Abstract
Background:
Aristolochic acid I (AAI) is an extract from Chinese herbs that causes progressive interstitial nephritis. The aim of this research is to know whether chymases play the crucial role in AAI-induced nephropathy.
Methods:
The mice were treated with AAI via intraperitoneal injection and the accumulated AAI dosages are 30 mg/kg of body weight for two, four, six, and eight weeks. The animals were sacrificed after another two or four weeks for nephropathy development. Collection of blood, urine, and kidney samples for the further biochemical analysis, hematoxylin–eosin (H and E) and Masson's trichrome stained to detected pathologic, and MMP2 and MMP9 activity assays.
Results:
After the treatment of AAI, of the mice, their body weights were decreased (P < 0.01), and concentration of creatinine and blood urea nitrogen (BUN) in serum (P < 0.01) and urine collection were increased (P < 0.01). In the renal tissue sections, high amount of inflammatory cells were found by H and E stain, and increased fibrosis in renal interstitial tissue were observed by Masson's trichrome stain. In mice kidney tissue, significantly increased chymase activity after treatment of AAI was found (P < 0.01), but ACE activity did not show significant changes. In ACE KO mice, increased MMP2 and decreased MMP9 activity were found in the AAI-treated mice compared with AAI-untreated control (P < 0.01).
Conclusions:
Moreover, it was also observed that the deficiency of ACE would accelerate the disease development of AAI-induced nephropathy. These results may help to know more information about the role of AAI-induced chronic kidney disease and can be applied in developing new drug targets for nephropathy.
Keywords: Aristolochic acid I, chronic kidney disease, chymases, peptidyl-dipeptidase A, renal insufficiency
Introduction
Chronic kidney disease (CKD) is a general term for heterogeneous disorders affecting the structure and function of the kidney.[1] The presence of kidney damage is usually regarded as an index.[2] CKD is kidneys damage that cannot excess water out of the blood finally to make urine.[3] In kidneys, localized renin–angiotensin system plays crucial role in physiological regulation, but ACE is the major enzyme converting Ang I to Ang II.[4] Increased chymase activity is found and proved that it replaces the function of ACE to convert Ang I to Ang II.[5] Therefore, chymases are also known to convert angiotensin I to angiotensin II and thus play a role in hypertension.[6] One of functions of chymase is thought to be involved in the degradation of ECM by activation of matrix metalloproteases-9 (MMP-9) or matrix metalloproteases-2 (MMP-2). Renal fibrosis is usually the final outcome of CKD, which is related to the disorder of ECM due to the imbalance of MMP-2 and MMP-9 activity.[7] The major MMPs in kidney are gelatinases (MMP-2 and MMP-9) that cleave denatured collagen. In normal human kidneys, chymase expression are faint in glomeruli and vascular smooth muscle cells of normal kidneys.[8] Aristolochic acid I (AAI) is a rapidly progressive interstitial nephritis associated with the intake of Chinese herbs.[9,10,11]
Methods
Animals
All experiments were carried out in accordance with institutional guidelines and the approval of the Institutional Animal Care and Use Committee. Male C57BL/6 mice were purchased from National Laboratory Animal Center (NLAC, Taipei, Taiwan), and ACE knockout mice were established and raised at the Laboratory Animal Center. The number of mice used in this study have been evaluated and minimized with best efforts based on the DNA genotyping and sex. The ACE KO mice of hemizygous and homozygous mutants were used in this study. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC), Fooyin University (107-RDH-002). The principles outlined in the Declaration of Helsinki should be followed (World Medical Association)
AAI-induced CKD in ACE KO mice
ACE KO mice of hemizygous mutants (seven- to eight-week old, 20–25 g) were used to establish CKD mice model. Mice were divided into two groups (n = 5 in each group) according to the disease development on the AAI treatment. The AAI groups were treated with a dose of 10 mg/kg/3 days AAI via I.P. injection in period of seven weeks and sacrificed after another two and four weeks of disease development. The sham groups were only treated with pH 7.4 PBS and sacrificed on the eight week.
Serum collection
Mice were sacrificed after injection of anesthesia (avertin 250 mg/kg), and blood were collected by direct cardiac puncture. The blood samples without adding heparin were then centrifuged (3,000 rpm, 15 min at 4°C) to acquire serum (supernatants). The kidney were excised after perfusion with 0.9% NaCl solution and kept on ice for further steps.
Biochemical analysis
Mice body weights were recorded. Mice urine was collected weekly by home-made metabolism cage for 24 h and the urine samples were store at −20°C after centrifugation (3,000 rpm, 15 min). To evaluate the mice renal function, the levels of creatinine, albumin, and blood urea nitrogen in urine and serum were measured by using Fujifilm clinical chemical analyzer.
Gelatin zymography assay
The gelatin zymography was used to detect the MMP-2 and MMP-9 activities with gelatin-containing gels. The procedure was reported in our previous research. The amount of 10-μg proteins was mixed with 2 × zymogram sample buffer (0.125 M Tris-HCl, pH 6.8, 20% (v/v) glycerol, 4% (w/v) SDS and 0.005% bromophenol blue). Samples were incubated for 10 min at room temperature and then loaded into the SDS-PAGE prepared with 10% acrylamide gels containing 0.1% gelatin (Sigma-Aldrich, St. Louis, MO, USA). The electrophoresis was run under the power supply of 100 V, and following electrophoresis, the gel was washed twice for 30 min in zymogram renaturing buffer (2.5% Triton X-100) with slight shake at room temperature to remove SDS. Then, the gel was incubated for 18 h at 37°C in reaction buffer (50 mM Tris-HCl, pH 7.4, 200 mM NaCl, 5 mM CaCl2). After incubation, the gel was stained with Coomassie blue for 30 min and destained with destain buffer (50% methanol, 10% acetic acid) for 12 h. The activities of MMP-2 and MMP-9 could be observed on the gels as clear zones or unstained zones. The bands were quantified by Scion Image software (NIH, Bethesda, MD, USA) based on the area of bands hydrolyzed by gelatinase.
ACE and chymase activity assay
ACE activity was measured by the commercial fluorogenic peptide substrate Mca-YVADAPK (Dnp)-OH (AnaSpec, San Jose, CA, USA). The assay was carried out in a micro-quartz cuvette containing 10-μL kidney tissue homogenous extract and 33-μM fluorogenic peptide substrate and protease inhibitor (1:150; Sigma-Aldrich) in a final volume of 300 μL filled up with ACE assay buffer. The specific inhibitor of ACE used in this study was captopril (Sigma-Aldrich). The samples were incubated at 37°C for 1 h and then the fluorescence signals were recorded at an excitation wavelength of 320 nm and an emission wavelength of 405 nm by a fluorescence spectrophotometer (Hitachi F-2700). Chymase activity was detected by the AuNPs-peptide probe developed by our laboratory (FITC-Acp-DRVYIHPFHLDDDDDC-AuNPs). In a micro-quartz cuvette, a total volume of 250 μL including AuNPs-peptide probe (125 μL), reactive buffer (pH 8 TTC buffer), and kidney tissue homogenous extract was incubated at 37°C for 15 min. The fluorescence intensity was recorded and analyzed at 515 nm with excitation wavelength of 495 nm by a fluorescence spectrophotometer.
Histological determination
Kidneys were collected after sacrifice and fixed in 10% formalin solution, and then embedded in paraffin for sectioning. Sections were stained with hematoxylin and eosin (H and E) and Masson's trichrome staining using standard pathology procedures, and photographed by a digital camera mounted on a microscope. All sections were observed using a computerized microscope equipped with a high-resolution video camera (BX 51, Olympus, Tokyo, Japan).
Statistical analysis
All values were expressed as the mean ± standard deviation (SD). Statistical significance used for one-way analysis of variance (ANOVA) evaluating differences among multiple weeks compared with beginning week (0 week) and treated AAI. Statistical significance between two samples was assessed using one-way analysis of variance (ANOVA) by Student's t-test when the data belong to more than two groups. Once the P value was less than 0.05 or 0.01, the differences were considered statistical significantly compared with treated AAI or wild-type (WT).
Results
Histology of kidney tissue sections after AAI treatment at two and four weeks in ACE KO mice by H and E staining and masson's trichrome staining
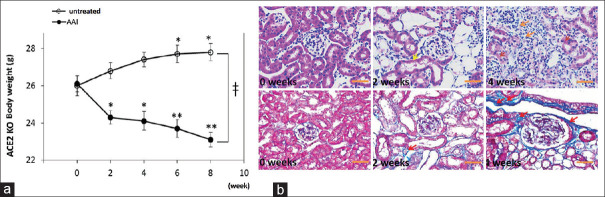
The body weight of WT and ACE KO mice are recorded once every two weeks. We found that the ACE KO mice without treated with AAI steady lose weight at the first two weeks, the lowers body weight on six and eight weeks (P < 0.001). However, WT body weights were increased after six and eight weeks [Figure 1]. However, compared with WT and ACE KO mice after eight weeks fed, we found that they have significant difference. The kidney tissue sections are stained with H and E to observe the white blood cells infiltration in ACE KO mice at four weeks after AAI treatment. The sections are from ACE KO mice treated with 10 mg/kg/3 days AAI for two and four weeks and then sacrificed after another two and four weeks. Compared with the without treated group, the AAI groups showed severe white blood cells infiltration in renal interstitium and the thicker vessel wall [Figure 1a]. On the other hand, the kidney fibrosis tissue sections were also observed stained with Masson's trichrome. Compared with the without AAI treated group, the AAI treatment group showed intrarenal fibrosis (blue color) and severe interstitial tissue damage [Figure 1b].
Figure 1.
Hematoxylin–eosin (H and E) and trichrome stained of ACE2 KO mice kidney tissue after the treatment of AAI. (a) The wild-type (WT) mice treated with PBS steady gained weight; however, ACE KO mice after the four-week treatment of AAI lost weight. All values are expressed as the mean ± SD from each group. * and ** indicate P < 0.05 and P < 0.01, respectively, compared with the mice body weight at first day. (b) H and E stained of kidney tissue sections were form ACE KO mice treated with AAI for two and four weeks and sacrificed after another two weeks. Compared with the AAI-untreated group, the AAI group showed severe red blood cells infiltration in AAI groups. The trichrome stained kidney tissue sections were from ACE2 KO mice treated with AAI for two and four weeks and sacrificed after another two weeks. Compared with the AAI-untreated group, the AAI group showed intrarenal fibrosis (red color arrowheads) and severe interstitial tissue damage (blue color tissue)
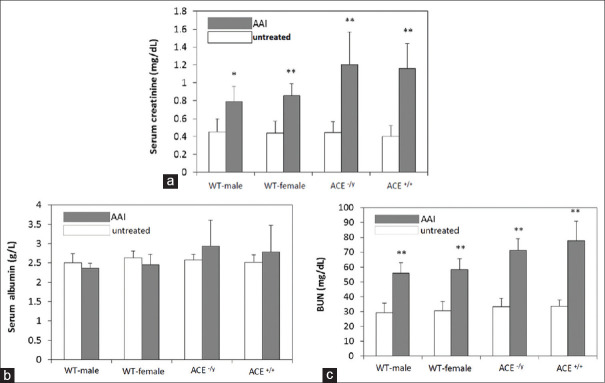
Blood collected samples after two weeks of AAI-treatment induced CKD
After 2 weeks of AAI treatment, mice were sacrificed and blood was collected from them to detect serum creatinine, albumin, and BUN concentration by biochemistry analysis. The serum creatinine was increased after two weeks of AAI treatment in WT mice. Using one-way analysis of variance (ANOVA) following student's t-test, we could find in WT mice that not only the serum creatinine increased in males, but also in females (P < 0.01) after AAI treatment. On the other hand, we also could find the same increases in the ACE KO mice either hemizygous or homozygous (P < 0.01). However, the serum creatinine in ACE KO after AAI group was found higher than that in the WT group [Figure 2a]. On serum albumin detection, we found that there was no significant difference in WT or ACE KO with or without AAI treatment [Figure 2b]. However, the serum BUN was increased in AAI treatment either WT or ACE KO mice (P < 0.01) using one-way analysis of variance (ANOVA) following student's t-test [Figure 2c]. All serum samples are collected from mice which are treated with 10 mg/kg/3 days AAI for four weeks and sacrificed after another two and four weeks.
Figure 2.
Serum biochemical analysis of mice treated with AAI. All serum samples were collected from mice which were treated with 10 mg/kg/3 days AAI for four weeks and sacrificed after another two and four weeks. (a) The serum creatinine in AAI group was found higher than in untreated-AAI group in WT and ACE KO mice. (b) The serum albumin was not found significant change in AAI group compared to that in untreated-AAI group in WT and ACE KO mice. (c) The serum BUN was found significantly increased in AAI group compared with untreated-AAI group. All values are expressed as the mean ± SD from each group. * and ** indicate and P < 0.05 and P < 0.01, respectively, compared with untreated-AAI group
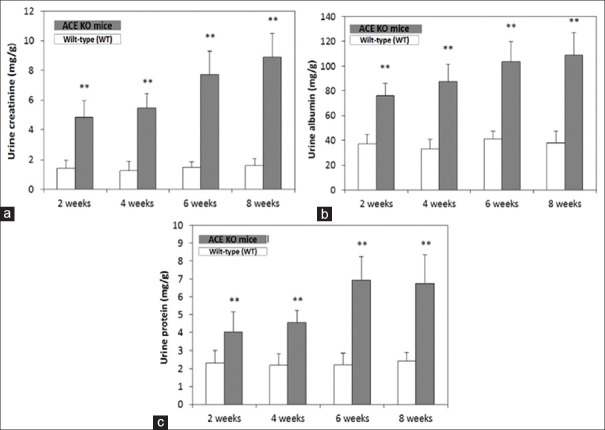
Urine samples analysis in ACE KO mice at two, four, six, and eight weeks by biochemistry analysis
The 24-h urine samples are collected once every two weeks with self-made metabolism cages. The urine creatinine was found increased in ACE KO mice at two, four, six, and eight weeks compared with WT (P < 0.01) [Figure 3a]. Increased urine albumin and protein were observed in ACE KO mice at two, four, six, and eight weeks compared with WT (P < 0.01) [Figure 3b and c].
Figure 3.
Urine biochemical analysis of ACE KO mice untreated with AAI. The ACE KO mice were fed at two, four, six, and eight weeks and sacrificed after another two and four weeks, and the urine samples were collected twice peer weeks with self-made metabolism cages. (a) The urine creatinine was found increased in ACE KO mice at two, four, six, and eight weeks untreated-AAI group. (b) Increased urine albumin was observed in ACE2 KO mice at two, four, six, and eight weeks untreated-AAI group. (c) Remarkably increase in urine protein was also observed in ACE KO mice at two, four, six, and eight weeks untreated-AAI group. All values are expressed as the mean ± SD from each group. ** indicates P < 0.01, compared with the WT group
The kidney tissue chymase and ACE activity in ACE KO mice after two and four weeks of AAI treatment
The kidney tissue chymase activity is detected in ACE KO mice by self-made AuNPs-peptide probe. After two and four weeks treatment of AAI, chymase activity of the delta fluorescence intensity detected by self-made AuNPs-peptide probe was found to increase at four weeks AAI treatment of ACE KO mice, when compared with untreated ones (P < 0.01) [Figure 4a]. However, the kidney tissue ACE activities at two and four weeks of AAI treatment were a little decreased in ACE KO mice [Figure 4b].
Figure 4.
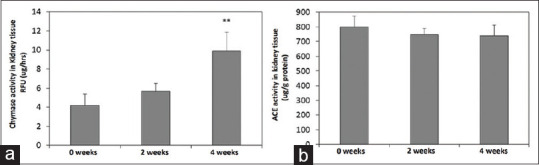
Chymase and ACE activity in kidney tissue isolated from ACE KO mice treated with AAI. The peptide was designed for chymase, the biosensor platform was proven, that it was specific for chymase, and the biochemical analysis showed the kidney injury. Experimental flowchart of synthesis of the application in CKD mice model. (a) After the treatment AAI, the chymase activity of mice kidney tissue was detected by self-made AuNPs-peptide probe. About increase in chymase activity was detected in four-week disease development AAI-treated ACE KO mice compared with without AAI. The mice were treated 10 mg/kg/3 days AAI for two and four weeks in ACE KO mice. (b) The kidney tissue ACE activity decreased after the two- and four-week treatment of AAI in ACE KO mice. All values are expressed as the mean ± SD from each group. ** indicates P < 0.01 compared with ACE KO mice group without treating AAI
The Kidney tissue MMP-2 and MMP-9 activity in ACE KO mice after two and four weeks of AAI treatment by gelatin zymography
The kidney tissues MMP-2 and MMP-9 activities were determined by gelatin zymography. They showed us that MMP-2 activity was increased at two and four weeks of AAI treatment (P < 0.01), compared with the untreated ones [Figure 5a]. However, MMP-9 activity was decreased at two and four weeks (P < 0.01) of AAI treatment [Figure 5b] in ACE KO mice, when compared with untreated AAI ones.
Figure 5.
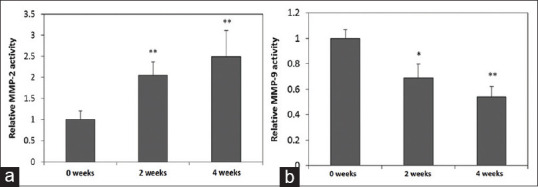
Relative of MMP-2 and-9 activity in kidney tissue isolated from ACE KO mice treated with AAI. (a) The kidney tissue MMP-2 activity was determined by gelatin zymography. About increased MMP-2 was found in the AAI-treated ACE KO mice compared with those detected in without treated. MMP-2 activity increased with the longer disease development. (b) Decreased kidney tissue MMP-9 was found in AAI treated ACE KO mice group compared with those without treated group, and longer disease development showed more decrease in MMP-9 activity. The mice were treated 10 mg/kg/3 days AAI for two and four weeks. All values are expressed as the mean ± SD from each group. * and ** indicate P < 0.05 and P < 0.01, respectively, compared with the ACE KO mice group without treating AAI
Discussion
In this study, we have established an AAI-induced CKD mice model during long-term eight-week experimental designs. The AAI has known to be nephrotoxic usually causes renal fibrosis associated with urothelial carcinoma.[12,13] First, we determined the different range of WT and ACE KO mice in CKD [Figure 1]. According to the results, we determined ACE KO mice beginning body weight reduced on two weeks fed, but we did not make sure what resulted in CKD.[14] Furthermore, we did treatment of AAI to determine CKD-induced. [Figure 2] showed that after the treatment of AAI, the level of serum creatinine and BUN immediately improved; however, serum albumin unchanged. The level of serum BUN is known to associate with other nonrenal events.[15,16] Increased serum creatinine and BUN level reflected the harmful body condition which may result from the treatment of the AAI; otherwise, the serum creatinine level might reflect more closely renal function conditions. Both serum creatinine and BUN were increased after the treatment of AAI, but the serum albumin unchanged showed the failed kidney function and kidney injury in WT and ACE KO mice, either ACE-/y or ACE+/+ [Figure 2].[17] Moreover, we detected creatinine, albumin, and urine protein in ACE KO mice by urinalysis. The kidney disease diagnosis can be analyzed depending on the kidney function by detecting the creatinine in urine to calculate the glomerular filtration rate and on kidney injury using detecting the albumin in urine.[1] In ACE KO mice compared with WT after feeding for two, four, six, and eight weeks, we found that their urine creatinine, urine albumin, and urine protein increased [Figure 3]. Therefore, according to Figures 1 and 3, we could prove kidney damage. An AuNPs-peptide probe is well established for chymase detection, but the peptide is designed to be negatively charged for the sensitivity and stability.[18] The level of chymase protein expression was found significant and interstitial fibrosis was observed. It was found that the mean number of chymase positive has significantly higher in nephrectomy specimens of kidneys than in normal kidneys.[19,20] In our study, the chymase activity expression of kidney tissue increased in long-term four-week treatment [Figure 4a]. This result accorded with Masson's trichrome staining kidney section result, which showed after four-week treatment with AAI, causing fibrosis. However, ACE activity in mice kidney is detected and the decrease in ACE activity is found in the AAI-induced CKD mice model at four weeks [Figure 4b]. It might mean that the generation of Ang II in normal kidney may not be through ACE to convert and which in injury kidney may be through non-ACE pathway to convert. There was another pathway of converting Ang I to Ang II, such as chymase. However, in some kidney diseases, MMP-9 activity was found decreased, such as in AAN.[21,22] In our result, MMP-2 activity of mice kidney in both two- and four-week treatments were increased, but decreased the MMP-9 activity [Figure 5]. Renal interstitial fibrosis was found associated with reduced levels of MMPs and ECM accumulation in some renal disease.[23,24] Because of the AAI, the upper regulation of MMP-9 synthesis is inhibited, causing downregulation of MMP-9 in AAI-induced kidney disease.
Authors contribution
J. P. Wu. contributes to the conception or design of the work. JP Wu analyzed the data for the work. All authors are in agreement to be accountable for all aspects of the work and final approval of the version.
Financial support and sponsorship
This work was supported by grants from Ministry of Science and Technology (MOST 105-2811-B-039-008 and MOST 106-2811-B-650-003).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Li W, Zhang S. Risk factors of parathyroid dysfunction in elderly patients with chronic kidney disease undergoing hemodialysis. Adv Clin Exp Med. 2015;24:1007–12. doi: 10.17219/acem/23439. [DOI] [PubMed] [Google Scholar]
- 2.Kim SM, Kim KM, Kwon SK, Kim HY. Erythropoiesis-stimulating agents and anemia in patients with non-dialytic chronic kidney disease. J Korean Med Sci. 2016;31:55–60. doi: 10.3346/jkms.2016.31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsiao FC, Tung YC, Chou SH, Wu LS, Lin CP, Wang CL, et al. Fixed-dose combinations of renin-angiotensin system inhibitors and calcium channel blockers in the treatment of hypertension: A comparison of angiotensin receptor blockers and angiotensin-converting enzyme inhibitors? Medicine (Baltimore) 2015;94:e2355. doi: 10.1097/MD.0000000000002355. doi: 10.1097/MD.0000000000002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aragão DS, de Andrade MC, Ebihara F, Watanabe IK, Magalhães DC, Juliano MA, et al. Serine proteases as candidates for proteolytic processing of angiotensin-I converting enzyme. Int J Biol Macromol. 2015;72:673–9. doi: 10.1016/j.ijbiomac.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Wang R, Chen J, Zhang Z, Cen Y. Role of chymase in the local renin-angiotensin system in keloids: Inhibition of chymase may be an effective therapeutic approach to treat keloids. Drug Des Devel Ther. 2015;9:4979–88. doi: 10.2147/DDDT.S87842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S, Bivona BJ, Ford SM, Jr, Xu S, Kobori H, de Garavilla L, et al. Direct evidence for intrarenal chymase-dependent angiotensin II formation on the diabetic renal microvasculature. Hypertension. 2013;6:465–71. doi: 10.1161/HYPERTENSIONAHA.111.202424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DI Carlo A. Matrix metalloproteinase-2 and -9 and tissue inhibitor of metalloproteinase-1 and -2 in sera and urine of patients with renal carcinoma. Oncol Lett. 2014;7:621–6. doi: 10.3892/ol.2013.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunel V, Antoine MH, Stévigny C, Nortier J, Duez P. New in vitro insights on a cell death pathway induced by magnolol and honokiol in aristolochic acid tubulotoxicity. Food Chem Toxicol. 2016;87:77–87. doi: 10.1016/j.fct.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Wang K, Feng C, Li C, Yao J, Xie X, Gong L, et al. Baicalin protects mice from aristolochic acid i-induced kidney injury by induction of CYP1A through the aromatic hydrocarbon receptor. Int J Mol Sci. 2015;16:16454–68. doi: 10.3390/ijms160716454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng C, Xie X, Wu M, Li C, Gao M, Liu M, et al. Tanshinone I protects mice from aristolochic acid I-induced kidney injury by induction of CYP1A. Environ Toxicol Pharmacol. 2013;36:850–7. doi: 10.1016/j.etap.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Xing G, Qi X, Chen M, Wu Y, Yao J, Gong L, et al. Comparison of the mutagenicity of aristolochic acid I and aristolochic acid II in the gpt delta transgenic mouse kidney. Mutat Res. 2012;743:52–8. doi: 10.1016/j.mrgentox.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Declèves AÉ, Jadot I, Colombaro V, Martin B, Voisin V, Nortier J, et al. Protective effect of nitric oxide in aristolochic acid-induced toxic acute kidney injury: An old friend with new assets. Exp Physiol. 2016;10:193–206. doi: 10.1113/EP085333. [DOI] [PubMed] [Google Scholar]
- 13.Nortier J, Pozdzik A, Roumeguere T, Vanher weghem JL. [Aristolochic acid nephropathy (“Chinese herb nephropathy”)] Nephrol Ther. 2015;11:574–88. doi: 10.1016/j.nephro.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Zeng Y, Yang X, Wang J, Fan J, Kong Q, Yu X. Aristolochic acid I induced autophagy extenuates cell apoptosis via ERK 1/2 pathway in renal tubular epithelial cells? PLoS One. 2012;7:e30312. doi: 10.1371/journal.pone.0030312. doi: 10.1371/journal.pone. 0030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwak DH, Lee S. Aristolochic Acid I causes testis toxicity by inhibiting Akt and ERK1/2 phosphorylation. Chem Res Toxicol. 2016;29:117–24. doi: 10.1021/acs.chemrestox.5b00467. [DOI] [PubMed] [Google Scholar]
- 16.Wu JP, Ho TJ, Tsai CC, Yeh YL, Lin CC, Lin KH, et al. Hepatoprotective effects of traditional chinese medicine on liver fibrosis from ethanol administration following partial hepatectomy. Chin J Physiol. 2015;58:393–403. doi: 10.4077/CJP.2015.BAD339. [DOI] [PubMed] [Google Scholar]
- 17.Tsai CC, Wu JP, Lin YM, Yeh YL, Ho TJ, Kuo CH, et al. The effect of elephantopus scaber L? on liver regeneration after partial hepatectomy. Evid Based Complement Alternat Med. 2013;2013:369180. doi: 10.1155/2013/369180. doi: 10.1155/2013/369180. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.McDonald JE, Padmanabhan N, Petrie MC, Hillier C, Connell JM, McMurray JJ. Vasoconstrictor effect of the angiotensin-converting enzyme-resistant, chymase-specific substrate [Pro (11) (D)-Ala (12)] angiotensin I in human dorsal hand veins: In vivo demonstration of non-ace production of angiotensin II in humans. Circulation. 2001;104:1805–8. doi: 10.1161/hc4001.097220. [DOI] [PubMed] [Google Scholar]
- 19.Vass DG, Shrestha B, Haylor J, Hughes J, Marson L. Inflammatory lymphangiogenesis in a rat transplant model of interstitial fibrosis and tubular atrophy. Transpl Int. 2012;25:792–800. doi: 10.1111/j.1432-2277.2012.01482.x. [DOI] [PubMed] [Google Scholar]
- 20.Freise C, Kim KY, Querfeld U. A lindera obtusiloba extract blocks calcium-/phosphate-induced transdifferentiation and calcification of vascular smooth muscle cells and interferes with matrix metalloproteinase-2 and metalloproteinase-9 and NF-κB? Evid Based Complement Alternat Med. 2015;2015:679238. doi: 10.1155/2015/679238. doi: 10.1155/2015/679238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bojic S, Kotur-Stevuljevic J, Kalezic N, Stevanovic P, Jelic-Ivanovic Z, Bilanovic D, et al. Diagnostic value of matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 in sepsis-associated acute kidney injury. Tohoku J Exp Med. 2015;237:103–9. doi: 10.1620/tjem.237.103. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Liu J, Wang W, Zhang Z, Li D, Lin K, et al. Matrix metalloproteinase 9 and vasodilator-stimulated phosphoprotein related to acute kidney injury in severe acute pancreatitis rats. Dig Dis Sci. 2015;60:3647–55. doi: 10.1007/s10620-015-3820-8. [DOI] [PubMed] [Google Scholar]
- 23.DuBose JJ, Azizzadeh A. Utilization of a tubularized cormatrix extracellular matrix for repair of an arteriovenous fistula aneurysm. Ann Vasc Surg. 2015;29:366.e1–4. doi: 10.1016/j.avsg.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Bai Y, Lu H, Hu L, Hong D, Ding L, Chen B. Effect of sedum sarmentosum BUNGE extract on aristolochic acid-induced renal tubular epithelial cell injury. J Pharmacol Sci. 2014;124:445–56. doi: 10.1254/jphs.13216fp. [DOI] [PubMed] [Google Scholar]