Abstract
Objective:
Antibody-dependent enhancement (ADE) affects host-virus dynamics in fundamentally different ways: i) enhancement of initial virus acquisition, and/or ii) increased disease progression/severity. Here we address the question whether anti-HIV-1 antibodies can enhance initial infection. While cell-culture experiments hinted at this possibility, in-vivo proof remained elusive.
Design:
We used passive immunization in nonhuman primates challenged with simian-human immunodeficiency virus (SHIV), a chimera expressing HIV-1 envelope. We purified IgG from rhesus monkeys with early-stage SHIV infection – before cross-neutralizing anti-HIV-1 antibodies had developed – and screened for maximal complement-mediated antibody-dependent enhancement (C’-ADE) of viral replication with a SHIV strain phylogenetically distinct from that harbored by IgG donor macaques. IgG fractions with maximal C’-ADE but lacking neutralization were combined to yield enhancing anti-SHIV IgG (enSHIVIG).
Results:
We serially enrolled naive macaques (Group 1) to determine the minimal and 50% animal infectious doses required to establish persistent infection after intrarectal SHIV challenge. The first animal was inoculated with a 1 : 10 virus-stock dilution; after this animal's viral RNA load was >104copies/ml, the next macaque was challenged with 10x less virus, a process repeated until viremia no longer ensued. Group 2 was pretreated intravenously with enSHIVIG 24 h before SHIV challenge. Overall, Group 2 macaques required 3.4-fold less virus compared to controls (P = 0.002). This finding is consistent with enhanced susceptibility of the passively immunized animals to mucosal SHIV challenge.
Conclusion:
These passive immunization data give proof of IgG-mediated enhanced virus acquisition after mucosal exposure – a potential concern for antibody-based AIDS vaccine development.
Keywords: ADE, complement-mediated antibody-dependent enhancement, enhancing antibodies, HIV, rhesus macaques, SHIV
Introduction
Antibodies not only protect against viral pathogens, but may also enhance disease extent/severity through antibody-dependent enhancement (ADE) – well known for example, for dengue virus [1,2], respiratory syncytial virus (RSV) [3,4], and measles virus [5] (reviewed in [6,7]). ADE is generally described as disease exacerbation with more rapid progression and/or involvement of different organ systems in the presence of enhancing antibodies. However, ADE may also occur when invading virus first interacts with hosts, leading to Antibody-Dependent Enhancement of Virus Acquisition (ADE-VA).
Since the start of the HIV/AIDS pandemic, in-vitro studies have raised the possibility of ADE for HIV [8–13] (reviewed in [14]) and revealed complement-mediated antibody-dependent enhancement (C’-ADE) as one possible mechanism for IgG-mediated ADE [15–17]. C’-ADE involves activation of complement pathways and requires expression of complement receptor 2 (CR2; CD21) and CD4 on target cells [15–18]. HIV envelope (Env)-mediated complement activation occurs by Env-C1q interaction, leading to deposition of C3 components and opsonization of virions, which then engage CD4 and CD21 along with coreceptors for target-cell entry [15,16]. Such enhancement has been demonstrated in lymphoblastoid cell lines [9,10,15–22]. Surprisingly high HIV C’-ADE levels were reported by Willey et al.[18] who tested plasma/serum samples, purified IgG, or IgM collected from individuals with early-stage HIV infection. C’-ADE assays were performed with autologous patient HIV isolates in a CD21-expressing cell line; heat inactivation or anti-CD21 monoclonal antibody (mAb) pretreatment abrogated HIV enhancement [18]. Whether in vitro C’-ADE by anti-HIV Env IgG results in enhanced virus acquisition in vivo remained unknown.
To address this question, we took advantage of chimeric simian-human immunodeficiency viruses (SHIVs) that replicate and cause disease in rhesus monkeys; SHIVs express HIV-1 envelope, rendering evaluating the biological activity of anti-HIV-1 Env antibodies possible. We isolated polyclonal IgG from macaques sampled repeatedly after SHIV infection/seroconversion; IgG fractions with significant C’-ADE activity but lacking neutralizing activity were pooled to yield a large prep termed enSHIVIG (Methods, Supplemental Digital Content).
Next, we employed a classical tool: passive immunization that establishes cause-and-effect between antibodies and clinical outcome. Using endpoint intrarectal virus titration, we asked whether intravenous enSHIVIG treatment prior to SHIV challenge would lower the minimal virus dose required to establish persistent systemic infection in macaques. Here we report that anti-HIV-1 Env IgG significantly enhanced mucosal virus acquisition.
Methods
Cell lines, reagents and virus
SupT1.R5 cells (CD4+CCR5+CR2+) were provided by J.A. Hoxie (University of Pennsylvania), A3R5.7 cells by D.C. Montefiori (Duke University), SHIV-1157ip [23] gp120 and gp160 by S.L. Hu (University of Washington), mAb Fm-6-IgG1 by W.A. Marasco (Dana-Farber Cancer Institute), and HIV-1MN gp41, consensus-clade C peptides, and CN54 gp140 [24] by the NIH AIDS Reagent Program. We generated reporter virus NL-LucR.1157ipd3N4 by cloning SHIV-1157ipd3N4 [25]env into plasmid pNL-LucR.T2A (provided by C. Ochsenbauer, University of Alabama). SHIV-1157ipd3N4 stock [grown in rhesus macaque peripheral blood mononuclear cells (PBMC)] contained 713 ng/ml of p27 and 7 × 106 50% tissue culture infectious doses (TCID50)/ml (measured in TZM-bl cells).
Isolation of polyclonal rhesus macaque IgG to generate the enSHIVIG prep
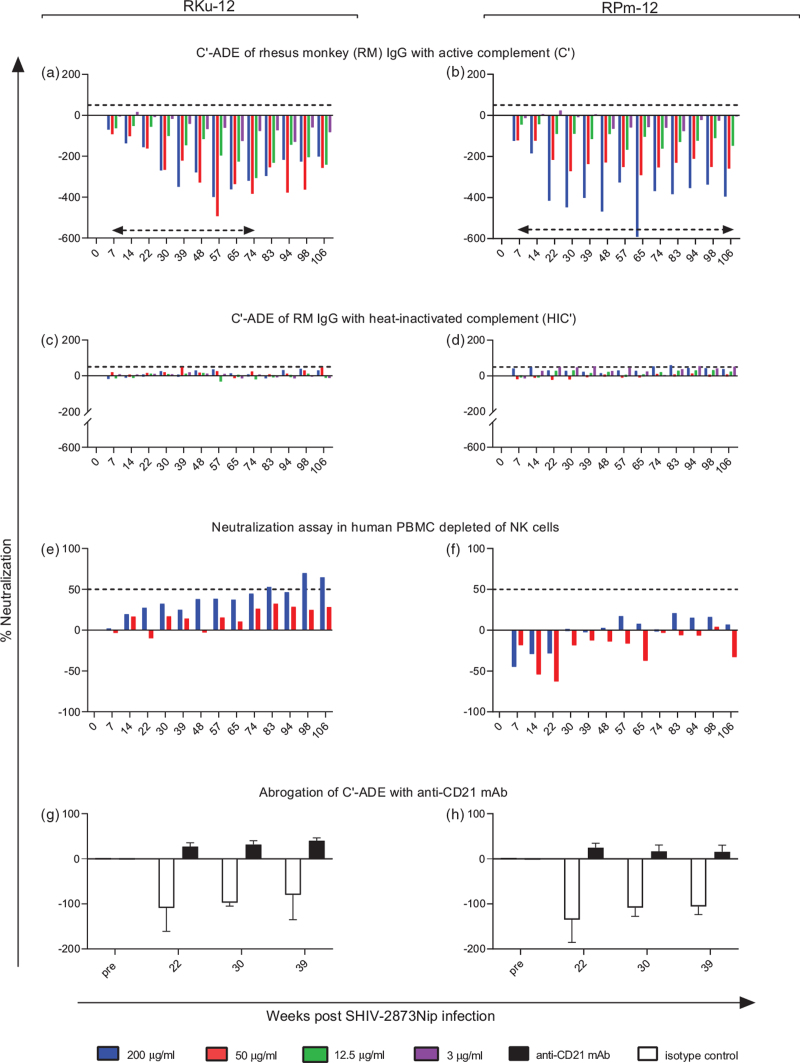
We isolated total serum IgG from virus-only controls of our previous study [26]; these macaques had early-stage SHIV-2873Nip [27] infection and seroconverted to HIV Env. IgG from individual animals/different time points were tested for C’-ADE/neutralizing activity using SupT1.R5 cells and A3R5 cells. Neutralization was also tested in human PBMC depleted of NK cells (Fig. 1, S1-S4). IgG preps of two donor macaques with the highest C’-ADE but no neutralization were pooled to yield enhancing anti-SHIV IgG (enSHIVIG), which was tested for purity (Fig. S5), sterility, and endotoxin content.
Fig. 1.
Anti-SHIV IgG responses in donor monkeys RKu-12 and RPm-12 at the weeks post SHIV-2873Nip challenge indicated.
(a and b) C’-ADE for purified IgG from donor RKu-12 (left panels) and donor RPm-12 (right panels) in the presence of human complement (C’); dashed horizontal arrows, timeframe within which IgG was pooled from each donor macaque to yield enSHIVIG (Methods, Supplemental Digital Content); (a–f), dashed horizontal lines on the positive y-axis indicate the 50% neutralization threshold. (c and d) assays with heat-inactivated C’ (HIC’); (e and f) neutralization in human PBMC depleted of NK cells; (g and h) abrogation of C’-ADE by preincubating Sup T1.R5 cells with an anti-CD21 mAb targeting complement receptor 2 (CR2/CD21); error bars represent SEM. All assays used the R5 tier 2 heterologous SHIV-1157ipd3N4 [25], our intended challenge virus for the current in-vivo studies. Negative neutralization indicates enhancement.
In-vivo end-point virus titration by mucosal SHIV-1157ipd3N4 challenge and passive immunization
All primate studies were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the USA (Methods, Supplemental Digital Content). Rhesus macaques were randomized into two groups (n = 8/group). Group 1 underwent intrarectal virus challenges at decreasing virus doses; Group 2 was pretreated with enSHIVIG (25 mg/kg) 24 h before intrarectal virus challenge. All macaques were atraumatically challenged intrarectally with decreasing virus doses using serial enrolment. Plasma samples for viral load determinations were obtained on the day of SHIV challenge and prospectively thereafter. An enSHIVIG pharmacokinetic study is described in Supplemental Digital Content.
Statistical analyses
Calculation of 50% animal infectious dose (AID50) values and statistical comparison of virus stock dilutions yielding systemic infection were performed using the Spouge method [28]. Peak viral RNA loads were compared using Wilcoxon rank-sum tests.
Results
C’-ADE versus neutralizing activity of IgG isolated from SHIV-2873Nip-infected macaques
We assessed C’-ADE and neutralizing activity for IgG isolated from individual macaques at different weeks post-challenge using a heterologous test virus; controls included heat-inactivated C’ (HIC’); early time-point IgG showed reproducible C’-ADE as indicated by negative neutralization abrogated after heat-inactivation of C’ (Fig. 1a-d). Neutralization using human NK cell-depleted PBMC revealed no neutralization ≥50% at early time points (Fig. 1e,f). To confirm C’ involvement, we blocked complement receptor CR2 with an anti-CD21 mAb which abrogated C’-ADE (Fig. 1 g,h). Overall, we screened eight SHIV-infected monkeys and selected the two with the highest enhancement in the absence of neutralization in human PBMC assays, animals RKu-12 and RPm-12; data for the entire macaque cohort, including assays for enhancement/neutralization of SHIV-1157ipd3N4 until week 106 post-inoculation in A3R5.7 cells are shown in Figs. S1-S4 (Supplemental Digital Content). Our data confirmed that IgG purified from early weeks post-SHIV inoculation yielded reproducible in-vitro enhancement that was CR2 dependent.
In-vitro enSHIVIG characterization with SHIV-1157ipd3N4 [23], the intended heterologous challenge virus
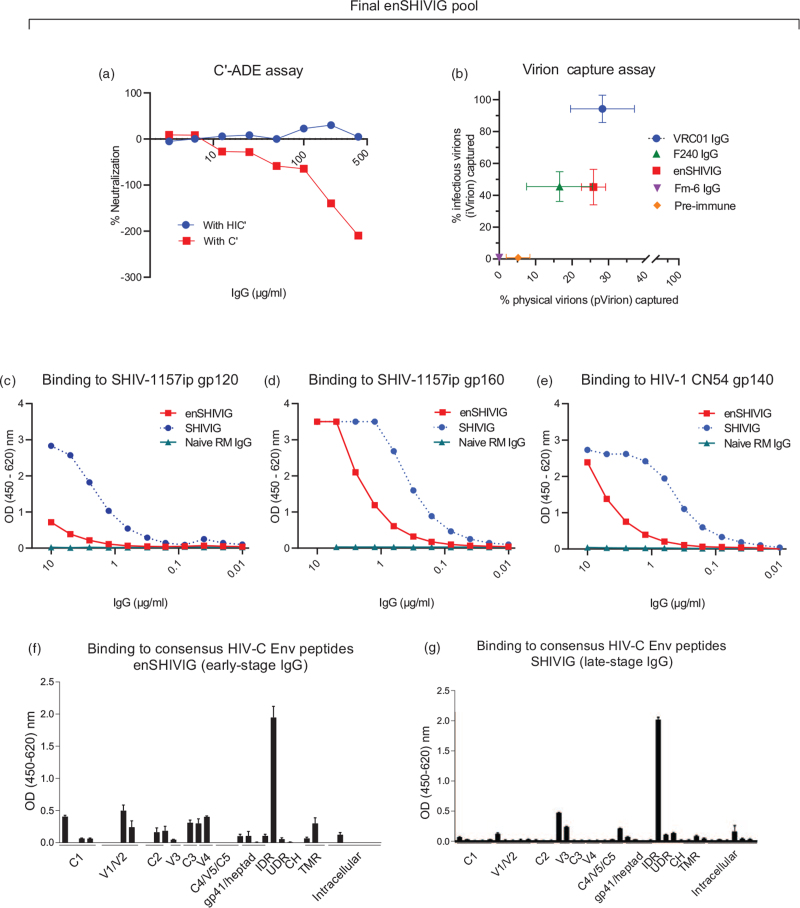
The enSHIVIG pool exhibited concentration-dependent C’-ADE that was abrogated by heat inactivation (Fig. 2a). We then assessed the ability of enSHIVIG to capture infectious or physical particles using virion capture assays (Fig. 2b) with SHIV-1157ipd3N4 [25], a phylogenetically distinct strain from SHIV-2873Nip [27], the virus harbored by enSHIVIG-donor animals. The neutralizing mAb VRC01 captured almost all infectious virions, but only ∼30% of physical particles, indicating that the virus stock contained a mixture of virions, the majority of which was noninfectious. enSHIVIG captured < 30% of physical and ∼45% of infectious SHIV-1157ipd3N4 particles, which was equivalent to the fraction captured by F240, a non-neutralizing anti-gp41 mAb (Fig. 2b). Neither preimmune IgG nor the irrelevant mAb Fm-6-IgG1 showed significant virion capture.
Fig. 2.
Characterization of pooled early-stage enhancing IgG, termed enSHIVIG.
The polyclonal rhesus monkey enSHIVIG prep (Methods, Supplemental Digital Content) was tested for C’-ADE against the heterologous reporter virus, NL-LucR.1157ipd3N4, in the presence of complement (C’) or heat-inactivated complement (HIC’). Negative neutralization indicates enhancement. (b) Capture of physical (x-axis) and infectious virions (y-axis); VRC01 IgG, neutralizing human anti-CD4 binding site mAb; F240 IgG, non-neutralizing anti-HIV gp41 mAb; Fm-6 IgG, irrelevant anti-SARS isotype control mAb; preimmune, rhesus monkey IgG isolated from naive macaques. (c) Binding to gp120 of SHIV-1157-ip [23], (d) binding to gp160 of SHIV-1157-ip, or (e) gp140 of CN54 [24] by ELISA for enSHIVIG (red squares) or SHIVIG (blue circles). SHIVIG, pooled rhesus monkey (RM) IgG from late-stage SHIV-C infection selected for high-titer cross-neutralizing IgG [21]. Binding to consensus HIV-C Env peptides by ELISA for enSHIVIG (f) or SHIVIG (g). X-axis, pools of peptides representing various HIV Env domains; C1-C5, gp120 constant regions 1–5; V1-V5, gp120 variable loops; IDR, gp41 immunodominant region; UDR, undefined region; CH, C-terminal heptad region; TMR, transmembrane region. (g) Reprinted with permission [21].
Next, we measured enSHIVIG binding to HIV gp120, gp160, or gp140 (Fig. 2c-e). We directly compared two IgG pools (i) enSHIVIG, polyclonal rhesus IgG isolated during early-stage SHIV infection and selected for maximal C’-ADE, and (ii) SHIVIG, polyclonal rhesus IgG isolated during late-stage SHIV infection and selected for maximal cross-neutralization of a heterologous tier 2 SHIV [21]. enSHIVIG bound significantly better to gp160 or gp140 (Fig. 2d,e) than to gp120 (Fig. 2c), implying predominant binding to gp41. This was less pronounced with late-stage SHIVIG (blue symbols, Fig. 2c-e). We then performed binding assays with individual consensus HIV clade C peptides; early-stage enSHIVIG (Fig. 2f) differed in epitope recognition from late-stage SHIVIG (Fig. 2 g), especially in the relative lack of anti-V3 binding. Interestingly, recognition of V1 and V2 peptides was remarkably better for enSHIVIG compared to late-stage SHIVIG. The relative absence of anti-V3 antibody responses in enSHIVIG explains the lack of neutralization in this early-stage anti-HIV Env IgG pool [29].
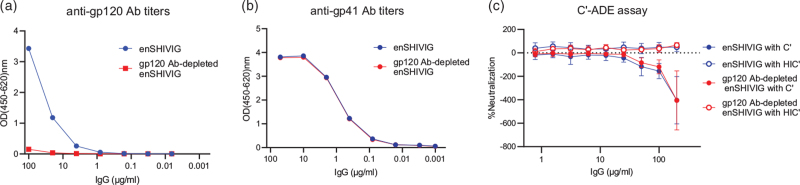
C’-ADE of enSHIVIG pool depleted of anti-gp120 antibodies
To assess the contribution of anti-HIV gp120 antibodies to C’-ADE, we depleted the enSHIVIG prep of anti-gp120 IgG with beads (Fig. 3a,b); such depletion did not change the pattern of enhancement (Fig. 3c). These data imply that C’-ADE was predominantly due to the action of anti-gp41 antibodies present as the major fraction in enSHIVIG; other investigators have identified antibodies against the immunodominant HIV gp41 region as responsible for ADE in vitro[30–32].
Fig. 3.
C’-ADE of enSHIVIG pool depleted of anti-gp120 antibodies.
Anti-HIV-C gp120 IgG was depleted from the enSHIVIG pool using the His-tagged gp120 protein (Methods, Supplemental Digital Content). (a and b) The resulting preparation (gp120 Ab-depleted enSHIVIG) and the original enSHIVIG were tested for binding activity to gp120 and gp41 by ELISA. (c) C’-ADE assays were performed in the presence of active complement (C’) or heat-inactivated complement (HIC’) for both enSHIVIG and the corresponding gp120 Ab-depleted enSHIVIG using Sup T1.R5 cells with NL-LucR.1157ipd3N4 as reporter virus as described in Methods. The experiment was repeated five times; error bars represent standard deviations.
Intrarectal SHIV-1157ipd3N4 challenge
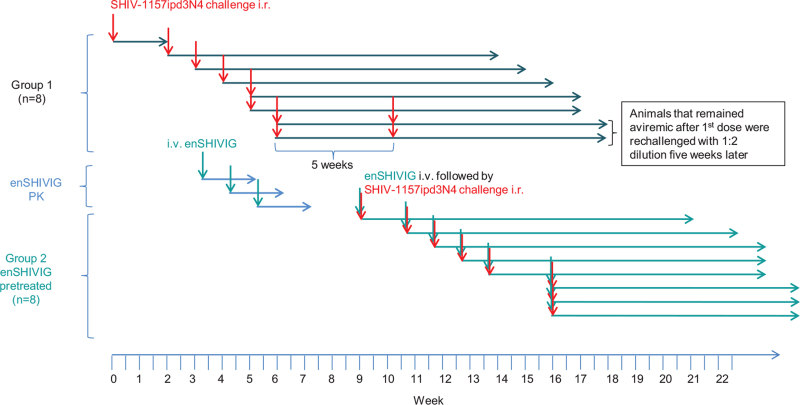
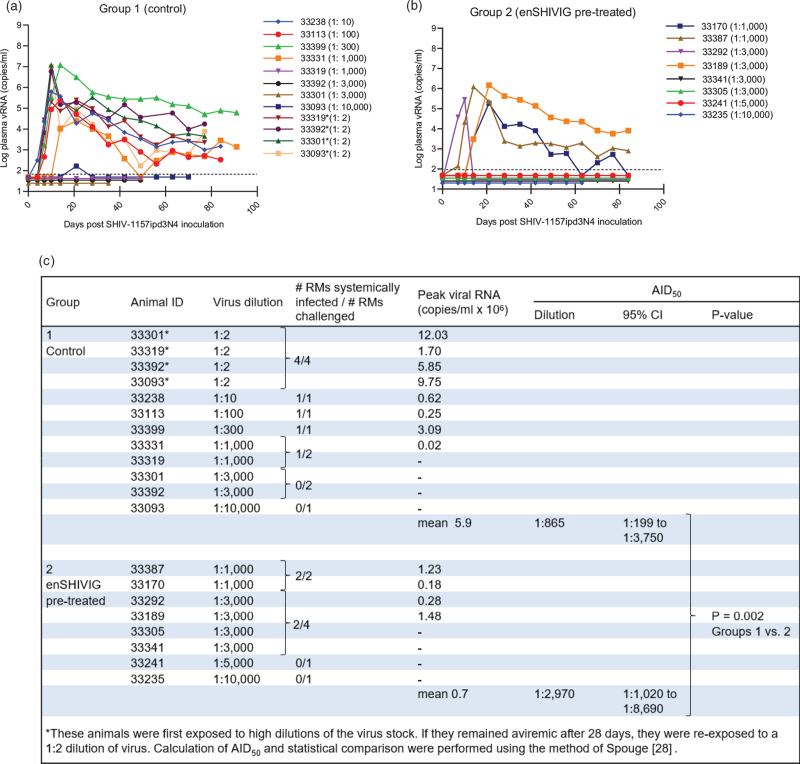
To test the hypothesis that early-stage anti-HIV Env IgG enhances in-vivo viral acquisition, we performed an end-point virus titration in macaques, using an upfront heterologous, R5 tier 2 clade C SHIV. To avoid confounding influences of different viral quasi-species, we selected an infectious molecular clone, SHIV-1157ipd3N4 [25]. We enrolled two groups of eight macaques. First, we determined minimal infectious and 50% animal infectious doses (AID50) in naive animals, which were sequentially exposed intrarectally to increasingly diluted SHIV stock (Fig. 4). After a given animal's viremia was >104 copies/ml, the next macaque was inoculated with a ten-fold higher virus-stock dilution, until a 1 : 10,000 dilution failed to infect. Subsequent animals were then exposed to intermediate dilutions; animals that remained aviremic on day 28 after initial challenge were re-exposed to a high virus inoculum (1 : 2 dilution of the stock); all such animals became viremic (Fig. 5a).
Fig. 4.
Animal study timeline.
Nineteen animals were enrolled in the study; eight in each Groups 1 and 2 and the remaining three macaques were enrolled in a pilot pharmacokinetic (PK) study of enSHIVIG. Eight animals in Group 1 were inoculated sequentially with different doses of the challenge virus, SHIV-1157ipd3N4 (red arrows). The three animals in the PK study were given different doses of enSHIVIG (green arrows); (Fig. S6, Supplemental Digital Content). All animals in Group 2 were also enrolled sequentially; each animal received 25 mg/kg of enSHIVIG 24 h before i.r. SHIV-1157ipd3N4 challenge with different doses (red and green arrows).
Fig. 5.
Plasma viral RNA (vRNA) loads after intrarectal SHIV-1157ipd3N4 challenge.
(a) Control, (b) enSHIVIG-pretreated rhesus monkeys (RMs). Dashed lines, RT-PCR assay limit of detection (50 vRNA copies/ml). The animals were enrolled sequentially. (c) Comparison of infection rate (number of RMs infected/number of RMs challenged) between Groups 1 and 2.
Next, we serially enrolled Group 2 animals (Fig. 5b); 24 h before intrarectal SHIV challenge, each was given intravenous enSHIVIG (25 mg/kg), a dose based on our earlier SHIVIG experiment [21] and a pilot pharmacokinetic study (Fig. S6, Supplemental Digital Content). The first macaque was exposed to a 1 : 1,000 virus-stock dilution, resulting in high viremia. After a 1 : 10,000 dilution failed to infect, dilutions between 1 : 1,000 and 1 : 3,000 were used. AID50 values were calculated for both groups and statistical comparisons were performed using the Spouge method [28]. For enSHIVIG-pretreated macaques, the AID50 corresponded to a 1 : 2,970 virus stock dilution, compared to a 1 : 865 dilution for naive controls. This translates to requiring 3.4x less virus for the enSHIVIG-treated animals compared to controls (P = 0.002). While the mean peak vRNA loads between the two groups differed by 0.9 logs, this difference was not significant (P = 0.202, Wilcoxon rank-sum test). We conclude that early-phase anti-HIV Env IgG significantly enhanced SHIV transmission and gave proof-of-concept for ADE-VA. Passive immunization established the polyclonal enSHIVIG as the sole cause for this increased virus acquisition.
Discussion
Here we showed: i) enSHIVIG, when passively administered to macaques, enhanced virus acquisition and significantly lowered the amount of virus needed to achieve viremia compared to naive controls; ii) ex-vivo enSHIVIG testing in the presence of active complement revealed significant C’-ADE activity that was abrogated by C’ heat inactivation or anti-CD21 mAb. These results indicate that antibodies generated during early-stage HIV/SHIV infection may increase host susceptibility and facilitate virus acquisition and early dissemination.
Previously [21], we had treated macaques biweekly with different intravenous doses of SHIVIG, the polyclonal high-titer neutralizing IgG, in order to link in-vitro neutralization titers with prevention of mucosal SHIV acquisition. Unexpectedly, animals pretreated with low-dose SHIVIG (25 mg/kg) had more viral quasispecies compared to untreated controls – implying increased SHIV transmission. Despite good SHIVIG neutralizing activity in TZM-bl cells, enhancement was observed in the presence of active complement in CR2/CD21-expressing SupT1.R5 cells that was abrogated by complement heat inactivation [21]. Together, these findings reinforce our current data that weakly or non-neutralizing neutralizing IgG may enhance mucosal SHIV acquisition through mechanisms dependent on complement activation.
It is intriguing to compare the 3.4-fold enhanced mucosal SHIV-1157ipd3N4 acquisition we report here with the magnitude of in-vitro HIV enhancement by Willey et al.[18] who measured C’-ADE in CR2-expressing SupT1/R5 cells using paired autologous early-stage sera/HIV isolates. Enhancement ranged from 8- to 236-fold and was lower when assessed with heterologous virus isolates. Differences in the order-of-magnitude of HIV C’-ADE reported [18] and our 3.4-fold lowering of the SHIV challenge dose needed to persistently infect enSHIVIG-pretreated macaques can be ascribed to CR2 expression by all SupT1.R5 cells used for in-vitro assays. In vivo, however, CR2 is expressed only by select cell populations, such as B cells, follicular dendritic cells, and according to a recent report [33], on naive CD4+ and CD8+ T cells.
In addition to C’-ADE, in-vitro assays have revealed another mechanism: Fc receptor-mediated ADE (FcR-ADE) [11,13,34–37] (reviewed in [38,39]). Monocyte/macrophage-derived cell lines expressing different FcRs were used to demonstrate FcR-ADE. Forthal et al.[40] provided indirect evidence of FcR-ADE from a Phase III AIDS vaccine trial; by subgroup analysis, a statistically significant association was noted between increased HIV acquisition and the FcγRIIIa allele in vaccinees given monomeric gp120.
Our present data as well as those summarized above from prior studies have one common denominator: the IgGs were polyclonal. As such, we cannot distinguish between two possibilities for ADE: i) polyclonal IgG consists of a mixture inherently neutralizing and inherently enhancing antibodies; and ii) a given IgG neutralizes in one situation and enhances in another. This key issue can only be addressed by using mAbs – done in a seminal study by Kliks et al.[41] who examined the interaction of two different human anti-V3 mAbs with three different HIV-1 strains. Depending on the virus tested, the results yielded either neutralization, enhancement, or neither. Thus, well characterized mAbs are unpredictable in their interactions with different HIV strains. Enhancing antibodies have also been implicated in mother-to-child transmission of HIV in a number of studies [42–44]; some reports raised the possibility that enhancement may be linked to antibodies targeting HIV-1 gp41 [43–45].
Although different investigators have shown HIV ADE in various cell line-based assays over the years, whether such in-vitro data would translate into Antibody-Dependent Enhanced Virus Acquisition – ADE-VA – remained unsolved. Passive immunization of macaques with early-stage anti-SHIV IgG followed by intrarectal SHIV challenge gave proof-of-principle for increased virus acquisition and host susceptibility. AIDS vaccine development should consider the potential of ADE-VA due to vaccine-induced antibodies during experimental vaccine trials. To rule out this possibility, passive immunization with vaccine-induced antibodies could be used as a tool in biologically relevant animal models, that is, models that reflect key aspects of HIV transmission among humans, including i) tier 2 R5 challenge viruses carrying HIV-1 Env, ii) a nonhuman primate species, and iii) antibodies that are heterologous to the challenge viruses. The latter point is important since matched homologous virus/antibody systems will exaggerate neutralization and thereby mask potential enhancement by weakly or non-neutralizing antibodies. In the realistic setting of human vaccinees’ exposure to circulating HIV strains, an exact match between immunogen composition and the myriad of HIV quasispecies can never be expected.
Indirect evidence that vaccine-induced antibodies can have adverse effects comes from a feline immunodeficiency virus (FIV) study, where cats were vaccinated with various recombinant envelope glycoproteins [46]. Although neutralization in cell-line based assays was observed in plasma samples from some vaccinated groups, no virus-neutralizing antibodies were detected in the feline lymphocyte assay. Upon FIV challenge, cell-associated FIV loads were increased in the groups vaccinated with recombinant FIV Env glycoproteins compared to other groups or controls. Passive transfer of unfractionated plasma from groups with increased cell-associated FIV enhanced viral infection parameters in the recipients. While these data imply ADE, an influence of other factor(s) present in unfractionated plasma cannot be ruled out.
In sum, AIDS virus C’-ADE is real – as our passive immunization showed significant lowering of the virus dose needed to achieve viremia indicative of ADE-VA. As such, the current study with early-stage enSHIVIG confirmed our unexpected finding with late-stage SHIVIG, selected for maximal in-vitro tier 2 SHIV cross-neutralization, where low-dose pretreatment yielded sub-neutralizing anti-HIV Env IgG levels that significantly increased the number of transmitted viral quasispecies. Together, our data imply that decreasing anti-HIV Env neutralizing antibody titers could bring vaccinated individuals into a situation where ADE-VA prevails.
ADE-VA may be of concern for other pathogens, especially rapidly mutating RNA viruses susceptible to neutralization escape. Vaccine development will need to consider potential enhancement of host susceptibility to infection due to ADE [47,48]. We propose that our strategy – passive immunization with purified polyclonal IgG isolated from previously infected/vaccinated individuals, combined with in-vivo end-point virus titration to assess the amount of virus needed to achieve infection of naïve versus passively immunized animals, can play an important role in assessing the potential for ADE-VA.
Acknowledgements
We thank F. Villinger and S. Gong (UL Lafayette/NIRC) for critical reading of the manuscript; A. Gray and E. Plake (Texas Biomed) for assistance with in vitro assays, J. Hoxie (University of Pennsylvania) for providing the SupT1.R5 cell line, C. Ochsenbauer (University of Alabama, Birmingham) for plasmid pNL-LucR.T2A. We thank K. Brasky and P. Frost for coordinating the primate studies and S. Joubran for assistance with the preparation of the manuscript. The following reagents were obtained through the NIH HIV Reagent Program, Division of AIDS, NIAID, NIH: HIV-1MN gp41 recombinant protein made in E. coli, ARP-12027 (contributed by DAIDS/NIAID; produced by ImmunoDX, LLC); recombinant HIV-1 CN54 gp140 from CHO cells, ARP-12064 (contributed by DAIDS/NIAID; produced by Polymun Scientific, Inc.).
Funding: This work was supported by NIH grants R01 DE023049 and U19 AI142636 to R.M.R.
Author contributions: H.K.V. generated the enSHIVIG pool, H.K.V, S.K.L, B.M., and A.A. characterized enSHIVIG and SHIVIG pools; D.H. determined viral RNA loads, S.J.R. performed statistical analyses, H.K.V., B.M., and R.M.R. generated the illustrations; R.M.R conceived and supervised the research; B.M. and R.M.R wrote the manuscript; all authors reviewed/edited the manuscript and approved the final version.
Conflicts of interest
The authors declare no competing financial interests.
Supplementary Material
Bishal Marasini, Hemant K. Vyas, and Samir K. Lakhashe equal contributions.
Supplemental digital content is available for this article.
References
- 1.Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol 2013; 158:1445–1459. [DOI] [PubMed] [Google Scholar]
- 2.Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, et al. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017; 358:929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 1969; 89:405–421. [DOI] [PubMed] [Google Scholar]
- 4.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89:422–434. [DOI] [PubMed] [Google Scholar]
- 5.Polack FP, Hoffman SJ, Crujeiras G, Griffin DE. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat Med 2003; 9:1209–1213. [DOI] [PubMed] [Google Scholar]
- 6.Arvin AM, Fink K, Schmid MA, Cathcart A, Spreafico R, Havenar-Daughton C, et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 2020; 584:353–363. [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni R. Bramhachari PV. Antibody-Dependent Enhancement of Viral Infections. Dynamics of immune activation in viral diseases.. Singapore: Springer Singapore; 2020. 9–41. [Google Scholar]
- 8.Robinson WE, Jr, Montefiori DC, Mitchell WM. A human immunodeficiency virus type 1 (HIV-1) infection-enhancing factor in seropositive sera. Biochem Biophys Res Commun 1987; 149:693–699. [DOI] [PubMed] [Google Scholar]
- 9.Robinson WE, Jr, Montefiori DC, Mitchell WM. Antibody-dependent enhancement of human immunodeficiency virus type 1 infection. Lancet 1988; 1:790–794. [DOI] [PubMed] [Google Scholar]
- 10.Robinson WE, Jr, Montefiori DC, Mitchell WM, Prince AM, Alter HJ, Dreesman GR, et al. Antibody-dependent enhancement of human immunodeficiency virus type 1 (HIV-1) infection in vitro by serum from HIV-1-infected and passively immunized chimpanzees. Proc Natl Acad Sci U S A 1989; 86:4710–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeda A, Tuazon CU, Ennis FA. Antibody-enhanced infection by HIV-1 via Fc receptor-mediated entry. Science 1988; 242:580–583. [DOI] [PubMed] [Google Scholar]
- 12.Homsy J, Meyer M, Levy JA. Serum enhancement of human immunodeficiency virus (HIV) infection correlates with disease in HIV-infected individuals. J Virol 1990; 64:1437–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homsy J, Tateno M, Levy J. Antibody-dependent enhancement of HIV infection. Lancet 1988; 1:1285–1286. [PubMed] [Google Scholar]
- 14.Gorlani A, Forthal DN. Antibody-dependent enhancement and the risk of HIV infection. Curr HIV Res 2013; 11:421–426. [DOI] [PubMed] [Google Scholar]
- 15.Prohaszka Z, Nemes J, Hidvegi T, Toth FD, Kerekes K, Erdei A, et al. Two parallel routes of the complement-mediated antibody-dependent enhancement of HIV-1 infection. AIDS 1997; 11:949–958. [DOI] [PubMed] [Google Scholar]
- 16.Robinson WE. Mechanism for complement-mediated, antibody-dependent enhancement of human immunodeficiency virus type 1 infection in MT2 cells is enhanced entry through CD4, CD21, and CXCR4 chemokine receptors. Viral Immunol 2006; 19:434–447. [DOI] [PubMed] [Google Scholar]
- 17.Robinson WE, Jr, Montefiori DC, Mitchell WM. Complement-mediated antibody-dependent enhancement of HIV-1 infection requires CD4 and complement receptors. Virology 1990; 175:600–604. [DOI] [PubMed] [Google Scholar]
- 18.Willey S, Aasa-Chapman MM, O’Farrell S, Pellegrino P, Williams I, Weiss RA, et al. Extensive complement-dependent enhancement of HIV-1 by autologous nonneutralising antibodies at early stages of infection. Retrovirology 2011; 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund O, Hansen J, Soorensen AM, Mosekilde E, Nielsen JO, Hansen JE. Increased adhesion as a mechanism of antibody-dependent and antibody-independent complement-mediated enhancement of human immunodeficiency virus infection. J Virol 1995; 69:2393–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson WE, Jr, Montefiori DC, Gillespie DH, Mitchell WM. Complement-mediated, antibody-dependent enhancement of HIV-1 infection in vitro is characterized by increased protein and RNA syntheses and infectious virus release. J Acquir Immune Defic Syndr 1989; 2:33–42. [PubMed] [Google Scholar]
- 21.Sholukh AM, Byrareddy SN, Shanmuganathan V, Hemashettar G, Lakhashe SK, Rasmussen RA, et al. Passive immunization of macaques with polyclonal anti-SHIV IgG against a heterologous tier 2 SHIV: outcome depends on IgG dose. Retrovirology 2014; 11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szabo J, Prohaszka Z, Toth FD, Gyuris A, Segesdi J, Banhegyi D, et al. Strong correlation between the complement-mediated antibody-dependent enhancement of HIV-1 infection and plasma viral load. AIDS 1999; 13:1841–1849. [DOI] [PubMed] [Google Scholar]
- 23.Humbert M, Rasmussen RA, Song R, Ong H, Sharma P, Chenine AL, et al. SHIV-1157i and passaged progeny viruses encoding R5 HIV-1 clade C env cause AIDS in rhesus monkeys. Retrovirology 2008; 5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su L, Graf M, Zhang Y, von Briesen H, Xing H, Kostler J, et al. Characterization of a virtually full-length human immunodeficiency virus type 1 genome of a prevalent intersubtype (C/B’) recombinant strain in China. J Virol 2000; 74:11367–11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song RJ, Chenine AL, Rasmussen RA, Ruprecht CR, Mirshahidi S, Grisson RD, et al. Molecularly cloned SHIV-1157ipd3N4: a highly replication- competent, mucosally transmissible R5 simian-human immunodeficiency virus encoding HIV clade C Env. J Virol 2006; 80:8729–8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakhashe SK, Byrareddy SN, Zhou M, Bachler BC, Hemashettar G, Hu SL, et al. Multimodality vaccination against clade C SHIV: partial protection against mucosal challenges with a heterologous tier 2 virus. Vaccine 2014; 32:6527–6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siddappa NB, Song R, Kramer VG, Chenine AL, Velu V, Ong H, et al. Neutralization-sensitive R5-tropic simian-human immunodeficiency virus SHIV-2873Nip, which carries env isolated from an infant with a recent HIV clade C infection. J Virol 2009; 83:1422–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spouge JL. Statistical analysis of sparse infection data and its implications for retroviral treatment trials in primates. Proc Natl Acad Sci U S A 1992; 89:7581–7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hioe CE, Wrin T, Seaman MS, Yu X, Wood B, Self S, et al. Anti-V3 monoclonal antibodies display broad neutralizing activities against multiple HIV-1 subtypes. PLoS One 2010; 5:e10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson WE, Jr, Gorny MK, Xu JY, Mitchell WM, Zolla-Pazner S. Two immunodominant domains of gp41 bind antibodies which enhance human immunodeficiency virus type 1 infection in vitro. J Virol 1991; 65:4169–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson WE, Jr, Kawamura T, Gorny MK, Lake D, Xu JY, Matsumoto Y, et al. Human monoclonal antibodies to the human immunodeficiency virus type 1 (HIV-1) transmembrane glycoprotein gp41 enhance HIV-1 infection in vitro. Proc Natl Acad Sci U S A 1990; 87:3185–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson WE, Jr, Kawamura T, Lake D, Masuho Y, Mitchell WM, Hersh EM. Antibodies to the primary immunodominant domain of human immunodeficiency virus type 1 (HIV-1) glycoprotein gp41 enhance HIV-1 infection in vitro. J Virol 1990; 64:5301–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith NA, Coleman CB, Gewurz BE, Rochford R. CD21 (Complement Receptor 2) Is the Receptor for Epstein-Barr Virus Entry into T Cells. J Virol 2020; 94:e00428-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haubrich RH, Takeda A, Koff W, Smith G, Ennis FA. Studies of antibody-dependent enhancement of human immunodeficiency virus (HIV) type 1 infection mediated by Fc receptors using sera from recipients of a recombinant gp160 experimental HIV-1 vaccine. J Infect Dis 1992; 165:545–548. [DOI] [PubMed] [Google Scholar]
- 35.Homsy J, Meyer M, Tateno M, Clarkson S, Levy JA. The Fc and not CD4 receptor mediates antibody enhancement of HIV infection in human cells. Science 1989; 244:1357–1360. [DOI] [PubMed] [Google Scholar]
- 36.Takeda A, Sweet RW, Ennis FA. Two receptors are required for antibody-dependent enhancement of human immunodeficiency virus type 1 infection: CD4 and Fc gamma R. J Virol 1990; 64:5605–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trischmann H, Davis D, Lachmann PJ. Lymphocytotropic strains of HIV type 1 when complexed with enhancing antibodies can infect macrophages via Fc gamma RIII, independently of CD4. AIDS Res Hum Retroviruses 1995; 11:343–352. [DOI] [PubMed] [Google Scholar]
- 38.Bournazos S, Gupta A, Ravetch JV. The role of IgG Fc receptors in antibody-dependent enhancement. Nat Rev Immunol 2020; 20:633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor A, Foo SS, Bruzzone R, Dinh LV, King NJ, Mahalingam S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol Rev 2015; 268:340–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forthal DN, Gabriel EE, Wang A, Landucci G, Phan TB. Association of Fcgamma receptor IIIa genotype with the rate of HIV infection after gp120 vaccination. Blood 2012; 120:2836–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kliks SC, Shioda T, Haigwood NL, Levy JA. V3 variability can influence the ability of an antibody to neutralize or enhance infection by diverse strains of human immunodeficiency virus type 1. Proc Natl Acad Sci 1993; 90:11518–11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kliks SC, Wara DW, Landers DV, Levy JA. Features of HIV-1 that could influence maternal-child transmission. JAMA 1994; 272:467–474. [PubMed] [Google Scholar]
- 43.Diomede L, Nyoka S, Pastori C, Scotti L, Zambon A, Sherman G, et al. Passively transmitted gp41 antibodies in babies born from HIV-1 subtype C-seropositive women: correlation between fine specificity and protection. J Virol 2012; 86:4129–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naiman NE, Slyker J, Nduati R, Overbaugh JM. Maternal Envelope gp41 Ectodomain-Specific Antibodies Are Associated With Increased Mother-to-Child Transmission of Human Immunodeficiency Virus-1. J Infect Dis 2020; 221:232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tranchat C, Van de Perre P, Simonon-Sorel A, Karita E, Benchaib M, Lepage P, et al. Maternal humoral factors associated with perinatal human immunodeficiency virus type-1 transmission in a cohort from Kigali, Rwanda, 1988–1994. J Infect 1999; 39:213–220. [DOI] [PubMed] [Google Scholar]
- 46.Siebelink KH, Tijhaar E, Huisman RC, Huisman W, de Ronde A, Darby IH, et al. Enhancement of feline immunodeficiency virus infection after immunization with envelope glycoprotein subunit vaccines. J Virol 1995; 69:3704–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwasaki A, Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol 2020; 20:339–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol 2020; 5:1185–1191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.