Abstract
Objective
To evaluate the evidence from randomised trials for the efficacy and safety of phytotherapeutic interventions in the management of biochemically recurrent (BCR) prostate cancer, indicated by prostate-specific antigen (PSA) progression, numbers progressing to/time to initiation of androgen-deprivation therapy or salvage therapy.
Patients and Methods
MEDLINE (Ovid), EMBASE (Ovid), AMED (Ovid), CINAHL (EBSCO) and the Cochrane Library databases were searched. Clinical trials investigating phytotherapeutic interventions as dietary supplements or dietary components, including multi-component herbal formulations, in men with BCR prostate cancer were located. Eight of nine authors contacted for further information responded. Methodological quality was assessed using the Cochrane Collaboration’s risk of bias assessment tool. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews was followed.
Results
Of 23 full-text articles assessed for eligibility, five met the criteria for inclusion. Two studies were placebo controlled; two were active control trials; and one a high-/low-dose trial. The interventions were administered as isolated phytochemicals (sulphoraphane), phytotherapeutic extracts [Pomi-T (pomegranate, turmeric, green tea and broccoli sprout extract), soy, lycopene, and POMx (pomegranate extract)], or plant-derived dietary items (soy and lycopene). All studies found serum PSA levels to stabilise, decrease or rise more slowly in a significant number of men, and three studies reported stabilising or lengthening of PSA-doubling time. Studies were generally of good quality, but sample sizes were predominantly small, and durations short.
Conclusions
High-quality studies in this area are lacking. Sulphoraphane, lycopene, soy isoflavones, POMx, and Pomi-T are safe and well tolerated. There is limited evidence that they can affect PSA dynamics. No recommendation can be made for the use of these agents in managing prostate cancer morbidity and mortality until high-quality, fully powered studies are available. Recommendations are made for improving reproducibility and translation of findings with regard to study population, study endpoints, design, and the reporting of phytotherapeutic interventions.
Keywords: prostate cancer, biochemical recurrence, phytotherapy, herbal medicine, systematic review, clinical trials
Introduction
Approximately 35% of men treated annually for localised prostate cancer with definitive local therapies, e.g. radical prostatectomy (RP), brachytherapy, and external beam radiation therapy (EBRT), will develop biochemical failure or biochemical recurrence (BCR) of disease [1], detected by a rising serum PSA level within 10 years of treatment [2]. This usually signifies the presence of incurable disease, and can indicate disease progression years before clinical signs or symptoms develop [2].
The small fraction (10–15%) of men with rapidly progressive BCR prostate cancer, signified by a PSA-doubling time (PSA-DT) of ≤3 months, have a high risk of clinical recurrence and cancer mortality. These are typically managed with early systemic therapy, usually in the form of androgen-deprivation therapy (ADT) initially [3]. For the remainder with more indolent PSA dynamics, however, there may be a period of several years of observation before embarking on salvage ADT [4], with its associated toxicities [5]. Substantial anxiety during this period of watchful waiting (WW) [6] often drives patients to seek non-hormonal treatments. Complementary medicine use among men with prostate cancer ranges from 8% to 90% (median 31%) with 8% to 50% (median 30%) using it specifically for cancer care [7]. For herbal medicine use, the prevalence ranges from 1.2% to 24.5%. Evidence for the efficacy of non-hormonal herbal treatments (phytotherapies) is needed from studies in humans.
Scientific investigation of phytotherapeutic agents for potential benefit in prostate cancer chemoprevention is increasing, especially for green tea (Camelia sinensis) catechins, lycopene and soy (Glycine max) isoflavones, curcumin from turmeric (Curcuma longa), sulphoraphane and indole-3-carbinol from broccoli (Brassica oleracea). Preclinical studies and/or clinical trials also suggest benefits for resveratrol from grape skins or Japanese knotweed (Polygonum cuspidatum), pomegranate (Punica granatum) extract (POMx), Silymarin (from St Mary’s/milk thistle, Silybum marianum), and mushrooms such as reishi (Ganoderma lucidum), turkey tail (Coriolus/Trametes), and shiitake (Lentinula edodes).
Potential anti-cancer mechanisms of these agents have been extensively reviewed elsewhere [8–17]. These include inhibition of proliferation, induction of apoptosis, and cell cycle arrest. Soy isoflavones, indole-3-carbinol and 3,3′-diindolylmethane from broccoli, lycopene (predominantly found in tomatoes), (−)-epigallocatechin-3-gallate (green tea polyphenols), and curcumin from turmeric, are known to downregulate the signal transductions in androgen receptor (AR), protein kinase B (Akt), nuclear factor-κB (NF-κB), and other signal transduction pathways [18]. In vitro, sulphoraphane can inhibit cancer cells through a variety of mechanisms including inflammation, angiogenesis, and metastasis, and in vivo, its administration inhibited prostate cancer progression and metastasis in transgenic adenocarcinoma of the mouse prostate (TRAMP) mice [11]. Pomegranate polyphenols modulate B-cell lymphoma 2 (Bcl-2) proteins, increase p21 and p27, and downregulate the cyclin-cyclin-dependent kinase (Cdk) complex network [12]. Silymarin (St Mary’s/milk thistle) and its main active constituent, silibinin may enhance IGF-binding protein 3 (IGFBP-3) action and inhibit IGF-1-induced growth or affect levels of AR-regulating genes [10]. Mushroom polysaccharides stimulate T-cells, B-cells, neutrophils, and macrophage dependent immune system responses [16]. Systemic bioavailability is poor for curcumin and silibinin [10,17].
The objective of the present systematic review was to evaluate the evidence from randomised trials of phytotherapeutic interventions in the management of BCR prostate cancer for delaying disease progression, indicated by PSA progression, numbers progressing to/time to initiation of ADT or other salvage therapies.
Patients and Methods
The following electronic databases were searched (earliest to May 2015): EMBASE (Ovid), MEDLINE (Ovid), AMED (Ovid), Cochrane Central Register of Controlled Trials (CENTRAL) and CINAHL (EBSCO).
In addition, further relevant papers were identified using the ‘related articles’ function in PubMed, and by hand-searching reference lists of relevant journal articles and conference proceedings.
Search Strategy
Search strategy in MEDLINE, adapted for other databases.
exp Prostatic neoplasms/
prostate cancer.mp.
1 or 2
(herb$ or medicinal plant$ or plant extract$ or plant drug$ or botanic$ or phytotherap$).mp
(citrus or pomegranate or Punica granatum or indol-3-carbinol or broccoli or diindolymethane or DIM or glucosinolate$ or sulphoraphane or polyphenol$ or resveratrol or grape or curcumin or turmeric or Curcuma longa or green tea or Camellia sinensis or catechin$ or isoflavone$ or phytoestrogen$ or genistein or daidzein or Glycine max or soy$ or lycopene or red clover or Trifolium or liquorice or licorice or Glycyrrhiza or garlic or Allium sativum or pycnogenol or shitake or mushroom$ or Ganoderma or Trametes or turkey tail or PSP or PSK or Coriolus or Lentinula or lentinan or Agaricus or silybin or Silybum or silymarin or silybin$ or flaxseed or linseed or Linum or Viscum or mistletoe or Iscador).mp
4 or 5
3 and 6
limit 7 to (English and clinical trials, all)
English language restriction was imposed.
Study Selection
Randomised clinical trials were eligible for inclusion in this review. Trials investigating phytotherapeutic extracts in combination with mainstream treatment were excluded.
Types of Participants
Patients with BCR hormone-sensitive prostate cancer after local therapy for histologically confirmed prostate cancer were included.
Types of Intervention
Trials investigating phytotherapeutic extracts, isolated phytochemicals, and plant-derived dietary components/items were included. Data from studies investigating herbal formulations containing additional vitamins or minerals were excluded. Studies of PC-SPES (mixture of eight different herbs, including chrysanthemum, liquorice and saw palmetto, plus the minerals selenium, calcium, magnesium, zinc and copper), which has been discredited due to adulteration with synthetic drugs, and of herbs administered via non-oral routes were excluded.
Types of Controls
Studies comparing interventions with inactive controls (e.g. placebo, no treatment, standard care or a waiting list control) or active control interventions (e.g. a different variant of the same intervention or a different herbal intervention) were included.
Types of Outcome Measures
The primary outcomes of the present review were
Disease progression denoted by changes in PSA levels and PSA kinetics (PSA velocity, i.e. rise rate; PSA-DT).
Secondary Outcomes Included
Numbers progressing/time to initiation of mainstream treatments, such as ADT or salvage therapies.
Changes to prostate symptoms.
Quality of life measures.
Adverse events (AEs).
Compliance.
Outcomes Excluded
Bioavailability/tissue levels.
Mechanisms of action.
Data Extraction and Methodological Quality Assessment
Two reviewers (D.v.D. and C.P. or K.B.) independently screened the titles and abstracts of all articles returned from the search strategy. When necessary, full-text articles were obtained to determine eligibility of the study. Any disagreement between the two authors was to be resolved by a third author (C.P. or K.B.). Data extraction from included studies was conducted by two investigators (D.v.D. and K.B.). Excluded studies were reviewed by D.v.D. and M.P.; discrepancies were checked by K.B.
The following data were extracted from the included articles: intervention, patient characteristics at baseline, trial design, sample size, duration, outcome measures, results, and AEs. Where necessary, authors were contacted for further details. The quality of each study was assessed using the Cochrane Collaboration’s risk of bias assessment tool [19]. Three reviewers (D.v.D., K.B. and C.P.) worked independently to determine selection, attrition, detection, performance, and reporting bias. Any discrepancies were resolved by discussion between the reviewers. To supplement the quality assessment, additional criteria were assessed according to the proposed elaboration of Consolidated Standards Of Reporting Trials (CONSORT) checklist item 4 for reporting randomised controlled trials (RCTs) of herbal medicines [20].
Data Analysis
Lack of homogeneity across trials precluded any meaningful meta-analysis.
Results
Description of Studies
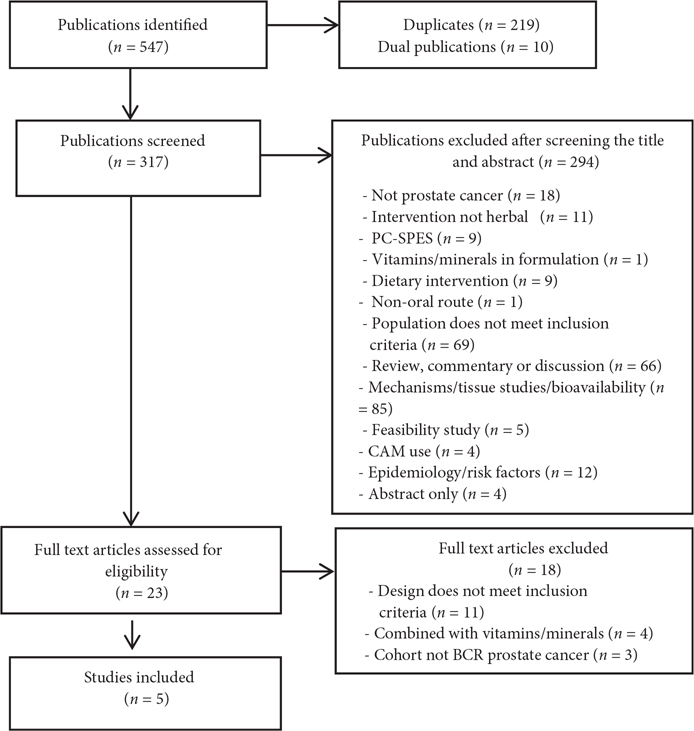
After the removal of duplicates, 318 articles were located and 23 full-text articles were assessed, five of which met the selection criteria (Fig. 1). Key data extracted from the identified studies are included in Table 1 [11,21–24]. Of the five studies, two were placebo controlled [11,22], two compared the intervention with a different herbal intervention [23,24], and one was a variant of the same intervention (high-/low-dose) trial [21]. Study durations were as follows: 2 months [24], three of 6 months [11,22,23], and one study of up to 18 months [21]. However in the latter, only 58% completed either 18 months or met the protocol-defined progression.
Fig. 1.
Flowchart of selection process.
Table 1.
Characteristics of identified randomised trials of phytotherapeutic extracts, isolated phytochemicals and dietary interventions in BCR prostate cancer.
| Reference and country | Intervention, extract and dosage vs comparator | Diagnosis and participant characteristics | Study design, no. of participants and duration | Endpoints | Main results | AES | Authors’ conclusions |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Intervention compared with an inactive control intervention (e.g. placebo, no treatment, standard care, or a waiting list control) | |||||||
| Placebo controlled | |||||||
| Monotherapies | |||||||
| Cipolla et al. [11] France |
Sulphoraphane (SF) 60 mg daily vs placebo |
BCR prostate cancer –rising PSA level (at three successive measurements) after RP (n = 29) or RP and RT (n = 39) or RP and RT and HT (n = 10) Current or prior HT? Yes, in conjunction with RT (n = 10) PSA level: >0.2 and <5 ng/mL; mean 0.76 ng/mL PSA-DT: >5 and <36 months; mean 14.4 months Gleason score: ≤8 Age, mean (SD): 69 (6) years |
Double-blind, randomised, placebo-controlled trial, n = 81/78 ITT population (SF, n = 38; placebo, n = 40) Duration: 6 months |
Primary: 0.012 log (ng/mL)/month decrease in the log PSA slopes in the SF arm vs placebo arm Secondary: Difference in PSA values from baseline to the end of month 6 PSA-DT Testosterone level |
Primary endpoint was not reached using four values (months 0, 1, 3, 6). The adjusted median log PSA slope in the SF group was 38.5% lower than in the placebo group. Mean PSA level change between the end of month 6 and baseline was significantly lower in the SF vs placebo group at a mean (SD) of 0.099 (0.34) vs 0.62 (1.47) ng/mL (P = 0.043). PSA-DT was 86% longer in the SF vs the placebo group, at 28.9 vs 15.5 months. Testosterone levels: no difference between arms Very good tolerance |
Only Grade I GI toxicity (bloating) in the SF arm (n = 17) but not statistically different from placebo (n = 10) | This stabilised SF was shown to significantly delay PSA progression after RP ± RT. The decrease in the PSA slope was most marked after 3 months treatment |
| Multi-component interventions | |||||||
| Thomas et al. [22] UK |
Pomi-T: pomegranate green tea, broccoli sprouts and turmeric – all 300 mg/day vs placebo | Localised prostate cancer managed with AS (n = 121, 60%) or WW (n = 78, 40%) following a PSA relapse after radical treatments (RT, n = 65; RP and RT, n = 8; brachytherapy, n = 9). Current or prior HT? No current use, prior use included. Mean PSA level: 6.5 ng/mL PSA-DT: not specified Gleason score, mean: 6.4 Age, mean: 73 years (active); 76 years (placebo) |
RCT – placebo-controlled, n = 203/199 (136/134 active; 67/65 placebo) Duration: 6 months |
PSA levels Average PSA change Number remaining on AS Cholesterol Blood glucose CRP BP |
PSA levels were stable or lower than baseline more often in the Pomi-T group (46% vs 14%; P < 0.001). In the AS sub-group (n = 121) the mean PSA level dropped by 0.14% (95% Cl −7.57 to 7.95) in the Pomi-T group, but rose by 46.98% in the placebo group (95% CI 28.51 to 68.31; P = 0.001); in the WW subgroup (n = 78) the mean PSA rose by 8.78% in the Pomi-T group (95% CI −6.32 to 26.62) vs 80.34% in the placebo group (95% CI 50.54 to 116.55; P = 0.001). Median increase in PSA level was 63.8% lower in the Pomi-T vs the placebo group (14.7% vs 78.5%; P < 0.001) at 6 months. More men in the Pomi-T group than in the placebo group continued on AS or WW (92% vs 72%). No significant differences between groups for cholesterol, serum glucose, CRP or BP. Compliance was excellent (96.5% Pomi-T, and 98.4% placebo) |
No statistically significant difference in AEs between arms: 34 (24%) in Pomi-T and 23 (34%) in placebo group. No Grade III–IV toxicities, but one Grade II (diarrhoea) with Pomi-T. GI events in 21 (15.5%) in Pomi-T vs 5 (7.5%) in the placebo group (NS). No effect on INR in 30 men on warfarin |
This study found a significant short-term, favourable effect on the percentage rise in PSA in men managed with AS and WW after ingestion of this food supplement |
| Intervention compared with an active control intervention (e.g. a different variant of the same intervention, a different drug, a different kind of therapy) | |||||||
| Comparator a different herbal intervention | |||||||
| Monotherapies | |||||||
| Grainger et al. [24] USA |
Dietary lycopene (tomatoes) vs soy protein Lycopene >25 mg/day or soy protein 40 g/day for 1 month, followed by combined tomato-rich diet and soy supplements for a further month (both arms) |
BCR prostate cancer after local therapy (at least two consecutive increases in serum PSA level); asymptomatic Current or prior HT? Yes, current: (n = 20 (tomato, n = 9 and soy n = 11) PSA level: not specified PSA-DT: 38% <4 months; 32% 4–9 months; 30% >9 months Gleason score: not specified Age, mean (SD): 70 (7) years |
Randomised trial, n = 41/40 (lycopene 20; soy protein 21) Duration: 2 months |
Serum PSA level PSA-DT VEGF IGF-1 Testosterone Cholesterol Compliance |
Serum PSA level decreased for 14/41 men (36%) over 2 months [tomato 5/20 (25%); soy 10/20 (50%)] Prolongation of PSA-DT occurred in 23/40 (58%) men over 2 months [tomato 13/20 (65%); soy 10/21 (51%)] Number of men with slowest PSA-DT increased from 12 (30%) to 19 (48%) (P = 0.08) over the 2 months. A reduction in mean serum VEGF at months 0–1, 1–2 (P < 0.04), with no effect of group seen. No significant changes in testosterone or IGF-1 for either HT naıve or refractory men. A clinically small decrease in serum total cholesterol (P = 0.009). Excellent compliance |
No Grade II–IV toxicities were seen. Constipation (Grade I) in three men (7%) while on soy protein | Patients with prostate cancer will consume diets rich in tomato products and soy with excellent compliance and bioavailability of phytochemicals |
| Multi-component interventions | |||||||
| Vaishampayan et al. [23] USA |
Lycopene 15 mg vs lycopene plus soy isoflavones 40 mg twice daily | Prostate cancer with rising serum PSA level (three successive elevations at ≥2-week intervals or at least two PSA readings of ≥10 ng/mL) after local therapy or while on HT (LHRH analogue) Current or prior HT? Yes, at least 1–1.5 months prior [lycopene, 14 (36%); lycopene plus soy isoflavones 11 (33%)] Median PSA level: 6.5 ng/mL. PSA-DT: not specified Gleason score: not specified Age, median: 75 years |
Randomised phase II clinical trial, n = 71/70 (lycopene, n = 38; lycopene plus soy isoflavones, n = 33) Duration: median 6 months in lycopene arm; 5.5 months in lycopene plus soy isoflavones arm |
Serum PSA PSA velocity |
No partial (50% reduction) or complete PSA (PSA level ≤4 ng/mL) response in either group. 35/37 (95%) patients in the lycopene group and 22/33 (67%) in the lycopene plus soy isoflavones group achieved stabilisation in serum PSA level. PSA velocity significantly declined from before to after therapy (P = 0.015) in the hormone-sensitive and hormone-refractory groups (P = 0.017); in the latter group, lycopene only resulted in a significantly greater decline than lycopene plus soy isoflavones (P = 0.02) |
No significant treatment-related toxicities Both regimens were very well tolerated. One patient reported a Grade I headache that was possibly related to therapy |
Lycopene and soy isoflavones may delay progression of both hormone-refractory and hormone-sensitive prostate cancer in men with BCR, but there may not be an additive effect when the two are co-administered |
| Randomised high-/low-dose trials | |||||||
| Monotherapies | |||||||
| Paller et al. [21] USA |
Pomegranate (Punica granatum) extract (POMx) 1 or 3 g of POMx (90% polyphenols), stratified by baseline PSA-DT and Gleason score | BCR prostate cancer (rising PSA on ≥3 time points at least a month apart, within previous year) after primary therapy for localised prostate cancer. (RP, n = 52; RP and RT, n = 12; RT, n = 54; cryotherapy, n = 2; brachytherapy, n = 19) Current or prior HT? Yes, at least 1 year prior: n = 28/101 (1-g, n = 11/50; 3-g n = 17/51) PSA level: 5.3 ng/mL (1-g 4.79; 3-g 5.74 ng/mL) PSA-DT, mean: 14.7 months Gleason score, mean: 6.5 Age, mean: 74.5 years |
Randomised, multicentre, double-blind phase II, dose-exploring trial, n = 104/101/95 ITT (n = 46 low; n = 49 high) Duration: up to 18 months (92% of patients completed 6 months, 70% completed 12 months; 58% completed 18 months or met the protocol) |
PSA-DT Testosterone Oestradiol SHBG |
The median PSA-DT lengthened from 11.9 months at baseline to 18.5 months after treatment (P < 0.001). In the low-dose group from a median of 11.9 to 18.8 months and median of 12.2 to 17.5 months in the high-dose group, with no significant difference between dose groups (P = 0.554). PSA-DT increases >100% of baseline were seen in 43% of patients. Declining PSA levels were seen in 13 patients (13%). No significant changes occurred in testosterone. Oestradiol trended higher in the 3-g group (28.0 to 32.3 pg/mL) SHBG increased in both groups (42.5–54.7 nmole/L in 3g-POMx group and 42.8–49.2 nmole/L in 1g-POMx), difference: NS |
No clinically significant toxicities were seen. Diarrhoea of Grade ≤II was seen in 1.9% and 13.5% of patients in the 1-g and 3-g dose groups, respectively, and deemed drug-related in only five patients (all 3-g POMx). In the 1-g group: one case of reflux disease; in the 3-g group: six reported GI events – nausea (four), abdominal pain, constipation, frequent bowel movements, stomach discomfort and vomiting (one case each) |
POMx treatment was associated with 6-month increases in PSA-DT independent of dose level without AEs. The significance of this on-study slowing of PSA-DT remains unclear |
BP, blood pressure; CRP, C-reactive protein; GI, gastrointestinal; INF, international normalisation ratio; ITT, intention to treat; HT, hormone therapy; NS, statistically non-significant.
Details of the 18 excluded studies are given in Table 2 [4,25–41].
Table 2.
Characteristics of the excluded studies, grouped by reason excluded.
| Reference and country | Intervention, extract and dosage vs comparator | Diagnosis and inclusion of ADT | Study design and duration | Endpoints | Results | AEs |
|---|---|---|---|---|---|---|
|
| ||||||
| Non-randomised studies of herbal extract or isolated phytochemicals | ||||||
| Clark et al. [25] USA |
Lycopene capsule of escalating doses: 15/30/45/60/90 or 120 mg/day | BCR prostate cancer after surgery or RT. Current or prior HT? Previous yes, but no new HT within 6 months pre-enrolment. Evidence of metastases? No PSA, median: 4.4 ng/mL PSA-DT: 3.7 years Gleason score: not specified Age, median: 74 years |
Phase I–II prospective dose-escalating (within-groups) trial n = 36 (six cohorts of six patients) Duration: 1 year |
PSA response ≥50% reduction. PSA response as a function of lycopene dose. Duration of PSA response. Time to PSA progression. PSA-DT or slope. Toxicity |
No PSA responses Median time to progression was not reached. PSA-DT before-to-after NS PSA slopes before-to-after NS. Toxicity was mild. Compliance 100% |
Well tolerated. One patient discontinued due to diarrhoea (Grade II toxicity). Seven Grade II transient abnormalities of serum glucose (five) or creatinine (two) |
| Flaig et al. [26] USA |
Silybinin from St. Mary’s thistle (Silybum marianum): Silybin phytosome (Siliphos®) 2.5–20 g daily | Prostate cancer, progressive disease after surgery, RT (±ADT), and life expectancy ≥3 months. Current or prior HT? Yes, prior; LHRH analogues permitted. Evidence of metastases? Uncontrolled brain metastases excluded. PSA, median: 4.3 ng/mL PSA-DT: not specified Gleason score, mean: 7.6 Age, median: 70 years |
Phase I dose-escalation and pharmacokinetic study, n = 13 Duration: 3–12 months (3–13 courses) |
PSA response (50% reduction). Toxicity. Tolerability |
No objective PSA responses. One case elevated ALT. Hyperbilirubinaemia in 10/24 courses (41.67%); at 15 g and 20 g dose levels, but only in two of 32 courses (6.25%) with dose reduction to 13 g; no Grade III–IV bilirubin toxicity. Grade I elevation in creatinine (six cases) usually resolved spontaneously. Grade I hypercalcaemia. (six cases), mild and self-limiting. Good follow-up compliance |
Well tolerated. Minor halitosis in two patients, attributed to silybin-phytosome |
| deVere White et al. [27] USA |
Shiitake mushroom extract polysaccharide/oligosaccharide complex, 8 g/day for a 70-kg man, adjusted at the rate of 1 g/10 kg body weight | Prostate cancer and two consecutive elevated PSA readings; AS; WW; HT; hormone refractory Current or prior HT? Yes Evidence of metastases? Yes (n = 21) PSA, mean: 10.5 ng/mL after RP; 12.3 ng/mL after RT; 13.9 ng/mL AS; hormonal 10.7 ng/mL; hormone refractory 41.5 ng/mL PSA-DT: not specified Gleason score: mean not calculable Age, mean: 71.5 years |
Non-randomised, open label study n = 62/61 Duration: 6 months |
Change in PSA levels: complete response (CR) = undetectable PSA; partial response (PR) = >50% decrease; stable response = 50% change or less in PSA level; progression = >50% increase | No effect: no appreciable difference in level of PSA or in the rate of change in the PSA. level after treatment initiation. 61/62 evaluable patients for PSA |
|
| Yoshimura et al. [28] Japan |
Mushrooms: Agaricus blazei Murill (Senseiro; KyowaWellness, Tokyo, Japan) vs Ganoderma lucidum mushroom (Rokkaku Reishi; Suntory Holdings, Osaka, Japan) | Men with BCR after radical treatment. Current or prior HT? Yes, prior to radical therapy only. PSA, mean: not specified PSA-DT not specified Gleason score: not specified Age, mean: not calculable |
Open label study n = 51 (Senseiro, n = 34; Reishi, n = 17). Duration: Senseiro: 6 months + 2–32 months; Rokkaku Reishi 7 months + 6–25 months |
PSA PR (>50% reduction). PSA-DT. Serum testosterone |
No patient showed a PR of serum PSA level. For both Senseiro and Rokkaku Reishi, PSA-DT at 6 months was significantly longer than that at study entry at a median of 8.3–9.6 months (P = 0.03) and 5.8–10.7 months (P = 0.02, respectively). 30% of patients on Senseiro and 12% on Rokkaku Reishi showed a ≥20% reduction in testosterone. Alteration of PSA-DT did not correlate with that of serum testosterone levels |
No serious AEs were seen, but three patients withdrew after a rapid rise in serum PSA level |
| Twardowski et al. [29] USA |
White button mushroom (Agaricus bisporus) at six doses: 4/6/8/10/12, and 14 g/day |
BCR prostate cancer/PSA failure after RP or RT. PSA ≥0.2 ng/mL after RP, or ≥2.0 ng/mL above post-RT nadir. Current or prior HT? Yes, prior. Up to 9 months of neoadjuvant or adjuvant treatment, completed at least 6 months before registration Evidence of metastases? No PSA, median: 1.9 ng/mL PSA-DT: not reported Gleason score, mean: not specified Age, median: 68 years |
Phase I dose-escalation study, n = 36 Duration: 3 months |
PSA progression. DHT, DHEA and oestrogen. Cytokine multiplex Myeloid-derived suppressor cells (MDSCs) in peripheral blood mononuclear cells. Toxicity |
11% overall PSA response rate. CR to undetectable levels in two patients on 8 and 14 g/day. PR in two patients on 8 and 12 g/day. 13 (36%) had some PSA decrease below baseline after 3 months. CR and PR demonstrated higher levels of baseline interleukin 15 than non-responders, and associated declines in MDSCs. PSA responses did not correlate with serum testosterone, DHT or DHEA levels |
Minimal side-effects, mostly limited to Grade I abdominal bloating. One case of Grade III hyponatraemia at 8 g/day dose, patient was taken off protocol |
| Guess et al. [30] USA |
Modified citrus pectin (MCP), Pecta-Sol, 14.4 g/day in divided doses | Recurrent adenocarcinoma of the prostate and low but progressively rising PSA after RP, EBRT, RP and RT or cryosurgery. Current or prior HT? Yes, previous. Evidence of metastases? No, up to stage 3c PSA, mean: 1.03 ng/mL PSA-DT, mean: 6.9 months Gleason score, mean: 6.9 Age, mean: 69.6 years |
Non-randomised phase II pilot study, n = 13/10 Duration: 12 months |
PSA level. PSA-DT |
No decreases in absolute PSA level. An increase in PSA-DT in eight of 10 evaluable patients, but only seven were statistically significant (P ≤ 0.05). Compliance excellent (patient subjective report) |
Well tolerated. No serious side-effects but three patients withdrew due to side-effects (two with mild abdominal cramps, one with mild diarrhoea) that resolved soon after stopping the MCP |
| Hussain et al. [31] USA |
Soy isoflavones, 200 mg/day orally | Prostate cancer and rising PSA (i) under WW; or (ii) after local therapy, or (iii) while receiving HT. Current or prior HT? Yes, prior. Current LHRH analogues permitted. Evidence of metastases? Yes PSA, mean: 13.3 ng/mL PSA-DT: not specified Gleason score: not specified Age, mean: 73 years |
Pilot study, n = 41 Duration: 0.8–6 months |
PSA levels. Biomarkers (IGF and oxidative stress markers). Toxicity |
No PSA CRs (normalisation of PSA) or PRs (≥50% reduction). PSA velocity decreased in whole group (P = 0.01). No significant change in the level of 5-hydroxymethyl-2′-deoxyuridine or plasma levels of IGF-1 and IGFBP-3. No observed changes in serum testosterone |
Very well tolerated, with no toxicity attributable to Novasoy treatment. No Grade ≥III AEs |
| deVere White et al. [32] USA |
Genistein-rich extract, 5 g/day: 40% isoflavones (Novasoy) added to a mushroom (G. lucidum) mycelia culture – a total of 450 mg/day of genistein, plus an additional 450 mg/day of other aglycone isoflavones | Five treatment groups: failed RP; failed RT; intermittent HT; combination of RP and RT; AS or WW. Current or prior HT? Not specified. Evidence of metastases? Yes PSA, mean: 7 ng/mL PSA-DT: not specified Gleason score, mean: not calculable Age, mean: 74.5 years |
Open-label pilot study, n = 52 Duration: 6 months |
PSA levels. Clinical chemistries (ALP, AST, total bilirubin, creatinine, cholesterol, and GGT) |
One AS patient had ≥50% reduction in PSA level. Seven AS patients had PSA level reductions of <50%. Patients in the AS group had PSA levels on average 27% lower than otherwise predicted (P = 0.026). Clinical chemistries were all unaltered |
Three patients withdrew due to AEs (diarrhoea) |
| Kwan et al. [33] Canada |
Soy beverage, 500 mL/day, equivalent to ≈50–100 mg isoflavones | Rising PSA after curative radical RT for prostate cancer but no clinical evidence of distant disease. Current or prior HT? Yes, during primary treatment. Evidence of metastases? No PSA, median: 5 ng/mL PSA-DT, median: 18.2 months Gleason score, median: 6 Age, median: 78 years |
Phase II non-randomised study, n = 34/29 Duration: 6 months |
PSA level. PSA-DT. Tolerability. Compliance |
PSA level showed a declining trend in four patients (13.8%). Eight patients (27.6%) had >100% prolongation of PSA-DT, but PSA-DT also showed a ≥50% shortening in five patients (17.2%). Mean consumption of the assigned soy beverage, 29 patients who had >1 month soy was 93% |
Well tolerated. Most common AE was Grade I GI complaints; Hives (one patient) and weight gain (four) also reported |
| Pendleton et al. [34] USA |
Soy milk containing 141 mg isoflavonoids/day | BCR prostate cancer after previous local therapy Current or prior HT? ADT not within 12 months of study entry Evidence of metastases? No PSA, median: 9.2 ng/mL PSA-DT, median: 15 months Gleason score, median: 7 Age, median: 73 years |
Phase II non-randomised clinical trial, n = 20 Duration: 12 months |
PSA level. PSA-DT. Serum testosterone, lipids. QoL |
PSA level reduced in six patients but increased in 13. PSA slope significantly decreased in six patients (P < 0.05), but significantly increased in two (P < 0.05). Improvements in PSA-DT in 14 patients (P = 0.044). Free testosterone (median 10.3 vs 9.7 ng/mL, P = 0.031), but no change in total testosterone or cholesterol. QoL: no significant change Compliance high; only two patients did not ingest the required amount of soy milk |
One patient (5%) withdrew due to diarrhoea. No other side-effects |
| Pantuck et al. [35] USA |
Pomegranate (Punica granatum). Wonderful variety, 570 mg total polyphenol gallic acid equivalents/250 mL of pomegranate juice daily | Rising PSA (PSA level of >0.2 and <5 ng/mL) after RP or RT. Current or prior HT? No. Evidence of metastases? No. PSA, mean: 2.23 ng/mL. PSA-DT, mean: 14.3 months Gleason score: 5–7 in 94% of patients. Age, mean: not specified |
Phase II, Simon two-stage clinical trial, n = 46 Duration: 6–40 months (until disease progression) |
PSA level. PSA-DT. Hormone levels (testosterone, androstenedione, oestradiol, SHBG, DHEA, IGF) |
From both stages, 16 (35%) achieved a PSA level decrease (mean, 27%). Four of these achieved a PSA level decline of >50%. Mean PSA-DT increased from 15 to 54 months after treatment (P < 0.001). No significant difference in hormone levels before and after treatment |
Well tolerated. No serious AEs |
| Intervention combined with vitamins/minerals | ||||||
| Schroder et al. [36] The Netherlands |
Soy isoflavones, lycopene, silymarin and antioxidants vs placebo | Prostate cancer and rising PSA after RP or RT. Current or prior HT? No. Evidence of metastases? Not specified. PSA, mean: 3.3 ng/mL. PSA-DT: not specified. Gleason score: not specified. Age, mean: 70 years |
RCT crossover study – tertiary prevention, n = 49/46 Duration: 2.5 months treatment phases separated by a 1-month washout |
PSA slope. PSA-DT. Hormone levels (testosterone, DHEA, SHBG, LH) |
Significant decrease in non-transformed PSA slope (P = 0.03) and (2)log PSA slope (P = 0.041) [PP analysis]. A 2.6-fold increase in the PSA-DT for placebo and supplement periods (PP). No changes in hormone levels. Two patients were withdrawn for <80% compliance. Compliance was >90% in 41/46 patients |
The few AEs (e.g. GI complaints) were judged not related to the dietary supplement |
| Kranse et al. [37] The Netherlands |
Isoflavones plus green tea extract, lycopene, antioxidants including carotenoids, selenium, phytosterol, α-tocopherol and margarine vs placebo | Hormonally untreated men with prostate cancer and rising PSA after RP or RT or under WW. Current or prior HT? No. Evidence of metastases? No. PSA, median: 3.24 ng/mL. PSA-DT: not specified. Gleason score: not specified. Age, median: 70 years |
RCT crossover study, n = 37 Duration: 1.5 month treatment phases separated by a 0.5 month washout |
PSA, total and free. PSA-DT. Male sex hormone levels |
Free PSA decreased during verum (P = 0.02). Total PSA-DT unaffected, but NS increase from 9.5 months during placebo period to 10 months during verum. In men in whom the free androgen index decreased (21/32), there was a significant decrease in the slopes of both total and free PSA (P = 0.04). Male sex hormone levels lower with verum (P = 0.02): DHT, −0.11 nmol/L (P = 0.005); testosterone, −1 nmol/L (P = 0.02) |
No serious AEs; one patient withdrew due to abdominal pains |
| Barber et al. [38] UK |
Lycopene whole-tomato lycopene supplementation 10 mg/day (two tablets of Lycoplus – each tablet contains 5 mg lycopene, 100 mg vitamin C, 1.25 mg vitamin E, 0.83 mg phytoene and phytofluene and 0.21 mg β-carotene) | Men with localised prostate cancer on clinical surveillance (AS or BCR/WW). Current or prior HT? Yes, at least 1 year prior. Evidence of metastases? No. PSA, mean: 23.3 ng/mL. PSA-DT: not specified. Gleason score, mean: 6. Age, mean: 73 years |
Phase II clinical within-groups pilot study, n = 37 Duration, mean: 10.4 months |
PSA velocity. PSA-DT. Toxicity |
Regression slopes of (log) PSA vs time decreased in 26/37 (70%) of patients (P < 0.001). PSA-DT: non-significant increase (median increase of 174 days). No toxicity |
Discolouration of faeces |
| Dorff et al. [4] USA |
Prostate Health Cocktail (PHC), a supplement containing vitamins D and E, saw palmetto, lycopene, green tea and soy extracts | BRC, rising PSA level after RT or RP or both with PSA-DT between 3 and 36 months. Current or prior HT? Yes, at least 3 months prior with testosterone recovery. Evidence of metastases? No. PSA, median: 2.8 ng/mL. PSA-DT, median: 9.4 months. Gleason score: 23% had Gleason >7. Age, median: 67 years |
Open label pilot study, n = 40/39 Duration: 4-week cycles (1–13 cycles, median 10) |
PSA response. PSA-DT. Circulating tumour cells. DHT. Testosterone |
No men had a PSA CR or PR. 15/39 men (38%) had a PSA decline (1.1–55% maximum decrease); median change at 3 months was 18.1%. PSA-DT median 6.7 months; 12/37 (32%) had slower PSA-DT. Circulating tumour cells in 16/33 patients. No change in testosterone or DHT |
Grade I or II liver enzyme elevations (transient), Grade I or II GI symptoms (nine), Grade I weakness/dizziness/pain (five), and Grade I fatigue (two) |
| Risk of BCR PCa | ||||||
| Bosland et al. [39] USA |
Soy protein isolate vs placebo | Men at high risk of recurrence after RP. Current or prior HT? Not specified. Evidence of metastases? Yes PSA level, mean before RP 7.4 ng/mL. PSA-DT: not specified. Gleason score, mean: 7 Age, mean: 61 years |
RCT – placebo controlled, n = 177/159 (81 soy; 78 placebo) Duration: up to 2 years |
BCR rate (defined as development of PSA >0.07 ng/mL) over first 2 years after randomisation. Time to recurrence. Adherence |
28.3% developed BCR within 2 years, close to the a priori predicted rate of 30% [22 (27.2%) in the intervention group and 23 (29.5%) in the placebo group]. Self-reported adherence was excellent, with 152 patients (96%) reporting having consumed >90% of the packets supplied |
Trial stopped after 45 men developed BCR due to lack of treatment effect. Six potentially related GI issues but no difference to placebo group |
| Vidlar et al. [40] Czech Republic |
Silymarin [St. Mary’s thistle; Silybum marianum (SM)] 570 mg, combined with selenium (Se) 240 μg as selenomethionine vs placebo | Men with prostate cancer after RP. Current or prior HT? Not specified. Evidence of metastases? Not specified. PSA, mean: not specified. PSA-DT: not specified. Gleason score: not specified. Age, mean: 63.8 years |
RCT – placebo controlled, n = 37 (n = 19, SM-Se group; n = 18, placebo group) Duration: 6 months |
QoL. Haematology and basic clinical chemistry (ALT, AST and GGT, cholesterol, TAG, ApoA1 and ApoB). Oxidative stress markers. Testosterone |
Improved QoL. Decreased LDLs and total cholesterol from baseline in SM-Se group (P < 0.05); increase from baseline in erythrocyte count and haemoglobin in SM-Se group (P < 0.05). No change to testosterone or antioxidant status |
No AEs reported. |
| Advanced PrCa | ||||||
| Stenner-Liewen et al. [41] Switzerland |
Pomegranate (Punica granatum) juice, 500 mL or 500 mL placebo beverage/day for 1 month, followed by 250 mL pomegranate juice/day (both arms) for a further month | Early PSA rise after initial therapy, and PSA level ≥5 ng/mL. Current or prior HT? Yes, both. Evidence of metastases? Yes PSA level, mean: 74.6 ng/mL PSA-DT: not specified Gleason score: ≥8 in 49% Age, mean: 72.5 years |
RCT – double-blind, placebo-controlled phase IIb, n = 103/98 Duration: 2 months |
PSA values. Pain scores. Adherence |
No differences between groups for PSA values or pain scores. Adherence to protocol was good, with 94 patients (96%) completing the first period (days 1–28), and 87 (89%) completing both periods (days 1–56) |
No Grade ≥III toxicities. Bowel disturbances (one case obstipation and one diarrhoea in verum group) most frequently reported |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; Apo, apolipoprotein AST, aspartate amino-transferase; CR, complete response; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone; GI, gastrointestinal; GT, γ-glutamyltransferase; HT, hormone therapy; LDL, low-density lipoprotein; MCP, modified citrus pectin; PP, per protocol analysis; PR, partial response; QoL, quality of life; SM, Silybum marianum; TAG, trigylcerides.
Patients
In all, 500 patients were randomised across these five studies, of which 489 were included in intention-to-treat analyses. Of these, 368 were patients with BCR prostate cancer. One placebo-controlled RCT was conducted in each of the UK [22] and France [11]; the other three were conducted in the USA [21,23,24].
One study (Pomi-T®; Power Heath Products Ltd, Pocklington, York, UK) included men before and after radical therapies, on active surveillance (AS) or WW. The others included men after RP and/or radiotherapy (RT) for localised prostate cancer [11,21,23,24]. One of these also included men on hormone therapy (LHRH analogue) [23].
Four studies reported mean ages, which ranged from 69 to 75 years [11,21,23,24], and the other reported a median age of 75 years [23]. The mean baseline Gleason scores reported in two studies were 6.4 [22] and 6.45 [21], respectively. The baseline PSA levels were specified in four studies: means of 0.76 ng/mL [11], 6.5 ng/mL [22], 5.3 ng/mL [21], and a median value of 6.5 ng/mL [23]. The baseline PSA-DT was reported in three trials: means were 14.4 [11] and 14.7 months [21]; percentages of men with slow, moderate and fast PSA velocities were 38% (<4 months), 32% (4–9 months), and 30% (>9 months), respectively [24].
The PSA inclusion criteria were specified in four studies: PSA level of >0.2 and <5 ng/mL after RP ± EBRT [11]; no PSA level limits [24]; a minimum PSA level of 10 ng/mL [23]; a PSA level of ≥0.4 ng/mL after RP or multiple therapies, PSA level of >1.5 ng/mL after primary RT or cryotherapy; PSA nadir plus 2 ng/mL after EBRT with neoadjuvant hormonal therapy [21].
Definitions of BCR were included in four studies: increasing PSA level at three successive measurements [11]; at least two consecutive increases in serum PSA level [24]; three successive elevations at a minimum interval of 2 weeks or at least two PSA values at least 2 weeks apart [23]; rising PSA level at three or more time points at least a month apart, within the year before enrolment [21].
PSA-DT calculations were reported in three of the studies [11,21,24], and PSA velocity calculations described in a fourth (linear mixed-effects modelling with logarithm of PSA level [23]). The PSA-DT was calculated either by fitting a simple linear regression model, using the slope, (the natural log of 2 divided by the slope obtained from fitting a linear regression of the natural log of PSA on time (months) [21], consistent with the Memorial Sloan Kettering Cancer Centre calculation [11]), or by using only the difference of the first and last PSA values (the natural log(2) × time interval/log final PSA − log initial PSA [24]).
Current users of hormone therapy were included in one trial [24]. One study permitted LHRH analogue but no other hormones within the previous 4 weeks [23]. Three other studies included prior but not current users [11,21,22]. Two allowed hormone use in conjunction with RT [11,22] and the other at least 1 year prior to enrolment [21]. There was no significant difference across groups for current or prior use of hormone/anti-androgen therapy in any of the trials including these baseline data [11,21,23,24].
Interventions
Three types of interventions were identified in the studies: phytotherapeutic extracts, isolated phytochemicals, and plant-derived dietary items. Interventions were delivered either as monotherapies or in combinations. Two studies investigated lycopene: dietary lycopene (tomatoes) >25 mg/day, calculated according to a food worksheet vs a powdered soy protein supplement 40 g/day (Solae Company, St. Louis, MO, USA; product Number AB20 Soy 1.2; each 40 g packet contained 24 mg genistein, 12 mg diadzein, and 2 mg glycitein. isoflavones were present in the aglycone form) for 1 month followed by the combination for a further month (both arms) [24]; lycopene 30 mg/day (Lyc-o-mato®,15 mg orally twice daily) vs a combination of lycopene at the same dose plus isoflavone capsules 80 mg daily (Solgen R 40 mg orally twice daily, LycoRed Company, Beer-Sheva, Israel) [23]. The other three studies investigated three different interventions: free stabilised sulphoraphane 60 mg daily (Nutrinov™) [11]; Pomi-T, a combination of pomegranate (Punica granatum) extract, green tea (Camellia sinensis), broccoli sprouts (Brassica oleracea) and turmeric (Curcuma longa) – all 300 mg/day; and pomegranate (Punica granatum) extract 1 g or 3 g/day (POMx capsules, POM Wonderful LLC; 1 or 3 capsules/day, each containing 1 000 mg of polyphenol extract, comparable to ≈250 mL pomegranate juice, 90% polyphenols) [21].
Controls/Comparators
Two studies were placebo-controlled trials (sulphoraphane [11] and Pomi-T [22]) of 6 months duration; the pomegranate (POMx) study was a high-/low-dose study of up to 18 months duration [21]; and the remaining two compared lycopene with a different phytotherapeutic agent (soy protein) for 2 months [24]/combination (lycopene plus soy isoflavones for up to 6 months) [23].
Effects of Interventions
PSA levels
With all five interventions investigated, serum PSA levels either stabilised, decreased, or increased less than with placebo in a significant number of men on verum [the active study substance(s)]. PSA levels rose significantly less with sulphoraphane than with placebo over 6 months (P = 0.03), and the log PSA slope was significantly lower between baseline and the end of month 6 (P = 0.036), and particularly at months 3 and 6 (P = 0.011) [11]. In the Pomi-T study, the mean PSA level rose by 8.78% in the WW group on verum (95% CI −6.32 to 26.62), compared with an 80.34% (95% CI 50.54–116.55) rise in the placebo group (P = 0.001) [22]. The serum PSA level decreased in 25% of men administered tomato/lycopene for 1 month followed by a combination of tomato and soy protein for a further month, compared with 43% men administered soy protein for 1 month, followed by the combination for a further month, (36% men overall) [24]. No partial (50% PSA reduction) or complete (PSA level of ≤4 ng/mL) responses were seen with lycopene or lycopene plus soy isoflavones over 6 months [23]. However, 95% of patients in the lycopene group and 67% of the lycopene-isoflavones arm achieved stabilisation of serum PSA levels. Additionally, the PSA velocity significantly declined over the 6 months for both men with hormone-sensitive (P = 0.015) and hormone-refractory (P = 0.017) prostate cancer. Lycopene-only resulted in a significantly greater decline than the combination for the men with hormone-refractory disease (P = 0.021). In the pomegranate study (POMx), declining post-baseline PSA levels were seen in 13% (six patients in the low-dose arm and nine patients in the high-dose arm) and stable disease was seen in 36 (78%) patients in the low-dose arm and 40 (82%) in the high-dose arm [21].
PSA-DT
All three studies reporting on PSA-DT found prolongation in a number of men [11,21,24]. An estimated 78% PSA-DT increase was seen with sulphoraphane (21.7 months) compared with placebo (12.2 months) [11]. The median PSA-DT extended from 14.26 to 28.9 months over 6 months. With tomato or soy protein for 1 month followed by a combination of the two for a further month, prolongation of the PSA-DT occurred in 58% of men, 65% in the tomato group, and 51% in the soy group [24]. The percentage of men whose PSA-DT was classified as slowest (>9 months) increased from 30% to 48% (P = 0.08), over the 2-month period. Administration of POMx capsules (1 or 3 g/day) resulted in a median lengthening of the PSA-DT from 11.9 to 18.5 months over the study period of up to 18 months (P < 0.001), with no significant difference between the dosage regimens [21]. In the 1g-POMx group, 76% of patients had a stable or lengthening PSA-DT and 46% had increases of ≥100% in PSA-DT. In the 3g-POMx group, 82% of patients had stable or lengthening PSA-DT, and 41% had ≥100% increase in PSA-DT.
Other
There was no significant effect on testosterone levels with sulphoraphane, POMx or lycopene and soy protein [11,21,24]. With administration of POMx capsules, oestradiol trended higher in the high-dose group from 28.0 to 32.3 pg/mL, but not in the low-dose group. Sex hormone-binding globulin (SHBG) increased in both groups (42.5–54.7 nmole/L in the 3g-POMx group and 42.8–49.2 nmole/L in the 1g-POMx) with no significant difference between groups [21]. Lycopene plus soy resulted in reductions in serum vascular endothelial growth factor (VEGF; P < 0.04) and total cholesterol, in both treatment arms [24].
AEs
AEs were predominantly Grade I gastrointestinal events that were not significantly more frequent than with placebo in the RCTs. As shown in Table 1, other events were minor in nature.
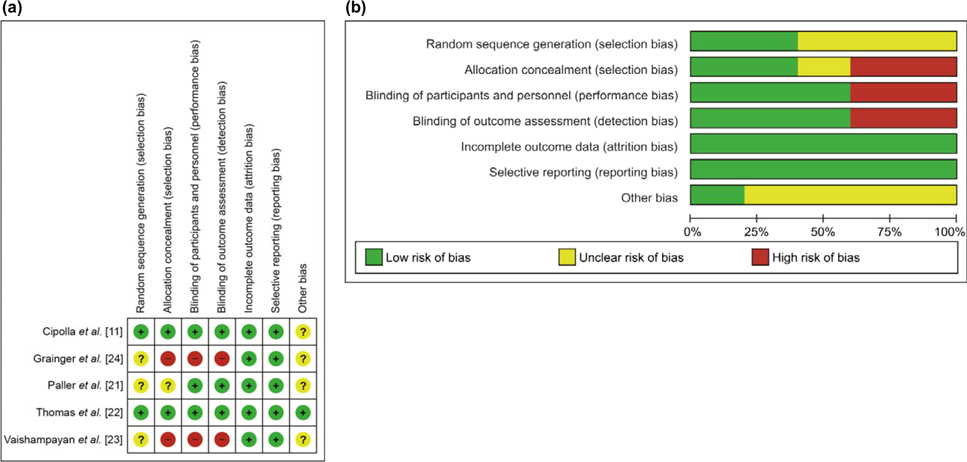
Risk of Bias
Risk of bias analysis was conducted in accordance with the Cochrane Collaboration guidelines for systematic reviews as an assessment of the validity of findings, and is summarised in Figure 2A,B [11,21–24]. Most were categorised as low risk (60%) or unclear (20%). Random sequence generation was only described in two of the five studies. The two unblinded active-comparator studies were assessed as high risk on selection, performance, and detection bias criteria [23,24]. In the sulphoraphane study, it would appear the trial product manufacturer had involvement in data analysis and interpretation [11].
Fig. 2.
Risk of bias summary (a) and graph (b) of included studies.
Reporting According to Elaboration of CONSORT for Trials of Herbal Interventions
All identified studies were assessed according to the elaborated CONSORT statement for trials of herbal interventions [20]. Generally compliance was low (Table 3). All studies reported the common names of the plant [4A.1(iv)], the propriety product name or extract name and name of manufacturer of the product [4A.(2)], and dose and duration of administration [4C(i) and (ii)], but none included the botanical family [4A.1(iii)], the type and concentration of extraction solvent (where relevant); or a description of the practitioners (4F).
Table 3.
Reporting according to the proposed elaboration of CONSORT checklist Item 4 for reporting RCTs of herbal medicine.
| Cipolla et al. [11] | Thomas et al. [22] | Grainger et al. [24] | Vaishampayan et al. [23] | Paller et al. [21] | |
|---|---|---|---|---|---|
|
| |||||
| 4A: Herbal medicinal product name | |||||
| 1. (i) Latin binomial; | Yes | Yes | |||
| (ii) Botanical authority; | Yes | ||||
| (iii) Family; | |||||
| (iv) Common names | Yes | Yes | Yes | Yes | Yes |
| 2. The propriety product name (brand name) or extract name and name of manufacturer of the product | Yes | N/A | Yes | Yes | |
| 3. Whether the product is authorised (licensed or registered) in the country in which the study was conducted. | Not required | N/A | |||
| 4B: Characteristics of the herbal product | |||||
| 1. Plant part used in extract | Yes/No | Yes/No | |||
| 2. Type of product e.g. raw, (fresh or dry) extract; | Yes | ||||
| 3. (i) Type and concentration of extraction solvent use (e.g. 80% ethanol etc.); and | N/A | ||||
| (ii) The ratio of herbal drug to extract (e.g. 2:1) | Green tea –Yes Others N/A | ||||
| 4. The method of authentication of raw material, the lot number of raw material; whether a voucher specimen was retained; details of where deposited and reference number. | Available on request | ||||
| 4C: Dosage regimen and quantitative description | |||||
| 1. (i) The dosage of the product; | Yes | Yes | Yes | Yes | |
| (ii) Duration of administration; and | Yes | Yes | Yes | Yes | |
| (iii) How these were determined. | Yes | Yes | |||
| 2. The content of all quantified herbal product constituents per dosage unit form. Added materials such as binders etc. | No/Yes | Yes | |||
| 3. For standardised products the quantity of active/marker constituents per dosage unit form. | N/A | N/A | N/A | N/A | |
| 4D: Qualitative testing | |||||
| 1. (i) Products chemical fingerprint and methods used; | Yes | ||||
| (ii) Who performed the chemical analysis; | Yes | ||||
| (iii) Whether a sample of the product was retained; (iv) if so, where | Yes | ||||
| 2. (i) Description of any special testing/purity testing undertaken | Presented to reviewers | ||||
| (ii) Which unwanted components were removed, and | |||||
| iii) how | |||||
| 3. (i) Standardisation: what to standardise; and | |||||
| ii) how | |||||
| 4E: Placebo/control group | |||||
| 1. The rationale for the type of control/placebo used | Yes | N/A | N/A | N/A | |
| 4F: Practitioner/s | |||||
| 1. A description of the practitioners (training and practice experience) who are a part of the intervention | |||||
Discussion
There is growing interest in researching phytotherapeutic interventions for BCR prostate cancer, especially ones that do not interfere with hormone receptors. Many men who experience PSA relapse after local treatment for prostate cancer turn to herbal and other complementary medicines in an attempt to delay the need for ADT, with its attendant side-effects, to prolong the time to metastases, and/or reduce prostate cancer-specific morbidity and mortality. We have presented the results of a systematic review of the evidence from randomised trials of phytotherapeutic extracts, isolated phytochemicals, and plant-derived dietary items in the context of BCR prostate cancer. To our knowledge, this is the first review of phytotherapeutic interventions in this cohort.
Of the 23 identified clinical studies, five met the criteria for inclusion. No two were identical in terms of intervention and comparator, thus precluding any meaningful meta-analysis. Overall, the quality of the studies was good, although generally sample sizes were small and durations short. Interventions investigated were sulphoraphane; lycopene vs soy (Glycine max) protein; lycopene with soy isoflavones; Pomi-T, which is a combination of pomegranate (Punica granatum), green tea (Camellia sinensis), broccoli sprouts (Brassica oleracea) and turmeric (Curcuma longa); and pomegranate (Punica granatum) extract (POMx). Despite small sample sizes, all studies found serum PSA levels to stabilise, decrease, or rise more slowly in a significant number of men. Statistically significant results were found for a decrease in the mean PSA slope vs placebo with sulphoraphane, the mean PSA change with Pomi-T, and PSA velocity in both hormone-sensitive and hormone-refractory prostate cancer with lycopene and lycopene plus soy isoflavones. Prolongation of the PSA-DT was seen in all three studies reporting on this endpoint [11,21,24], and reached statistical significance with sulphoraphane, and POMx. In the third, the 2-month study of tomato vs soy protein, it should be noted that PSA was not the main endpoint of interest, but rather metabolism and absorption [24]. Importantly, in the three studies that monitored testosterone levels across the treatment phase, no significant changes were found. With POMx [21], both dosage regimens resulted in non-significant increases in SHBG levels. Only one study measured changes in VEGF, which found significant reductions with both dietary lycopene and soy protein [24]. Safety and tolerability were very good for all interventions. However, these findings should be treated with caution pending replication of results from further adequately powered studies.
Limitations
Consistent with the findings of other reviews on phytotherapeutic interventions in prostate cancer [42–44], limitations include the number of studies identified, sample sizes, duration of follow-up, and lack of power in some studies to detect clinically meaningful changes in PSA or other biomarkers of disease progression. One study included men on both AS (before local therapy) and WW (after local therapy), without consistent reporting of sub-population analysis, thereby limiting our ability to determine the impact on the rising PSA population after local therapy [22].
In addition, comparison of results across studies is hindered by the variety of ways used to report PSA endpoints: mean change in PSA/percentage change from baseline; percentage lower than control arm; percentage of men with a decrease in PSA; number of/percentage of men with a partial (>50% reduction) or complete PSA response; time to PSA progression. The PSA-DT was defined as the mean or median log slope; the number of men moving from one risk category (slow, moderate or fast) to another; the median or mean number of months’ prolongation; and percentage change from baseline.
For adherence to the proposed elaboration of CONSORT checklist item 4 (Table 3) for trials of herbal medicine, reporting was inadequate for information relating to the herb name, characteristics of the herbal product, quantitative description, and qualitative testing. A lack of complete and transparent reporting impacts on reproducibility of studies, as well as translation into clinical practice.
Abstracts published in proceedings of professional meetings were searched but not included in the review. As none of these was a negative study, this is unlikely to reflect publication bias. However, publication bias cannot be excluded. The key issues identified with phytotherapeutic research in this area are summarised in Table 4.
Table 4.
Key limitations identified with phytotherapeutic research in this area.
| Small sample sizes |
| Short follow-up periods |
| Inadequate power |
| Significant variations in study populations |
| Significant variations in intervention |
| Significant variations in design |
| Significant variations in endpoints |
| Publication bias cannot be excluded |
| Bias sometimes difficult to establish due to incomplete reporting |
| Some manufacture driven trial data |
| Type and concentration of extraction solvent used not commonly reported |
| Extraction methods and actual bioavailability of compounds are variable |
| The ratio of herbal drug to extract not commonly reported |
| Equivalence of product with expected bioactivity is variable |
The overall aim of the present review was to examine the evidence for phytotherapeutic interventions in prolonging metastasis-free survival in men with BCR prostate cancer. The attempt to pool or compare results from the identified studies highlighted several barriers to comparability of findings from phytotherapy research in this cohort; namely variations in study population, intervention, design and endpoints. As most studies do not concurrently measure other markers of tumour progression in participants, the possibility of the interventions masking PSA kinetics cannot be excluded.
Recommendations
Greater consensus among researchers designing clinical trials is needed to permit meta-analyses and meaningful comparisons of studies that would assist clinicians in advising patients of the risks and/or benefits of phytotherapeutic interventions in BCR prostate cancer. Four areas are candidates for such consensus:
Definitions of BCR, and the number and frequency of PSA readings over time: The authors propose that researchers use the AUA definition of BCR after RP (PSA level of >0.2 ng/mL measured 6–13 weeks after RP, followed by a confirmatory test showing a persistent PSA level of >0.2 ng/mL [45]. Researchers should also follow the AUA recommendation for defining BCR after RT by using the American Society for Radiation Oncology (ASTRO) definition of BCR (the midpoint between PSA nadir and the first of three consecutive rises in PSA level [5]).
Stratification: Because metastasis-free survival is strongly influenced by the PSA-DT and Gleason score, it is important to stratify patients on these two factors (PSA-DT of <9 vs ≥9 months and Gleason score ≤3 + 4 vs ≥4 + 3 [2,3]).
Methods of calculating the PSA-DT: As recommended by the Prostate-Specific Antigen Working Group’s Guidelines on PSA-DT, the calculation of PSA-DT is the natural log of 2 divided by the slope obtained from fitting a linear regression of the natural log of PSA on time (months) for at least three values of PSA all >0.2 ng/mL and all taken within 12 months [46].
Reporting of results: Results should include, where available, change in the PSA-DT, 30% and/or 50% decline in PSA level and metastasis-free survival in accordance with the Prostate Cancer Working Group definitions [47].
Endpoints
Although the PSA-DT is probably the most important prognostic factor in metastases-free survival and overall survival [2], further evidence is needed to confirm findings that changes in PSA-DT after initiation of a therapy are prognostic for metastasis-free survival in patients with BCR disease [5,48]. Inclusion of metastases-free survival as an endpoint is encouraged, despite necessitating lengthy follow-up periods. To determine whether effects endure, adequate follow-up periods, and non-treatment follow-up beyond the treatment phase are needed. Where interventions have potential phytoestrogenic activity, the inclusion of sex hormones and SHBG is recommended.
To ensure that interventions are not simply suppressing PSA, but also impacting on cancer progression, routine prostate imaging by CT and bone scans every 1–2 years, depending on PSA kinetics, is recommended.
Design
The importance of a placebo arm and adequate sample size to overcome the possibility of type II errors due to natural PSA variability is emphasised by the finding that calculated PSA-DT in BCR prostate cancer may naturally increase over time in the absence of therapy and may be influenced by the duration of PSA follow-up [49].
Intervention
In 2006, an elaboration of CONSORT item 4 was proposed for reporting on clinical trials of herbal interventions [20]. As biologically defined agents, crude phytotherapeutic drugs vary in chemical composition according to agricultural practices and growing conditions. Other factors that determine whether different phytotherapeutic extracts achieve phyto-equivalence (the same therapeutic activity), include plant part used, extraction methods, solvents, ratio of crude herbal drug to solvent, presence or absence of standardisation, and the quantity of herbal product constituents per dosage unit form. Disclosure of this information is essential for researchers wishing to replicate studies, as well as for clinicians prescribing based on published findings to have confidence that they are using an equivalent agent/product. However, reporting according to this checklist remains poorly done [50]. Improved reporting according to the proposed elaboration of CONSORT item 4 for reporting on clinical trials of herbal interventions is therefore urgently required.
Conclusion
There is growing interest in finding safe and effective phytotherapeutic therapies that can delay time to metastases and reduce prostate cancer-specific mortality and morbidity in men with BCR after local treatment. High-quality studies in this area are lacking. Sulphoraphane, lycopene, soy isoflavones, POMx, and Pomi-T are safe and well-tolerated. There is limited evidence that they can affect PSA dynamics. No recommendation can be made for the use of these agents in managing prostate cancer morbidity and mortality until high-quality, fully powered, placebo-controlled studies are available.
Abbreviations:
- ADT
androgen-deprivation therapy
- AE
adverse event
- AR
androgen receptor
- AS
active surveillance
- BCR
biochemical recurrence/biochemically recurrent
- CONSORT
Consolidated Standards Of Reporting Trials
- IGFBP-3
IGF-binding protein 3
- POMx
pomegranate extract
- PSA-DT
PSA doubling time
- RCT
randomised controlled trial
- RP
radical prostatectomy
- (EB)RT
(external beam) radiotherapy
- SHBG
sex hormone-blinding globulin
- VEGF
vascular endothelial growth factor
- WW
watchful waiting
Footnotes
Conflict of Interest
Prof. Kerry Bone works as a consultant director of Research and Development for MediHerb (Integria) Australia, manufacturers of herbal medicines. He and Dr Diana van Die are in-laws.
References
- 1.Klotz LH. PSA recurrence: definitions, PSA kinetics, and identifying patients at risk. Can J Urol 2006; 13(Suppl. 2): 43–7 [PubMed] [Google Scholar]
- 2.Antonarakis ES, Feng Z, Trock BJ et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int 2012; 109: 32–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedland SJ, Humphreys EB, Mangold LA et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005; 294: 433–9 [DOI] [PubMed] [Google Scholar]
- 4.Dorff TB, Groshen S, Tsao-Wei DD et al. A Phase II trial of a combination herbal supplement for men with biochemically recurrent prostate cancer. Prostate Cancer Prostatic Dis 2014; 17: 359–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol 2013; 11: 14–23 [PMC free article] [PubMed] [Google Scholar]
- 6.Ames SC, Tan WW, Ames GE et al. Quality of life of men with biochemical recurrence of prostate cancer. J Psychosoc Oncol 2008; 26: 17–34 [DOI] [PubMed] [Google Scholar]
- 7.Bishop FL, Rea A, Lewith H et al. Complementary medicine use by men with prostate cancer: a systematic review of prevalence studies. Prostate Cancer Prostatic Dis 2011; 14: 1–13 [DOI] [PubMed] [Google Scholar]
- 8.Athar M, Back JH, Tang X et al. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol 2007; 224: 274–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhuvaneswari V, Nagini S. Lycopene: a review of its potential as an anticancer agent. Curr Med Chem Anticancer Agents 2005; 5: 627–35 [DOI] [PubMed] [Google Scholar]
- 10.Cheung CW, Gibbons N, Johnson DW, Nicol DL. Silibinin – a promising new treatment for cancer. Anticancer Agents Med Chem 2010; 10: 186–95 [DOI] [PubMed] [Google Scholar]
- 11.Cipolla BG, Mandron E, Lefort JM et al. Effect of sulforaphane in men with biochemical recurrence after radical prostatectomy. Cancer Prev Res (Phila) 2015; 8: 712–9 [DOI] [PubMed] [Google Scholar]
- 12.Faria A, Calhau C. The bioactivity of pomegranate: impact on health and disease. Crit Rev Food Sci Nutr 2011; 51: 626–34 [DOI] [PubMed] [Google Scholar]
- 13.Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci 2007; 64: 1105–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahmoud AM, Yang W, Bosland MC. Soy isoflavones and prostate cancer: a review of molecular mechanisms. J Steroid Biochem Mol Biol 2014; 140: 116–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey M, Gupta S. Green tea and prostate cancer: from bench to clinic. Front Biosci (Elite Ed) 2009; 1: 13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren L, Perera C, Hemar Y. Antitumor activity of mushroom polysaccharides: a review. Food Funct 2012; 3: 1118–30 [DOI] [PubMed] [Google Scholar]
- 17.Shehzad A, Lee J, Lee YS. Curcumin in various cancers. BioFactors 2013; 39: 56–68 [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Ahmad A, Kong D, Bao B, Sarkar FH. Recent progress on nutraceutical research in prostate cancer. Cancer Metastasis Rev 2014; 33: 629–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.0.1). Chichester, West Sussex, UK: John Wiley & Sons Ltd, 2008 [Google Scholar]
- 20.Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med 2006; 144: 364–7 [DOI] [PubMed] [Google Scholar]
- 21.Paller CJ, Ye X, Wozniak PJ et al. A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer Prostatic Dis 2013; 16: 50–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas R, Williams M, Sharma H, Chaudry A, Bellamy P. A double-blind, placebo-controlled randomised trial evaluating the effect of a polyphenol-rich whole food supplement on PSA progression in men with prostate cancer – The UK NCRN Pomi-T study. Prostate Cancer Prostatic Dis 2014; 17: 180–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaishampayan U, Hussain M, Banerjee M et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer 2007; 59: 1–7 [DOI] [PubMed] [Google Scholar]
- 24.Grainger EM, Schwartz SJ, Wang S et al. A combination of tomato and soy products for men with recurring prostate cancer and rising prostate specific antigen. Nutr Cancer 2008; 60: 145–54 [DOI] [PubMed] [Google Scholar]
- 25.Clark PE, Hall MC, Borden LS Jr et al. Phase I-II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology 2006; 67: 1257–61 [DOI] [PubMed] [Google Scholar]
- 26.Flaig TW, Gustafson DL, Su LJ et al. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs 2007; 25: 139–46 [DOI] [PubMed] [Google Scholar]
- 27.deVere White RW, Hackman RM, Soares SE, Beckett LA, Sun B. Effects of a mushroom mycelium extract on the treatment of prostate cancer. Urology 2002; 60: 640–4 [DOI] [PubMed] [Google Scholar]
- 28.Yoshimura K, Kamoto T, Ogawa O et al. Medical mushrooms used for biochemical failure after radical treatment for prostate cancer: an open-label study. Int J Urol 2010; 17: 548–54 [DOI] [PubMed] [Google Scholar]
- 29.Twardowski P, Kanaya N, Frankel P et al. A phase I trial of mushroom powder in patients with biochemically recurrent prostate cancer: roles of cytokines and myeloid-derived suppressor cells for Agaricus bisporus–induced prostate-specific antigen responses. Cancer 2015; 121: 2942–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guess BW, Scholz MC, Strum SB, Lam RY, Johnson HJ, Jennrich RI. Modified citrus pectin (MCP) increases the prostate-specific antigen doubling time in men with prostate cancer: a phase II pilot study. Prostate Cancer Prostatic Dis 2003; 6: 301–4 [DOI] [PubMed] [Google Scholar]
- 31.Hussain M, Banerjee M, Sarkar FH et al. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer 2003; 47: 111–7 [DOI] [PubMed] [Google Scholar]
- 32.deVere White RW, Hackman RM, Soares SE, Beckett LA, Li Y, Sun B. Effects of a genistein-rich extract on PSA levels in men with a history of prostate cancer. Urology 2004; 63: 259–63 [DOI] [PubMed] [Google Scholar]
- 33.Kwan W, Duncan G, Van Patten C, Liu M, Lim J. A phase II trial of a soy beverage for subjects without clinical disease with rising prostate-specific antigen after radical radiation for prostate cancer. Nutr Cancer 2010; 62: 198–207 [DOI] [PubMed] [Google Scholar]
- 34.Pendleton JM, Tan WW, Anai S et al. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer 2008; 8: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pantuck AJ, Leppert JT, Zomorodian N et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res 2006; 12: 4018–26 [DOI] [PubMed] [Google Scholar]
- 36.Schroder FH, Roobol MJ, Boeve ER et al. Randomized, double-blind, placebo-controlled crossover study in men with prostate cancer and rising PSA: effectiveness of a dietary supplement. Eur Urol 2005; 48: 922–31 [DOI] [PubMed] [Google Scholar]
- 37.Kranse R, Dagnelie PC, Van Kemenade MC et al. Dietary intervention in prostate cancer patients: PSA response in a randomized double-blind placebo-controlled study. Int J Cancer 2005; 113: 835–40 [DOI] [PubMed] [Google Scholar]
- 38.Barber NJ, Zhang X, Zhu G et al. Lycopene inhibits DNA synthesis in primary prostate epithelial cells in vitro and its administration is associated with a reduced prostate-specific antigen velocity in a phase II clinical study. Prostate Cancer Prostatic Dis 2006; 9: 407–13 [DOI] [PubMed] [Google Scholar]
- 39.Bosland MC, Kato I, Zeleniuch-Jacquotte A et al. Effect of soy protein isolate supplementation on biochemical recurrence of prostate cancer after radical prostatectomy: a randomized trial. JAMA 2013; 310: 170–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vidlar A, Vostalova J, Ulrichova J et al. The safety and efficacy of a silymarin and selenium combination in men after radical prostatectomy – a six month placebo-controlled double-blind clinical trial. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2010; 154: 239–44 [DOI] [PubMed] [Google Scholar]
- 41.Stenner-Liewen F, Liewen H, Cathomas R et al. Daily pomegranate intake has no impact on PSA levels in patients with advanced prostate cancer – results of a phase IIb randomized controlled trial. J Cancer 2013; 4: 597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilic D, Misso M. Lycopene for the prevention and treatment of benign prostatic hyperplasia and prostate cancer: a systematic review. Maturitas 2012; 72: 269–76 [DOI] [PubMed] [Google Scholar]
- 43.van Die MD, Bone KM, Williams SG, Pirotta MV. Soy and soy isoflavones in prostate cancer: a systematic review and meta-analysis of randomized controlled trials. BJU Int 2014; 113: E119–30 [DOI] [PubMed] [Google Scholar]
- 44.Van Patten CL, de Boer JG, Tomlinson Guns ES. Diet and dietary supplement intervention trials for the prevention of prostate cancer recurrence: a review of the randomized controlled trial evidence. J Urol 2008; 180: 2314–22 [DOI] [PubMed] [Google Scholar]
- 45.Cookson MS, Aus G, Burnett AL et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol 2007; 177: 540–5 [DOI] [PubMed] [Google Scholar]
- 46.Arlen PM, Bianco F, Dahut WL et al. Prostate Specific Antigen Working Group guidelines on prostate specific antigen doubling time. J Urol 2008; 179: 2181–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scher HI, Halabi S, Tannock I et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antonarakis ES, Zahurak ML, Lin J, Keizman D, Carducci MA, Eisenberger MA. Changes in PSA kinetics predict metastasis- free survival in men with PSA-recurrent prostate cancer treated with nonhormonal agents: combined analysis of 4 phase II trials. Cancer 2012; 118: 1533–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paller CJ, Olatoye D, Xie S et al. The effect of the frequency and duration of PSA measurement on PSA doubling time calculations in men with biochemically recurrent prostate cancer. Prostate Cancer Prostatic Dis 2014; 17: 28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Die MD, Burger HG, Teede HJ, Bone KM. Vitex agnus-castus extracts for female reproductive disorders: a systematic review of clinical trials. Planta Med 2013; 79: 562–75 [DOI] [PubMed] [Google Scholar]