, 57.1) | <.001 |
| Predialysis BP (mm Hg) a | |||||
| Systolic | 150.9 (148.7, 153.1) | 152.3 (148.5, 156.1) | 151.8 (148.2, 155.3) | 148.3 (144.4, 152.2) | 0.2 |
| Diastolic | 76.6 (74.5, 78.8) | 72.2 (68.5, 75.9) | 80.6 (77.2, 84.0) | 76.4 (72.7, 80.2) | <.001 |
| Postdialysis BP (mm Hg) a | |||||
| Systolic | 145.4 (142.7, 148.2) | 142.4 (137.7, 147.0) | 142.9 (138.5, 147.2) | 151.7 (146.9, 156.4) | <.001 |
| Diastolic | 76.7 (74.7, 78.7) | 71.7 (68.5, 75.0) | 77.9 (74.8, 80.9) | 80.4 (77.1, 83.8) | <.001 |
| Pre- minus postdialysis BP change (mm Hg) a | |||||
| Systolic | 7.3 (4.7, 9.9) | 10.0 (5.4, 14.6) | 9.3 (5.0, 13.6) | −3.3 (−8.0, 1.4) | <.001 |
| Diastolic | 0.7 (−0.6, 2.0) | 0.3 (−2.1, 2.7) | 2.3 (0.0, 4.5) | −4.2 (−6.7, −1.7) | <.001 |
| Home BP* (mm Hg) a | |||||
| Systolic | 145.3 (142.2, 148.3) | 132.8 (128.7, 136.9) | 146.8 (143.0, 150.7) | 156.6 (152.3, 160.9) | <.001 |
| Diastolic | 76.5 (74.1, 78.9) | 68.2 (64.4, 71.9) | 80.3 (76.8, 83.8) | 80.4 (76.6, 84.2) | <.001 |
| ABPM daytime BP# (mm Hg)a | |||||
| Systolic | 143.0 (137.9,148.1) | 135.6 (127.9, 143.3) | 146.6 (138.2, 154.9) | 149.0 (140.3, 157.8) | 0.05 |
| Diastolic | 79.3 (75.9, 82.7) | 72.8 (68.0, 77.7) | 81.1 (75.9, 86.4) | 86.0 (80.4, 91.5) | <.001 |
| ABPM nocturnal BP# (mm Hg)a | |||||
| Systolic | 142.3 (136.8, 147.7) | 137.7 (129.1, 146.3) | 145.3 (136.0, 154.6) | 144.9 (135.0, 154.8) | 0.4 |
| Diastolic | 76.2 (72.8, 79.6) | 71.0 (66.0, 76.0) | 77.6 (72.2, 83.1) | 81.5 (75.8, 87.3) | 0.02 |
|
| |||||
| Dialysis Treatment Characteristics e | |||||
| Length of dialysis session (minutes) a | 219.8 (214.7, 224.8) | 217.1 (208.0, 226.2) | 220.4 (211.9, 228.8) | 222.1 (212.8, 231.5) | 0.7 |
| Intradialytic hypotensiond (rate per 100 treatments) | 3.5 (2.6, 4.7) | 4.9 (2.9, 8.1) | 3.2 (2.3, 4.3) | 1.8 (1.0, 3.1) | 0.04 |
| Intradialytic Cramps (rate per 100 treatments) | 7.2 (5.8, 8.9) | 7.5 (5.1, 11.0) | 7.2 (5.8, 9.0) | 7.0 (4.7, 10.3) | 0.9 |
| Interdialytic weight gain (kg) a | 2.7 (2.5, 2.8) | 2.5 (2.2, 2.8) | 2.9 (2.6, 3.1) | 2.6 (2.3, 2.9) | 0.2 |
Missing ABPM data n=21, n=24, and n=18, for clusters 1, 2, and 3, respectively.
LS Mean (95% confidence interval)
median (interquartile range)
The p-value represents the results of comparing factor by cluster using generalized linear mixed effects models adjusted for treatment arm
Defined as the occurrence of 1 or more episode of intradialytic systolic BP <90 mm Hg. Derived from n=126,158 dialysis treatments.
The median (IQR) number of dialysis treatments from which these variables were derived was as follows: 42(33,46) for treatment duration, 144(126,158) for intradialytic hypotension and cramps, and 152(138,157) for interdialytic weight gain.
Abbreviations: ABPM, ambulatory blood pressure monitoring
Left Ventricular Hypertrophy (LVH) and Mass Index (LVMI) by Cluster
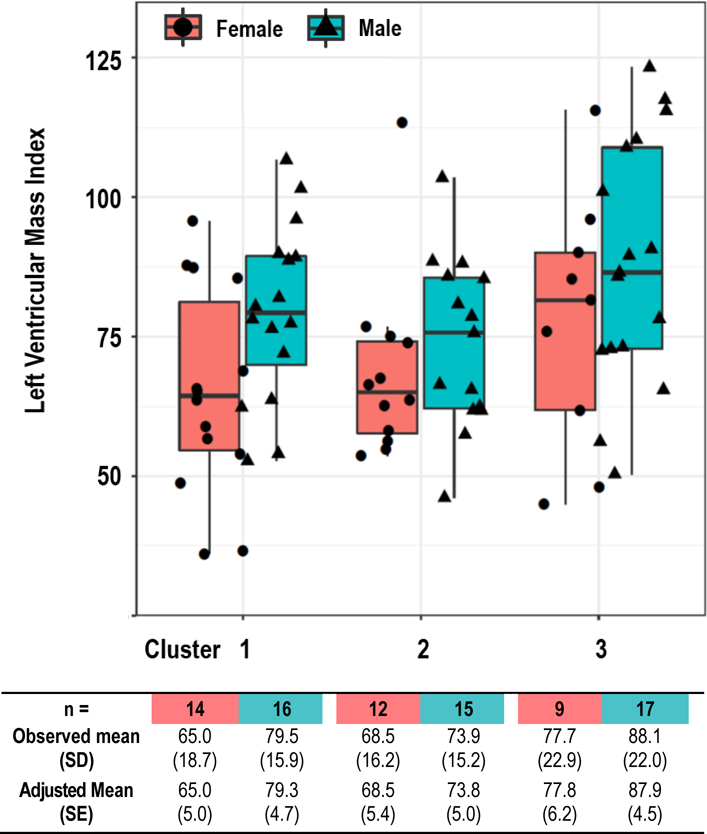
The prevalence of LVH tended to be higher in Cluster 3 (61.5%) vs. Clusters 1 (36.7%) and 2 (37.0%), although these differences did not attain statistical significance. Figure 2 shows boxplots of LVMI at 12 months after study end, stratified by sex and cluster. After adjusting for sex (p=0.02) and treatment arm (p=0.53), LVMI was higher in Cluster 3 versus Clusters 1 and 2 with differences in LS means of 10.6 gm/m2 (SE 4.96, p=0.04) and 12.0 gm/m2 (SE 5.08, p=0.02), respectively. The difference between clusters 1 and 2 was 1.42 gm/m2 (SE 4.89, p=0.8). In a simple linear regression relating the one year averaged predialysis-to-home systolic BP difference with LVMI, there was an increase in LVMI with declining negative values of the predialysis-home BP difference (Fig S2). This is consistent with the results of the cluster analysis showing higher LVMI in Cluster 3 (‘home higher’).
Figure 2. Observed and Adjusted Mean Left Ventricular Mass Index in Quarter 4 by Sex and Cluster.
Figure 2. Boxplots with jitter of left ventricular mass index by sex and cluster. The observed and adjusted LS means (SE) are shown in the table. LS means (SE) were calculated from a general linear regression model, adjusting for sex, cluster, treatment arms, and sex X cluster interaction. LVMI was higher in cluster 3 versus clusters 1 and 2 with differences in LS means of 10.6 gm/m2 (SE 4.96, p=0.04) and 12.0 gm/m2 (SE 5.08, p=0.02), respectively.
Predialysis-to-Home Systolic BP Difference and Change in Left Ventricular Mass Index Over 12 months
In a model adjusted for sex and treatment arm, there was no difference by Cluster in the change in LVMI over 12 months (predicted change (95% confidence) in Cluster 1, 2 and 3 of 1.41 (−3.33, 6.14), 2.60 (−2.38, 7.58), and 1.17 (−3.93, 6.26) g/m, respectively, p=0.91). The predialysis-to-home BP difference treated as a continuous variable was also not significantly related to the change in LVMI over 12 months (Figure S3).
DISCUSSION
Past studies indicate that on average, BP runs lower at home than predialysis and this is also seen in this study. However, when we examine the home-to-predialysis BP difference within individuals over repeated measures of each obtained over the course of a year, we find that for nearly one third of patients, BP was usually higher at home than predialysis. Moreover, this group of patients (Cluster 3) had higher LVMI than those in Cluster 1 (‘home lower’) or 2 (‘home and predialysis similar’). The clusters were indistinguishable with regards to predialysis SBP and interdialytic weight gain. The ‘home higher’ group had a small increase in BP during dialysis, in contrast to a significant decline in BP during dialysis that was seen in the other patients. Postdialysis BP was also significantly higher in Cluster 3 than Clusters 1 and 2. The lack of a decline in BP with dialysis may be a signal identifying a patient who will exhibit a ‘home higher’ BP pattern. The higher LVMI among patients in Cluster 3, despite similar predialysis BPs, indicates that home and postdialysis BPs may better reflect the average BP load than the predialysis BP. The higher LVMI in this group suggests that their high blood pressure at home is under recognized and undertreated.
The present study supports the European Renal Association guidelines recommending home over dialysis unit BPs to guide management of dialysis patients.15 The evidence to support such recommendations is quite limited. A study of predominantly African Americans (n=140) who underwent a single ABPM session and two weeks of intermittent home and dialysis unit BP readings showed higher predictive accuracy for LVH of home followed by ABPM, pre- and postdialysis readings6. In largely the same population, the risk for cardiovascular and all-cause mortality increased monotonically with increasing quartile of home BP and ABPM, which was not found for pre- and postdialysis readings16. From these results, one does not appreciate that there is a group of patients, (sizable in the present study at 30%) that run higher BPs at home than in the dialysis unit and have higher LVMI than other patients. Such findings provide further evidence of the value of home readings and are interpreted in clinical terms more readily than a series of ROC curves or the linearity of the relationship of quartiles of BP with an outcome.
Pertaining to whether intermittent home readings vs. ABPM should be the preferred method of out-of-dialysis-unit BP measurement, this study found poor adherence with quarterly ABPM, with completion rates of a single ABPM during Q1–4 of 32%, 29%, 28% and 58%, respectively.8 This was also found in the Hypertension in Hemodialysis Patients Treated with Atenolol or Lisinopril Study.17 ABPM certainly poses additional logistical challenges, and, given our experience, it may not be practical. A recent US study testing the feasibility of home BP monitoring, found a high rate of adherence (97.4%) with intermittent home readings measured twice daily for one day every two weeks over 4 months34.
The pattern of home vs. predialysis unit BP seen in Cluster 3 is similar in concept to masked hypertension in the general population, in which BP is elevated at home but not in the office. Masked hypertension, estimated to affect 13% of the general population,18 is associated with increased LVMI19 and an increased risk of major adverse cardiovascular events as compared with normotension or white coat hypertension.20,21 Given the strong and consistent relationship of LVMI with cardiovascular events, and that this risk is altered with a change in LVMI,22–26 we suspect that the patients in Cluster 3 are at increased risk. Whether more aggressive BP lowering could reduce this risk remains to be proven.
Why for some patients BP is higher at home than in the office, or in this case, the dialysis unit, is not clear. Smoking and use of a greater number of antihypertensive agents have been associated with masked hypertension in the general population.27 We found no difference in smoking status, the number of antihypertensive drugs used or the frequency at which drugs were withheld prior to dialysis by cluster. That BP is higher at home the next day than predialysis is particularly striking as the predialysis BP is measured at the highest degree of fluid overload in the dialytic cycle. One may postulate that Cluster 3 patients would have higher IDWGs driving the higher BP at home however, we found no difference in IDWG by cluster. Alcohol use (not measured in this study), stress, endothelial dysfunction, other factors, as discussed in the masked hypertension and intradialytic hypertension, may be operative.28–30
This study has unique strengths. Data was collected over one year as opposed to previous studies that examined only a few weeks of BP measurements.6,7 This permitted characterization of direction, magnitude and variability of the dialysis unit minus home BP differences within individuals and assessment of long term adherence. Great care was taken to measure the predialysis BPs per a standardized protocol, including use of stopwatches to time the 5 minute rest period prior to the first measurement, attention to other aspects of the recommended procedure for measuring BP,10 and certification of staff and patients. The BP measurements on which these analyses are based are likely to be more accurate than routine dialysis unit readings. As standardized BP readings are usually lower than routine readings, the predialysis-to-home BP difference may be greater than that reported here. LVMI was assessed with MRI, which is more accurate than echocardiogram.31
The study also has several limitations. The study population was relatively small which limited the statistical power to detect differences across clusters. The smaller sample size also restricted our choice of methods for forming the clusters. Although we recognize that latent mixture modeling methods are often preferred over a hierarchical approach, these require significantly larger sample sizes.32,33 However, when we attempted to use the latent class methods, the clusters displayed a moderate level of agreement with the clusters reported in the manuscript. The present study was done within the framework of a randomized trial, raising the possibility that differences in clusters might be due to the intervention. We think this is less likely as the proportions by treatment arm did not differ across clusters (Table 3), analyses were adjusted for treatment arm and there was a non-significant trend towards a higher proportion with LVH in Cluster 3 patients at baseline, prior to randomization (Table 3). The number of paired readings per individual varied, mainly due to missing home readings. There was no difference by the quantity of paired readings (≤12 vs. >12 pairs) in the proportion of patients within Cluster 3 (Table S1B) or in the predialysis-to-home BP difference (Table S1C). Nonetheless, we cannot exclude the possibility that the missing data may have introduced bias. The study participants did not represent the general hemodialysis population since eligibility required a 2 week standardized predialysis SBP >155 mmHg and the absence of frequent intradialytic hypotension. The prevalence estimates of the home higher pattern, may be lower in a less hypertensive population. The present study did not demonstrate that treating patients based on home readings as compared with dialysis unit readings would improve outcomes or identify the optimal BP target.
In summary, this study finds that for that in close to one-third of hypertensive dialysis patients, BP runs higher at home than predialysis and these patients had a higher LVMI than the other patients. Accordingly, these patients may be at higher risk for cardiovascular events. Dialysis facilities should educate patients and staff on obtaining out-of-dialysis-unit BP readings and undertake regular programs to enhance and maintain adherence.
Supplementary Material
Acknowledgements:
There was no direct financial support for this manuscript. The BID Study was supported by National Institute of Diabetes and Digestive and Kidney Diseases (R01DK083424) and Dialysis Clinic, Inc.
Disclosures
Drs. Miskulin and Pankratz receive salary support and Dr. Shaffi receives research support from Dialysis Clinic, Inc. (DCI); H. Jiang, S. Paine, Drs. Gul, Harford, and Zager are employees of DCI; Drs. Jhamb, Kwong, Negrea, Gassman and Ploth have no disclosures.
Reference List
- 1.Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG: Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med. 2003;115(4):291–297. [DOI] [PubMed] [Google Scholar]
- 2.Dinesh K, Kunaparaju S, Cape K, Flythe JE, Feldman HI, Brunelli SM. A model of systolic blood pressure during the course of dialysis and clinical factors associated with various blood pressure behaviors. Am J Kidney Dis. 2011;58(5):794–803. [DOI] [PubMed] [Google Scholar]
- 3.Karpetas A, Loutradis C, Bikos A, et al. Blood pressure variability is increasing from the first to the second day of the interdialytic interval in hemodialysis patients. J Hypertens. 2017;35(12):2517–2526. [DOI] [PubMed] [Google Scholar]
- 4.K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1–153. [PubMed] [Google Scholar]
- 5.Jindal K, Chan CT, Deziel C, et al. Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology. J Am Soc Nephrol. 2006;17(3 Suppl 1):S1–27. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal R, Brim NJ, Mahenthiran J, Andersen MJ, Saha C: Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension. 2006;47(1):62–68. [DOI] [PubMed] [Google Scholar]
- 7.Moriya H, Ohtake T, Kobayashi S. Aortic stiffness, left ventricular hypertrophy and weekly averaged blood pressure (WAB) in patients on haemodialysis. Nephrol Dial Transplant. 2007;22:1198–1204. [DOI] [PubMed] [Google Scholar]
- 8.Miskulin DC, Gassman J, Schrader R, et al. BP in dialysis: results of a pilot study. J Am Soc Nephrol. 2018;29(1):307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gul A, Miskulin D, Gassman J, et al. Design of the blood pressure goals in dialysis pilot study. Am J Med Sci. 2014;347(2):125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716. [DOI] [PubMed] [Google Scholar]
- 11.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17(3):323–29. [DOI] [PubMed] [Google Scholar]
- 12.Chan CT, Greene T, Chertow GM, et al. Determinants of left ventricular mass in patients on hemodialysis: Frequent Hemodialysis Network (FHN) Trials. Circ Cardiovasc Imaging. 2012;5(2):251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murtagh F, Legendre P. Ward’s hierarchical agglomerative clustering method: which algorithms implement Ward’s criterion? J Classif. 2014;31(3):274–295. [Google Scholar]
- 14.Ward JH Jr. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58(301):236–244. [Google Scholar]
- 15.Sarafidis PA, Persu A, Agarwal R, et al. Hypertension in dialysis patients: a consensus document by the European Renal and Cardiovascular Medicine (EURECA-m) working group of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) and the Hypertension and the Kidney working group of the European Society of Hypertension (ESH). Nephrol Dial Transplant. 2017;32(4):620–640. [DOI] [PubMed] [Google Scholar]
- 16.Alborzi P, Patel N, Agarwal R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol. 2007;2(6):1228–1234. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dial Transplant. 2014;29(3):672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ. 2020;29(1):102–111. [DOI] [PubMed] [Google Scholar]
- 19.Liu JE, Roman MJ, Pini R, Schwartz JE, Pickering TG, Devereux RB. Cardiac and arterial target organ damage in adults with elevated ambulatory and normal office blood pressure. Ann Intern Med. 1999;131(8):564–72. [DOI] [PubMed] [Google Scholar]
- 20.Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25(11):2193–2198. [DOI] [PubMed] [Google Scholar]
- 21.Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta analysis. Am J Hypertens. 2011;24(1):52–58. [DOI] [PubMed] [Google Scholar]
- 22.Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE. Long-term evolution of cardiomyopathy in dialysis patients. Kidney Int. 1998;54(5):1720–1725. [DOI] [PubMed] [Google Scholar]
- 23.London GM, Pannier B, Guerin AP, et al. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J Am Soc Nephrol. 2001;12(12):2759–2767. [DOI] [PubMed] [Google Scholar]
- 24.Paoletti E, Bellino D, Signori A, et al. Regression of asymptomatic cardiomyopathy and clinical outcome of renal transplant recipients: a long-term prospective cohort study. Nephrol Dial Transplant. 2016;31(7):1168–1174. [DOI] [PubMed] [Google Scholar]
- 25.Pierdomenico SD, Cuccurullo F. Risk reduction after regression of echocardiographic left ventricular hypertrophy in hypertension: a meta-analysis. Am J Hypertens. 2010;23(8):876–881. [DOI] [PubMed] [Google Scholar]
- 26.Trinh E, Chan CT. Intensive home hemodialysis results in regression of left ventricular hypertrophy and better clinical outcomes. Am J Nephrol. 2016;44(4):300–307. [DOI] [PubMed] [Google Scholar]
- 27.Verberk WJ, Kessels AG, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens. 2008;21(9):969–975. [DOI] [PubMed] [Google Scholar]
- 28.Inrig JK. Intradialytic hypertension: a less-recognized cardiovascular complication of hemodialysis. Am J Kidney Dis. 2010;55(3):580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickering TG, Eguchi K, Kario K. Masked hypertension: a review. Hypertens Res. 2007;30(6):479–488. [DOI] [PubMed] [Google Scholar]
- 30.Takeno K, Mita T, Nakayama S, et al. Masked hypertension, endothelial dysfunction, and arterial stiffness in type 2 diabetes mellitus: a pilot study. Am J Hypertens. 2012;25(2):165–170. [DOI] [PubMed] [Google Scholar]
- 31.Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens. 1995;8(3):221–228. [DOI] [PubMed] [Google Scholar]
- 32.Jaki T, Kim M, Lamont A, et al. The Effects of Sample Size on the Estimation of Regression Mixture Models. Educ Psychol Meas. 2019;79(2):358–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S. Sample Size Requirements in Single- and Multiphase Growth Mixture Models: A Monte Carlo Simulation Study. Struct Equ Modeling. 2012;19(3):457–476. [Google Scholar]
- 34.Bansal N Glidden DV, Mehrotra M, Townsend RR, Cohen J, Linke L, Palad F, Larson H, and Hsu C. Treating Home Versus Predialysis Blood Pressure Among In-Center Hemodialysis Patients: A Pilot Randomized Trial. Am J Kidney Dis. 2020; 77:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.