Abstract
Purpose of review
To date, prognostication of patients after acute traumatic spinal cord injury (SCI) mostly relies on the neurological assessment of residual function attributed to lesion characteristics. With emerging treatment candidates awaiting to be tested in early clinical trials, there is a need for wholistic high-yield prognostic biomarkers that integrate both neurogenic and nonneurogenic SCI pathophysiology as well as premorbid patient characteristics.
Recent findings
It is becoming clearer that effective prognostication after acute SCI would benefit from integrating an assessment of pathophysiological changes on a systemic level, and with that, extend from a lesion-centric approach. Immunological markers mirror tissue injury as well as host immune function and are easily accessible through routine blood sampling. New studies have highlighted the value of circulating white blood cells, neutrophils and lymphocytes in particular, as prognostic systemic indicators of SCI severity and outcomes.
Summary
We survey recent advances in methods and approaches that may allow for a more refined diagnosis and better prognostication after acute SCI, discuss how these may help deepen our understanding of SCI pathophysiology, and be of use in clinical trials.
Keywords: acute phase, clinical research, lymphopenia, neurological recovery, neutrophilia, spinal shock
INTRODUCTION
Acute traumatic spinal cord injury (SCI) is a devastating neurological condition, typically resulting in severe long-term physical disability and a drastically reduced overall quality of life; an estimated 27 million people are believed to be presently living with the consequences of SCI globally [1]. Management of SCI patients and their overall health and wellbeing comprises early spine surgery [2▪] and supportive treatment that focuses mostly on rehabilitation and secondary complications.
The arrival of the molecular era and a more concerted research effort around the globe have accelerated our understanding as to what happens inside the spinal cord once it becomes injured, including the various factors that contribute to its regenerative failure. This has led to the generation of many promising therapeutic targets and interventions strategies, and an increasing number of these are now beginning to find their way into the clinic via experimental treatment trials. Although this provides great hope that a cure for SCI will one day be a reality, a significant area of concern is the fact that a great many of these new putative treatment options appear to ‘fail’ in clinical trials based on an apparent lack of evidence for efficacy [3,4]. Numerous factors can contribute to poor translation of basic science, and many of these have already been extensively discussed in the literature [4]. However, there is increasing awareness that current approaches for diagnosing the extent of spinal cord damage at presentation are unsatisfactory, thereby likely hampering our ability to demonstrate efficacy of new neuroprotective and pro-regenerative treatments in human patients. It could be argued that this applies not only to experimental treatments given during the very early stages of SCI, i.e., when there is still much overall uncertainty due to e.g. the presence of spinal shock (see below), but also those administered well beyond that, as it is widely accepted for some level of spontaneous recovery to occur during at least the first year postaccident [5]. More consideration also needs to be given to SCI patient population heterogeneity, recognizing that the term ‘heterogeneity’ here applies not only to differences in the cause, level and severity of the initial SCI, but likely also to variations in individual genetics, presence or absence of additional traumatic injuries, existing co-morbidities, postinjury complications, response to medication and the pattern of pathological progression. All of these factors are currently underrepresented in clinical research despite their impact on outcome trajectories [6–9,10▪▪]. This mini-review will discuss the current state of the field regarding the diagnostic and prognostic modalities that are being used and/or developed, followed by a brief discussion of what we believe the future holds for prognostication of SCI.
Box 1.
no caption available
ACUTE NEUROLOGICAL SYMPTOMS AND SPINAL SHOCK: WHAT DO THEY HIDE?
Neurological symptoms in acute SCI patients typically reflect the level and severity of spinal cord damage; however, the likely presence of acute spinal shock, which describes the transient depression of all reflex activity and sensorimotor function below the level of injury, also precludes the accurate assessment of any residual neurological function associated with structurally intact neural tissue [11,12]. Paralysis due to spinal shock is typically initiated immediately after injury, and can last for many days, if not weeks, as determined via assessment of the bulbocavernosus reflex [13]. With a significant proportion of SCIs being anatomically incomplete, it is important to consider the areflexic time period in conjunction with the progression of early SCI pathophysiology. Specifically, while the primary injury – that is, the damage caused to the spinal cord by mechanical forces at the time of the accident – is a key determinant for recovery [3], secondary injury cascades involving both neurogenic and inflammatory components cause a further spread of damage into the neighbouring spinal cord tissue [14]. Acute spinal shock can thus not only skew the initial presentation of lesion severity but also mask its secondary progression. Clinical observations add another perspective here. Sir Ludwig Guttmann noted that the duration of the spinal shock is not uniform and can be influenced by secondary events. Acquired infections in particular were associated with a prolonged, extended spinal shock phase [15]. A delayed resolution of spinal shock in case of acquired infection corroborates a direct and early impact of the latter on the residual circuitry.
EXTRAPOLATING PROGNOSIS FROM DIAGNOSIS – AN ONGOING ART OF EXPLORATION
Clinical diagnosis for SCI has largely been unchanged for the past few decades, with the American Spinal Injury Association (ASIA) International Standards for Neurological Classification of SCI (ISNCSCI) remaining the globally accepted test of choice for categorising SCI-associated neurological impairments in motor and sensory function [11,16,17]. Clinical scores extrapolated from this early diagnostic assessment, typically performed within days of injury onset, are often also used to gain at least some level of prognostic insight [11,17,18]. Indeed, the neurological level and severity of the initial injury, as described by ASIA Impairment Scale (AIS) grading, are amongst the strongest predictors of long-term neurological outcome [19–21]. The Spinal Cord Independence Measure III (SCIM III) can provide additional insights in terms of an individual's practical level of disability when attempting everyday activities [16]. Although both tools are indispensable for early clinical assessment and documenting a patient's progression over time, there are obvious caveats in relation to prognostication. Specifically, conducting these examinations soon after patient admission is often impractical, too time-consuming and unreliable in emergency situations, especially if the patient is unresponsive or compromised [9]. Furthermore, and as alluded to earlier, broadly categorising SCIs via these grading systems can overlook its complexity, particularly in the acute phase where spinal shock may interfere with the very neurological assessments designed to obtain clinical evidence for spinal cord tissue sparing and residual function [22].
Imaging techniques such as MRI can reveal additional detail of the extent of damage to the spinal cord and thus hold promise for prognostication. For example, intramedullary MRI signal abnormalities can be informative when the patient is unconscious, and they also correlate with AIS grading [23–25]. Unfortunately, the clinical utility of these surrogate indicators over existing clinical examinations is disputed [19,24], and a reliable correlation (or translation) of imaging findings to functional impairments is hence not guaranteed [22]. Advanced MRI techniques may provide more detailed insights into microstructural integrity of the spinal cord [17,26], but they are not routinely used yet in clinical practice due to time, significant postprocessing considerations, and a lack of consistent clinical guidelines. At least some of these hurdles may now be overcome by the establishment of consensus acquisition protocols for quantitative MRI of the human spinal cord, which should generate reproducible and reliable data for analysis [27▪▪,28]. Time considerations around the processing and analysis of quantitative MRI data remain an issue, however, at least if required for patient selection in early intervention trials where treatments may need to commence within 6–12 h post-SCI to combat early secondary injury progression.
Overall, the limitations associated with existing neurological examinations and imaging hinder translational investigations that are targeting these critical early hours. This poses a risk for premature conclusions on intervention efficacy and the translatability of preclinical research efforts more generally, not because of poor experimental design and/or cross-species differences, but rather due to significant constraints in the sensitivity and use of both neurological examination and imaging for prognostication. To combat these limitations, randomised controlled trials often use large sample sizes to account for the uncertainty around in natural trajectory of recovery between SCI patients [4,9]. This brings its own challenges, however, in terms of meeting recruitment targets [29], and also does not necessarily overcome pitfalls associated with an ultimately imbalanced study design due to our present inability to more accurately stratify patients by SCI severity at the outset. Lastly, and perhaps most crucially, both clinical examination and imaging reflect the product of the initial trauma and any additional spread of tissue damage (i.e. resultant pathology), but not the drivers of injury progression (i.e. underlying pathophysiology), and they will therefore always be inescapably restricted in prognostic utility.
EMERGING MOLECULAR BIOMARKERS FOR STRATIFICATION AND PROGNOSIS OF SPINAL CORD INJURY
There is growing awareness that the pathophysiological changes induced by SCI itself may provide rich and mostly untapped opportunities for assessing injury severity and patient prognostication [30]. At the molecular level, pioneering work by Kwon et al. demonstrated the predictive utility of several proteins (these being either cytokines or intracellular proteins) in the cerebrospinal fluid (CSF) of SCI patients as candidate biomarkers for detecting local inflammation and tissue destruction at the lesion site [9,31,32▪▪]. A significant number of other studies have also described links between nontraditional biomarkers of injury and neurological recovery from SCI, with varying strengths of association and clinical utility (reviewed in detail in [9,33]). However, none of these have as of yet been accepted and incorporated into routine clinical practice [22]. A contributing factor here may be the fact that their prognostic ability is yet to be conclusively demonstrated [22,34] (reviewed in [22]). In addition, there are also impracticalities around inserting indwelling intrathecal catheters and having to sample CSF very acutely from unstable patients for baseline measurements in early intervention trials where treatment typically must be initiated within hours of injury [35,36]; blood contamination may also be an issue. In summary, although candidate CSF protein biomarkers continue to be of interest and certainly hold promise for predicting longer-term patient outcomes [37,38], there is an ongoing pressing need for biomarkers that are derived from routinely collected data that can be easily incorporated into clinical care protocols, particularly so during acute SCI patient management.
Patient blood samples may provide a convenient alternative to CSF here. Blood-spinal cord barrier breach following traumatic injury to the cord means that many biomarkers found in CSF are often also detectable in serum, albeit at a lower concentration. It is important to note here that each ‘CSF-derived’ biomarker should be carefully considered, as not all will translate in terms of their usefulness for SCI diagnosis and/or prognosis when measured systemically. Specifically, protein biomarkers in the CSF will better reflect local central nervous system (CNS) sources and likely also be more informative about evolving pathophysiological changes, including metabolic alterations and inflammation [22,34,39]. In the blood, the specificity and value of at least some of these protein biomarkers may be blurred, or lost even, in the systemic acute phase response, that is, the generalised response of the body to a traumatic injury, or contributions from non-CNS sources. The same considerations apply to nonprotein-based biomarkers such as cell-free DNA [40] and microRNAs [41]. We would add that these limitations, real or perceived, may also be time-dependent, with emerging research suggesting that cytokine measurements can be more useful during the postacute phase [42]. The risk of confounding peripheral responses can be easily mitigated, however, by focusing on nongeneric molecular biomarkers that have high CNS tissue specificity. Indeed, intracellular proteins released from damaged CNS neurons or glia tend to correlate better with injury severity and outcomes following a major neurotraumatic event like SCI, with glial fibrillary acidic protein (GFAP), S100 calcium-binding protein B (S100B), neurofilament-light (NfL), neuron-specific enolase (NSE), tau and ubiquitin C-terminal hydrolase-L1 (UCH-L1) being amongst the most promising [43,44]. Irrespective of their potential, none are currently measured as part of routine care; considerations around the time it takes to run specialised assays for these markers and analyse the data again also factor into their practicality for prognostication and patient selection in early intervention trials.
CIRCULATING LEUKOCYTES: AN UNTAPPED SOURCE FOR SPINAL CORD INJURY PROGNOSTICATION?
There is long-standing awareness that SCI (and trauma in general) impacts on circulating white blood cell (WBC) numbers and differentials. Any potential use of such changes for SCI prognostication would be both practical and convenient as full blood counts (FBCs) are fast, routinely conducted upon patient admission, and often also measured frequently during the first-week postinjury, at least for critically ill patients. We recently conducted a retrospective study to carefully examine the WBC response to SCI, its relationship to lesion level, overall trauma severity and patient outcomes, and evaluated whether these associations may hold value for prognostication [45▪▪]. Using both an exploration and a validation cohort, we uncovered a strong relationship between significantly elevated neutrophil numbers in the blood of patients with an acute traumatic SCI and the likelihood of little to no functional improvement at hospital discharge, that is, no AIS grade conversion compared to admission data; the odds ratio for an unfavourable outcome in association with acute neutrophilia remained significantly increased after adjusting for age, sex, overall trauma severity, initial AIS grade and infectious complications.
In contrast to neutrophils, circulating lymphocytes did not increase in number in response to traumatic SCI. Rather, and consistent with previous reports [46,47], they instead often fall to below their normal clinical reference range during the first-week postaccident [45▪▪]. Although traditionally considered a hallmark of SCI-immune depression syndrome (SCI-IDS) – a neurogenic condition of immune compromise that can be experienced by individuals with high-level SCI [47–49], we did not find a lesion level dependency for early lymphopenia in this particular study. Intriguingly, however, we did observe that patients presenting with an isolated SCI and clinical lymphopenia during the first 3 days postaccident were more likely to have a substantial improvement in their neurological status (i.e. AIS grade conversion) during their hospital stay [45▪▪]. A further probing of lymphocyte subtypes and function (vide infra) is now required to better understand their putative contribution(s) and/or causative involvement in intraspinal inflammation, lesion site development and SCI-IDS.
Irrespective of these knowledge gaps, receiver-operator characteristics and precision-recall analyses confirmed that, when modelled appropriately, both neutrophilia and lymphopenia can serve as independent prognostic markers of long-term neurological recovery in acute SCI [45▪▪]. These latter findings are consistent with the reports of others that increased neutrophil-to-lymphocyte ratios may be predictive of poor neurological recovery after SCI [50▪▪,51]. Possible use of blood products in acute care may require some additional consideration here, as transfusion-related leukocytosis can occur in critically ill patients [52], although that also does not necessarily confound the use of WBC differentials as biomarkers for SCI prognostication. Lastly, incorporating platelet counts and other blood parameters may refine the value of models using systemic WBC responses to predict SCI outcomes [53▪,54]. The next steps will be to prognostically study WBC types of interest at much greater resolution, aiming to better appreciate their functional roles, capacity and predictive value in relation to SCI recovery.
Propelled by modern omics, multidimensional analysis and integrated bioinformatics approaches, this work has already begun. Specifically, and taking advantage of the fact that WBCs both sense and rapidly response to damage-associated molecular patterns (DAMPs), Kyritsis et al. analysed the transcriptome of circulating WBC that were harvested from SCI patients [50▪▪]. Unbiased gene expression analysis indeed showed that patients with SCIs have divergent gene expression profiles, not only from healthy controls but also nonneurological trauma, with nearly 200 differentially expressed genes; these genes were also associated with SCI severity. Indeed, extensive modelling and gene network analyses showed that co-expression signatures of WBCs such as natural killer cells, mast cells and granulocytes could predict an AIS grade of A and D. Although limited by sample size and an as of yet unknown value of the identified transcriptional fingerprints for predicting AIS grade conversion, this seminal work highlights the enormous potential for reliable and even more advanced prognostication in human SCI patients via RNA-based blood biomarkers. It also demonstrates how the unbiased analysis of such large omics datasets can be both hypothesis-generating and used to uncover novel biomarkers. That said, a drawback of modern ‘omics’ approaches is that data analysis can be tedious and time-consuming, making them again less suitable at present for patient stratification and selection purposes in early intervention trials. Nevertheless, these methods extend significantly from those used in Jogia et al.[45▪▪], in that they allow for a more global understanding of how WBCs respond to and/or are modulated by SCI.
TOWARDS BETTER DIAGNOSIS AND PROGNOSIS FOR ACUTE SPINAL CORD INJURY: THE ROAD AHEAD
Current clinical assessment methods for SCI diagnosis have limited predictive power to be of value for prognostication, at least not in the context of early intervention trials where individuals presenting with virtually identical injuries can have very divergent trajectories in terms of their natural recovery [3,9,55▪]. Going forward, a systematic framework for the selection of valuable biomarkers and prognostic tools is needed to maximally improve prediction accuracy. Undoubtedly, this will require multivariate considerations to account for SCI heterogeneity and other patient-specific factors more generally [19]; such approaches are now beginning to emerge as discussed. Ideally, biomarkers for SCI severity and prognosis would be (1) easily extractable from clinical data, (2) reliable and routinely used across different healthcare systems, and (3) sensitive to patient-specific variables, including the consequences of treatment, to allow for optimal translation. Our own recent work [45▪▪] and that of independent others [50▪▪] has widened the horizons of SCI biomarker research by demonstrating the utility of WBC responses to SCI for the purpose of patient stratification and prognosis. However, more work is needed to better understand if and/or how generalised immune cell responses to tissue damage are altered by SCI itself, quite possibly in a lesion level- and severity-dependent manner [49]. The importance of this is highlighted by the findings of Prüss et al. who showed that reversing acute SCI-induced lymphopenia does not necessarily alleviate the increased susceptibility to infections in this condition [47]. This observation is in agreement with our own findings that, although limited by sample size, lymphopenia itself was not associated with infectious complications early after SCI [45▪▪]. How altered immunity and infectious complications may ultimately interfere with rehabilitation [6,7,56] remains incompletely understood, but ongoing efforts involving deep immunophenotyping, gene expression profiling and other assessments of WBC function will likely provide answers to these outstanding questions.
Digging deeper into putative changes in WBC function after SCI will also require careful consideration of a range of extraneous factors. The gut microbiota has already received considerable attention because of its immunomodulatory influence (for review, see [57]). Indeed, analysis of faecal samples from animals with SCI demonstrate SCI-associated metabolic alterations, including changes in short-chain fatty acids [58,59▪]; manipulating these may positively affect immune function after SCI [60,61]. Beyond the gut microbiota, hepatic inflammation and associated metabolic changes following SCI were recently also demonstrated to increase tissue inflammation and secondary damage at the lesion site [62,63]. On the topic of metabolic (dys)regulation, the importance of immunometabolism needs to be recognised and explored [64], with inflammatory processes dictating the energetic and substrate needs of WBCs [65]. Immunometabolism is a relatively untapped concept in neurotrauma that may yield additional intrinsic and functionally relevant biomarkers that could also be manipulatable to improve patient outcomes.
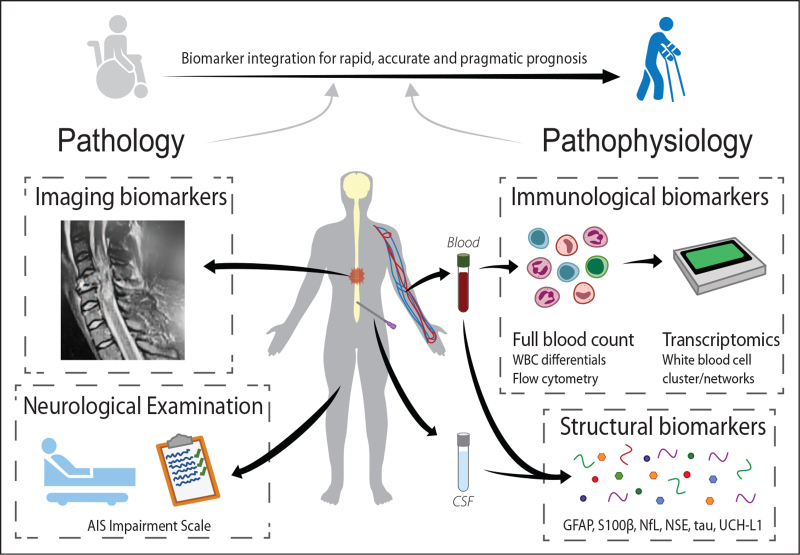
Ultimately, the future of SCI prognostication lies in the development of highly sensitive biomarker panels that can provide a detailed general assessment of injury severity and temporal evolution of the lesion site, and also help facilitate selection of the right treatment option(s) as part of personalised medicine approaches to acute SCI. We envisage that immunological biomarkers will be used and integrated here with structural biomarkers of tissue damage (DAMPs), along with quantitative imaging methods that are in the pipeline for eventual clinical use [26,33,66,67], yielding an all-encompassing and empirically tested panel that can account for the complexity of SCI pathophysiology and patient heterogeneity (Fig. 1). Ultimately, and complementary to markers of structural damage (proxy for the ‘lesion affected network’), genetic biomarkers may also need to be included to characterize the individual capacity to respond to an injury of the CNS. Genetic disposition can determine the capacity to form new neuroaxonal connections, and for these to be integrated into a ‘recovery related network’. A key modification known thus far affects the brain-derived neurotrophic factor (BDNF) gene, a well-known denominator of activity-dependent neuroplasticity. Early work suggests that modifications of BDNF (val66 polymorphism) are associated with restricted plasticity and worsened outcomes in SCI, a finding corroborated by observations in other acute CNS injury paradigms [68–70]. Another example relates to the apolipoprotein E polymorphism (ApoE), with subjects carrying the ApoE 4 allele having blunted recovery profiles as well [71].
FIGURE 1.
Complementing systemic biomarkers integrate neurogenic and nonneurogenic pathophysiology as well as individual premorbid patient characteristics. Diagram showing proposed multidimensional integration of clinical data (e.g. neurological examination, imaging and full blood counts) with emerging molecular biomarkers in the blood and/or CSF, to allow for better diagnosis of SCI severity and prediction of outcomes. For simplicity, only select immunological and structural biomarkers are shown; other promising molecular candidates are referred to in the main text. CSF, cerebrospinal fluid; SCI, spinal cord injury.
Lastly, most emerging biomarkers presently require specialised research service, and data analysis can be both costly and prohibitively time consuming, hampering their acceptance and integration into routine clinical laboratory operations. Yet, if not adopted into routine prognostication, we posit that many future SCI trials may fail, not necessarily because of weaknesses in the preclinical data, but rather due to the inherent heterogeneity of SCI, our current inability to stratify patients accordingly, and to accurately predict what level of spontaneous recovery they are likely to experience. For these reasons, the design of early intervention trials in acute SCI may need to be reconsidered, given that the required level of informed decision-making around patient selection may simply not be feasible or practical due to time constraints. Randomised controlled trials remain the gold standard in evidence-based medicine, but the adoption of studies with a nested case-control design where each test subject is matched to a selection of study controls, i.e., those with very similar individual characteristics based on a comprehensive panel of biomarkers, may overcome some of the issues that currently hamper early intervention trials because of prognostic uncertainty. Alternatively, suitable control subjects could be randomly selected from prospective, purposely build registries with associated specimen biobanks (cohort study design). In both instances, the risk of bias could be reduced by matching test and control subjects without knowledge of the (final) outcome measures.
CONCLUSION
Irrespective of what the future holds for clinical trial design in acute SCI, it is without question that there is pressing need for improved prognosis of patient outcomes. The fast-moving multidisciplinary fields of cellular, molecular and neuroimaging biomarker research will likely address this need, providing clarity on the effectiveness of experimental treatments, and also what interventions should be pursued by treating teams and their patients for optimal rehabilitation and enhanced quality of life.
Acknowledgements
None.
Financial support and sponsorship
M.J.R. is supported by SpinalCure Australia, the Wings for Life Spinal Cord Research Foundation (Grants WfL AU-19/17 and WfL-AU-18/21), and the National Health and Medical Research Council of Australia (Grant 1163835). T.J. was supported by a Research Training Program Scholarship (Australian Government). The work of MAK receives funding support from the Wings for Life Spinal Cord Research Foundation (Grants WfL-DE-16/16 and WfL-DE-11/20).
J.M.S. is supported by the National Institutes of Neurological Disorders-NIH (Grant R01NS118200), the European Union (EU Era Net – Neuron Program, SILENCE Grant 01EW170A and SCI-NET 01EW1710), the Craig H Neilsen Foundation (Grant 596764), the Wings for Life Foundation and the Hunt and Curtis endowment. J.M.S. is a Discovery Theme Initiative Scholar (Chronic Brain Injury) of the Ohio State University.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.James SL, Theadom A, Ellenbogen RG, et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18:56–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2▪.Badhiwala JH, Wilson JR, Witiw CD, et al. The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol 2021; 20:117–126. [DOI] [PubMed] [Google Scholar]; Landmark study demonstrating both the importance of, and critical time window for, early surgical intervention in acute SCI in relation to patient outcomes.
- 3.Khorasanizadeh M, Yousefifard M, Eskian M, et al. Neurological recovery following traumatic spinal cord injury: a systematic review and meta-analysis. J Neurosurg Spine 2019. 1–17. doi: 10.3171/2018.10.SPINE18802. [DOI] [PubMed] [Google Scholar]
- 4.Elizei SS, Kwon BK. The translational importance of establishing biomarkers of human spinal cord injury. Neural Regen Res 2017; 12:385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drazin D, Boakye M. Spontaneous recovery patterns and prognosis after spinal cord injury. In: Fehlings MG, Vaccaro AR, Boakye M, et al. Essentials of spinal cord injury: basic research to clinical practice. New York: Thieme Medical Publishers 2013; p. 75–83. [Google Scholar]
- 6.Failli V, Kopp MA, Gericke C, et al. Functional neurological recovery after spinal cord injury is impaired in patients with infections. Brain 2012; 135 (Pt 11):3238–3250. [DOI] [PubMed] [Google Scholar]
- 7.Kopp MA, Watzlawick R, Martus P, et al. Long-term functional outcome in patients with acquired infections after acute spinal cord injury. Neurology 2017; 88:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liebscher T, Ludwig J, Lübstorf T, et al. Cervical spine injuries with acute traumatic spinal cord injury - spinal surgery adverse events and their association with neurological and functional outcome. Spine 2021; doi:10.1097/BRS.0000000000004124. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon BK, Bloom O, Wanner IB, et al. Neurochemical biomarkers in spinal cord injury. Spinal Cord 2019; 57:819–831. [DOI] [PubMed] [Google Scholar]
- 10▪▪.Fouad K, Popovich PG, Kopp MA, Schwab JM. The neuroanatomical-functional paradox in spinal cord injury. Nat Rev Neurol 2021; 17:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent seminal review highlighting SCI complexity and the multitude of variables influencing outcomes in both preclinical and clinical studies.
- 11.Hachem LD, Ahuja CS, Fehlings MG. Assessment and management of acute spinal cord injury: From point of injury to rehabilitation. J Spinal Cord Med 2017; 40:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evaniew N, Sharifi B, Waheed Z, et al. The influence of neurological examination timing within hours after acute traumatic spinal cord injuries: an observational study. Spinal Cord 2020; 58:247–254. [DOI] [PubMed] [Google Scholar]
- 13.Ditunno JF, Little JW, Tessler A, Burns AS. Spinal shock revisited: a four-phase model. Spinal Cord 2004; 42:383–395. [DOI] [PubMed] [Google Scholar]
- 14.Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers 2017; 3:17018.doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 15.Guttmann L. Spinal cord injuries: comprehensive management and research. 2nd ed. ed. Oxford: Blackwell Scientific Publications Philadelphia: distributed by J.B. Lippincott; 1976. [Google Scholar]
- 16.Hadley MN, Walters BC, Aarabi B, et al. Clinical assessment following acute cervical spinal cord injury. Neurosurgery 2013; 72: (suppl_3): 40–53. [DOI] [PubMed] [Google Scholar]
- 17.Sharif S, Jazaib Ali MY. Outcome prediction in spinal cord injury: myth or reality. World Neurosurg 2020; 140:574–590. [DOI] [PubMed] [Google Scholar]
- 18.van Middendorp JJ, Hosman AJF, Donders ART, et al. A clinical prediction rule for ambulation outcomes after traumatic spinal cord injury: a longitudinal cohort study. Lancet 2011; 377:1004–1010. [DOI] [PubMed] [Google Scholar]
- 19.Mputu Mputu P, Beauséjour M, Richard-Denis A, Mac-Thiong JM. Early predictors of neurological outcomes after traumatic spinal cord injury: a systematic review and proposal of a conceptual framework. Am J Phys Med Rehabil 2021; 100:700–711. [DOI] [PubMed] [Google Scholar]
- 20.Elsevier, Burns AS, Marino RJ, Flanders AE, Flett H. Verhaagen J, McDonald JW. Chapter 3 - Clinical diagnosis and prognosis following spinal cord injury. Handbook of clinical neurology. Vol 109 2012. 47–62. [DOI] [PubMed] [Google Scholar]
- 21.Betz R, Biering-Sørensen F, Burns SP, et al. The 2019 revision of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)—What's new? Spinal Cord 2019; 57:815–817. [DOI] [PubMed] [Google Scholar]
- 22.Snyder R, Verla T, Ropper AE. Practical application of recent advances in diagnostic, prognostic, and therapeutic modalities for spinal cord injury. World Neurosurg 2020; 136:330–336. [DOI] [PubMed] [Google Scholar]
- 23.Aarabi B, Sansur CA, Ibrahimi DM, et al. Intramedullary lesion length on postoperative magnetic resonance imaging is a strong predictor of ASIA Impairment Scale Grade Conversion Following Decompressive Surgery in Cervical Spinal Cord Injury. Neurosurgery 2017; 80:610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martineau J, Goulet J, Richard-Denis A, Mac-Thiong J-M. The relevance of MRI for predicting neurological recovery following cervical traumatic spinal cord injury. Spinal Cord 2019; 57:866–873. [DOI] [PubMed] [Google Scholar]
- 25.Mummaneni N, Burke JF, DiGiorgio AM, et al. Injury volume extracted from MRI predicts neurologic outcome in acute spinal cord injury: a prospective TRACK-SCI pilot study. J Clin Neurosci 2020; 82:231–236. [DOI] [PubMed] [Google Scholar]
- 26.Zaninovich OA, Avila MJ, Kay M, et al. The role of diffusion tensor imaging in the diagnosis, prognosis, and assessment of recovery and treatment of spinal cord injury: a systematic review. Neurosurg Focus 2019; 46:E7. [DOI] [PubMed] [Google Scholar]
- 27▪▪.Cohen-Adad J, Alonso-Ortiz E, Abramovic M, et al. Generic acquisition protocol for quantitative MRI of the spinal cord. Nat Protoc 2021; doi: 10.1038/s41596-021-00588-0. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; Detailed protocols for standardised quantitative MRI of the human spinal cord that can be now be universally applied by both researchers and clinicians to study SCI.
- 28.Cohen-Adad J, Alonso-Ortiz E, Abramovic F, et al. Open-access quantitative MRI data of the spinal cord and reproducibility across participants, sites and manufacturers. Sci Data 2021; 8:219.doi:10.1038/s41597-021-00941-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fletcher B, Gheorghe A, Moore D, et al. Improving the recruitment activity of clinicians in randomised controlled trials: a systematic review. BMJ Open 2012; 2:e000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawryluk G, Whetstone W, Saigal R, et al. Mean arterial blood pressure correlates with neurological recovery after human spinal cord injury: analysis of high frequency physiologic data. J Neurotrauma 2015; 32:1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon BK, Stammers AMT, Belanger LM, et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma 2009; 27:669–682. [DOI] [PubMed] [Google Scholar]
- 32▪▪.Skinnider MA, Rogalski J, Tigchelaar S, et al. Proteomic portraits reveal evolutionarily conserved and divergent responses to spinal cord injury. Mol Cell Proteomics 2021; 20:100096. doi:10.1016/j,mcpro.2021.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identification of biomarkers conserved in both human patients and a large animal model of SCI, and use of machine learning to develop a prognostic model for predicting neurological improvements.
- 33.Schading S, Emmenegger TM, Freund P. Improving diagnostic workup following traumatic spinal cord injury: advances in biomarkers. Curr Neurol Neurosci Rep 2021; 21:49.doi: 10.1007/s11910-021-01134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Streijger F, Skinnider MA, Rogalski JC, et al. A Targeted proteomics analysis of cerebrospinal fluid after acute human spinal cord injury. J Neurotrauma 2017; 34:2054–2068. [DOI] [PubMed] [Google Scholar]
- 35.Casha S, Zygun D, McGowan MD, et al. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 2012; 135:1224–1236. [DOI] [PubMed] [Google Scholar]
- 36.Fehlings MG, Wilson JR, Frankowski RF, et al. Riluzole for the treatment of acute traumatic spinal cord injury: rationale for and design of the NACTN Phase I clinical trial. J Neurosurg Spine 2012; 17: (Suppl1): 151–156. [DOI] [PubMed] [Google Scholar]
- 37.Capirossi R, Piunti B, Fernández M, et al. Early CSF biomarkers and late functional outcomes in spinal cord injury. A Pilot Study. Int J Mol Sci 2020; 21: doi: 10.3390/ijms21239037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernández M, Baldassarro VA, Capirossi R, et al. Possible strategies to optimize a biomarker discovery approach to correlate with neurological outcome in patients with spinal cord injury: A Pilot Study. J Neurotrauma 2020; 37:431–440. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y, Streijger F, Wang Y, et al. Parallel metabolomic profiling of cerebrospinal fluid and serum for identifying biomarkers of injury severity after acute human spinal cord injury. Sci Rep 2016; 6:38718.doi: 10.1038/srep38718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang HC, Lin YT, Hsu SY, et al. Serial plasma DNA levels as predictors of outcome in patients with acute traumatic cervical spinal cord injury. J Transl Med 2019; 17:329.doi: 10.1186/s12967-019-2084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tigchelaar S, Gupta R, Shannon CP, et al. MicroRNA biomarkers in cerebrospinal fluid and serum reflect injury severity in human acute traumatic spinal cord injury. J Neurotrauma 2019; 36:2358–2371. [DOI] [PubMed] [Google Scholar]
- 42.Ogurcov S, Shulman I, Garanina E, et al. Blood serum cytokines in patients with subacute spinal cord injury: a pilot study to search for biomarkers of injury severity. Brain Sci 2021; 11:322.doi: 10.3390/brainsci11030322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhle J, Gaiottino J, Leppert D, et al. Serum neurofilament light chain is a biomarker of human spinal cord injury severity and outcome. J Neurol Neurosurg Psychiatry 2015; 86:273.doi: 10.1136/jnnp-2013-307454. [DOI] [PubMed] [Google Scholar]
- 44.Ahadi R, Khodagholi F, Daneshi A, et al. Diagnostic value of serum levels of GFAP, pNF-H, and NSE compared with clinical findings in severity assessment of human traumatic spinal cord injury. Spine 2015; 40:E823–E830. [DOI] [PubMed] [Google Scholar]
- 45▪▪.Jogia T, Lübstorf T, Jacobson E, et al. Prognostic value of early leukocyte fluctuations for recovery from traumatic spinal cord injury. Clin Transl Med 2021; 11:e272. [DOI] [PMC free article] [PubMed] [Google Scholar]; Retrospective study of SCI patients across two independent cohorts establishing the prognostic value of select early changes in circulating WBC counts that are statistically associated with long-term recovery.
- 46.Riegger T, Conrad S, Liu K, et al. Spinal cord injury-induced immune depression syndrome (SCI-IDS). Eur J Neurosci 2007; 25:1743–1747. [DOI] [PubMed] [Google Scholar]
- 47.Prüss H, Tedeschi A, Thiriot A, et al. Spinal cord injury-induced immunodeficiency is mediated by a sympathetic-neuroendocrine adrenal reflex. Nat Neurosci 2017; 20:1549–1559. [DOI] [PubMed] [Google Scholar]
- 48.Kopp MA, Druschel C, Meisel C, et al. The SCIentinel study--prospective multicenter study to define the spinal cord injury-induced immune depression syndrome (SCI-IDS)--study protocol and interim feasibility data. BMC Neurol 2013; 13:168.doi: 10.1186/1471-2377-13-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brommer B, Engel O, Kopp MA, et al. Spinal cord injury-induced immune deficiency syndrome enhances infection susceptibility dependent on lesion level. Brain 2016; 139 (Pt 3):692–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50▪▪.Kyritsis N, Torres-Espín A, Schupp PG, et al. Diagnostic blood RNA profiles for human acute spinal cord injury. J Exp Med 2021; 218:e20201795.doi: 10.1084/jem.20201795. [DOI] [PMC free article] [PubMed] [Google Scholar]; Seminal study demonstrating the robust utlity of gene expression analysis in circulating white blood cells after acute SCI, and how statistical modelling can be used to examine changes in gene expression that are associated with long-term patient outcomes.
- 51.Zhao JL, Lai ST, Du ZY, et al. Circulating neutrophil-to-lymphocyte ratio at admission predicts the long-term outcome in acute traumatic cervical spinal cord injury patients. BMC Musculoskelet Disord 2020; 21:548.doi: 10.1186/s12891-020-03556-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Izbicki G, Rudensky B, Na’amad M, et al. Transfusion-related leukocytosis in critically ill patients. Crit Care Med 2004; 32:439–442. [DOI] [PubMed] [Google Scholar]
- 53▪.Leister I, Linde LD, Vo AK, et al. Routine blood chemistry predicts functional recovery after traumatic spinal cord injury: a post hoc analysis. Neurorehabil Neural Repair 2021; 35:321–333. [DOI] [PubMed] [Google Scholar]; This study shows that blood parameters other than white blood cells may also hold prognostic value for predicting outcomes after a traumatic SCI.
- 54.Xie Y, Wang Y, Zhou Y, et al. A nomogram for predicting acute respiratory failure after cervical traumatic spinal cord injury based on admission clinical findings. Neurocrit Care 2021; doi:10.1007/s12028-021-01302-4. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55▪.Jaja BNR, Badhiwala J, Guest J, et al. Trajectory-based classification of recovery in sensorimotor complete traumatic cervical spinal cord injury. Neurology 2021; 96:e2736–e2748. [DOI] [PubMed] [Google Scholar]; Identification of patient-specific factors or characteristics that influence neurological recovery in addition to the severity of SCI at baseline.
- 56.Jaja BNR, Jiang F, Badhiwala JH, et al. Association of pneumonia, wound infection, and sepsis with clinical outcomes after acute traumatic spinal cord injury. J Neurotrauma 2019; 36:3044–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jogia T, Ruitenberg MJ. Traumatic spinal cord injury and the gut microbiota: current insights and future challenges. Front Immunol 2020; 11:704.doi: 10.3389/fimmu.2020.00704. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt EKA, Torres-Espin A, Raposo PJF, et al. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. PLoS One 2020; 15:e0226128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59▪.Jing Y, Yu Y, Bai F, et al. Effect of fecal microbiota transplantation on neurological restoration in a spinal cord injury mouse model: involvement of brain-gut axis. Microbiome 2021; 9:59.doi: 10.1186/s40168-021-01007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Preclinical work in mice with SCI showing that fecal microbiota transplants confer neuroprotection via an increase of immunomodulatory short-chain fatty acids and immunological reprogramming at the site of SCI.
- 60.Wang Q, Liu Y, Han L, et al. Risk factors for acute stroke-associated pneumonia and prediction of neutrophil-to-lymphocyte ratios. Am J Emerg Med 2021; 41:55–59. [DOI] [PubMed] [Google Scholar]
- 61.David S, López-Vales R. Bioactive lipid mediators in the initiation and resolution of inflammation after spinal cord injury. Neuroscience 2021; 466:273–297. [DOI] [PubMed] [Google Scholar]
- 62.Goodus MT, Carson KE, Sauerbeck AD, Dey P, et al. Liver inflammation at the time of spinal cord injury enhances intraspinal pathology, liver injury, metabolic syndrome and locomotor deficits. Exp Neurol 2021; 342:113725.doi: 10.1016/j.expneurol.2021.113725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yates AG, Jogia T, Gillespie ER, et al. Acute IL-1RA treatment suppresses the peripheral and central inflammatory response to spinal cord injury. J Neuroinflamm 2021; 18:15.doi: 10.1186/s12974-020-02050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Neill LAJ, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol 2016; 16:553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Devanney NA, Stewart AN, Gensel JC. Microglia and macrophage metabolism in CNS injury and disease: The role of immunometabolism in neurodegeneration and neurotrauma. Exp Neurol 2020; 329:113310.doi: 10.1016/j.expneurol.2020.113310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shabani S, Meyer BP, Budde MD, Wang MC. Diagnostic imaging in spinal cord injury. Neurosurg Clin N Am 2021; 32:323–331. [DOI] [PubMed] [Google Scholar]
- 67.Shanmuganathan K, Zhuo J, Bodanapally UK, et al. Comparison of acute diffusion tensor imaging and conventional magnetic resonance parameters in predicting long-term outcome after blunt cervical spinal cord injury. J Neurotrauma 2020; 37:458–465. [DOI] [PubMed] [Google Scholar]
- 68.Lamy JC, Boakye M. BDNF Val66Met polymorphism alters spinal DC stimulation-induced plasticity in humans. J Neurophysiol 2013; 110:109–116. [DOI] [PubMed] [Google Scholar]
- 69.Siironen J, Juvela S, Kanarek K, et al. The Met allele of the BDNF Val66Met polymorphism predicts poor outcome among survivors of aneurysmal subarachnoid hemorrhage. Stroke 2007; 38:2858–2860. [DOI] [PubMed] [Google Scholar]
- 70.Leech KA, Hornby TG. High-Intensity Locomotor Exercise Increases Brain-Derived Neurotrophic Factor in Individuals with Incomplete Spinal Cord Injury. J Neurotrauma 2017; 34:1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jha A, Lammertse DP, Coll JR, et al. Apolipoprotein E epsilon4 allele and outcomes of traumatic spinal cord injury. J Spinal Cord Med 2008; 31:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]