Abstract
Objective:
We developed and validated a set of composite scores that combine quantitative magnetic resonance (MR)-based measurements of hyaline cartilage damage, bone marrow lesions (BMLs), and effusion-synovitis into composite scores.
Methods:
We selected 300 participants (development cohort=100, validation cohort=200) from the Osteoarthritis Initiative with complete clinical, radiographic, and MR data at baseline and 24 months. We used semi-automated programs to quantify tibiofemoral and patellar cartilage damage, BML volume, and whole-knee effusion-synovitis volume. The candidate composite scores were formed by summing changes from baseline to 24 months based on pre-specified methods. We evaluated the candidate composite scores 1) ability to differentiate groups with and without KOA progression (17 radiographic and patient-reported definitions), 2) sensitivity to change (standardized response means), and 3) relative performance relating to legacy outcome measures of KOA progression.
Results:
Three out of 13 developed composite scores qualified for testing in the validation cohort (ranked by sensitivity to change): 1) whole-knee cumulative cartilage damage, 2) unweighted total knee score, and 3) BML+effusion-synovitis volume. Change in cumulative cartilage damage associated with radiographic progression (Kellgren-Lawrence: OR=1.84; Joint Space Width Progression: OR=2.11). Changes in the unweighted total knee score (OR=1.97) and BML+effusion-synovitis score (OR=1.92) associated with WOMAC knee pain progression
Conclusion:
Two composite scores emerged, reflecting discrete domains of KOA progression. First, cumulative damage, which is measured by a whole-knee cartilage damage score, reflects the damage accrued over time. Second, dynamic disease activity, which is measured by a BML+effusion-synovitis score, relates with changes in a patient’s state of disease and symptoms.
Keywords: Magnetic Resonance Imaging (MRI), Osteoarthritis, Cartilage, Disease Activity, Synovitis
Optional Terms: Knee, Bone Marrow Lesions, Effusion
Osteoarthritis of the knee (KOA) is a highly prevalent age-related disorder and a leading cause of pain and functional impairment in the population(1). Despite this, there are few effective interventions for KOA and none accepted to reduce its structural progression(2). One of the most significant obstacles to testing and developing disease-modifying therapies for KOA is the absence of measures of disease progression that meaningfully reflect a change in patient status(3). For regulatory agencies to accept an imaging biomarker of KOA progression the biomarker must: 1) reflect the complex pathology throughout the joint, 2) address the apparent discordance between structural changes and signs/symptoms/function, 3) offer a standardized definition of disease progression, and 4) reliably predict meaningful changes in symptoms and function(3).
Semiquantitative scales are comprehensive, but the approach is inherently insensitive to change(4) and limited by the absence of a validated composite measurement reflecting severity in the whole knee(5, 6). Quantitative measurements in knees have also been limited by a focus on single structures (e.g., hyaline cartilage).
Over the past decade, there has been a paradigm shift from conceptualizing KOA as a single-structure disorder, based in hyaline cartilage, to a multi-tissue ‘whole-organ’ failure of diarthrodial joints(2). MR imaging has revealed structural features, such as BMLs and effusion-synovitis, which are clinically relevant(12). BMLs reflect altered peri-articular bone morphology and density(13, 14) and associate with hyaline cartilage damage and pain(12, 15, 16). BMLs appear to be responsive to clinical intervention(17–19) and have been proposed as a therapeutic target for KOA disease modification(18, 20). Effusion-synovitis is also common in KOA and is strongly associated with pain(12, 21, 22) and hyaline cartilage loss(23, 24).
Combining these relevant features into a single quantitative composite knee score, rather than measuring each feature separately could advance the field towards by addressing 1) the multifactorial and complex etiopathogenesis of KOA, 2) the discordance between structural changes and signs/symptoms/function, and 3) the lack of consensus for the best definitions of disease progression. However, while this is an appealing concept, there are methodological issues that need to be addressed to determine if a composite score is practical, feasible, and valid. The first question is whether measures of each feature can be combined in a way that is internally (heuristically) consistent. For example, it is unclear if change in BMLs, which relates with change in pain, can or should be combined with change in hyaline cartilage, which relates to joint space narrowing. A second challenge is to determine how to mathematically combine the individual features into a single composite score that retains construct validity and optimizes the ability to detect a treatment difference (discriminative validity). Therefore, the goals of this study were to develop and validate candidate composite scores that combine quantitative measurements of hyaline cartilage damage, BMLs, and effusion/synovitis (Figure 1 and 2) into composite scores that are heuristically consistent; and then determine sensitivity to change, and identify the best performing composite scores in relation to established legacy measures of KOA progression (i.e., joint space with [JSW], Kellgren-Lawrence [KL] grade, WOMAC pain).
Figure 1: Measurement of medial tibiofemoral cartilage damage index (CDI) on paired baseline and follow-up magnetic resonance (MR) images.
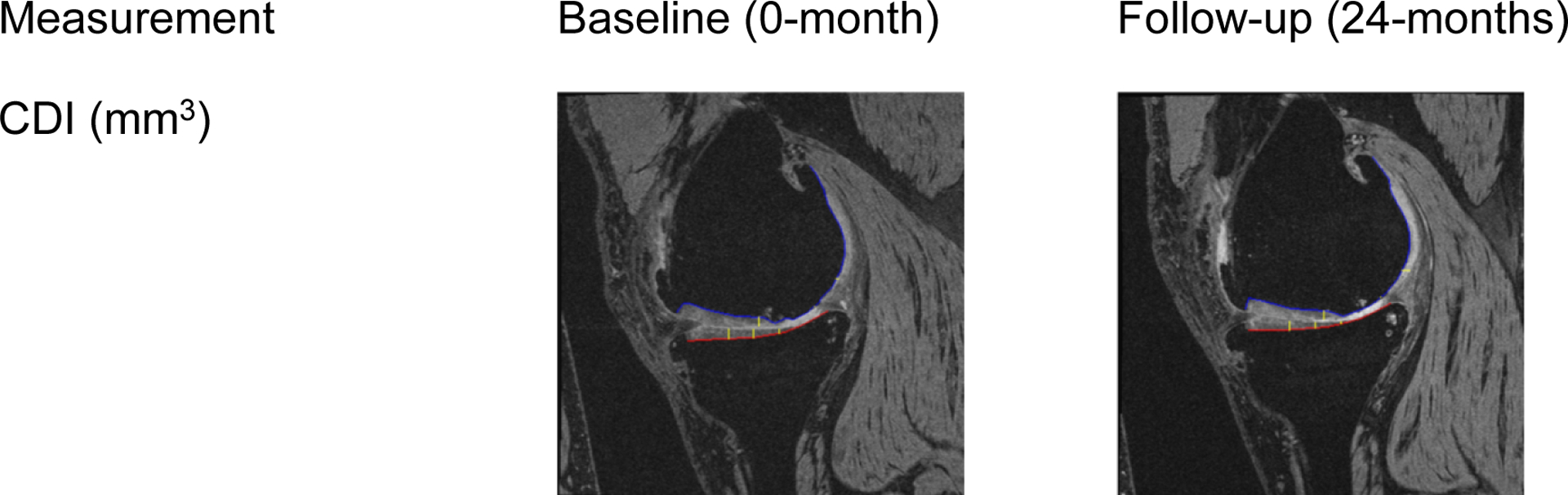
The images are examples of the medial tibiofemoral CDI with cartilage thickness being assessed at 3 out of 9 informative locations (yellow lines) on a tibia (red line) and femur (blue line). These measurements contribute to the whole-knee cumulative cartilage damage.
Figure 2: Measurement of medial tibiofemoral bone marrow lesion (BML) and whole knee effusion-synovitis on paired baseline and follow-up magnetic resonance (MR) images.
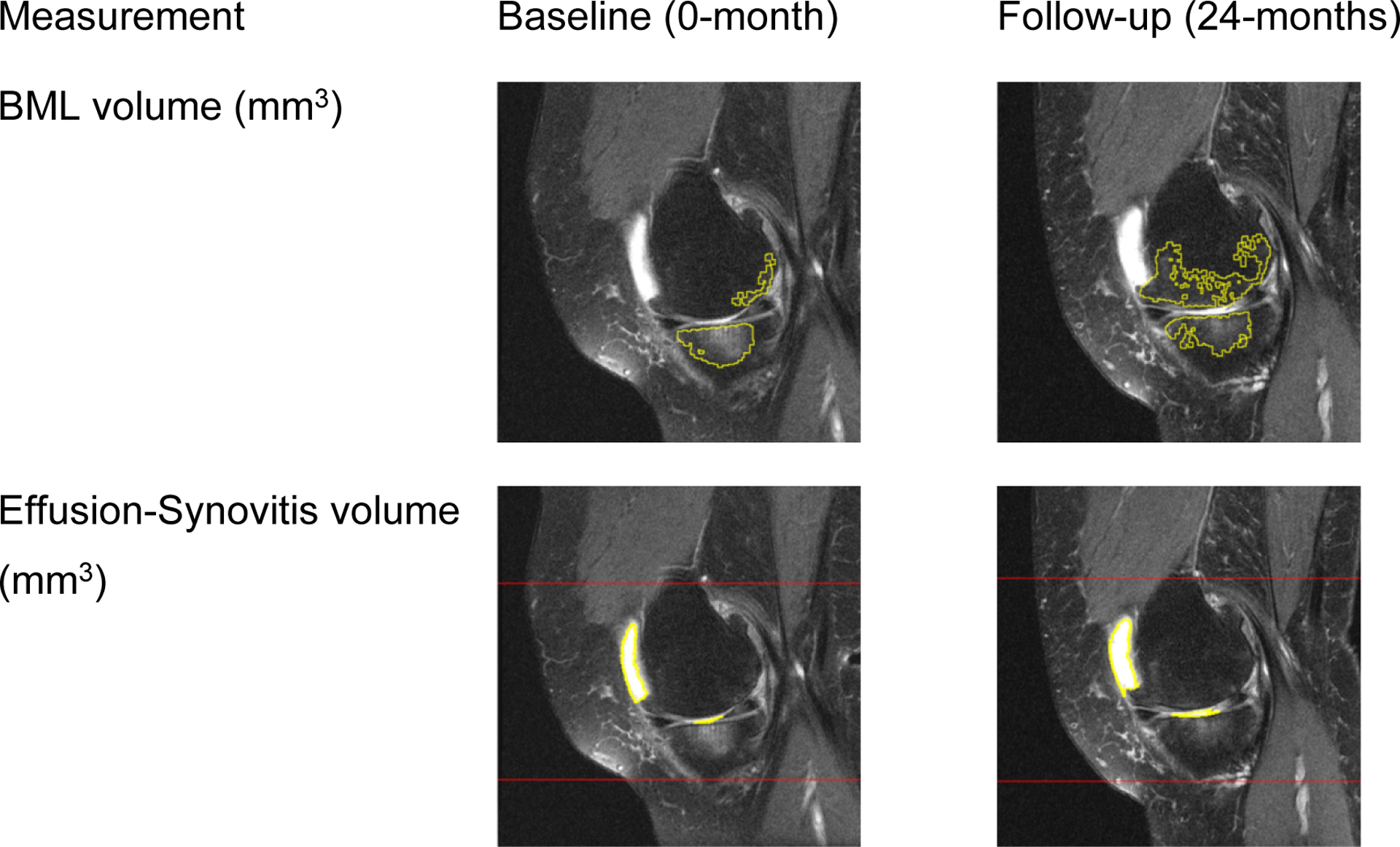
The top row are example MR images of a segmented BML (yellow lines). The bottom row are examples of effusion-synovitis segmentation (yellow lines). These measurements are components of the score based on BML+effusion-synovitis volume.
MATERIALS AND METHODS
Overview
To develop and validate composite, quantitative, MR-based outcome scores we selected 2 subcohorts from the Osteoarthritis Initiative (OAI), a multicenter cohort study of individuals with or at risk for symptomatic KOA in the United States. We used a development cohort (n=100) to develop and perform preliminary analyses to assess construct validity of candidate composite scores based on measures of radiographic progression as well as changes in quality of life and knee pain (Table 1). Then we used a validation cohort (n=200) to confirm the construct validity of the best-performing composite scores by determining which ones exhibit the best discriminative ability and sensitivity to change.
Table 1:
Binary Outcomes used for Construct Validity Steps in the Development and Validation Cohorts
| Outcome | Details of change from baseline to 24-month visit |
|---|---|
| Radiographic | |
| Kellgren-Lawrence Grade Progression | Any change in Kellgren-Lawrence grade |
| Lateral JSN Progression | Any change in lateral JSN |
| Medial JSN Progression | Any change in medial JSN |
| JSN Progression | Any change in medial or lateral JSN |
| Medial JSW progression | Change ≥ median change in the development dataset |
| Quality of Life | |
| KQOL 1 improvement | KQOL item 1 improves from 2 worst responses to 3 best responses (how often aware of problems with knee(s)) |
| KQOL 1 worsens | KQOL item 1 worsens from 3 best responses to 2 worst responses (how often aware of problems with knee(s)) |
| KQOL 2 improvement | KQOL item 2 improves from 2 worst responses to 3 best responses (modified lifestyle to avoid potentially damaging activities to knee(s)) |
| KQOL 2 worsens | KQOL item 2 worsens from 3 best responses to 2 worst responses (modified lifestyle to avoid potentially damaging activities to knee(s)) |
| KQOL 3 improvement | KQOL item 3 improves from 2 worst responses to 3 best responses (how much troubled with lack of confidence in knee(s)) |
| KQOL 3 worsens | KQOL item 3 worsens from 3 best responses to 2 worst responses (how much troubled with lack of confidence in knee(s)) |
| KQOL 4 improvement | KQOL item 4 improves from 2 worst responses to 3 best responses (in general, how much difficulty have with knee(s)) |
| KQOL 4 worsens | KQOL item 4 worsens from 3 best responses to 2 worst responses (in general, how much difficulty have with knee(s)) |
| Pain | |
| WOMAC pain progression | Pain increased by at least 2 points on 0–20 scale |
| WOMAC pain progression | Pain increased by at least 3 points on 0–20 scale |
| WOMAC pain improvement | Pain decreased by at least 2 points on 0–20 scale |
| WOMAC pain improvement | Pain decreased by at least 3 points on 0–20 scale |
JSN = joint space narrowing, KQOL = KOOS Quality of Life.
Selection of Subcohorts
The OAI included 4 clinical sites (Memorial Hospital of Rhode Island, The Ohio State University, the University of Maryland and Johns Hopkins University, and the University of Pittsburgh) and recruited between February 2004 and May 2006. Each clinical site and the coordinating center obtained institutional review board approval for the OAI. Informed consent was obtained from every participant.
In the course of prior analyses, the OAI investigators formed a Core Image Assessment sample that reflected people typically enrolled in clinical trials. This convenience sample consisted of one knee from 600 participants with symptomatic KOA at baseline (frequent knee pain and KL grade = 2 or 3) and complete baseline and 24-month data for clinical, radiographic, and MR outcomes(34). The current project used newer central radiographic readings, which explained why a few people had KL grade = 0, 1, or 4. We used the Core Image Assessment sample to assemble our 2 subcohorts for these analyses. We first selected participants for the development cohort. Within each KL grade, we randomly sampled participants with and without radiographic progression (any increase in KL grade over 24 months). The process was then repeated for the validation cohort after excluding the participants in the development cohort.
Magnetic Resonance Images
Each OAI site acquired knee MR images at baseline and 24-month follow-up visits using identical Siemens 3.0 Tesla Trio MR systems and knee coils. The OAI used study-certified, licensed MR technologists and completed comprehensive quality control measurements to promote quality, provide consistency across sites, and longitudinal uniformity(25, 26).
Acquisitions included a 3-dimensional dual echo steady state (3D DESS) sequence (field of view=140mm, slice thickness=0.7mm, skip=0mm, flip angle=25 degrees, echo time=4.7ms, recovery time=16.3ms, 307×384 matrix, x-resolution=0.365mm, y-resolution=0.456mm) and an intermediate-weighted fat suppressed (IWFS) sequence (field of view=160mm, slice thickness=3mm, skip=0mm, flip angle=180 degrees, echo time=30ms, recovery time=3200ms, 313×448 matrix, x-resolution=0.357mm, y-resolution=0.511mm).
Radiographic Outcomes
OAI participants had annual bilateral weight-bearing, fixed-flexion posterior-anterior knee radiographs. Central readers provided KL grades, medial and lateral joint space narrowing (JSN) grades, and medial JSW measurements. The intra-rater agreement for KL and JSN grades using the weighted kappa ranged from 0.70 to 0.88 and the intraclass correlation coefficient (ICC) for JSW was >0.9(27). We used the baseline and 24-month data to define KL, JSN, and JSW progression. The operational definitions of progression in these constructs are in Table 1. Data are publicly available at https://data-archive.nimh.nih.gov/oai (kxr_sq_bu00 (version 0.8), kxr_sq_bu00 (version 3.7), kxr_qjsw_duryea00 (version 0.8), and kxr_qjsw_duryea03 (version 3.7)).
Patient-Centered Outcomes
The Western Ontario & McMaster Universities Osteoarthritis Index (WOMAC) questionnaire measuring knee-specific pain (0 to 20 scale) was obtained at each OAI visit. We used baseline and 24-month WOMAC pain scores to calculate pain progression. We used the 4 Knee Injury and Osteoarthritis Outcomes Scores (KOOS) quality of life questions because we were interested in the unique concepts they reflect: 1) awareness of knee problem, 2) modifying activities, 3) lack of confidence, and 4) difficulty with a knee. The operational definitions of progression in these constructs are in Table 1. Data are publicly available at https://data-archive.nimh.nih.gov/oai (allclinical00 (version 0.2.2) and allclinical03 (version (3.2.1)).
Quantitative Measurement of Change in Cartilage Damage
We used a validated approach(28–30) to quantify cartilage damage on the 3D DESS MR images from the baseline and 24-month visits (Figure 1). The Cartilage Damage Index (CDI) generates a single measure from measurements of cartilage thickness obtained from predetermined regions of interest distributed across 6 regions in the knee joint: medial and lateral tibia, femur, and patella. We deployed a semi-automated interface to delineate the bone-cartilage boundary on the automatically selected MR images. We also used the interface to automatically identify the predefined informative locations to measure cartilage thickness in each of the 6 regions (9 locations in each tibiofemoral region, 12 locations in patellar regions). The CDI summary measure is calculated by summing the products of cartilage thickness, cartilage length, and voxel size from each of the informative locations. Intra-reader reliability (ICC) for the baseline measures ≥0.86 with >72 hours between read and re-read(28–32).
Quantitative Measurement of Change in Bone Marrow Lesion Volume
We measured BML volume in the medial and lateral tibia, femur, and patella regions using the IWFS MR sequence and a validated semi-automated software (Figure 2)(33). We defined BML as regions of high-signal intensity within a bone that appeared on >2 slices and were within 10mm of the articular surface(15, 34, 35). The software uses thresholding to delineate suspected BML regions. The reader (AC) manually adjusted the threshold and removed artifacts. All BML segmentation results were reviewed for quality by a second reader (JBD) and adjusted when necessary. Our reader had good intra-reader reliability among 20 knees measured twice with >48 hours between readings: ICC=0.98 for baseline BML volume.
Quantitative Measurement of Change in Effusion-Synovitis Volume
We measured whole knee effusion-synovitis volume using the IWFS MR sequence and a validated semi-automated software, using an approach that has been described previously (Figure 2)(36–38). Effusion-synovitis segmentation results were read by one reader (RS) and reviewed for quality by a second reader (MZ), with changes made when necessary. Our reader had good intra-reader reliability among 15 knees measured twice with >48 hours between readings: ICC=0.87 for baseline effusion-synovitis volume.
Analytic Approach
Standardizing MR-Based Measures of Change to the Same Scale
We generated 13 MR-based structural measurements: articular cartilage damage measured by CDI (6 measurements: medial and lateral femur, tibia, and patella), BML volume (6 measurements: medial and lateral femur, tibia, and patella), and effusion-synovitis volume (single whole-knee measurement). We adjusted each structural measure for knee size based on femur width (medial to lateral femoral epicondyle). Next, we subtracted the baseline mean and divided by the baseline standard deviation ([measure – baseline mean]/baseline standard deviation) to transform all measurements to the same scale (mean=0 and standard deviation=1). We calculated change in each structural measurement by subtracting the baseline from the 24-month value. We multiplied the CDI change measurements by (–1) to ensure greater change represented worsening disease burden among all the structural measurements.
Development of Candidate Composite Knee Scores
In the development cohort, we implemented 4 approaches to select features to combine into 13 candidate composite knee scores.
For the first approach, we created whole-knee composite scores, which included all 13 measures of change in cartilage, BML, and effusion-synovitis. We used 3 mathematical methods to calculate total MR-based scores: (1) unweighted summation, (2) inverse variance weighting, and (3) weighting according to the eigenvectors from a principal component analysis (PCA).
For the second approach, we used expert judgment based on the existing literature to select MR-based features that might reasonably be combined: (1) a cumulative cartilage damage score comprising all 6 measures of cartilage change and (2) a BML+effusion-synovitis composite using all 7 measures of change in BML and effusion-synovitis volumes. We used an unweighted sum (simple addition) to calculate these 2 candidate composite scores.
For the third approach, we used a data-driven approach based on a PCA to identify principal components (i.e., structural measures that cluster together based on correlated measures of change in MR-based features). The PCA enabled us to determine if structural measures group based on anatomic location (e.g., medial femoral BML volume and cartilage change) or within a given tissue (e.g., all BML volumes). Each principal component with an Eigenvalue>1 was considered a candidate composite knee score. For each component with Eigenvalue>1, we calculated a candidate composite knee score by multiplying standardized measures of each MR-based feature that had a factor loading >0.4 (absolute value) in a principal component by its eigenvector divided by the sum of the eigenvectors of the contributory components.
For the fourth approach, we calculated 2 variables using an unweighted sum of BML or effusion-synovitis volumes in the whole knee.
Preliminary Evaluation of the Candidate Composite Scores in the Development Cohort.
To prune the set of candidate composite scores before advancing these to the validation sample, we performed preliminary analyses in the development cohort. We evaluated the performance of each candidate composite score to differentiate KOA progressors from nonprogressors based on 2-year change in 17 dichotomous measures of pain, quality of life, and radiography (Table 1) using Wilcoxon Rank Sum tests.
Our decision rule to identify the best-performing composite scores that warranted further validation was that a candidate composite score needed to differentiate (Wilcoxon Rank Sum p-value<0.20(39)) between groups with and without KOA progression for at least one measure in each of the 3 constructs: 5 measures of radiographic progression, 8 measures of quality of life, and 4 measures of knee pain (Table 1).
Confirmation of Validity of the Best-Performing Composite Scores.
In the validation cohort, we examined the best performing candidate composite scores from the development cohort. Specifically, we re-assessed their performance based on 3 criteria: 1) an ability to differentiate groups with and without KOA progression (radiographic and patient-reported), 2) sensitivity to change, and 3) associations with several commonly used outcome measures of KOA progression. First, to confirm whether a candidate quantitative composite knee score differentiated groups we evaluated the same 17 dichotomized measures tested in the development cohort (requiring a Wilcoxon Rank sum p-value<0.05). Second, we calculated sensitivity to change for each candidate composite knee score using a standardized response mean (SRM) and interquartile range (IQR). Finally, we compared the performance of each candidate composite score using the area under the receiver-operating characteristics curve (AUC) and odds ratios (OR) in relation to commonly used outcome measures of KOA progression: change in KL Grade, JSW, and pain. To help compare our results with a prior biomarker study we adjusted for the same variables they selected: sex, race, age, body mass index (BMI), KL grade, WOMAC pain measure, use of pain medications, and minimum joint space width(40). The ORs and 95% confidence intervals were calculated using one standard deviation as the unit of analysis.
The assumption of linearity of the candidate composite scores with the outcomes was assessed. We used model diagnostics to identify potential outliers and influential points in the multivariable models. P-values were not adjusted to account for multiple comparisons. We performed all analyses using SAS 9.4 (Cary, NC).
RESULTS
We removed one participant from the development cohort and 3 participants from the validation cohort due to concerns with MR image quality (e.g., metal artifact, pathologic changes unrelated to KOA). The 99 participants in the development cohort had a mean age of 62 years, mean BMI of 30 kg/m2, and 60% were female (Table 2). Similarly, the 197 participants in the validation cohort had a mean age of 61 years, mean BMI of 30 kg/m2, and 54% were female (Table 2).
Table 2:
Participant Characteristics
| Development Cohort (n=99) | Validation Cohort (n=197) | |
|---|---|---|
| Female (n, %) | 59 (59.6) | 107 (54.3) |
| Age (years) | 61.5 (8.6) | 61.2 (8.8) |
| BMI (kg/m2) | 30.2 (4.6) | 30.1 (5.1) |
| KL grade (n, %) | ||
| 0 | 1 (1.0) | 1 (0.5) |
| 1 | 9 (9.1) | 9 (4.6) |
| 2 | 40 (40.4) | 83 (42.1) |
| 3 | 47 (47.5) | 101 (51.3) |
| 4 | 2 (2.0) | 3 (1.5) |
| Progression, KL grade (n, %) | 29 (29.9) | 56 (28.9) |
| Progression, medial JSW (n, %) | 47 (47.5) | 92 (46.7) |
| WOMAC Pain, baseline | 4.4 (3.6) | 5.0 (3.6) |
| WOMAC Pain, worsen at least 3 points (n, %) | 20 (20.2) | 31 (15.7) |
Reported as mean (SD) unless otherwise indicated. KL progression is any worsening in the KL score. Medial JSW progression is more change than the median change.
Development and Preliminary Evaluation of the Candidate Composite Scores in the Development Cohort.
The development process produced 13 candidate composite scores of structural changes (Table 3). Four of the 13 candidate composite scores met our decision rule based on discriminatory ability to advance as a best-performing composite score (Supplementary Table S1). These included (1) the unweighted total knee score (2) cumulative cartilage damage (3) BML+effusion-synovitis volume, and (4) change in the first principal component (patellar damage component).
Table 3:
Candidate Quantitative Composite Knee Scores in the Development Cohort
| Score | Calculation |
|---|---|
| Principal Component 1** | Sum of CDI in the lateral and medial patella and BML in the medial patella |
| Principal Component 2* | Sum of BML in lateral and medial tibia |
| Principal Component 3* | Sum of CDI in the lateral patella, effusion-synovitis volume, and BML in the lateral patella |
| Principal Component 4* | Sum of CDI in the medial femur and tibia and BML in the medial femur |
| Principal Component 5* | Sum of BML in lateral femur, medial femur, and lateral patella |
| Principal Component 6* | Sum of CDI in the lateral femur and tibia |
| PCA-Weighted Total | Sum of all 13 measurements, weighted by the principal component loading |
| Inverse variance weighting Total | Sum of all 13 measures, Inverse variance weighted |
| Unweighted Total** | Sum of all 13 measurements (simple addition) |
| Cumulative Cartilage Damage** | Sum of all 6 CDI measures |
| BML+Effusion-Synovitis** | Sum of all 6 BML and effusion-synovitis measures |
| Whole knee BML volume | Sum of all 6 BML measures |
| Whole knee Effusion-Synovitis Volume | Sum of all effusion-synovitis volumes |
All knee composite scores based on the principal components analysis are multiplied by the eigenvector for the component and divided by the sum of the eigenvectors of the 6 components.
Met criteria for further testing in the validation cohort.
PCA = principal component analysis, BML = bone marrow lesion, CDI = cartilage damage index
Confirmation of Validity of the Best-Performing Composite Scores in the Validation Cohort.
Change in the BML+effusion-synovitis score was the only candidate composite knee score to differentiate progressors and nonprogressors for at least one measure within each of the 3 constructs (Supplementary Table S1). Specifically, change in the BML+effusion-synovitis score differentiated progressors and nonprogressors for 1 out of 5 radiographic measures, 1 out of 6 quality of life measures, and 3 out of 4 knee pain measures. Change in the cumulative cartilage damage differentiated groups for 4 radiographic measures, 1 quality of life measures, but none of the knee pain measures. Change in the unweighted total knee score differentiated groups for 3 radiographic measures, none of the quality of life measures, and 3 knee pain measures. Finally, change in the first principal component only differentiated progressors and nonprogressors for one measure of radiographic progression. Hereafter, we omitted the first principal component from the analyses.
Sensitivity to Change in the Validation Cohort.
The cumulative cartilage damage had the greatest sensitivity to change (SRM=1.19; mean (SD)=0.88 (0.74)), followed by the unweighted total knee score (SRM=0.48, mean (SD)=1.52 (3.16)), and BML+effusion-synovitis (SRM=0.22, mean (SD)=0.65 (2.96)). Alternatively, the unweighted total knee score had the largest IQR of change (IQR=2.49; 25th%=0.12, 75th%=2.61) followed by BML+effusion-synovitis (IQR=1.90; 25th%=−0.43, 75th%=1.47) and cumulative cartilage damage (IQR=0.89; 25th%=0.40, 75th%=1.29).
Associations with Commonly Used KOA Progression Outcomes
Table 4 summarizes the associations between the candidate composite scores and progression in KL, medial JSW, and WOMAC pain. In adjusted models, change in cumulative cartilage damage was the only candidate associated with KL progression (OR=1.84) or JSW progression (OR=2.11). Changes in the unweighted total knee score (OR=1.97) and BML+effusion-synovitis score (OR=1.92) associated with WOMAC knee pain progression.
Table 4:
Odds ratios and Area under the ROC curve for Composite Scores in the Validation Cohort
| Unadjusted | Adjusted* | |||
|---|---|---|---|---|
| Knee Measure | Odds Ratio (95% CI) | AUC | Odds ratio (95% CI) | AUC |
| Kellgren-Lawrence Grade Progression | ||||
| Unweighted total score | 1.29 (0.94, 1.77) | 0.60 | 1.38 (0.97, 1.96) | 0.71 |
| Cumulative cartilage damage | 1.54 (1.12, 2.11) | 0.64 | 1.84 (1.27, 2.69) | 0.72 |
| BML+effusion-synovitis | 1.17 (0.85, 1.61) | 0.59 | 1.21 (0.86, 1.70) | 0.70 |
| Medial Joint Space Width Progression | ||||
| Unweighted total score | 1.20 (0.90, 1.61) | 0.56 | 1.21 (0.89, 1.64) | 0.63 |
| Cumulative cartilage damage | 1.93 (1.38, 2.72) | 0.67 | 2.11 (1.44, 3.07) | 0.70 |
| BML+effusion-synovitis | 1.05 (0.79, 1.39) | 0.52 | 1.05 (0.78, 1.41) | 0.63 |
| WOMAC Pain Progression | ||||
| Unweighted total score | 1.72 (1.18, 2.52) | 0.63 | 1.97 (1.26, 3.08) | 0.76 |
| Cumulative cartilage damage | 1.28 (0.89, 1.84) | 0.55 | 1.31 (0.86, 1.98) | 0.72 |
| BML+effusion-synovitis | 1.69 (1.15, 2.48) | 0.65 | 1.92 (1.23, 3.01) | 0.76 |
BML = bone marrow lesion; AUC = area under the ROC curve; 95% CI = 95% confidence intervals.
Adjusted for age, sex, BMI, race, WOMAC pain, KL, JSW and pain med use.
DISCUSSION
We showed that quantitative composite knee scores using quantitative measurements of cartilage damage, BML, and effusion-synovitis have construct validity with radiographic structural progression (cartilage damage score) and worsening of knee pain (unweighted total score and BML+effusion-synovitis score). We also considered a data-driven approach to identify novel candidate composite scores (e.g., tibial BMLs, patellar pathology); however, these failed to demonstrate adequate performance in the development dataset. Furthermore, despite our presumption that one definition of KOA structural progression may be ideal, we found that 2 composite scores of disease progression may provide a more pragmatic approach to standardize the definitions of KOA progression. By adopting 2 definitions of disease progression – one for cumulative disease burden and another for more dynamic processes related to patient-centered outcomes – we can overcome some significant obstacles to testing disease-modifying therapies for KOA. Specifically, these definitions, and their respective scores, address the multifactorial and complex nature of KOA, represent structural changes in the whole knee, the discordance between structural changes and signs/symptoms/function, and offer a new framework to define KOA progression(3).
This work builds on the potential brought by MR imaging as a powerful outcome tool to measure structures throughout a joint. Thus far, no assessment strategy has met the demand to define structural severity in the whole joint or to reflect changes in important patient-relevant outcomes. Semiquantitative scales have great utility but are insensitive to change(4) and lack a validated composite score to reflect severity in the whole knee(5, 6). To overcome these challenges, we deployed our parsimonious validated methods to quantify cartilage loss, BMLs, and effusion-synovitis, which are clinically relevant features of KOA. Furthermore, the cartilage damage score (representing tibial, femoral, and patellar regions) and BML+effusion-synovitis score (representing tibial, femoral, and patellar regions and effusion-synovitis throughout the knee) reflect pathological changes throughout the knee. These new composite scores rely on quantitative measurements, which addresses concerns about combining semiquantitative scores to create a whole-knee score(5, 6). Furthermore, these new whole-knee scores offer an alternative to the typical focus on a single region or compartment of the knee (e.g., medial tibiofemoral) with burdensome quantitative methods.
Based on our findings, the cumulative cartilage damage and BML+effusion-synovitis composite scores reflect different constructs of KOA progression. Cumulative cartilage damage represents hyaline cartilage damage throughout the knee, relates to radiographic severity, and reflects the damage attributable to KOA over the course of the disease. The relationship between cartilage damage with radiographic severity complements prior evidence that regional and compartment-specific semiquantitative and quantitative MR-based assessments of cartilage damage progression discriminate and predict structural progression defined by radiographs better than progression in patient-reported outcomes(12, 41, 42). The novelty of the cumulative cartilage damage score is that it is a fully quantitative composite score representing key informative locations throughout the knee, which is optimized to discriminate change. Future studies may clarify if short-term changes in the cumulative cartilage damage score predict long-term symptoms – a hypothesis supported by regional measures of cartilage loss modestly predicting future total knee replacements(43, 44). In contrast to cumulative cartilage damage, the BML+effusion-synovitis composite score may reflect a more dynamic disease activity process that relates to knee pain and reflects a patient’s current state of disease and symptoms. These findings are consistent with prior reports that effusion-synovitis and BMLs relate to changes in patient-reported outcomes(12, 33, 45). The novelty of the BML+effusion-synovitis composite score is that it is a fully quantitative composite score that combines BML volumes and effusion-synovitis volumes throughout the knee to maximize the potential to discriminate change. Overall, these findings are consistent with the heuristic used in other rheumatologic diseases that separately measure damage and disease activity(46).
Our study does have some limitations. Meniscal damage, an important but complex component of KOA, is not included in our composite scores. There is merit in determining the optimal measures of meniscal change among people with KOA and then working on including the meniscus into future composite scores. However, the exclusion of a meniscal measure does not undermine our primary finding that KOA progression may be conceptualized as 2 constructs. Future research will clarify if meniscal measurements contribute to the cumulative damage score or if meniscal changes represent another unique construct of KOA progression. Osteophytes were also not included in our composite scores because their progression is slow and unlikely to change meaningfully in 24 months. We also observed modest AUCs, possibly reflecting the imprecision in using construct validity in the absence of a gold standard for KOA progression. For example, we were limited to using radiographic outcomes focused on the tibiofemoral joint despite our focus on MR-based changes throughout the joint. We also did not account for multiple testing in our significance tests. While we used a sequential testing strategy with development and validation subcohorts to reduce the number of falsely recommended quantitative composite scores, these composite scores need further testing on other data for full validation, including evaluating the internal consistency of the scores. Finally, we demonstrated that the new composite scores outperform whole-knee BML or effusion-synovitis volumes and relate to radiographic outcomes (e.g., JSW change, KL change). However, future studies are needed to compare these new scores to traditional measures of structural progression (e.g., JSW change, mean cartilage thickness change) in regard to predicting patient-centered outcomes and discriminating group differences in clinical trials.
Our study has several strengths. First, we used a robust, longitudinal cohort with high-quality MR images. Second, we used validated semi-automated quantitative measures of cartilage damage(30, 32), BML volume(33), and effusion-synovitis volume(36–38) that are less burdensome than other measurement methods. Furthermore, these software programs can be disseminated for use by technicians with a modest training requirement. Finally, we used separate development and validation subcohorts for more rigorous validation of the composite knee scores.
In conclusion, we showed that quantitative composite knee scores based on MR measures of cartilage damage, BML, and effusion-synovitis are associated with KOA progression. Our analyses offer a new perspective on KOA progression reflected in the two different domains. First, cumulative damage, which is measured by a cumulative cartilage damage score, is associated with radiographic progression and reflects the damage attributable to KOA over the course of the disease. Second, dynamic disease activity, which is measured by a BML+effusion-synovitis score, relates to changes in a patient’s state of disease and symptoms. These domains of KOA progression, and their respective scores, address the multifactorial and complex nature of KOA, the discordance between structural changes and signs/symptoms/function, offer a new framework to define KOA progression, and have promise as MR-based biomarkers.
Supplementary Material
SIGNIFICANCE AND INNOVATION.
Clinical trials for knee osteoarthritis urgently need quantitative composite whole-knee scores that address 1) the multifactorial and complex etiopathogenesis of knee osteoarthritis, 2) the discordance between structural changes and signs/symptoms/function, and 3) the lack of consensus for the best definitions of disease progression.
We offer 2 composite scores that reflect discrete domains of KOA progression.
Cumulative damage: the joint damage accrued over time, reflected by a whole-knee cartilage damage score.
Disease activity: a patient’s state of disease and symptoms that may ebb and flow, reflected by a bone marrow lesion + effusion-synovitis score.
Acknowledgements
We would like to thank Rachel Sachs for her work in analyzing the effusion-synovitis data.
Grants or Other Financial Supporter(s):
This work was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number U01-AR067168. Furthermore, the project was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR002544. The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. Dr. Lo is supported by K23 AR062127, an NIH/NIAMS funded mentored award, providing support for design and conduct of the study, analysis, and interpretation of the data. This work is supported in part with resources at the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413), at the Michael E. DeBakey VA Medical Center, Houston, TX. Dr. Harkey was supported by the National Institutes of Health (grant no. 5 TL1 TR 1454-3). The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the National Institutes of Health. The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; nor in the decision to submit the article for publication. The authors declare they have no other conflicts of interest to report.
Contributor Information
Jeffrey B. Driban, Tufts Medical Center, Rheumatology.
Lori Lyn Price, Tufts Medical Center, Institute of Clinical Research and Health Policy Studies; Tufts University, Clinical and Translational Science Institute.
Michael P. LaValley, Boston University, Biostatistics.
Grace H. Lo, Baylor College of Medicine, Medicine.
Ming Zhang, Tufts Medical Center, Division of Rheumatology, Allergy, & Immunology.
Matthew S. Harkey, Tufts Medical Center, Rheumatology.
Amanda Canavatchel, Tufts Medical Center, Division of Rheumatology, Allergy, and Immunology.
Timothy E. McAlindon, Tufts Medical Center, Chief, Division of Rheumatology.
REFERENCES
- 1.Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG. Alternative Methods for Defining Osteoarthritis and the Impact on Estimating Prevalence in a US Population-Based Survey. Arthritis Care Res (Hoboken) 2016; 68: 574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane NE, Brandt K, Hawker G, Peeva E, Schreyer E, Tsuji W, et al. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage 2011; 19: 478–82. [DOI] [PubMed] [Google Scholar]
- 3.Guidance for Industry: Osteoarthritis: Structural Endpoints for the Development of Drugs, Devices, and Biological Products for Treatment. Rockville, MD: United States Food and Drug Administration; 2018. [Google Scholar]
- 4.Eckstein F, Nevitt M, Gimona A, Picha K, Lee JH, Davies RY, et al. Rates of change and sensitivity to change in cartilage morphology in healthy knees and in knees with mild, moderate, and end-stage radiographic osteoarthritis: results from 831 participants from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2011; 63: 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage; 19: 990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conaghan PG, Tennant A, Peterfy CG, Woodworth T, Stevens R, Guermazi A, et al. Examining a whole-organ magnetic resonance imaging scoring system for osteoarthritis of the knee using Rasch analysis. Osteoarthritis Cartilage 2006; 14 Suppl A: A116–21. [DOI] [PubMed] [Google Scholar]
- 7.Hunter DJ, Conaghan PG, Peterfy CG, Bloch D, Guermazi A, Woodworth T, et al. Responsiveness, effect size, and smallest detectable difference of Magnetic Resonance Imaging in knee osteoarthritis. Osteoarthritis Cartilage 2006; 14 Suppl A: A112–5. [DOI] [PubMed] [Google Scholar]
- 8.Duryea J, Neumann G, Niu J, Totterman S, Tamez J, Dabrowski C, et al. Comparison of radiographic joint space width with magnetic resonance imaging cartilage morphometry: analysis of longitudinal data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2010; 62: 932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter DJ, Niu J, Zhang Y, Totterman S, Tamez J, Dabrowski C, et al. Change in cartilage morphometry: a sample of the progression cohort of the Osteoarthritis Initiative. Ann Rheum Dis 2009; 68: 349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wirth W, Nevitt M, Hellio Le Graverand MP, Benichou O, Dreher D, Davies RY, et al. Sensitivity to change of cartilage morphometry using coronal FLASH, sagittal DESS, and coronal MPR DESS protocols--comparative data from the Osteoarthritis Initiative (OAI). Osteoarthritis Cartilage 2010; 18: 547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckstein F, Cotofana S, Wirth W, Nevitt M, John MR, Dreher D, et al. Painful knees have greater rates of cartilage loss than painless knees after adjusting for radiographic disease stage: Data from the OA initiative. Arthritis Rheum 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter DJ, Zhang W, Conaghan PG, Hirko K, Menashe L, Li L, et al. Systematic review of the concurrent and predictive validity of MRI biomarkers in OA. Osteoarthritis Cartilage 2011; 19: 557–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazakia GJ, Kuo D, Schooler J, Siddiqui S, Shanbhag S, Bernstein G, et al. Bone and cartilage demonstrate changes localized to bone marrow edema-like lesions within osteoarthritic knees. Osteoarthritis Cartilage 2013; 21: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanetti MBE, Romero J, Hodler J. Bone marrow edema pattern in osteoarthritis knees: correlation between MR imaging and histologic findings. Radiology 2000; 215: 835–40. [DOI] [PubMed] [Google Scholar]
- 15.Driban JB, Lo GH, Lee JY, Ward RJ, Miller E, Pang J, et al. Quantitative bone marrow lesion size in osteoarthritic knees correlates with cartilage damage and predicts longitudinal cartilage loss. BMC Musculoskelet Disord 2011; 12: 217–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med 2001; 134: 541–9. [DOI] [PubMed] [Google Scholar]
- 17.Callaghan MJ, Parkes MJ, Hutchinson CE, Gait AD, Forsythe LM, Marjanovic EJ, et al. A randomised trial of a brace for patellofemoral osteoarthritis targeting knee pain and bone marrow lesions. Ann Rheum Dis 2015; 74: 1164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laslett LL, Dore DA, Quinn SJ, Boon P, Ryan E, Winzenberg TM, et al. Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomised controlled trial. Ann Rheum Dis 2012; 71: 1322–8. [DOI] [PubMed] [Google Scholar]
- 19.Pelletier JP, Roubille C, Raynauld JP, Abram F, Dorais M, Delorme P, et al. Disease-modifying effect of strontium ranelate in a subset of patients from the Phase III knee osteoarthritis study SEKOIA using quantitative MRI: reduction in bone marrow lesions protects against cartilage loss. Ann Rheum Dis 2015; 74: 422–9. [DOI] [PubMed] [Google Scholar]
- 20.Carbone LD, Nevitt MC, Wildy K, Barrow KD, Harris F, Felson D, et al. The relationship of antiresorptive drug use to structural findings and symptoms of knee osteoarthritis. Arthritis Rheum 2004; 50: 3516–25. [DOI] [PubMed] [Google Scholar]
- 21.Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis and Cartilage 2006; 14: 1033–40. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf E, Kortekaas MC, Watt I, Huizinga TW, Kloppenburg M. Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann Rheum Dis 2011; 70: 60–7. [DOI] [PubMed] [Google Scholar]
- 23.Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis 2011; 70: 1804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roemer FW, Kwoh CK, Hannon MJ, Green SM, Jakicic JM, Boudreau R, et al. Risk factors for magnetic resonance imaging-detected patellofemoral and tibiofemoral cartilage loss during a six-month period: the joints on glucosamine study. Arthritis Rheum 2012; 64: 1888–98. [DOI] [PubMed] [Google Scholar]
- 25.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage 2008; 16: 1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Osteoarthritis Initiative. [cited 2019; Available from: https://nda.nih.gov/oai/
- 27. cited; Available from: https://data-archive.nimh.nih.gov/oai.
- 28.Zhang M, Canavatchel AR, Driban JB, Price L, Moreno-koehler A, Lo GH, et al. Patellar cartilage change is not associated with difficulty with stairs and crepitus: a two year longitudinal study of data from the osteoarthritis initiative. Osteoarthritis Cartilage 2016; 24: S356–S7. [Google Scholar]
- 29.Zhang M, Driban JB, Price LL, Harper D, Lo GH, Miller E, et al. Development of a rapid knee cartilage damage quantification method using magnetic resonance images. BMC Musculoskelet Disord 2014; 15: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang M, Driban JB, Price LL, Lo GH, Miller E, McAlindon TE. Development of a Rapid Cartilage Damage Quantification Method for the Lateral Tibiofemoral Compartment Using Magnetic Resonance Images: Data from the Osteoarthritis Initiative. BioMed research international 2015; 2015: 634275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin 1979; 86: 420–8. [DOI] [PubMed] [Google Scholar]
- 32.Zhang M, B. DJ, Price LL, Harpe rD, Lo GH, Miller E, et al. Development of a rapid knee cartilage damage quantification method using magnetic resonance imaging. BMC Musculoskelet Disord 2014; 15:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang M, Driban JB, Price LL, Lo GH, McAlindon TE. Magnetic Resonance Image Sequence Influences the Relationship between Bone Marrow Lesions Volume and Pain: Data from the Osteoarthritis Initiative. BioMed research international 2015; 2015: 731903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roemer FW, Frobell R, Hunter DJ, Crema MD, Fischer W, Bohndorf K, et al. MRI-detected subchondral bone marrow signal alterations of the knee joint: terminology, imaging appearance, relevance and radiological differential diagnosis. Osteoarthritis Cartilage 2009; 17: 1115–31. [DOI] [PubMed] [Google Scholar]
- 35.Roemer FW, Khrad H, Hayashi D, Jara H, Ozonoff A, Fotinos-Hoyer AK, et al. Volumetric and semiquantitative assessment of MRI-detected subchondral bone marrow lesions in knee osteoarthritis: a comparison of contrast-enhanced and non-enhanced imaging. Osteoarthritis Cartilage 2010; 18: 1062–6. [DOI] [PubMed] [Google Scholar]
- 36.Davis JE, Ward RJ, MacKay JW, Lu B, Price LL, McAlindon TE, et al. Effusion-synovitis and infrapatellar fat pad signal intensity alteration differentiate accelerated knee osteoarthritis. Rheumatology (Oxford) 2019; 58: 418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harkey MS, Price LL, McAlindon TE, Davis JE, Stout AC, Lu B, et al. Association between declining walking speed and increasing bone marrow lesion and effusion volume in individuals with accelerated knee osteoarthritis. Arthritis Care Res (Hoboken) 2019; 71: 259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stout AC, Barbe MF, Eaton CB, Amin M, Al-Eid F, Price LL, et al. Inflammation and glucose homeostasis are associated with specific structural features among adults without knee osteoarthritis: a cross-sectional study from the osteoarthritis initiative. BMC Musculoskelet Disord 2018; 19: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heinze G, Dunkler D. Five myths about variable selection. Transpl Int 2017; 30: 6–10. [DOI] [PubMed] [Google Scholar]
- 40.Collins JE, Losina E, Nevitt MC, Roemer FW, Guermazi A, Lynch JA, et al. Semiquantitative imaging biomarkers of knee osteoarthritis progression: Data From the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol 2016; 68: 2422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eckstein F, Collins JE, Nevitt MC, Lynch JA, Kraus VB, Katz JN, et al. Brief Report: Cartilage Thickness Change as an Imaging Biomarker of Knee Osteoarthritis Progression: Data From the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis & rheumatology (Hoboken, NJ: ) 2015; 67: 3184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins JE, Losina E, Nevitt MC, Roemer FW, Guermazi A, Lynch JA, et al. Semiquantitative Imaging Biomarkers of Knee Osteoarthritis Progression: Data From the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis & Rheumatology 2016; 68: 2422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwoh CK, Guehring H, Aydemir A, Hannon MJ, Eckstein F, Hochberg MC. Predicting knee replacement in participants eligible for disease-modifying osteoarthritis drug treatment with structural endpoints. Osteoarthritis Cartilage 2020; 28: 782–91. [DOI] [PubMed] [Google Scholar]
- 44.Hitzl W, Wirth W, Maschek S, Cotofana S, Nevitt M, John MR, et al. Greater Lateral Femorotibial Cartilage Loss in Osteoarthritis Initiative Participants With Incident Total Knee Arthroplasty: A Prospective Cohort Study. Arthritis Care Res (Hoboken: ) 2015; 67: 1481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Driban JB, Price L, Lo GH, Pang J, Hunter DJ, Miller E, et al. Evaluation of bone marrow lesion volume as a knee osteoarthritis biomarker--longitudinal relationships with pain and structural changes: data from the Osteoarthritis Initiative. Arthritis Res Ther 2013; 15: R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fransen J, van Riel PL. Outcome measures in inflammatory rheumatic diseases. Arthritis Res Ther 2009; 11: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


