Abstract
Background:
A low level of vitamin B6 may theoretically cause symptoms of depression.
Aims:
To investigate the effect of vitamin B6 on the prevention of postpartum depression (PPD) among mothers at risk for PPD.
Methods:
This single-blind, placebo-controlled clinical trial was conducted on 81 pregnant women who were at risk of PPD from February to July 2016 at six selected health centers in Isfahan, Iran. A simple random sampling method was adopted. Forty cases and 41 controls received 80 mg vitamin B6 and placebo, respectively from the 28th week until the end of pregnancy. The risk of PPD was assessed as the main inclusion criteria using a structured clinical interview using hospital anxiety-depressive scale (HADS), social support appraisals scale (SS-A), and Holmes and Rahe life change and stress evaluation questionnaire (HRLCSEQ). The Edinburgh postpartum depression scale (EPDS) was used to assess the rate of depression prior to and 1.5 months after the intervention (end of pregnancy). Data were analyzed using SPSS 20 and statistical tests (Chi-square, independent t-test, Mann-Whitney's, and Exact Fisher Test).
Results:
Forty-three subjects were assigned to each group and the final analysis comprised 81 subjects (40 in the case and 41 in the control groups), the mean age of the case and control groups being 5.8 ± 29.6 and 4.6 ± 28.2, respectively. The mean depression score was 10.4 ± 1.4 in the case and 9.3 ± 4.2 in control groups (P = 0.34) before and 4.2 ± 2.7 in the case and 10.4 ± 3.4 in control groups (P < 0.001) after intervention.
Conclusions:
Vitamin B6 has a positive effect on reducing postpartum depression scores among mothers at risk for PPD. These may be clinically useful for preventing PPD in high-risk women.
Keywords: Clinical trial, depression, postpartum, primary care, vitamin B6 supplementation
Introduction
Despite some debate regarding the definition of postpartum depression (PPD), the revised version of the Diagnostic and Statistical Manual of Mental Disorders V defined PPD as a major depressive episode that occurs within 4 weeks after delivery.[1] However, the incidence of mood disorder dramatically increases after delivery; the first 90 days postpartum being associated with the highest risk of PPD that persists for 2 years after delivery.[2] The prevalence rate of PPD is reported as about 13%.[3]
The main symptoms of PPD are mood changes, sleep and appetite disorders, fatigue, concentration reduction, feelings of guilt, and an overall reduction in daily activities.[4] The afflicted mother cannot play its maternal and marital roles leading to family problems, as well as distorted child growth and development, and if this disorder is not treated it may result in suicide or infanticide.[5]
The PPD must be differentiated from baby blues that are characterized by over crying, excitability, wakefulness, and the emotional reactions of the mother.[6] Since this condition of sadness and PPD is not known by 80% of mothers, they do not report it to physicians.[7] The main risk factors for PPD include former psychiatric disorders, marital conflict, antenatal anxiety and depression, lack of social support, as well as recent stressful life events.[8]
There is a hypothesis regarding the association between serum vitamin B6 concentration and depression symptoms.[9] According to one study, plasma pyridoxal 5′-phosphate concentrations were significantly reduced in the second and third trimesters of pregnancy and returned to the first trimester levels 1 month after delivery.[10]
Shrimp reported using a high dose of vitamin B6 in the first trimester demonstrated the absence of side effects.[11] In this study, the dose of vitamin B6 used was similar to that used for the treatment of nausea or vomiting during the first trimester that was prescribed 25 mg three times a day.[12] Vitamin B6 is considered a cofactor in the tryptophan/serotonin pathway and its active metabolite form in plasma pyridoxal 5′-phosphate has an inverse association with depression symptoms.[13,14]
In several studies, a significant correlation was observed between vitamin B6 intake and depression symptoms.[15,16,17,18] The observed depletion in plasma concentration of pyridoxal 5′-phosphate in oral contraceptive users may reflect decreased body reserves of vitamin B6.[19] In a systematic review, the use of vitamin B6 was suggested as a complementary treatment for depression.[20]
To date, the role of vitamin B6 has only been confirmed in the treatment of premenstrual dysphoric disorder.[21] Several cross-sectional studies have been conducted considering the correlation between depression and vitamin B6. The present single-blind, placebo-controlled clinical trial was conducted to examine the preventive role of vitamin B6 when prescribed in the third trimester of pregnancy for cases at risk of PPD.
Methods
This single-blind, placebo-controlled clinical trial was approved by the Ethics Committee of Isfahan University of Medical Sciences and received the approval code of the Iranian Registry of Clinical Trials (IRCT2016091929873N1). The main eligibility was being a pregnant woman in the third trimester of pregnancy with reasonable physical health and a lack of major depressive disorder. The inclusion criterion was having at least one risk factor for PPD (history of psychiatric disorders, marital relationship, antenatal anxiety and depression, absence of depression and clinical anxiety in present pregnancy, lack of social support, recent stressful life events, as well as an unplanned pregnancy).[22] This inclusion was based on a structured interview using a checklist of major PPD predictors, hospital anxiety and depression Scale (HADS), social support appraisals scale (SS-A), as well as Holmes and Rahe life change and stress evaluation questionnaire (HRLCSEQ).
The exclusion criteria were developing major depression or severe anxiety after being assigned to the study for which medication or psychotherapy was needed. Moreover, pregnant women who were unwilling to continue with the study were excluded. To perform this study, an official recommendation letter was obtained from the Research Deputy of Isfahan University of Medical Sciences. Later, the researchers referred to the midwifery wards of six randomly selected health centers.
The participants were selected using a simple random sampling technique according to the inclusion criteria and were informed about the purpose and method of the study. The subjects were assured that the information would remain confidential. The consent forms were obtained and participants could withdraw at any stage of the project.
The case group received two 40 mg pills of vitamin B6 daily from the 28th week to the end of pregnancy and then one 40 mg pill of vitamin B6 for 1 month after delivery. The control group received two placebo pills daily from the 28th week to the end of pregnancy and then one placebo pill for 1 month after delivery. The placebo pill contains starch with a similar shape to the vitamin B6 pill. Every 2 weeks, the researcher calls on the subjects to remind them of taking pills. All participants who were reluctant to continue or stop eating the pills were excluded from the study.
According to the possible delivery date, they were reminded of phone calls to continue taking vitamin B6 and placebo one pill per day after childbirth. The follow-up was set for 1.5 months after childbirth concurrently with the first postpartum routine care session in the health centers. Moreover, the PPD questionnaires were completed by the participants. Out of 140 eligible pregnant women, 134 completed the baseline survey questionnaires (i.e., HADS, SS-A, and HRLCSEQ) and had a structured interview for the assessment of PPD risk factors (as mentioned in inclusion criteria).
In addition, a complete psychiatric, medical, and midwifery history, complete physical exams, as well as routine blood tests, including a complete blood count, thyroid-stimulating hormone, blood urea nitrogen, creatinine, urinalysis, hemoglobin, and fasting blood sugar tests were to be conducted. The Edinburgh postnatal depression scale (EPDS) was used to assess depression rates prior to and 1.5 months after the last intervention (day of pregnancy) as the primary outcome measure.
The HADS is a self-administered scale consisting of 14 items with anxiety and depression subscales. This instrument is rated on a four-point Likert scale. To reduce the risk of a false positive bias, the scale does not assess the symptoms of anxiety and depression associated with physical disorder, such as fatigue and insomnia. The HADS has been shown to have adequate diagnostic accuracy. In a recent meta-analysis including diagnostic accuracy tests, it was reported that the HADS depression scale had 82% sensitivity and 74% specificity for detecting major depressive disorder with a score of 8 or more as a cutoff value.
In addition, the anxiety scale had a sensitivity of 78% and a specificity of 74% for detecting generalized anxiety disorder.[23] In a psychometric study conducted by Montazeri et al. concerning internal consistency using Cronbach's alpha coefficient, it was reported that the Iranian version of HADS was considered acceptable. Cronbach's alpha coefficients were obtained as 0.78 and 0.86 for the HADS anxiety and depression subscales, respectively. The validity using known group comparison analysis showed satisfactory results. The cutoff level for detecting depression among the Iranian population was determined as 11.[24]
The SS-A is a 23-item questionnaire, including three domains of family, friends, and others.[25] A Persian translation of SS-A was used in the present study.[26] The correlation of the subscales scores with the total score was used to calculate the reliability of this questionnaire. Correlation coefficients were obtained as 0.76, 0.55, and 0.74 for the total social support score in the family, friends, and other subscales, respectively. P value was considered statistically significant in all subscales (P = 0.0001). The reliability (or internal consistency) of this instrument was confirmed by a Cronbach's alpha coefficient of 0.77. The cutoff of level 11 was proposed for social support screening.[26]
The HRLCSEQ consists of 41 items with a score for any item. The minimum and maximum scores for one item are 11 and 100, respectively. Maybe a participant had many items. The criterion for the study participants was the total score of more than 150. The subjects with a total score higher than 300 were referred to a psychiatrist. Using Cronbach's alpha coefficient and split-half techniques, the reliability coefficient for this tool was calculated as 0.72 and 0.64, respectively, it shows reasonable reliability for the questionnaire.[27]
The EPDS is among the standard instruments with a sensitivity of 65%, a specificity of 76.5%, a positive predictive value of 62.22%, and a negative predictive value of 79.28%. Kheirabadi et al. stated that this tool is useful for Iranian women. According to the aforementioned study, maximum sensitivity and maximum specificities are 78% and 75%, respectively. Depression threshold is considered 13 for Iranian women. This tool can be used in prenatal and postpartum periods.[28]
The sample size was estimated as 39 for each group according to the sample size calculation formula for the comparison of the two communities, considering a 95% confidence interval, 80% power, and 20% sample attrition.[29] The women who met the inclusion criteria were assigned to case and control groups using a simple random sampling technique, assigning each sample into intervention and control groups alternately.
All subjects were blinded to their group assignment and completed EPDS to assess the baseline depression score. Three subjects in the case group and two subjects in the control group were excluded according to their own requirements. All cases that remained until the end of the EPDS study were completed 6 weeks after delivery. The final analysis was performed on 81 participants.
Among the cases, 10 women were excluded and referred to a psychiatrist for probable major depression. In this study, 86 pregnant women who were diagnosed at risk of PPD from February to July 2016 participated [Diagram 1].
Diagram 1.
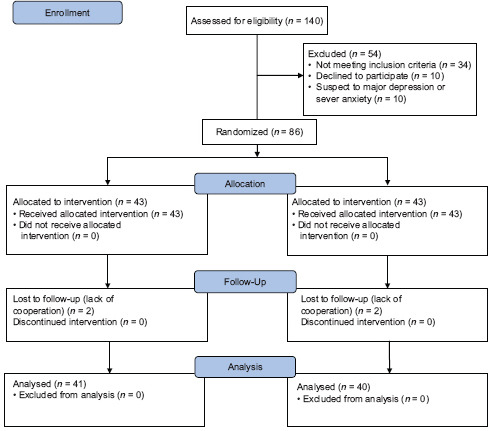
CONSORT 2010 Flow Diagram
Data analysis was performed using SPSS software (version 20) and statistical tests, namely Chi-square (occupation, income, unwanted pregnancy, and marriage relationship rating), independent t-test (age, body mass index, and child number scoring, and PPD scoring), Mann-Whitney U test (educational rating), Fisher's exact test (formerly psychiatric disorder), and covariance analysis (ANCOVA) (for variable control).
Results
Forty-three subjects were randomly assigned to each group, 40 and 41 subjects in the case and control groups, respectively were cooperated to the end of the study and analyzed for the primary outcome. The two groups were not significantly different in terms of demographic characteristics and midwifery history [Table 1].
Table 1.
Comparison of demographic variables between the case and control groups
| Variable | Case Group | Control Group | P | Test | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean | Standard deviation | Mean | Standard deviation | |||
| Age | 28.2 | 4.6 | 29.6 | 5.8 | 0.23 | Independent t-test |
| Husband’s Age | 33.2 | 5.2 | 33.3 | 5.2 | 0.93 | |
| BMI | 25.1 | 3.04 | 24.9 | 2.7 | 0.80 | |
| Pregnancy Number | 2 | 1.1 | 1.9 | 0,8 | 0.81 | |
| Child Number | 1.5 | 0.9 | 1.2 | 0.4 | 0.12 | |
|
| ||||||
| Variable | Number | % | Number | % | P | |
|
| ||||||
| Education | ||||||
| Below High School | 9 | 24.3 | 15 | 36.5 | 0.36 | Mann-Whitney test |
| High School | 19 | 51.4 | 17 | 41.5 | ||
| University | 9 | 24.3 | 9 | 22 | ||
| Job | ||||||
| Housewife | 32 | 82.1 | 36 | 92.3 | 0.18 | Chi-square test |
| Working | 7 | 17.9 | 3 | 7.7 | ||
| Income | ||||||
| Acceptable | 31 | 83.8 | 31 | 77.5 | 0.49 | |
| Unacceptable | 6 | 16.2 | 9 | 22.5 | ||
According to Table 2, the distribution of risk factors for PPD was the same in both groups.
Table 2.
Comparison of postpartum depression risk factors between case and control groups
| Variable | Case Group | Control group | P | Test | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Number | % | Number | % | |||
| Former Psychic Disorders | 2 | 12.5 | 2 | 4.9 | 0.02 | Fischer exact test |
| Weak Marriage Relationship | 11 | 28.2 | 8 | 20 | 0.39 | Chi-square test |
| Unwanted Pregnancy | 12 | 30 | 11 | 27.5 | 0.80 | |
|
| ||||||
| Mean | Standard Deviation | Mean | Standard Deviation | P | t-test | |
|
| ||||||
| Anxiety | 7.6 | 3.2 | 8.3 | 3 | 0.35 | |
| Social Support | 17 | 3 | 16.4 | 3.8 | 0.43 | |
| Stress | 162.1 | 60.8 | 162.3 | 80.8 | 0.99 | |
| Depression | 5.1 | 2.8 | 5.9 | 2.9 | 0.23 | |
Independent t-test results showed that there was no significant difference between the two groups regarding the mean score of pre-intervention depression (P = 0.34).
According to paired t-test results, it was found that the mean score of depression in the post-intervention group was significantly lower than that of the pre-intervention group (P < 0.001). In the control group, the mean score of depression was not significantly different pre- and post-intervention (P > 0.05) [Table 3]. No reportable complications were observed in both groups.
Table 3.
Depression score before and after the intervention within and between the two groups
| Depression Score | Case Group | Control Group | Between groups* | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Mean | Standard Deviation | Mean | Standard Deviation | P | t | |
| Before Intervention | 10.1 | 1.4 | 9.3 | 4.2 | 0.34 | 0.96 |
| After Intervention | 4.2 | 2.7 | 10.4 | 3.4 | <0.001 | 7.74 |
| Within groups** | P<0.001, t=12.71 | P=0.10, t=1.69 | ||||
*Independent t-test. **Paired t-test
Discussion
In this study, there was no significant difference between the two groups in terms of depression mean pre-intervention score; however, the mean score of the case group was significantly lower than the control group post-intervention.
No significant differences were observed regarding PPD risk factors between the case and control groups so that a significant difference in the post-intervention depression score could be related to the exclusive intervention effect. According to the obtained results considering the significant reduction of PPD score in the intervention group, vitamin B6 had a positive effect on decreasing PPD score which is consistent with the findings of the following studies on the effect of vitamin B6 on depression symptoms.
In a study conducted by Hvas et al. an inverse association was observed between vitamin B6 levels and depression symptoms.[13] Nanri stated that a higher vitamin B6 level may be associated with a lower risk of depression symptoms in Japanese subjects.[30] Furthermore, a significant relationship was suggested between vitamin B6 with depressive symptomatology in Massachusetts elders.[14]
In a study conducted by Murakami, it was observed that higher intake of dietary B vitamins, particularly folic acid and vitamin B6, were independently associated with a lower prevalence of depressive symptoms in early adolescence.[16] In another study, there was a longitudinal correlation of vitamin B6, folic acid, and vitamin B12 with depression symptoms among older adults.[31]
There are some studies of the results which are inconsistent with the findings of the present study in this regard. Miyake et al. reported a nonsignificant association between pyridoxine intake and PPD risk.[32] Personal and family psychiatric history and sociocultural factors were not controlled in this study and these may be the source of confounding factors.
Sanchez reported no significant relationship between vitamin B6/B12 intake and depression.[18] In a clinical trial conducted by Valentina, no beneficial effects of long-term low-dose supplementation with B vitamins or omega-3 fatty acids on depression symptoms were shown in cardiovascular disease survivors.[33]
The metabolite of vitamin B6 pyridoxal 5′-phosphate is a cofactor in the tryptophan/serotonin pathway and the synthesis of serotonin and other catecholamines is dependent on pyridoxal 5′-phosphate.[11] Depression has been associated with a deficiency of serotonin and other catecholamines. Vitamin B6 is an essential cofactor for tryptophan metabolism and facilitates the conversion of tryptophan to the monoamine neurotransmitter and serotonin.[34]
Based on the active role of pyridoxine phosphate in neurotransmitter synthesis, vitamin B6 may have a beneficial role in the prevention and treatment of depression.[30] A limited number of studies investigating the benefit of vitamin B6 supplementation focused on depression as part of premenstrual syndrome.[20] Low-dose oral contraceptive pills may adversely affect vitamin B6 levels and observed depression in plasma pyridoxal 5′-phosphate concentrations in oral contraceptive pill users may reflect decreased body reserves of vitamin B6.[19]
According to a study conducted by a researcher in available resources, the effect of vitamin B6 on PPD prevention had not yet been investigated. Perhaps, this study was the first one with this approach. Hence, it is recommended that further studies need to be conducted to determine how vitamin B6 plays a preventive role in PPD. For future studies, taking a blood sample to measure the plasma levels of vitamin B6 would be recommended.
Limitations
Uncontrolled dietary B6 intake and physical activity as confounding variables are the main limitations of this study.
Conclusions
The prescription of vitamin B6 may be an effective preventive approach towards PPD.
Declaration of patient consent
The authors certify that they have obtained consent forms from all the participants. In the form, the participants have given their consent for their images and other clinical information to be reported in the journal. The patients understood that their names and initials will not be published and due efforts will be made to conceal their identity.
Financial support and sponsorship
This study is supported by a research grant from the Vice Chancellor for Research of Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Hereby we thank all pregnant mothers and the managers and staff of selected health centers as the field of study who participated in the study patiently.
References
- 1.Cooper R. Diagnosing the diagnostic and statistical manual of mental disorders. Routledge; 2018. [Google Scholar]
- 2.Harris B. Postpartum depression. Psychiatr Ann. 2002;32:405–16. [Google Scholar]
- 3.Wisner KL, Parry BL, Piontek CM. Postpartum depression. N Engl J Med. 2002;347:194–9. doi: 10.1056/NEJMcp011542. [DOI] [PubMed] [Google Scholar]
- 4.Piteo AM, Roberts RM, Nettelbeck T, Burns N, Lushington K, Martin AJ, et al. Postnatal mediates relationship between infant and maternal sleep disruption and family dysfunction. Early Hum Dev. 2013;89:69–74. doi: 10.1016/j.earlhumdev.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Salary P, Banafshe EL, Hebrani PA, Nooghabi JJ. On the relationship between maternal fatigue and postpartum depression. J Fundam Mental Health. 2010;114:302–11. [Google Scholar]
- 6.Hirst KP, Moutier CY. Postpartum majordepression. Women. 2010;100:17–9. [Google Scholar]
- 7.Halbreich U, Karkun S. Cross-cultural and social diversity of prevalence of postpartum depression and depressive symptoms. J Affect Disord. 2006;91:97–111. doi: 10.1016/j.jad.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 8.Norhayati MN, Hazlina NN, Asrenee AR, Emilin WW. Magnitude and risk factors for postpartum symptoms: A literature review. J Affect Disord. 2015;175:34–52. doi: 10.1016/j.jad.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 9.Havas AM, Juul S, Bech P, Nexo E. Vitamin B6 level is associated with symptoms of depression. Psychother Psychosom. 2004;73:340–3. doi: 10.1159/000080386. [DOI] [PubMed] [Google Scholar]
- 10.Shibata K, Tachiki A, Mukaeda K, Fukuwatari T, Sasaki S, Jinno Y. Changes in plasma pyridoxal 5′-Phosphate concentration during pregnancy stages in Japanese women. J Nutr Sci Vitaminol. 2013;59:343–6. doi: 10.3177/jnsv.59.343. [DOI] [PubMed] [Google Scholar]
- 11.Shrim A, Boskovic R, Maltepe C, Navios Y, Garcia–Bournissen F, Koren G. Pregnancy outcome following use of large doses of vitamin B6 in the first trimester. J Obstet Gynaecol Res. 2006;26:749–51. doi: 10.1080/01443610600955826. [DOI] [PubMed] [Google Scholar]
- 12.Smith JA, Refuerzo J, Ramin S. Treatment and outcome of nausea and vomiting of pregnancy. UpToDate. Waltham, MA: 2014. [Last accessed on 2013 Jun 08]. [Google Scholar]
- 13.Hvas AM, Juul S, Bech P, Nexø E. Vitamin B6 level is associated with symptoms of depression. Psychother Psychosom. 2004;73:340–3. doi: 10.1159/000080386. [DOI] [PubMed] [Google Scholar]
- 14.Merete C, Falcon LM, Tucker KL. Vitamin B6 is associated with depressive symptomatology in Massachusetts elders. J Am Coll Nutr. 2008;27:421–7. doi: 10.1080/07315724.2008.10719720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamphuis MH, Geerlings MI, Grobbee DE, Kromhout D. Dietary intake of B (6-9-12) vitamins, serum homocysteine levels and their association with depressive symptoms: The Zutphen elderly study. Eur J Clin Nutr. 2008;62:939–45. doi: 10.1038/sj.ejcn.1602804. [DOI] [PubMed] [Google Scholar]
- 16.Murakami K, Miyake Y, Sasaki S, Tanaka K, Arakawa M. Dietary folate, riboflavin, vitamin B-6, and vitamin B-12 and depressive symptoms in early adolescence: The Ryukyus child health study. Psychosom Med. 2010;72:763–8. doi: 10.1097/PSY.0b013e3181f02f15. [DOI] [PubMed] [Google Scholar]
- 17.Murakami K, Mizoue T, Sasaki S, Ohta M, Sato M, Matsushita Y, et al. Dietary intake of folate, other B vitamins, and omega-3 polyunsaturated fatty acids in relation to depressive symptoms in Japanese adults. Nutrition. 2008;24:140–7. doi: 10.1016/j.nut.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Villegas A, Doreste J, Schlatter J, Pla J, Bes-Rastrollo M, Martinez-Gonzalez MA. Association between folate, vitamin B (6) and vitamin B (12) intake and depression in the SUN cohort study. J Hum Nutr Diet. 2009;22:122–33. doi: 10.1111/j.1365-277X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 19.Wilson SM, Bivins BN, Russell KA, Bailey LB. Oral contraceptive use: Impact on folate. Vitamin B6, and vitamin B1 status. Nutr Rev. 2011;69:572–83. doi: 10.1111/j.1753-4887.2011.00419.x. [DOI] [PubMed] [Google Scholar]
- 20.Williams A-L, Cotter A, Sabina A, Girard C, Goodman J, Katz DL. The role for vitamin B-6 as treatment for depression: A systematic review. Fam Pract. 2005;22:532–7. doi: 10.1093/fampra/cmi040. [DOI] [PubMed] [Google Scholar]
- 21.Nevatte T, O’Brien PM, Bäckström T, Brown C, Dennerstein L, Endicott J, et al. Consensus on the management of premenstrual disorders. Arch Womens Ment Health. 2013;16:279–91. doi: 10.1007/s00737-013-0346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shorey S, Chee CY, Ng ED, Chan YH, San Tam WW, Chong YS. Prevalence and incidence of postpartum depression among healthy mothers: A systematic review and meta-analysis. J Psychiatr Res. 2018;104:235–48. doi: 10.1016/j.jpsychires.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Brennan C, Worrall-Davies A, McMillan D, Gilbody S, House A. The hospital anxiety and depression scale: A diagnostic meta-analysis of case-finding ability. J Psychosom Res. 2010;69:371–8. doi: 10.1016/j.jpsychores.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Montazeri A, Vahdaninia M, Ebrahimi M, Jarvandi S. The Hospital anxiety and depression scale (HADS): Translation and validation study of the Iranian version. Health Qual Life Out. 2003;1:14–9. doi: 10.1186/1477-7525-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Reilly BK. The social support appraisals scale: Construct validation for psychiatric inpatients. J Clin Psychol. 1995;51:37–42. doi: 10.1002/1097-4679(199501)51:1<37::aid-jclp2270510107>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Abdollahzade Rafi M, Hassanzadeh M, Ahmadi S, Taheri M, Hosseini MA. Relationship between social support with depression and anxiety during third trimester pregnancy. Iranian J Nurs Res. 2012;7:1–10. Persian. [Google Scholar]
- 27.Heidari A. Comparison of alexithymia and stress between male and female. New Find Psychol. 2010;5:21–40. In Persian. [Google Scholar]
- 28.Masaeli N, Kheirabadi GR, Maracy MR, Akbaripour S. Psychometric properties and diagnostic accuracy of the edinburgh postnatal depression scale in a sample of Iranian women. Iran J Med Sci. 2012;37:32–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Alavi Majd H. Tehran: Shahid Behashti Med Sci University Publishing; 2006. Sample Size in Clinical Research; pp. 58–61. Persian. [Google Scholar]
- 30.Nanri A, Pham NM, Kurotani K, Kume A, Kuwahara K, Sato M, et al. Serum pyridoxal concentrations and depressive symptoms among Japanese adults: Results from a prospective study. Eur J Clin Nutr. 2013;67:1060–5. doi: 10.1038/ejcn.2013.115. [DOI] [PubMed] [Google Scholar]
- 31.Skarupski KA, Tangney C, Li H, Ouyang B, Evans DA, Morris MC. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am J Clin Nutr. 2010;92:330–5. doi: 10.3945/ajcn.2010.29413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyake Y, Sasaki S, Tanaka K, Yokoyama T, Ohya Y, Fukushima W, et al. Dietary folate and vitamins B12, B6, and B2 intake and the risk of postpartum depression in Japan: The Osaka maternal and child health study. J Affect Disord. 2006;96:133–8. doi: 10.1016/j.jad.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 33.Andreeva VA, Galan P, Torrès M, Julia C, Hercberg S, Kesse-Guyot E. Supplementation with B vitamins or n23 fatty acids and depressive symptoms in cardiovascular disease survivors: Ancillary findings from the supplementation with folate, vitamins B-6 and B-12 and/or OMega-3 fatty acids (SU.FOL.OM3) randomized trials. Am J Clin Nutr. 2012;96:208–14. doi: 10.3945/ajcn.112.035253. [DOI] [PubMed] [Google Scholar]
- 34.Sechi G, Sechi E, Fois C, Kumar N. Advances in clinical determinants and neurological manifestations of B vitamin deficiency in adults. Nutr Res. 2016;74:281–300. doi: 10.1093/nutrit/nuv107. [DOI] [PubMed] [Google Scholar]


