Abstract
Background:
Up to 20% of patients with acute myeloid leukemia (AML) present with hyperleukocytosis, usually defined as a white blood cell count (WBC) >100 ×109/L. Given the high early mortality rate, emergent cytoreduction with either leukapheresis, hydroxyurea or chemotherapy is indicated but the optimal strategy is unknown.
Study design and Methods:
For this systematic review and meta-analysis we searched MEDLINE and EMBASE via Ovid, Scopus, COCHRANE registry of clinical trials (CENTRAL), and Web of Science from inception through 03/2020 for multi-arm studies comparing early mortality rates of AML patients treated with leukapheresis and those who were not. The risk ratio (RR) of early death for patients who received leukapheresis vs. patients who did not was estimated using a sum of the log-ratio of individual study estimates weighted by sample size.
Results:
Among 13 two-arm, retrospective studies with 1743 patients (486 leukapheresis and 1257 non-leukapheresis patients) leukapheresis did not improve the primary outcome of early mortality compared to treatment strategies that they did not employ leukapheresis (RR: 0.88 [95% CI 0.69–1.13, p=0.321]) without statistically significant heterogeneity between studies (Cochran’s Q: 18 [p=0.115]; I2: 33.4%). Patients presenting with clinical leukostasis tended to be more likely to receive leukapheresis (OR: 2.01 [95% CI 0.99–4.08, p=0.052]).
Conclusion:
As we did not find evidence of a short-term mortality benefit and considering the associated complications and logistic burden, our results argue against the routine use of leukapheresis for hyperleukocytosis among AML patients.
Keywords: Leukapheresis, hyperleukocytosis, leukostasis, meta-analysis, acute myeloid leukemia, AML
Introduction:
Acute myeloid leukemia (AML) is the most common form of adult acute leukemia in the United States (US) and up to 20% of AML patients present with a white blood cell count (WBC) of greater than 100 ×109/L, often referred to as hyperleukocytosis.(1–3) Mortality rates as high as 8% and 29% within 24-hour and one-week periods, respectively, have been reported.(4–6) Therefore, hyperleukocytosis constitutes a hematologic emergency and emergent cytoreduction is often indicated. The high early mortality rate in patients with hyperleukocytosis has been attributed to the greater frequency of leukostasis, disseminated intravascular coagulation (DIC), and tumor lysis syndrome (TLS).(1, 3)
Cytoreduction can be achieved either by mechanical removal of WBC via leukapheresis or pharmacologic strategies such as hydroxyurea or immediate initiation of intensive chemotherapy, but the ideal therapeutic approach in the absence of randomized clinical trials remains controversial.(1, 7–10) As hydroxyurea can rapidly lower the blast cell count within a few days in most patients and has limited side effects,(8) guidelines issued by the National Comprehensive Cancer Network (NCCN) and the European LeukemiaNet (ELN) recommend considering hydroxyurea for pre-chemotherapy cytoreduction.(7, 11) Conversely, leukapheresis is associated with significant logistical obstacles both in terms of personnel and equipment as well as procedural risks related to large bore venous access, anticoagulation, and electrolyte and fluid shifts, which requires careful consideration of its risks and benefits.(10) In the 2019 consensus guidelines from the American Society for Apheresis, leukapheresis is considered a category II recommendation (acceptable second-line therapy) for patients with symptomatic hyperleukocytosis (i.e. leukostasis) and a category III (role not established) recommendation in cases of asymptomatic hyperleukocytosis.(12)
In a recent online survey among Eastern Cooperative Oncology Group (ECOG) members, 79.2% of respondents stated that they would use leukapheresis followed by induction chemotherapy in hyperleukocytic AML patients presenting with leukostasis and 32.8% would do so even in the absence of symptoms of leukostasis.(13) Given the ongoing debate regarding the use of leukapheresis and the expanding literature on this controversial topic, we conducted a systematic review and meta-analysis to synthesize the current evidence on the efficacy of leukapheresis for cytoreduction in AML patients presenting with hyperleukocytosis. Our meta-analysis included 1743 AML patients presenting with hyperleukocytosis (486 leukapheresis and 1257 non-leukapheresis patients) and is the largest such study in this patient population to date.
Study design and Methods:
Search strategy:
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines.(14) We used a combination of free-text terms linked by Boolean operators ([Acute myeloid leukemia OR AML] AND [Leukapheresis OR Leukocytapheresis]) to search MEDLINE and EMBASE via Ovid, the COCHRANE registry of clinical trials (CENTRAL), Scopus and the Web of Science electronic databases without language restriction from inception through March 18th, 2020. We also conducted a manual search of the reference lists of included studies.
After removal of duplicates, the titles and abstracts of all retrieved studies were reviewed for eligibility. Based on predefined criteria, studies were excluded during abstract review if they were (I) not on AML or therapeutic leukapheresis, (II) review articles, (III) basic research articles, (IV) commentaries without reporting of original data, or (V) if an English full-text was unavailable. Subsequently, full texts of the remaining studies were reviewed for eligibility and studies were excluded if they (I) were duplicate publications from the same patient cohort, (II) were clinical trials without published results, (III) were technical reports without clinical data, (IV) had insufficient reporting of the primary endpoint (e.g. single-arm studies without control group), (V) only reported results of leukapheresis combined with other modalities (e.g. exchange transfusion), or (VI) were case series with < 5 patients. Figure 1 illustrates the study selection process. We did not explicitly exclude studies on patients with acute promyelocytic leukemia (APL) and studies on both newly diagnosed and relapsed AML were eligible for inclusion.
Figure 1: Study selection flow chart:
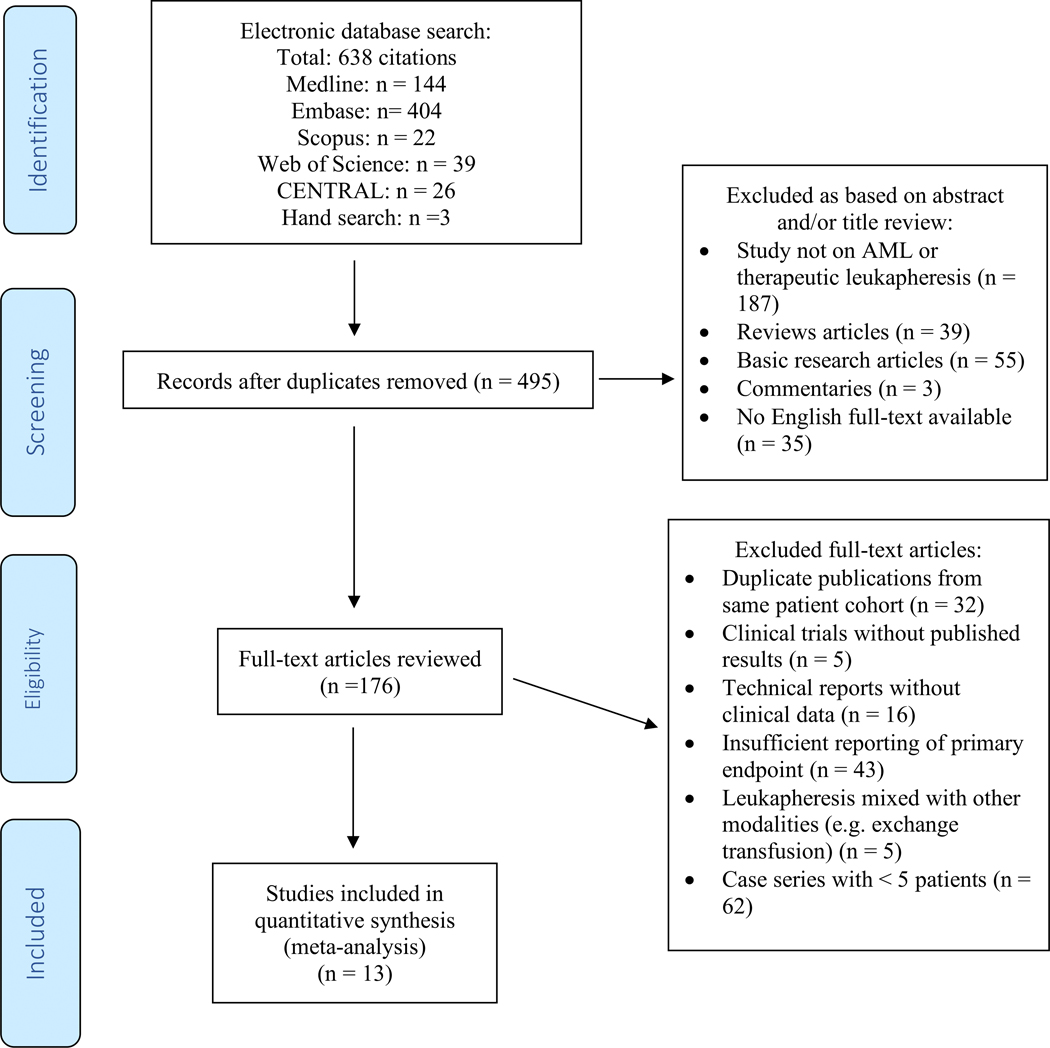
MEDLINE and EMBASE via Ovid, the COCHRANE registry of clinical trials (CENTRAL), Scopus and the Web of Science electronic databases were searched using a combination a combination of the following free-text terms linked by Boolean operators: [Acute myeloid leukemia OR AML] AND [Leukapheresis OR Leukocytapheresis]. We also conducted a manual search of the reference lists of included studies. After removal of duplicates, the titles and abstracts were reviewed and based on predefined criteria studies were excluded during this stage if they were (I) not on AML or therapeutic leukapheresis, (II) review articles, (III) basic research articles, (IV), commentaries without reporting of original data, or (V) if an English full-text was unavailable. Subsequently, full texts of the remaining studies were reviewed for eligibility and studies were excluded if they (I) were duplicate publications from same patient cohort, (II) were clinical trials without published results, (III) were technical reports without clinical data, (IV) had insufficient reporting of primary endpoint (e.g. single-arm studies), (V) only reported results of leukapheresis combined with other modalities (e.g. exchange transfusion), or (VI) were case series with < 5 patients. The final cohort for inclusion in this meta-analysis included 13 studies.
Quality assessment:
Data was collected using a standardized data-extraction form. Study quality was assessed using a Downs and Black checklist as published previously.(15, 16) The Downs and Black checklist contains 27 items with a maximum score of 28 points (with higher scores representing higher methodological quality) and has been validated for quality assessment for both randomized and non-randomized studies.(15) Quality of evidence for the primary outcome and risk of bias were assessed using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach.
Definition of endpoints:
The primary outcome was the risk ratio (RR) of early death for AML patients with hyperleukocytosis who underwent leukapheresis compared to AML patients with hyperleukocytosis who did not undergo leukapheresis. We used the definitions of hyperleukocytosis and leukostasis provided by the included studies. Early death was defined by the authors of the original studies with definitions ranging from mortality within a timespan of up to 30 days following admission to death during induction chemotherapy (Table 1). We only included studies that reported rates of death in patients who underwent leukapheresis and those who did not to construct a RR among the two groups subject to an identical outcome definition. Secondary outcome was the odds ratio (OR) of clinical leukostasis among patients treated with leukapheresis compared to patients who did not undergo leukapheresis.
Table 1:
Treatment characteristics, outcomes and adverse effects of studies of leukapheresis in AML
| Author | Group assignment | N (patients) | Median patient age (years) | Male sex (%) | Median baseline Hgb (g/dL) | Median baseline WBC (x109/L) | Median baseline platelets (x109/L) | Presence of leukostasis (%, [pulm, CNS]) | Pre-leukapheresis cytoreduction | Subsequent treatment | WBC reduction post-leukapheresis | Leukapheresis sessions (Median [Range]) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bug et al.(17) | Leukapheresis | 25 | 49.9 | 40.0% | 9.4 | 186; 100% with WBC >100 | 57 | 52% (52%, not reported) | 0% | 72% intensive chemo | 47% | 1 [1–5] | 21-day mortality: 16% |
| Non-leukapheresis | 28 | 49.4 | 50.0% | 8.9 | 166; 100% with WBC >100 | 49 | 43% (43%, not reported) | 0% | 93% intensive chemo | N/A | N/A | 21-day mortality: 32% | |
| Choi et al.(18) | Leukapheresis | 22 | 52 | 40.9% | 7.6 | 186; 100% with WBC >100 | 59 | 86% (18%, 27%) | Not reported | 100% intensive chemo | Not reported | 1 [1–3] | 14-day mortality: 18% |
| Non-leukapheresis | 22 | 56 | 59.1% | 6.8 | 142; 100% with WBC >100 | 51 | 86% (32%, 59%) | Not reported | 100% intensive chemo | N/A | N/A | 14-day mortality: 23% | |
| Giles et al.(19) | Leukapheresis | 71 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 100% intensive chemo | Not reported | 1 [1–4] | 28-day mortality: 23% |
| Non-leukapheresis | 75 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 100% intensive chemo | N/A | N/A | 28-day mortality: 25% | |
| Kuo et al.(20) | Leukapheresis | 41 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 44% hydroxyurea | 93% intensive chemo | 34% | Not reported | 28-day mortality: 32% |
| Non-leukapheresis | 47 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 47% hydroxyurea | 96% intensive chemo | 34% | N/A | 28-day mortality: 21% | |
| Malkan et al.(21) | Leukapheresis | 10 | 48 | 50.0% | 9.05 | 152.5; 100% with WBC >100 | 44.5 | 40% (not reported) | Not reported | Not reported | 31% | 1 [1–3] | 14-day mortality: 50% |
| Non-leukapheresis | 18 | 54.5 | 61.1% | 8.95 | 128.5; 100% with WBC >100 | 42 | Not reported | Not reported | Not reported | N/A | N/A | 14-day mortality: 28% | |
| Martinez-Cuadron et al.(22) | Leukapheresis | 18 | 55 | 44.4% | 9.1 | 198; 100% with WBC >100 | 38 | 61% (not reported) | Not reported | 56% intensive chemo | Not reported | Not reported | 28-day mortality: 50% |
| Non-leukapheresis | 36 | 63 | 52.8% | 9.7 | 189 | 53 | 50% (not reported) | Not reported | 53% intensive chemo | N/A | N/A | 28-day mortality: 50% | |
| Nan et al.(23) | Leukapheresis | 26 | 60 | 69.2% | 8.3 | 163.5 | 22.5 | 85% (not reported) | 81% hydroxyurea | 77% intensive chemo, 15% low-intensity treatment | 55% | 1 [1–3] | 28-day mortality: 38% |
| Non-leukapheresis | 26 | 64.5 | 38.5% | 9.2 | 101.3 | 36 | 65% (not reported) | 65% hydroxyurea | 65% intensive chemo, 19% low-intensity treatment | N/A | N/A | 28-day mortality: 62% | |
| Pastore et al.(24) | Leukapheresis | 20 | 62 | Not reported | Not reported | 208; 100% with WBC >100 | Not reported | 70% (70%, 20%) | Not reported | 100% intensive chemo | 77% | 1 [1–3] | 28-day mortality: 30%; CR: 50%; median OS: 8.8 months |
| Non-leukapheresis | 32 | 60 | Not reported | Not reported | 142; 100% with WBC >100 | Not reported | 23% (23%, 0%) | Not reported | 100% intensive chemo | N/A | N/A | 28-day mortality: 22% | |
| Shallis et al.(6) | Leukapheresis | 32 | 71 | 46.9% | 8.3 | 177 | 45 | 63% (63%, 25%) | Not reported | All non-intensive therapy | Not reported | Not reported | 30-day mortality: 52% |
| Non-leukapheresis | 187 | 76 | 59.9% | 9.1 | 118 | 32 | 28% (50%, 14%) | Not reported | All non-intensive therapy | N/A | N/A | 30-day mortality: 58% | |
| Stahl et al.(25) | Leukapheresis | 113 | 55 | 50.4% | 9.3 | 175 | 46.5 | 56% (45%, 45%) | 2.7% hydroxyurea, 97.3% none | 100% intensive chemo | Not reported | Not reported | 30-day mortality: 13% |
| Non-leukapheresis | 666 | 55 | 51.2% | 9.2 | 103 | 31 | 27% (42%, 44%) | 47% hydroxyurea | 100% intensive chemo | N/A | N/A | 30-day mortality: 17% | |
| Sung et al.(26) | Leukapheresis | 16 | Not reported (100% pediatric) | Not reported | Not reported | Not reported; 100% with WBC >100 | Not reported | Not reported | Not reported | 100% intensive chemo | Not reported | Not reported | 6% death during induction |
| Non-leukapheresis | 73 | Not reported (100% pediatric) | Not reported | Not reported | Not reported; 100% with WBC >100 | Not reported | Not reported | Not reported | 100% intensive chemo | N/A | N/A | 4% death during induction | |
| Ventura et al.(5) | Leukapheresis | 61 | Not reported | Not reported | Not reported | Not reported; 100% with WBC >100 | Not reported | 31% (31%, not reported) | Not reported | 100% intensive chemo | Not reported | Not reported | 11% death during induction |
| Non-leukapheresis | 24 | Not reported | Not reported | Not reported | Not reported; 100% with WBC >100 | Not reported | 63% (63%, not reported) | Not reported | 100% intensive chemo | N/A | N/A | 42% death during induction | |
| Wong et al.(27) | Leukapheresis | 31 | Not reported | Not reported | Not reported | Not reported; 100% with WBC >100 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 28-day mortality: 26% |
| Non-leukapheresis | 23 | Not reported | Not reported | Not reported | Not reported; 100% with WBC >100 | Not reported | Not reported | Not reported | Not reported | N/A | N/A | 28-day mortality: 13% |
Statistical analysis:
The RR of early death and the OR of presence of clinical leukostasis for patients who underwent leukapheresis vs. patients who did not undergo leukapheresis were estimated using a sum of the log-ratio of individual study estimates, weighted by sample size. Pooled effect size and 95% confidence interval (CI) were calculated using a random-effects model. Heterogeneity of studies was determined using Cochran’s Q and I2 indices and significant heterogeneity was defined as I2 > 60%. Sensitivity analyses were performed for the overall summary effects by removing the study contributing the most to study heterogeneity and re-running the meta-analysis excluding this study. Univariate meta-regression analysis was performed to statistically compare the effect sizes of studies based on whether these included a matching process of patients who received and patients who did not receive leukapheresis. All analyses were performed with Comprehensive Meta-Analysis (CMA 2.2, Biostat).
Results:
Results of literature search:
Using our search strategy described above, 638 citations were identified with 495 unique citations remaining after removal of duplicates. Applying the exclusion criteria outlined in the methods section, 319 publications were excluded based on title and abstract review. The remaining 176 manuscripts were reviewed in full for eligibility and another 163 publications were excluded as outlined in Figure 1 to derive at the final sample of 13 publications that were included in this meta-analysis.
Description of included studies:
All 13 publications used a retrospective design and compared short-term mortality of AML patients who underwent leukapheresis and those who did not. Studies included were published between 1988 and 2020 and conducted in Europe, Asia, and North America.(5, 6, 17–27) Four studies used various approaches to match baseline patient and disease characteristics between the two groups to reduce the potential influence of selection bias;(18, 22, 23, 25) with two of those studies using propensity score matching.(18, 25) The decision which patients should undergo leukapheresis was driven by department policies,(18, 23) the discretion of the treating physician,(5, 19, 20, 22) or a combination of both (17, 21, 24) in two, three, and four studies, respectively. The indication for leukapheresis was not specified in four studies.(6, 25–27)
Baseline patient characteristics:
There were 1,743 patients (486 leukapheresis and 1,257 non-leukapheresis patients) among the 13 studies included. All studies except for the study by Sung et al. were performed exclusively in adult patients.(26) In seven studies all patients had a WBC of greater than 100 ×109/L on presentation independent of whether they subsequently underwent leukapheresis or not.(5, 17, 18, 21, 24, 26, 27) Baseline cytogenetic risk and presence of selected somatic mutations were reported by four and two studies, respectively.(6, 17, 23, 25) Patients who underwent leukapheresis appeared to be younger (mean median age: 56.6 years vs. 59.8 years) and to have had higher WBC counts (mean median WBC: 180.9 × 109/L vs. 137.1 × 109/L) than patients who were not treated with leukapheresis, respectively (Table 1). A formal statistical comparison was not feasible, since measures of dispersion of age and WBC counts (standard deviation or inter-quartile range) were not consistently reported across studies and a pooled sample variance could not be generated.
Treatment characteristics:
In six studies all patients received intensive chemotherapy in both the leukapheresis and non-leukapheresis group,(5, 18, 19, 24–26) while no patient in the study by Shallis et al. received intensive chemotherapy.(6)
In all studies that reported the number of leukapheresis sessions per patient, patients underwent a median of one leukapheresis procedure with a maximum of three to five sessions in individual studies.(17–19, 21, 23, 24) Among these studies leukapheresis was stopped once the WBC dropped below 100 ×109/L (17, 23) or after clinical improvement (18, 23) in two studies each. Three studies did not specify the criteria for discontinuation of leukapheresis.(19, 21, 24)
Assessment of study quality:
Using a Downs and Black checklist, scores ranged from 16 to 22 points.(15) Four studies used various matching strategies to minimize the risk of selection bias with regard to leukapheresis receipt.(18, 22, 23, 25) Results of those studies, including two studies that used propensity score matching for baseline demographic and clinical factors including the presence of leukostasis were overall in line with the results from all studies combined.(18, 25) Quality assessments for individual studies are provided in Supplemental Table 1.
Due to the observational retrospective study design, the quality of evidence based on GRADE approach was low. Additionally, eight,(5, 6, 17, 19–21, 24, 26) one,(27) and four studies (18, 22, 23, 25) were deemed to be at high, unknown, and low risk of bias, respectively (Supplemental Table 2).
Relative risk for early mortality:
The early mortality rate was reported by all 13 studies. The pooled estimate of RR of early mortality of patients who received leukapheresis compared to patients who did not receive leukapheresis was 0.88 (95% CI 0.69–1.13, p=0.321) indicating that leukapheresis did not statistically significantly reduce the risk for early mortality of AML patients presenting with hyperleukocytosis (Figure 2). Heterogeneity among the various studies was low with a Cochran’s Q statistic of 18 (p=0.115) and an I2 statistic of 33.4%.
Figure 2:
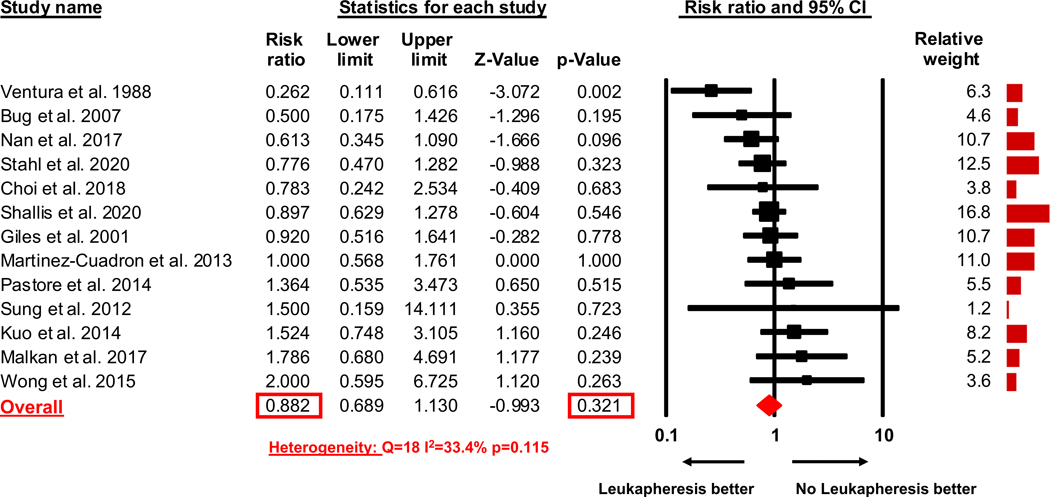
Meta-analysis risk ratio for early death
Odds of presence of leukostasis:
The percentage of patients with evidence of clinical leukostasis at the time of presentation was reported by eight studies.(5, 6, 17, 18, 22–25) The pooled estimate of the OR of presence of leukostasis at the time of presentation for patients who received leukapheresis compared to patients who did not receive leukapheresis was 2.01 (95% CI 0.99–4.08, p=0.052) indicating that clinical leukostasis was twice as likely to be present in patients who received leukapheresis compared to patients who did not receive leukapheresis; although this difference did not quite reach the level of statistical significance (Figure 3). Heterogeneity among the various studies was high with a Cochran’s Q statistic of 30.1 (p<0.001) and an I2 statistic of 76.7%.
Figure 3:
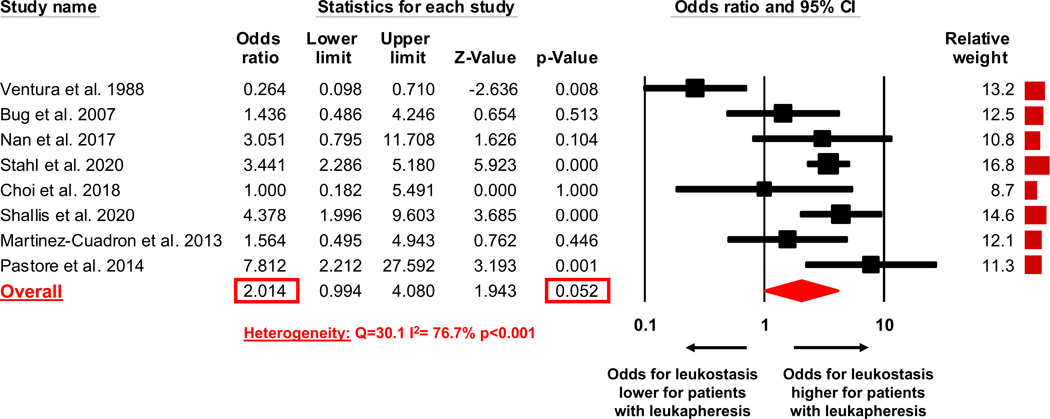
Odds ratio of clinical leukostasis at time of presentation in treated patient population
Sensitivity analysis:
The study with the largest impact on the heterogeneity between studies was the study by Ventura et al.(5) Therefore, meta-analyses of RR of early death and OR of presence of leukostasis at time of presentation were repeated with exclusion of the study by Ventura et al.(5) Notably, the study by Ventura et al. was the only one in which patients with leukostasis were less likely to undergo leukapheresis compared to patients without leukostasis and was published more than a decade prior to any other study.(5)
The pooled estimate of the RR of early mortality of patients who underwent leukapheresis compared to patients who did not undergo leukapheresis was 0.93 (95% CI 0.77–1.12, p=0.446), which similarly to the prior analyses showed no significant risk reduction of early death with leukapheresis (Supplemental Figure 1). Heterogeneity among the various studies was reduced with a Cochran’s Q statistic of 9.9 (p=0.446) and an I2 statistic of 9.9%.
The pooled estimate of the OR of presence of leukostasis at the time of presentation for patients who received leukapheresis compared to patients who did not receive leukapheresis was 2.99 (95% CI 1.98–4.51, p<0.001) indicating that when the study by Ventura et al. was excluded clinical leukostasis was three times as likely to be present in patients who underwent leukapheresis compared to patients who did not undergo leukapheresis (Supplemental Figure 2).(5) Heterogeneity among the various studies was substantially reduced with a Cochran’s Q statistic of 8.0 (p=0.24) and an I2 statistic of 25.3%.
As above, some studies used a formal matching process of patients who underwent leukapheresis and patients who did undergo leukapheresis,(18, 22, 23, 25) whereas other studies did not use a matching process to compare these two patient groups. Therefore, we conducted a univariate meta-regression analysis of the RR of early death based on whether studies used matching or not. The pooled estimate of the RR of early mortality of patients who received leukapheresis compared to patients who did not receive leukapheresis was 0.79 (95% CI 0.54–1.15) in the studies that used a formal matching process, which was not statistically significantly lower than the RR of early mortality of patients who received leukapheresis compared to patients who did not receive leukapheresis (0.92, 95% CI 0.67–1.28) in the studies that did not use a formal matching process (p=0.59) (Supplemental Figure 3). Heterogeneity was low both among the studies which used a formal matching process (Q=1.41, I2=0%, p<0.001) and among the studies that did not (Q=16.2, I2=44.5%, p=0.062).
Discussion
To our knowledge, this is the largest systematic review and meta-analysis on the effect of leukapheresis for the treatment of AML patients presenting with hyperleukocytosis. In our analysis of 13 studies with 1743 patients (486 leukapheresis patients and 1257 non-leukapheresis patients) leukapheresis did not lead to an improvement in the primary outcome of early mortality compared to treatment strategies which did not employ leukapheresis (RR: 0.88 [95% CI: 0.69 – 1.13]; p=0.32). The absence of heterogeneity in the meta-analysis supports the generalizability of this finding with only the study by Ventura et al. reporting a mortality benefit among patients receiving leukapheresis.(5) The study by Ventura et al. differed from the other studies in various ways as it was the only study in which patients with leukostasis were less likely to receive leukapheresis than patients without leukostasis and that it was conducted more than a decade earlier than any other study included in this meta-analysis.(5) Furthermore, in a pre-specified sensitivity analysis that excluded the study by Ventura et al. heterogeneity among studies was further reduced and there was again no significant mortality benefit observed with leukapheresis (Supplemental Figure 1).
In our meta-analysis, patients with leukostasis were two-fold as likely to receive leukapheresis than patients without signs or symptoms of leukostasis although this did not meet the threshold for statistical significance (OR: 2.01 [95% CI: 0.99–4.08]; p=0.052).(12) In a sensitivity analysis that excluded the study by Ventura et al.(5) this difference reached statistical significance (OR: 2.99 [95% CI: 1.98–4.51]; p<0.001). Additionally, comparing the baseline characteristics of our patients, younger patients appeared to be more likely to undergo the procedure. The age difference could be due to the perception that leukapheresis without subsequent intensive chemotherapy is futile and should therefore not be offered to patients who are ineligible for definitive leukemia-directed treatment.
The fact that leukostasis has been shown to be an adverse prognostic factor in multiple studies (20, 25, 28) and the finding that in our study patients with leukostasis were more likely to receive leukapheresis could have led to selection bias favoring the non-leukapheresis group. In contrast, a younger median age of the patients in the leukapheresis group could have biased results positively towards a benefit for leukapheresis. While controlling for baseline patient and disease characteristics should ideally be done through a randomized controlled trial, the logistic burden and the rarity of potentially eligible patients have prevented conduct of such a trial to date and it is very unlikely that such a trial will ever be conducted.
Four studies tried to minimize selection bias via various matching strategies for baseline demographic and disease characteristics.(18, 22, 23, 25) While this does not fully exclude the risk of selection bias, it is reassuring that there was no statistically significant difference in early mortality rates compared to studies that did not match patients (Supplemental Figure 3). Furthermore, a more stringent propensity score matching approach has been used to control for differences in baseline characteristics including the presence of leukostasis and the extent of hyperleukocytosis by Stahl et al. and Choi et al. between the two groups.(6, 18) Both studies did not show an early mortality benefit for leukapheresis and were in line with the results of the entire meta-analysis, further supporting the validity of our findings and arguing against selection bias with regard to leukostasis and leukapheresis receipt as the cause for the absent mortality benefit with leukapheresis.
As single arm retrospective studies have a significant risk of bias which can complicate the interpretation of results and cross-study comparisons, a major strength of our study was the fact that we only included studies with a control group of patients who did not receive leukapheresis. While Oberoi et al. conducted a meta-analysis on leukapheresis in AML in 2014, results from that study are difficult to interpret given the inclusion of studies that reported pooled results from various cytoreductive strategies (e.g. leukapheresis, exchange transfusion) and cranial irradiation.(2, 29, 30). Based on our more stringent inclusion and exclusion criteria to ensure cross-study comparability, we excluded two studies from our meta-analysis that were included by Oberoi et al.(29, 30) In our meta-analysis, there was no statistically significant heterogeneity among studies for the primary endpoint, which strengthens the validity of our results.
Our study has several limitations. First, leukostasis is a clinical diagnosis without an established diagnostic standard. As we had to rely on the data provided by the original studies, we cannot exclude the risk for confounding based on inconsistent definitions and misclassification of leukostasis. However, given that studies using matching strategies for baseline disease and patient characteristics including the presence of leukostasis also did not show an early mortality benefit, selection bias based on the presence of leukostasis is unlikely to explain the absence of an early mortality benefit with leukapheresis. Second, we were unable to conduct meta-analyses on other clinically relevant outcomes such as long-term survival or adverse event rates due to the heterogeneity of subsequent therapies and the reporting of endpoints. Third, the decision of which patients should receive leukapheresis was not standardized in most studies and could have differed between the studies included in this meta-analysis. Finally, in the absence of randomized controlled clinical trials, quality of evidence is derived from observational studies with an inherently lower quality of evidence.
Conclusion:
In this systematic review and meta-analysis of 13 studies with 1743 patients (486 leukapheresis and 1257 non-leukapheresis patients) we did not find any evidence for an improvement in the primary outcome of early mortality for leukapheresis compared to treatment strategies that they did not employ leukapheresis. The quality of evidence was limited by the observational study design and risk of bias as patients presenting with leukostasis were more likely to receive leukapheresis. However, in the absence of evidence from a randomized clinical trial, which is unlikely to be conducted in the future, our results provide the highest quality of evidence available to date and argue against the routine use of leukapheresis for management of hyperleukocytosis among AML patients, especially if it delays initiation of leukemia-directed chemotherapy.
Supplementary Material
Acknowledgments:
AMZ is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a NCI’s Cancer Clinical Investigator Team Leadership Award (CCITLA). Maximilian Stahl received funding from the MSKCC Clinical Scholars T32 Program under award number 2T32 CA009512–31. Research reported in this publication was supported by the NCI of the National Institutes of Health under Award Number P30 CA016359 to Yale Comprehensive Cancer Center and Cancer Center Support Grant/Core Grant to Memorial Sloan Kettering Cancer Center (P30 CA008748). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding sources: Amer Zeidan is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a NCI’s Cancer Clinical Investigator Team Leadership Award (CCITLA). Maximilian Stahl received funding from the MSKCC Clinical Scholars T32 Program under award number 2T32 CA009512–31. Research reported in this publication was supported by the NCI of the National Institutes of Health under Award Number P30 CA016359 to Yale Comprehensive Cancer Center and Cancer Center Support Grant/Core Grant to Memorial Sloan Kettering Cancer Center (P30 CA008748). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest: M.S.T. has received research funding from Abbvie, Cellerant, Orsenix, ADC Therapeutics, and Biosight. M.S.T. has received honoraria for advisory board membership from Abbvie, BioLineRx, Daiichi-Sankyo, Orsenix, KAHR, Rigel, Nohla, Delta Fly Pharma, Tetraphase, Oncolyze, and Jazz Pharma. M.S.T. received patents and royalties from UpToDate. A.M.Z. received research funding (institutional) from Celgene/BMS, Abbvie, Astex, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Novartis, Aprea, and ADC Therapeutics. A.M.Z participated in advisory boards, and/or had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene/BMS, Jazz, Incyte, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Trovagene, Takeda, Ionis, Amgen, and Epizyme. A.M.Z served on steering and independent data review committees for clinical trials for Novartis and Janssen. A.M.Z received travel support for meetings from Pfizer, Novartis, and Trovagene. None of these relationships were related to the development of this manuscript. All other authors report no relevant disclosures/competing interests.
References:
- 1.Röllig C, Ehninger G. How I treat hyperleukocytosis in acute myeloid leukemia. Blood. 2015;125(21):3246–52. [DOI] [PubMed] [Google Scholar]
- 2.Oberoi S, Lehrnbecher T, Phillips B, Hitzler J, Ethier M-C, Beyene J, et al. Leukapheresis and low-dose chemotherapy do not reduce early mortality in acute myeloid leukemia hyperleukocytosis: A systematic review and meta-analysis. Leuk Res. 2014;38(4):460–8. [DOI] [PubMed] [Google Scholar]
- 3.Shallis RM, Stahl M, Bewersdorf JP, Hendrickson JE, Zeidan AM. Leukocytapheresis for patients with acute myeloid leukemia presenting with hyperleukocytosis and leukostasis: a contemporary appraisal of outcomes and benefits. Expert Rev Hematol. 2020:null-null. [DOI] [PubMed] [Google Scholar]
- 4.Zuckerman T, Ganzel C, Tallman MS, Rowe JM. How I treat hematologic emergencies in adults with acute leukemia. Blood. 2012;120(10):1993–2002. [DOI] [PubMed] [Google Scholar]
- 5.Ventura GJ, Hester JP, Smith TL, Keating MJ. Acute myeloblastic leukemia with hyperleukocytosis: Risk factors for early mortality in induction. American Journal of Hematology. 1988;27(1):34–7. [DOI] [PubMed] [Google Scholar]
- 6.Shallis RM, Stahl M, Wei W, Montesinos P, Lengline E, Neukirchen J, et al. Patterns of care and clinical outcomes of patients with newly diagnosed acute myeloid leukemia presenting with hyperleukocytosis who do not receive intensive chemotherapy. Leuk Lymphoma. 2020:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tallman MS, Wang ES, Altman JK, Appelbaum FR, Bhatt VR, Bixby D, et al. Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(6):721–49. [DOI] [PubMed] [Google Scholar]
- 8.Mamez AC, Raffoux E, Chevret S, Lemiale V, Boissel N, Canet E, et al. Pre-treatment with oral hydroxyurea prior to intensive chemotherapy improves early survival of patients with high hyperleukocytosis in acute myeloid leukemia. Leuk Lymphoma. 2016;57(10):2281–8. [DOI] [PubMed] [Google Scholar]
- 9.Chen KH, Liu HC, Liang DC, Hou JY, Huang TH, Chang CY, et al. Minimally early morbidity in children with acute myeloid leukemia and hyperleukocytosis treated with prompt chemotherapy without leukapheresis. J Formos Med Assoc. 2014;113(11):833–8. [DOI] [PubMed] [Google Scholar]
- 10.Korkmaz S The management of hyperleukocytosis in 2017: Do we still need leukapheresis? Transfus Apher Sci. 2018;57(1):4–7. [DOI] [PubMed] [Google Scholar]
- 11.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice - Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J Clin Apher. 2019;34(3):171–354. [DOI] [PubMed] [Google Scholar]
- 13.Stahl M, Pine A, Hendrickson JE, Litzow MR, Luger SM, Stone RM, et al. Beliefs and practice patterns in hyperleukocytosis management in acute myeloid leukemia: a large U.S. web-based survey(.). Leuk Lymphoma. 2018;59(11):2723–6. [DOI] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 15.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stahl M, Bewersdorf JP, Giri S, Wang R, Zeidan AM. Use of Immunosuppressive therapy for management of myelodysplastic syndromes: a systematic review and meta-analysis. Haematologica. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bug G, Anargyrou K, Tonn T, Bialleck H, Seifried E, Hoelzer D, et al. Impact of leukapheresis on early death rate in adult acute myeloid leukemia presenting with hyperleukocytosis. Transfusion. 2007;47(10):1843–50. [DOI] [PubMed] [Google Scholar]
- 18.Choi MH, Choe YH, Park Y, Nah H, Kim S, Jeong SH, et al. The effect of therapeutic leukapheresis on early complications and outcomes in patients with acute leukemia and hyperleukocytosis: a propensity score-matched study. Transfusion. 2018;58(1):208–16. [DOI] [PubMed] [Google Scholar]
- 19.Giles FJ, Shen Y, Kantarjian HM, Korbling MJ, O’Brien S, Anderlini P, et al. Leukapheresis reduces early mortality in patients with acute myeloid leukemia with high white cell counts but does not improve long term survival. Leukemia and Lymphoma. 2001;42(1–2):67–73. [DOI] [PubMed] [Google Scholar]
- 20.Kuo KHM, Callum JL, Panzarella T, Jacks LM, Brandwein J, Crump M, et al. A retrospective observational study of leucoreductive strategies to manage patients with acute myeloid leukaemia presenting with hyperleucocytosis. British Journal of Haematology. 2015;168(3):384–94. [DOI] [PubMed] [Google Scholar]
- 21.Malkan UY, Ozcebe OI. Leukapheresis do not improve early death rates in acute myeloid leukemia patients with hyperleukocytosis. Transfusion and Apheresis Science. 2017;56(6):880–2. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Cuadron D, Montesinos P, Moscardo F, Lopez L, Martin G, Solves P, et al. Treatment with leukapheresis in patients diagnosed with hyperleukocytic acute myeloid leukemia. Blood Conference: 55th Annual Meeting of the American Society of Hematology, ASH. 2013;122(21). [Google Scholar]
- 23.Nan X, Qin Q, Gentille C, Ensor J, Leveque C, Pingali SR, et al. Leukapheresis reduces 4-week mortality in acute myeloid leukemia patients with hyperleukocytosis-a retrospective study from a tertiary center. Leukemia and Lymphoma. 2017;58(9):2110–7. [DOI] [PubMed] [Google Scholar]
- 24.Pastore F, Pastore A, Wittmann G, Hiddemann W, Spiekermann K. The role of therapeutic leukapheresis in hyperleukocytotic AML. PLoS ONE. 2014;9 (4) (no pagination)(e95062). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stahl M, Shallis RM, Wei W, Montesinos P, Lengline E, Neukirchen J, et al. Management of hyperleukocytosis and impact of leukapheresis among patients with acute myeloid leukemia (AML) on short- and long-term clinical outcomes: a large, retrospective, multicenter, international study. Leukemia. 2020;04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung L, Aplenc R, Alonzo TA, Gerbing RB, Gamis AS. Predictors and short-term outcomes of hyperleukocytosis in children with acute myeloid leukemia: a report from the Children’s Oncology Group.. Haematologica. 2012;97(11):1770–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong GC. Hyperleukocytosis in acute myeloid leukemia patients is associated with high 30-day mortality which is not improved with leukapheresis. Annals of Hematology. 2015;94(12):2067–8. [DOI] [PubMed] [Google Scholar]
- 28.Porcu P, Danielson CF, Orazi A, Heerema NA, Gabig TG, McCarthy LJ. Therapeutic leukapheresis in hyperleucocytic leukaemias: lack of correlation between degree of cytoreduction and early mortality rate. Br J Haematol. 1997;98(2):433–6. [DOI] [PubMed] [Google Scholar]
- 29.Inaba H, Fan Y, Pounds S, Geiger TL, Rubnitz JE, Ribeiro RC, et al. Clinical and biologic features and treatment outcome of children with newly diagnosed acute myeloid leukemia and hyperleukocytosis. Cancer. 2008;113(3):522–9. [DOI] [PubMed] [Google Scholar]
- 30.Chang M-C, Chen T-Y, Tang J-L, Lan Y-J, Chao T-Y, Chiu C-F, et al. Leukapheresis and cranial irradiation in patients with hyperleukocytic acute myeloid leukemia: No impact on early mortality and intracranial hemorrhage. Am J Hematol. 2007;82(11):976–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


