Abstract
Background:
Subcutaneous adipose tissue (SAT) relative to the other adipose tissues may have different roles in health and insulin resistance. The purpose of this study was to investigate the effectiveness of aerobic exercise on SAT thermogenesis indices, serum orexin-A (OXA), and insulin resistance in high-fat diet-induced obesity male Wistar rats.
Methods:
Thirty-two male Wistar rats with an average weight of 180–200 g were randomly assigned into 4 equal groups: normal fat diet (NFD), high-fat diet obesity (HFDO), normal fat diet after high-fat diet obesity (HFDO-NFD), and aerobic exercise group with normal fat diet after high-fat diet obesity (HFDO-AEX). Fasting levels of serum OXA, insulin, FBS, high-density lipoproteins, low-density lipoproteins, cholesterol and gene expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and UCP1 in SAT were evaluated. Samples were taken in the HFDO group after obesity-induced and in other groups 48 h after 8 weeks of aerobic exercise.
Results:
The results showed that HFD significantly decreased serum levels of OXA, HDL-c and gene expression of PGC1α and UCP1 in SAT. In addition, it caused a significant increase in Lee index, FBS, insulin resistance, and serum lipid profile in comparison with the NFD group (P ≤ 0.001). Aerobic exercise significantly modified the changes caused by HFD to the normal levels (P ≤ 0.001).
Conclusions:
These data suggest that aerobic exercise caused an improvement in insulin resistance and blood lipid profiles through an increase in the serum level of OXA and alteration in the SAT phenotype from white to brown or beige.
Keywords: Aerobic exercise, insulin resistance, obesity, orexin A, subcutaneous adipose tissue
Introduction
Obesity is a major public health concern, with social and economic implications, which are associated with increased mortality and morbidity.[1] It has been shown that subcutaneous adipose tissue (SAT) transplantation from exercise subjects to untrained obese rats improves glucose homeostasis and insulin sensitivity and reduces the risk of metabolic diseases and improves blood lipid profile.[2] However, in these years more attention has been paid to the role of muscle contraction and their myokines secreted during exercise. It is believed that there is another type of fat cell in the white adipose tissue (WAT) that causes these effects.[2] These cells, which called “beige,” have thermogenic properties close to brown adipose tissue (BAT) and emerge from WAT after birth and many factors are involved in regulation, differentiation, and proliferation of their thermogenic properties.[3]
It is well described that uncoupling protein 1 (UCP1) as a specific mitochondrial protein in brown and beige adipocytes converts chemical energy into heat without ATP production in the mitochondrial electron transport chain. In this regard, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) as an activator of peroxisome proliferator-activated receptor gamma is an important factor in mitochondrial biogenesis and plays an important role in increasing the thermogenic activity of adipocytes, and PGC-1α also stimulates the expression of UCP1 and other thermogenic compounds.[3,4] In addition, it was shown very recently that one of the most important factors influencing the thermogenic properties of adipose tissues is the orexin (hypocretin) hormones.[5] Orexin is a peptide produced in neurons of the peripheral, lateral, and posterior hypothalamus.[5,6,7] Due to the widespread distribution of orexin neurons in the central nervous system and the presence of large protein G receptors in various tissues, and relatively long half-life of this neuropeptide, it is involved in many physiological functions.[6,8] It has been shown that there is a specific axonal neural network between the central nervous system and the adipose tissue, which suggests the sympathetic regulation of adipose tissues by orexin neurons.[5,7] Moreover, the rapid release of OXA from the blood-brain barrier is an additional effect of this hormone, which influences various tissues.[9] Evidence suggests that obesity reduces the amount of OXA by various mechanisms.[5,6] Following obesity, hyperglycemia and decreased insulin sensitivity of the neurons in the lateral hypothalamus area (LHA) caused reduced expression of pre-pro orexin gene.[6,10] On the other hand, it seems that aerobic exercise with an effect on the orexin system increases the neuronal excitation of these neurons in the LHA and, as described above, can lead to altered thermogenic properties of the SAT. It is also worth noting that most studies about orexin system have almost focused on the effects of this neurohormone on appetite, depression, addiction, and insomnia, and studies about the effectiveness of exercise on the orexin system and its possible changes in thermogenic indices such as PGC-1α and UCP1, especially in SAT are few. Therefore, considering the wide extent of the SAT in the body and its potential role in insulin resistance, as well as the effect of exercise on the orexin system, and the possible change in the SAT phenotype, the aim of this study was to investigate the effectiveness of 8-week aerobic exercise on SAT thermogenesis indices, serum OXA, and insulin resistance in high-fat diet-induced obesity male Wistar rats.
Methods
Thirty-two male Wistar rats weighing 190–200 g were randomly divided into four groups (n = 8). Group 1: Normal fat diet (NFD), Group 2: High-fat diet obesity (HFDO), Group 3: Normal fat diet after high-fat diet obesity (HFDO-NFD), and Group 4: Aerobic exercise with normal fat diet after high-fat diet obesity (HFDO-AEX). This study was carried out in accordance with the ethical codes of working with laboratory animals (in accordance with the Helsinki declaration), under IR.UI.REC.1396.010 code of ethics from the University of Isfahan. Rats were purchased from the Royan Institute of Isfahan and were housed in a facility at 21 to 24°C with 50% humidity, under 12/12 h light/dark cycle (7 am to 19 pm) with free access to water and food.
High-fat diet-induced obesity and normal fat diet
After acclimatization, in order to induce obesity, HFD containing 60% of calorie intake of fat-tailed sheep, 20% carbohydrates, and 20% protein was used for 8 weeks. The diet then changed to a standard normal diet (NFD) until the end of the study.[11] Both of these products were purchased from the Royan Institute of Isfahan.
Assessment of body composition
Lee index was calculated as an indicator of body composition using the following formula.[12]
Lee Index = [weight (gr)0.33 ÷ nasoanal length (mm)] × 103
Exercise protocol
The rats were habituated with the treadmill apparatus to minimize the novelty stress (15 min a day and 20 m/min for 3 days). The aerobic exercise protocol was 5 days a week for 8 weeks. Exercise intensity set based on 60% of the average maximum training capacity followed by a 5-min warm-up period with 30% of the average maximum training capacity for 60 min or until animals reached fatigue. Maximum training capacity assessment was carried out for all rats in the HFDO-AEX group. The animals run on the treadmill as a warm-up at 6 m/min for 5 min and then speed of treadmill increased 3 m/min every 3 min until the point of exhaustion (failure of rats to continue running after 3 electrical stimuli).[13] Maximum speed was recorded as maximum exercise capability (100%) for each animal. All procedures occurred between 9:30 to 11 Am on a 5-line treadmill for the rodent. Animals in non-exercised (sedentary) groups were left on the treadmill for 5 min without running.
Sampling
In HFDO group, samples were taken after obesity-induced and in other groups 48 h after 8 weeks of aerobic exercise. Blood samples were carried out by anesthetizing with ether directly from the heart of animals. For serum isolation, 5 cc of blood was collected in tubes containing CLOT gel and were centrifuged for 5 min at 1200 × g in room temperature and stored at −80°C for future analysis. SAT samples were taken from inguinal adipose tissue.
RNA extraction and gene expression (Real-Time PCR)
RNA isolation from EAT homogenized samples was performed using an RNA extraction kit (EZ-10 spin column total RNA mini preps super kit, by BIO BASIC INC, Canada.) The cDNA synthesis was performed using the Japanese-made TaKaRa cDNA synthesis kits (PrimeScript ™ RT reagent kit Cat. #RR037A). All procedures were carried out according to the manufacturer's protocol, in a PCR CG1-96 by Corbett Corporation in Australia. Measurement of the relative expressions of PGC-1α, UCP1, and GAPDH (as housekeeping gene) was performed using Real-Time PCR BIO-RAD CFX96 and the designed specific primers are shown in Table 1. The relative mRNA expression was calculated according to the comparative cycle threshold (Ct) method, using ΔCT values.
Table 1.
Primer sequences for real time PCR
| Gene | Primer F | Primer R |
|---|---|---|
| UCP 1 | 5′-GTACCCAGCTGTGCAATGAC-3′ | 5′-GATGACGTTCCAGGATCCGA-3′ |
| PGC- 1α | 5′-CGGGATGGCAACTTCAGTAAT-3 | 5′-AAGAGCAAGAAGGCGACACA-3′ |
| GAPDH | 5′- TGCTGGTGCTGAGTATGTCGTG-3′ (F) | 5′- TGCTGACAATCTTGAGGGAGTTG-3′ (R) |
RT-PCR mix for PGC-1α was the following: 2 μl of cDNA, 1 μl of each primer (1 μM final concentration), 10 μl Ampliqon Real Q plus Master Mix Green-high Rox, and 6 μl RNase Free dH2O for a final volume of 20 μl. The RT-PCR protocol was 15 s at 95°C, 30 s at 55°C, and 40 s at 72°C for 40 cycles.
RT-PCR mix for UCP1 was composed as follows: 1 μl of cDNA, 1 μl of each primer (1 μM final concentration), 13-μl Ampliqon Real Q plus Master Mix Green-high Rox, and 10-μl RNase Free dH2O for a final volume of 26 μl. The RT-PCR protocol was 10 s at 95°C and 40 s at 60°C for 40 cycles.
RT-PCR mix for GAPDH was composed as follows: 2 μl of cDNA, 1 μl of each primer (1 μM final concentration), 10-μl Ampliqon Real Q plus Master Mix Green-high Rox, and 6-μl RNase Free dH2O for a final volume of 20 μl. In the RT-PCR protocol the binding temperature for the primer to the target sequence was 55° for PGC-1α and 60° for UCP.
Serum analysis
Serum concentrations of triglyceride (TG), high-density lipoproteins, low-density lipoproteins, and total cholesterol levels were measured using ELISA kits (Pishtaz Teb Zaman Diagnostic). Serum OXA, insulin, and fasting blood sugar were measured according to the protocol of the Rat OXA ELISA Kit (Kit. CSB-E08860), Rat Insulin ELISA Kit (CSB-E05070r), and Rat Glucose-6-phosphate ELISA kit (CSB-EL009118RA), respectively. Insulin resistance was calculated by HOMA-IR equation.[14]
Statistical analysis
The normality of data and homogeneity of variances were checked by Shapiro–Wilk's and Levene's tests. A one-way ANOVA with Tukey post hoc test and Pearson correlation coefficient statistics were used for the statistical analysis of the data. All statistical calculations were performed using Excel version 2016 and SPSS version 24 software, and the significance level was set at α < 0.05.
Results
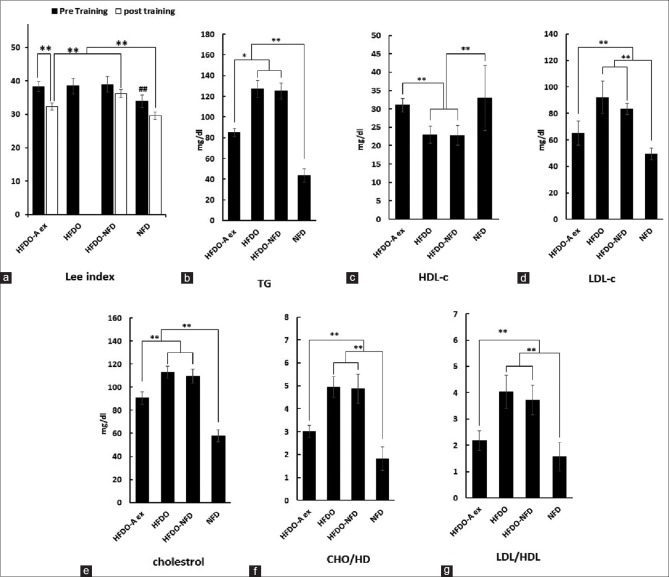
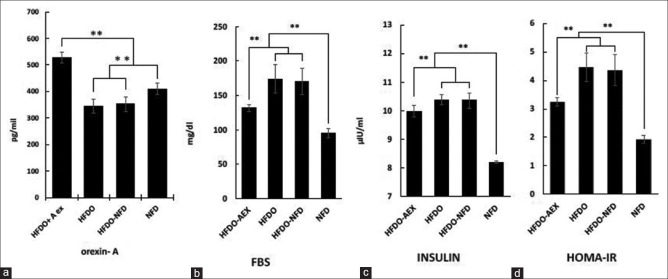
As shown in Figures 1 and 2, there were significant differences between the baseline values of FBS [Figure 2b], insulin resistance [Figure 2c], Lei index [Figure 1a] serum lipid profile contained, total cholesterol [Figure 1e], LDL [Figure 1d], TG [Figure 1b], Cho/HDL [Figure 1f], LDL/HDL [Figure 1g], and HDL [Figure 1c] following HFD compared to NFD protocols (P < 0.001). Serum OXA levels after HFD also decreased significantly compared to the NFD group [Figure 2a] (P < 0.001). After 8 weeks of aerobic exercise, these values significantly modified to the baseline in the HFDO-AEX group (In all cases P = 0.001 except in HDL, P = 0.031). These values did not change significantly in the HFDO-NFD group after the normal diet.
Figure 1.
Some variables alterations in different groups. Lee index (a), predictive cardiovascular disease risk factors; TG (b), HDL-c (c), LDL-c (d), cholesterol (e), CHO/HDL (f), and LDL/HDL (g). (NFD): Normal fat diet, (HFDO): High-fat diet obesity, (HFDO-NFD): Normal fat diet after high-fat diet obesity, and (HFDO-AEX): Aerobic exercise group with normal fat diet after high-fat diet obesity
Figure 2.
Alteration of serum orexin-A (a), fasting blood sugar, FBS (b), insulin (c), insulin resistance, HOMA-IR (d). (NFD): Normal fat diet, (HFDO): High-fat diet obesity, (HFDO-NFD): Normal fat diet after high-fat diet obesity, and (HFDO-AEX): Aerobic exercise group with normal fat diet after high-fat diet obesity
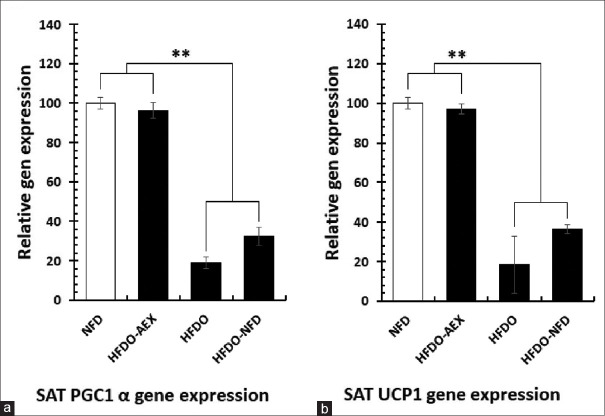
There were also significant differences between relative gene expression of PGC-1α and UCP1 in SAT following high-fat diet-induced obesity in HFDO group (P < 0.001). However, aerobic exercise in the HFDO-AEX group significantly increased the relative gene expression values of both PGC-1α and UCP1 genes [Figure 3a and b] (P < 0.001).
Figure 3.
Relative changes of PGC-1α (a) and UCP1 (b) in subcutaneous adipose tissue. (NFD): Normal fat diet, (HFDO): High-fat diet obesity, (HFDO-NFD): Normal fat diet after high-fat diet obesity, and (HFDO-AEX): Aerobic exercise group with normal fat diet after high-fat diet obesity
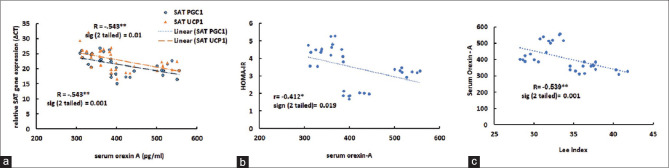
A significant and reversed correlation was observed between the OXA and the level of Lee index [Figure 4c] and HOMA-IR [Figure 4b]. Moreover, the correlation between serum OXA and relative gene expression (ΔCT) of desired genes was significant [Figure 4a].
Figure 4.
Relationship between relative SAT gene expression of UCP1 and PGC-1α with orexin A (a), HOMA-IR with orexin A (b) Lee index with orexin A (c). less Δ CT = more gene expression
Discussion
The main findings of the present study were that following high-fat diet-induced obesity, 8-week aerobic exercise intervention through an increase in the serum level of OXA and alteration in the SAT relative gene expression of PGC-1α and UCP1 caused an improvement in insulin resistance and blood lipid profile. As shown in [Figures 1 and 2], HFD period caused a significant increase in Lee index (23.9%), insulin resistance (56.78%), TG (65.78%), LDL (46.47%), and total cholesterol (48.67%). It is believed that concomitant with obesity, several inflammatory pathways are induced in adipose tissue leading to increased health risks and being linked to various metabolic disorders,[15] and it is well established that adipose tissue as an endocrine organ has wide-reaching effects on other organs, such as brain, and plays a major regulatory role in energy balance and glucose homeostasis.[15] Concomitant with obesity, excess insulin secretion promotes differentiation of preadipocytes to adipocytes and reduces triglyceride breakdown by preventing lipolysis,[16] furthermore in hepatocytes, insulin by stimulating the uptake of lipoprotein-derived fatty acids and glucose promotes lipogenesis.[16]
Consistent with the findings of the present study, a significant increase in blood glucose and insulin resistance after 3 and 8 weeks of high-fat diets containing 45% and 60% fat has also been reported.[17] However, some studies have reported inconsistent results,[18] and it seems that the reason for the discrepancy of insulin response may be related to the ratio of saturated and unsaturated fatty acids used in the diets and their role in glucose-dependent insulin secretion.
In addition, in this study, after HFD period, the relative expression of PGC-1α and UCP1 decreased (81.01 and 81.75%, respectively) and lee index increased significantly. Researchers believe that excessive calorie intake is one of the powerful contributing factors in differentiating preadipocytes into white adipocytes, and alterations in adipose tissue phenotypes are procedures to save and store extra calories.[4]
We have also found that HFD period caused a significant decrease in OXA levels compared to the NFD group (15.8%, [Figure 2a]). A previous report suggested that substantial weight gain may suppress the expression of the prepro-orexin in the LHA,[19] and also changes in blood glucose are negatively correlated with the expression of the gene encoding pre-pro-orexin in the hypothalamus of mice.[20] Another issue is that obesity by affecting cannabinoid receptors in the LHA can suppress orexinergic neurons and reduce the levels of OXA secretion.[21] On the other hand, the orexin system and obesity status are also affected by the exercise.[6,22,23] In this study, consistent with the previous findings,[11,24] after the end of the training period in the HFDO-AEX group, values were significantly modified [Figures 1 and 2]. It seems that aerobic exercise with effect on irisin,[25] lactate,[26] heart's natriuretic peptides,[4] Cannabinoid receptors,[21] CO2,[27] and glucose hemostasis[28] has been affecting the secretion of OXA in the central nervous system. One plausible explanation for this findings is that aerobic exercises are the most potent factors contributing to the improvement of serum OXA,[23] in addition OXA through an Orexin receptor 1/cAMP signaling pathway potentiates glucose-stimulated insulin secretion and decreased the amount of circulating glucose.[29]
On the other hand, orexin can affect insulin resistance by an alteration in the SAT phenotype.[30] Suggested that, the stimulation of beta-adrenergic receptors through the sympathetic stimulation increases the expression of PGC-1α, UCP1, and thermogenesis in SAT.[4,7] In the current study, after the aerobic exercise period, relative gene expression of the thermogenic indices increased significantly [Figure 3]. This finding confirmed the direct and significant correlation between serum OXA and relative gene expression of the SAT thermogenic indices PGC-1α and UCP1 [Figure 4a]. In this regard, contrary to the findings of this study, it has been reported that the injection of OXA in PVH had no effect on the expression of the UCP1 gene,[31] while in a previous study, it was suggested that a 12-week aerobic training in young subjects increased the amount of UCP1 mRNA in the SAT.[2]
Some studies have shown that the elevation of UCP1 gene expression in response to some stimuli such as neurohormones can improve the hydrolysis of the triglycerides stored in the lipid droplets and can decrease the obesity and various metabolic risk factors.[32] In our study, after 8 weeks of aerobic exercise, the values of the blood lipids profile were improved and circulating triglycerides (96.4%), LDL (54.6%), and total cholesterol (38.5%) were decreased significantly [Figure 1]. Although there are various mechanisms through which adipose tissue can contribute to changes in insulin sensitivity,[33] Tsuneki et al. demonstrated a novel role for orexin in hypothalamic insulin signaling, which is likely to be responsible for preventing the development of peripheral insulin resistance with age.[20] Similarly, we found that a period of aerobic exercise caused significant decrease in insulin (4.8%), FBS (44.6%), and HOMA-IR (63.21%). Also it should be taken into account that during exercise the vast peripheral vasodilation and central vasoconstriction (mechanisms for heat loss) causes the transfer of more blood from muscles to SAT.[34] This excess blood flow and associated endocrine factors such as OXA, catecholamine, and other circulating factors may affect SAT more than other adipose tissues depots. Therefore, this could be likely one of the reasons for the greater link between SAT and metabolic processes following physical activity.
Conclusions
In conclusion, according to above findings, it can be suggested that aerobic exercise and its potential impact on the orexin neurons might be one of the practical ways to induce alterations in adipose tissue phenotype from white to brown or beige. Therefore, our recommendation is to focus on increasing the thermogenic capacity of SAT, following aerobic exercise with different volume and intensities to reduce insulin resistance and metabolic disorders in obese patients, in future studies.
Ethical approval
The study protocol conformed to the Declaration of Helsinki and was approved by the Ethical Committee supervising procedures on experimental animals at University of Isfahan (Ethical code IR.UI.REC.1396.10).
Financial support and sponsorship
This work was supported by the vice dean for research and technology of the University of Isfahan for doctoral students.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This work was supported by the vice dean for research and technology of the University of Isfahan for doctoral students. Therefore, it is my duty to express my gratitude for his unconditional support.
References
- 1.Meldrum DR, Morris MA, Gambone JC. Obesity pandemic: Causes, consequences, and solutions—but do we have the will? Fertil Steril. 2017;107:833–9. doi: 10.1016/j.fertnstert.2017.02.104. [DOI] [PubMed] [Google Scholar]
- 2.Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes. 2015;64:2002–14. doi: 10.2337/db14-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inagaki T, Sakai J, Kajimura S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat Rev Mol Cell Biol. 2016;17:480–95. doi: 10.1038/nrm.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harms M, Seale P. Brown and beige fat: Development, function and therapeutic potential. Nat Med. 2013;19:1252–63. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 5.Messina G, Monda V, Moscatelli F. Role of orexin system in obesity. Biol Med. 2015;07:248–54. [Google Scholar]
- 6.Chieffi S, Carotenuto M, Monda V, Valenzano A, Villano I, Precenzano F, et al. Orexin system: The key for a healthy life. Front Physiol. 2017;8:357–66. doi: 10.3389/fphys.2017.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Leighton CE, Billington CJ, Kotz CM. Orexin modulation of adipose tissue. Biochim Biophys Acta. 2014;1842:440–5. doi: 10.1016/j.bbadis.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Ehrstrom M, Naslund E, Levin F, Kaur R, Kirchgessner AL, Theodorsson E, et al. Pharmacokinetic profile of orexin A and effects on plasma insulin and glucagon in the rat. Regul Pept. 2004;119:209–12. doi: 10.1016/j.regpep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Kastin AJ, Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther. 1999;289:219–23. [PubMed] [Google Scholar]
- 10.Zink AN, Bunney PE, Holm AA, Billington CJ, Kotz CM. Neuromodulation of orexin neurons reduces diet-induced adiposity. Int J Obes (Lond) 2018;42:737–45. doi: 10.1038/ijo.2017.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gollisch KS, Brandauer J, Jessen N, Toyoda T, Nayer A, Hirshman MF, et al. Effects of exercise training on subcutaneous and visceral adipose tissue in normal-and high-fat diet-fed rats. Am J Physiol Endocrinol Metab. 2009;297:E495–504. doi: 10.1152/ajpendo.90424.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novelli E, Diniz Y, Galhardi C, Ebaid G, Rodrigues H, Mani F, et al. Anthropometrical parameters and markers of obesity in rats. Lab Anim. 2007;41:111–9. doi: 10.1258/002367707779399518. [DOI] [PubMed] [Google Scholar]
- 13.Caponi PW, Lehnen AM, Pinto GH, Borges J, Markoski M, Machado UF, et al. Aerobic exercise training induces metabolic benefits in rats with metabolic syndrome independent of dietary changes. Clinics. 2013;68:1010–7. doi: 10.6061/clinics/2013(07)20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antunes LC, Elkfury JL, Jornada MN, Foletto KC, Bertoluci MC. Validation of HOMA-IR in a model of insulin-resistance induced by a high-fat diet in Wistar rats. Arch Endocrinol Metab. 2016;60:138–42. doi: 10.1590/2359-3997000000169. [DOI] [PubMed] [Google Scholar]
- 15.Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220:T47–59. doi: 10.1530/JOE-13-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–81. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ickin Gulen M, Guven Bagla A, Yavuz O, Hismiogullari A. Histopathological changes in rat pancreas and skeletal muscle associated with high fat diet induced insulin resistance. Biotech Histochem. 2015;90:495–505. doi: 10.3109/10520295.2015.1021380. [DOI] [PubMed] [Google Scholar]
- 18.Suk M, Shin Y. Effect of high-intensity exercise and high-fat diet on lipid metabolism in the liver of rats. J Exerc Nutrition Biochem. 2015;19:289–95. doi: 10.5717/jenb.2015.15122303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Ueta Y, Date Y, Nakazato M, Hara Y, Serino R, et al. Down regulation of the prepro-orexin gene expression in genetically obese mice. Brain Res Mol Brain Res. 1999;65:14–22. doi: 10.1016/s0169-328x(98)00320-9. [DOI] [PubMed] [Google Scholar]
- 20.Tsuneki H, Murata S, Anzawa Y, Soeda Y, Tokai E, Wada T, et al. Age-related insulin resistance in hypothalamus and peripheral tissues of orexin knockout mice. Diabetologia. 2008;51:657–67. doi: 10.1007/s00125-008-0929-8. [DOI] [PubMed] [Google Scholar]
- 21.Flores Á, Maldonado R, Berrendero F. Cannabinoid-hypocretin cross-talk in the central nervous system: What we know so far. Front Neurosci. 2013;7:1–17. doi: 10.3389/fnins.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chieffi S, Messina G, Villano I, Messina A, Esposito M, Monda V, et al. Exercise influence on hippocampal function: Possible involvement of orexin-A. Front Physiol. 2017;8:1–8. doi: 10.3389/fphys.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messina G, Di Bernardo G, Viggiano A, De Luca V, Monda V, Messina A, et al. Exercise increases the level of plasma orexin A in humans. J Basic Clin Physiol Pharmacol. 2016;27:611–6. doi: 10.1515/jbcpp-2015-0133. [DOI] [PubMed] [Google Scholar]
- 24.Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, et al. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–6. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrante C, Orlando G, Recinella L, Leone S, Chiavaroli A, Di Nisio C, et al. Central inhibitory effects on feeding induced by the adipo-myokine irisin. Eur J Pharmacol. 2016;791:389–94. doi: 10.1016/j.ejphar.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Hao YY, Yuan HW, Fang PH, Zhang Y, Liao YX, Shen C, et al. Plasma orexin-A level associated with physical activity in obese people. Eat Weight Disord. 2016;19:69–77. doi: 10.1007/s40519-016-0271-y. [DOI] [PubMed] [Google Scholar]
- 27.Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–90. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alizadeh A A, Rahmani-Nia F, Mohebbi H, Zakerkish M. Acute aerobic exercise and plasma levels of orexin A, insulin, glucose, and insulin resistance in males with type 2 diabetes. Jundishapur J Health Sci. 2016;8:15–19. [Google Scholar]
- 29.Park JH, Shim HM, Na AY, Bae JH, Im SS, Song DK. Orexin A regulates plasma insulin and leptin levels in a time-dependent manner following a glucose load in mice. Diabetologia. 2015;58:1542–50. doi: 10.1007/s00125-015-3573-0. [DOI] [PubMed] [Google Scholar]
- 30.Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, et al. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–49. doi: 10.1111/febs.12619. [DOI] [PubMed] [Google Scholar]
- 31.Russell S, Small C, Sunter D, Morgan I, Dakin C, Cohen M, et al. Chronic intraparaventricular nuclear administration of orexin A in male rats does not alter thyroid axis or uncoupling protein-1 in brown adipose tissue. Regul Pept. 2002;104:61–8. doi: 10.1016/s0167-0115(01)00349-4. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira BA, Pinhel MA, Nicoletti CF, Oliveira CC, Quinhoneiro DC, Noronha NY, et al. UCP1 and UCP3 expression is associated with lipid and carbohydrate oxidation and body composition. PLoS One. 2016;11:e0150811. doi: 10.1371/journal.pone.0150811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen Y, Zhao Y, Zheng D, Chang X, Ju S, Guo L. Effects of orexin A on GLUT4 expression and lipid content via MAPK signaling in 3T3-L1 adipocytes. J Steroid Biochem Mol Biol. 2013;138:376–83. doi: 10.1016/j.jsbmb.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Farrell PA, Joyner M, Caiozzo V. ACSM's advanced exercise physiology: Wolters Kluwer Health Adis (ESP) 2011 [Google Scholar]