Abstract
Purpose of Review:
Organophosphate esters (OPEs) are applied to a variety of consumer products, primarily as flame retardants and plasticizers. OPEs can leach out of products over time and are consequently prevalent in the environment and frequently detected in human biomonitoring studies. Exposure during pregnancy is of particular concern as OPEs have recently been detected in placenta, suggesting they may be transferred to the developing infant. Also, studies have now shown that children experience higher exposure to several OPEs compared to adults, indicating they may be disproportionately impacted by these compounds. This review summarizes the current literature on reproductive and child health outcomes of OPE exposures and highlights areas for future research.
Recent Findings:
Experimental animal studies demonstrate potential for OPEs to adversely impact health and a limited number of epidemiologic studies conducted in adult cohorts suggest that OPEs may interfere with the endocrine system. Neurodevelopment is currently the most well-characterized children’s health endpoint, and several studies indicate that prenatal OPE exposures impact both cognitive and behavioral development. Associations have also been reported with reproductive outcomes (e.g., fertilization and pregnancy loss) and with the timing of parturition and preterm birth. Cross-sectional studies also demonstrate associations between OPEs and respiratory health outcomes and measures of adiposity.
Summary:
A rapidly expanding body of research demonstrates that OPEs are associated with adverse reproductive health and birth outcomes, asthma and allergic disease, early growth and adiposity, and neurodevelopment. Still, additional research is urgently needed to elucidate the full impact of OPEs on children’s health.
Keywords: organophosphate esters, flame retardants, children’s health, neurodevelopment, birth outcomes, asthma
Organophosphate Esters (OPEs) Introduction
In order to comply with U.S. (state and federal) and international flammability regulations, manufacturers of consumer goods, including building materials, furniture, and electronic devices, routinely apply chemical flame retardants to their products (1–3). Until the mid-2000s, a class of brominated flame retardants (BFRs) known as polybrominated diphenyl ethers (PBDEs) were among the most commonly used commercial chemical flame retardants (1–3). Amid concerns of their environmental fate and potential toxicity, however, PBDEs were phased out of production and manufacturers have increasingly used organophosphate esters (OPEs) as alternatives to many BFRs (1–3). As a result, production of OPEs has increased in recent years (3–8). OPEs are applied as “additive” flame retardants (as opposed to “reactive”), meaning they are not chemically bound to their products, and are vulnerable to volatilization and leaching into the environment (4, 6, 7). Although OPEs are perhaps best known for their use as flame retardants in polyurethane foam (6, 7), they are also used as plasticizers, solvents, and in other industrial applications (4, 6, 7, 9), and are applied to electronic devices (10, 11), baby products (12), food packaging (13), recreational equipment (14, 15), and nail polishes (16).
OPE Exposure and Metabolism
Due to their application to a wide variety of consumer products and their capacity to volatilize and leach from these materials, OPEs are present at detectable concentrations in many human environments (4–7, 17–21), including residential housing (2, 17, 19, 21–26), office spaces (17, 19–21, 27, 28), and child care environments (17, 20, 21, 29, 30). In particular, OPEs are frequently detected in indoor air and in the dust of indoor environments (7, 19, 20, 22–24, 26, 30–35); consequently, inhalation and inadvertent ingestion of indoor dust are significant sources of exposure [e.g. (35)], although dermal absorption (14, 15, 36–38), respiration of contaminated air (21, 28, 39), ingestion of contaminated food (32, 40), consumption of contaminated water (41), and other pathways can also contribute to exposure (7, 34).
Inside the body, OPEs are often metabolized to their respective mono- or diesters (34, 42–47) (Figure 1), which are primarily excreted in urine (43, 44, 48), though other metabolic and excretion pathways also exist, such as hydroxylation and conjugation (34, 46, 49). Urinary OPE diester metabolites (and other biological markers of OPE exposure) are consistently detected with high frequencies in biomonitoring surveys and observational studies (21, 22, 24, 25, 32, 33, 50–61), demonstrating widespread exposure to these compounds. Biological half-lives of OPEs are much shorter than PBDEs and are likely on the order of hours to days (43, 44, 48, 62). Studies of intra-individual variability in OPE metabolite concentrations, however, have reported intraclass correlation coefficients (ICCs) for OPEs typically ranging between 0.3 and 0.8, depending on the metabolite, study population, and time period of interest (55, 63–66). These ICC values indicate moderate reproducibility over time, suggesting exposure is also reasonably consistent over time.
Figure 1:
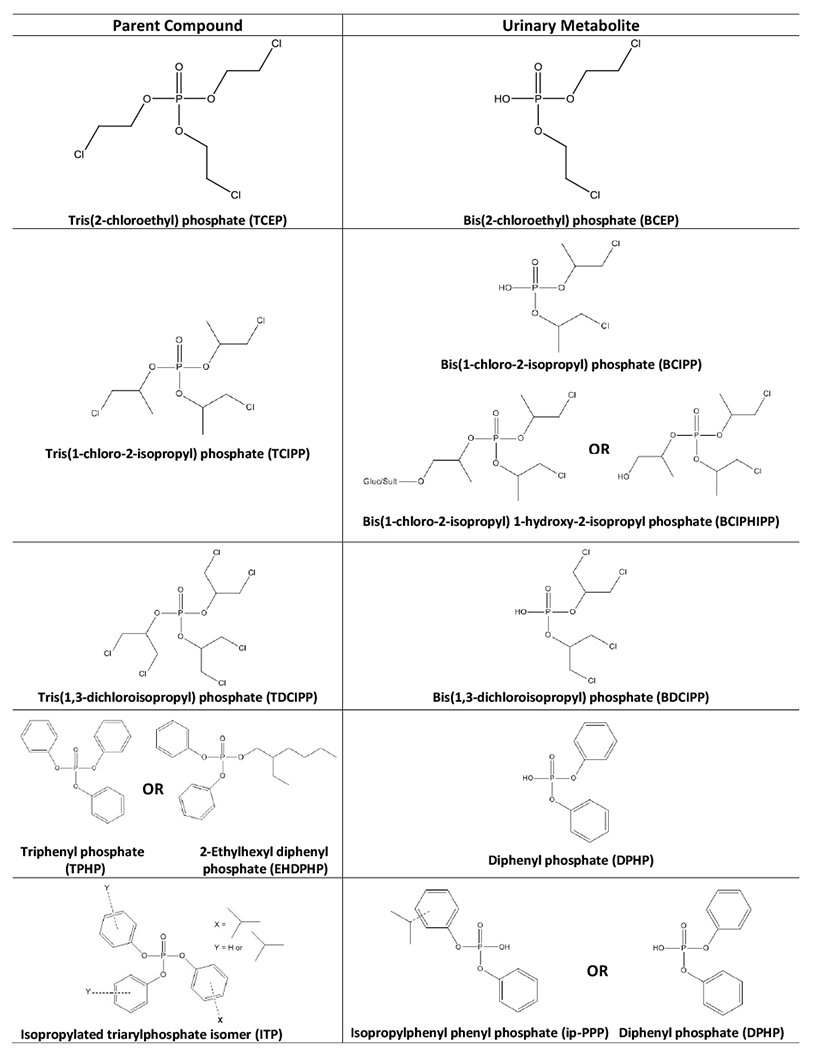
Select organophosphate esters and their urinary metabolites.
Consistent with findings among the general population, exposure to OPEs among pregnant women occurs with a similar high frequency (22, 52, 53, 55, 56, 63). Though data remain limited, available evidence suggests that maternal-fetal transfer of OPEs may occur. For example, Zhao et al. (67) measured several OPEs and their metabolites in human chorionic villi and deciduae, indicating potential maternal-fetal transfer in early gestation, prior to the development of a mature placenta. Other evidence suggests that placental accumulation and transplacental transfer of OPEs may occur. For example, Ding et al. measured triphenyl phosphate (TPHP) and tris(1,3-dichloroisopropyl)phosphate (TDCIPP) in 86% and 44% (respectively) of placental tissue samples obtained from 50 pregnant women living in China (52). Interestingly, an experimental study by Baldwin et al. (68) observed sex-specific accumulation of TPHP in rat placentas (a potential mechanism for sex-specific effects), but did not observe transfer to pups; a similar study also did not observe gestational transfer of OPEs to rat pups (69).
Higher levels of OPE exposures have been reported for young children and adolescents compared to other age groups (12, 32, 35, 50, 51, 60, 70–73). For example, a pair of investigations of mother-child pairs from California and New Jersey identified higher concentrations of two urinary OPE metabolites (diphenyl phosphate (DPHP), a metabolite of triphenyl phosphate (TPHP); bis(1,3-dichloro-2-propyl) phosphate, a metabolite of tris(1,3-dicholoro-2-propyl) phosphate (TDCIPP)) in children than mothers, including DPHP and BDCIPP concentrations that were 5.9 and 15.0 times greater in children than mothers in the California cohort (50, 51). More recently, Phillips and Hammel at al. (35)reported that urinary metabolites of TDCIPP and isopropylated triarylphosphate esters (ITPs) were universally detected in urine samples from children 3-6 years of age (n=181, collected 2014-2016), and metabolite levels were generally higher than those observed in other temporally and geographically similar cohorts of adults. The four other OPE metabolites assessed in this study were detected in urine samples from greater than 80 percent of children [i.e., mono-tert-butyl phenyl phenyl phosphate (tb-PPP), diphenyl phosphate (DPHP), bis(1-chloro-2-propyl) 1-hydroxy-2-propyl phosphate (BCIPHIPP), and bis(1-chloro-2-isopropyl) phosphate (BCIPP)]. Strong correlations between urinary metabolites and hand wipe samples observed by Phillips and Hammel et al. suggest that elevated hand-to-mouth contact may explain higher levels of exposure experienced by children (35). Additional factors potentially leading to higher levels of OPE exposure for children include the treatment of children’s products with flame retardants or physiological differences between children and adults (12, 74).
Reproductive and Child Health Outcomes
Epidemiologic investigations into the potential health impacts of OPEs have been limited; however, investigations into the potential for OPEs to adversely impact children’s health have increased in recent years (summarized in Table 1). Notably, OPEs have been linked to endocrine disruption in experimental animal and epidemiologic studies (25, 75–86), raising concerns about reproductive toxicity and the health impacts of early-life exposures. Here, we highlight recent studies which examine the relationship between OPE exposures and reproductive and children’s health outcomes and highlight areas for future research.
Table 1:
Epidemiologic studies investigating OPE exposures and impacts on reproductive and children’s health.
| Study Sample | Location | Study Design | Exposure Metric | Year of Sampling | Outcomes | Summary Findings | |
|---|---|---|---|---|---|---|---|
|
Reproductive Health
| |||||||
| Meeker et al. 2010 | 50 male partners in an infertile couple | Massachusetts, USA | cross-sectional | house dust | 1999-2003 | semen quality: concentration, motility and morphology; serum hormones: FSH, LH, inhibin B, SHBG, testosterone, free androgen index, free T4, total T3, and TSH | TDCPP associated with decline in free thyroxine and increase in prolactin; TPP was associated positively associated with prolactin and inversely associated with sperm concentration |
| Soubry et al. 2017 | 67 men in the TIEGER Study | North Carolina, USA | cross-sectional | urine | 2012-2013 | Sperm: differentially methylated regions (DMRs) of imprinted genes | OPES were associated with a higher fraction of aberrantly methylated sperm cells |
| Carignan et al. 2017 | 211 women in the EARTH Study | Massachusetts, USA | prospective IVF cohort | preconception urine | 2005-2015 | IVF outcomes: successful fertilization, implantation, clinical pregnancy and live birth | inverse associations between the sum of three OPE metabolites (BDCIPP, ip-PPP and DPHP) and proportions of fertilization, implantation, clinical pregnancy and live birth |
| Carignan et al. 2018 | 201 couples in the EARTH Study | Massachusetts, USA | prospective IVF cohort | preconception urine | 2005-2015 | IVF outcomes: successful fertilization, implantation, clinical pregnancy and live birth | male partner urinary BDCIPP was associated with reduced fertilization |
| Ingle et al. 2018 | 220 men in the EARTH Study | Massachusetts, USA | prospective IVF cohort | preconception urine | 2005-2015 | sperm count, concentration, motility, and morphology | odds of having a low sperm count decreased with increasing BDCIPP concentrations; other associations were weak and inconsistent |
| Messerlian et al. 2018 | 155 women with 179 pregnancies | Massachusetts, USA | prospective IVF cohort | preconception urine | 2005-2015 | pregnancy loss | preconception DPHP and the molar sum of OPEs associated with elevated risk of biochemical pregnancy loss |
|
| |||||||
|
Birth Outcomes
| |||||||
| Feng et al. 2016 | 23 pregnant women (14 delivering term infants) | Shanghai, China | prospective pregnancy cohort | prenatal urine | 2015 | miscarriages, neonatal birthweight, gestational diabetes | no reported associations |
| Hoffman et al. 2018 | 349 women in the PIN Study | North Carolina, USA | prospective pregnancy cohort | prenatal urine | 2002-2005 | birth weight, gestational age, and birthweight for gestational age from medical records | Females: ip-PPP and BDCIPP inversely related to gestational age and odds of preterm birth Males: ip-PPP associated with decreased odds of preterm birth and DPHP suggestively associated with longer gestational age |
|
| |||||||
|
Physical Growth and Adiposity
| |||||||
| Boyle et al. 2019 | 784 children age 6-19 years from NHANES | USA | cross-sectional | urine | 2013-2014 | obesity, body mass index, and waist circumference | inverse associations between DBUP and the prevalence odds of being obese, lower BMI z-scores and WC; BCEP was associated with increased prevalence odds of being overweight vs. normal weight among children |
|
| |||||||
|
Asthma and Allergy
| |||||||
| Araki et al. 2013 | 516 all ages (126 children) | Japan | cross-sectional | house dust | 2006 | report of medical treatment for bronchial asthma, atopic dermatitis, allergic rhinitis, or allergic conjunctivitis during the preceding 2 years | TCIPP and TDCIPP in dust was positively associated with prevalence of atopic dermatitis; dust TBP was associated with asthma and allergic rhinitis |
| Canbaz et al. 2016 | 220 children 4-8 years of age in the BAMSE cohort | Sweden | nested case-control with exposure at age 2 months | mother’s mattress dust | 1994-2006 | asthma at 4 or 8 years | TPHP and meta, meta, para-tricresyl phosphate (mmp-TMPP) for children that did not develop asthma |
| Araki et al. 2018 | 128 elementary school-aged children | Japan | cross-sectional | house dust and first-morning urine voids | 2009-2010 | parent-reported symptoms of wheeze, rhinoconjunctivitis, and eczema; evaluated using the International Study of Asthma and Allergies in Childhood questionnaire | TDCIPP in house dust, and metabolites of TDCIPP, TBOEP and TCIPP were associated with children’s allergic symptoms |
| Bi et al. 2018 | 54 children approximately 10 years of age | Texas, USA | cross-sectional | HVAC filter dust and settled floor dust | 2014-2015 | asthma severity measured with the validated Severity of Chronic Asthma scale | no reported associations for OPEs |
|
| |||||||
|
Neurodevelopment
| |||||||
| Castorina et al. 2017 | 248 to 282 children at age 7 years; CHAMACOS | California, USA | prospective pregnancy cohort | prenatal urine | 1999-2000 | neurodevelopment at age 7 years: Wechsler Intelligence Scale for Children, 4th ed. (WISC-IV); Behavior Assessment System for Children (BASC-2) | OPE metabolites positively associated with worse scores on the WISC-IV (particularly the Working Memory scale), and higher scores on the BASC-2 Hyperactivity scale |
| Lipscomb et al. 2017 | 72 preschool children | Oregon, USA | cross-sectional | silicone wristbands | 2012-2013 | Social Skills Improvement Rating Scale, a teacher-rated social behavior assessment | children with higher ΣOPFR were rated as having less responsible behavior and more externalizing behavior problems |
| Doherty et al. 2019a | 149 to 227 children at age 2-3 years; PIN | North Carolina, USA | prospective pregnancy cohort | prenatal urine | 2002-2005 | cognitive development at age 2-3 years: MacArthur-Bates Communicative Development Inventories (MB-CDI) and the Mullen Scales of Early Learning (MSEL) | Higher levels ip-PPP associated with worse cognitive assessment scores on the MSEL and MB-CDI |
| Doherty et al. 2019b | 199 children at age 3 years; PIN | North Carolina, USA | prospective pregnancy cohort | prenatal urine | 2002-2005 | behavioral development at age 3 years: Behavioral Assessment System for Children 2nd Ed (BASC-2) | BDCIPP associated with more withdrawal and attention problems; DPHP associated with greater hyperactivity and attention problems |
Reproductive Health
Even prior to birth of the child, there is evidence to suggest that OPE exposure may impact fertilization and conception. In one study, 211 women were recruited from an academic fertility clinic as a part of the Environment and Reproductive Health Study (EARTH)(65) and their exposure to OPEs was assessed by measuring urinary biomarkers. The investigators reported that increases in the sum of three OPE metabolites measured preconception [bis(1,3-dichloroisopropyl) phosphate (BDCIPP), mono-isopropyl phenyl phenyl phosphate (ip-PPP), and DPHP] was associated with decreased rates of successful fertilization, implantation, clinical pregnancy, and live birth (65). In another study of 155 women in the EARTH cohort, preconception urinary DPHP were associated with increased risk of biochemical pregnancy loss as was the molar sum of DPHP, BDCIPP an ip-PPP (87). Consistent with these findings, experimental animal studies also found that OPE exposures decreased egg production, egg quality, hatching and survival among zebrafish and delayed hatching among chicken embryos (77, 78, 86, 88).
OPEs have also been implicated in male fecundity and reproductive health in experimental and epidemiologic studies (25, 78, 89–93). For example, exposure to OPEs, particularly when considering mixtures, was associated with aberrant DNA methylation at imprinted genes in sperm (n=67 men). Although differences in methylation with OPE exposure were small in this study, successful fertilization with a sperm cell that is aberrantly methylated may present detrimental consequences for offspring (90). Among men recruited from a fertility clinic (n=50), TDCIPP and TPHP in residential house dust were inversely associated with sperm concentration and motility (25). More recently, larger-scale research in the EARTH study participants using urinary exposure biomarkers did not support findings of an association between OPE exposure and semen parameters (92) but did find associations between urinary BDCIPP concentrations measured preconception and reduced fertilization (93)(n=220 and 201, respectively).
Past research in the EARTH study participants suggests that, in comparisons with female partners, male partner exposure appeared less relevant to adverse pregnancy outcomes (65, 93); however, it is important to note that available evidence relating OPE exposures to reproductive health has been limited to fertility treatment cohorts which may limit the generalizability of these findings to the general population.
Gestational Length and Infant Size at Birth
Adverse birth outcomes, including premature birth and low birthweight, represent the leading causes of neonatal mortality in developed countries, and while most babies survive, those born too early or too small are at increased risk of chronic health conditions throughout their lifetimes (94). Experimental studies in animals suggest that exposure to OPEs may affect early-life growth and development (77, 82, 84, 86, 95–99). For example, chicken embryos exposed to TDCIPP were observed to have a 7% decrease in weight at hatching (86), and prenatal exposure to TDCIPP has been shown to increase the number of visibly small rat pups (i.e., runt pups) and significantly impacted weight gain through weaning (84). Gestational duration and potential impacts of OPEs on preterm birth risk have not been investigated in toxicological studies, due in part to the tightly controlled timing of parturition in most animal species.
Epidemiologic evidence for impacts on birth outcomes is limited to two studies. In a small study (n=14 term infants) of pregnant women from Shanghai, China, no associations were observed between birth weight and urinary DPHP measured in second trimester urine samples (median DPHP 1.1 ng/mL; BDCIPP assessed but detected infrequently) (53). Among a subset of women in the Pregnancy Infection and Nutrition Study (PIN), we recently reported sex-specific associations between OPEs assessed in second-trimester urine samples and birth outcomes (n=349). Women with the highest ip-PPP and BDCIPP concentrations in urine delivered girls earlier and were more likely to deliver preterm infants than women with lower exposure levels. Among males, maternal ip-PPP was associated with decreased odds of preterm birth and DPHP was suggestively associated with longer gestational age (100). Similar associations were observed with birthweight, but associations were attenuated when accounting for gestational age. Sex-specific impacts on gestational age are particularly interesting in the context of Baldwin et al. (68) which reported sex-specific accumulation of TPHP in rat placentas. Though not yet evaluated in human cohorts, it is possible that sex-specific accumulation of OPEs in the placenta is impacting placental function and as a result, the timing of parturition.
Physical Growth and Adiposity
Evidence linking OPE exposure with impacts on growth and adiposity is largely derived from in vitro and experimental animal studies. For example, four OPEs (i.e., TDCIPP, TCIPP, TPHP and TCEP) were positively correlated with triglyceride accumulation in a cell culture (3T3-L1) commonly use to investigate adipogenesis (i.e., fat cell development) (85). Furthermore, isopropylated triphenyl phosphate (ip-TPP) exposure was associated with increased total and HDL cholesterol, increased fructosamine (suggestive of hyperglycemia), and hypertrophy and neutral lipid accumulation in the adrenal gland in exposed rats (101). Further, perinatal exposure to Firemaster® 550, a flame retardant mixture containing OPEs [TPHP and isopropylated triaryl phosphates (ITPs)] and brominated compounds (2-ethylhexyl-2,3,4,5-tetrabromobenzoate and bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate) was associated with rapid weight gain in rat pups and obesity in adult rats (95).
Studies of adults and pregnant women have generally shown positive associations between measures of adiposity and OPE exposure (56, 65, 102). The epidemiologic literature includes a single cross-sectional evaluation of OPEs and children’s adiposity. Boyle et al. (102) reported that among children in the National Health and Nutrition Examination Survey (2013-2014 cycle), OPEs were associated with indicators of adiposity; however, the direction of the association differed by compound. Urinary di-n-butyl phosphate (DBUP) concentrations, for example, were inversely associated with the prevalence odds of obesity as well as body mass index z-scores. Conversely, urinary bis(2-chloroethyl) phosphate (BCEP) concentrations were suggestively associated with increased prevalence odds of being overweight, and similar relationships were observed with BMI z-scores and waist circumference (102). As noted by Boyle et al., these findings may be due to reverse causality. For example, if obese individuals consume more OPE-contaminated foods or spend more time in contact with OPE-containing furniture than lean individuals, higher levels of OPEs could be expected in overweight individuals. Although few studies have evaluated food as a source of OPE exposure, diets high in fresh foods have been associated with lower OPE metabolite concentrations in adults (63, 103), and OPEs have been reported in a greater proportion of packaged foods (89%) than non-processed foods (11%) (104).
Asthma and Allergy
Few studies have assessed OPE impacts on children’s immune and allergic outcomes, though allergic dermatitis has been reported following exposure (105–107). In a cross-sectional study evaluating allergic symptoms (i.e., rhinoconjunctivitis, wheeze, and eczema) among Japanese school children (n=128), TDCIPP in house dust was associated with eczema (108). Rhinoconjunctivitis and having at least one allergy symptom was more frequently observed among children who had the highest quartile of TCIPP urinary metabolites compared to the lowest quartile. Greater concentrations of metabolites of tris(2-butoxyethyl) phosphate (TBOEP) and TDCIPP were also observed to be associated with eczema and at least one allergy symptom. Previous work in Japan (n=516, including children and adults) found significant and positive associations between TCIPP and TDCIPP in house floor dust and the prevalence of atopic dermatitis (109). Tributyl phosphate (TBP) in both floor and multi-surface dust samples were also associated with asthma and allergic rhinitis. A matched case-control study conducted in Sweden evaluated asthma among 220 children at either 4 or 8 years of age with OPEs measured in mattress dust. Maternal mattress dust collected when children were 2 months of age was higher in TPHP and meta, meta, para-tricresyl phosphate (mmp-TMPP) for children that did not develop asthma (110). In addition, a null result was observed in the U.S. between OPEs measured in settled and HVAC filter dust and the severity of childhood asthma among 54 children (average age 10 years) (111). Overall, there is some suggestion that OPE exposure could be related to children’s allergy symptoms; however, more thorough evaluations are needed to determine if a relationship exists between OPEs and asthma. Possible differences in findings in the current literature could be due to the use of household dust as an indicator of exposure in many studies. While the home environment and household dust are likely sources of exposure, it is worth noting that these measures do not capture information about other microenvironments (i.e., the car, other areas of the home, the workplace or school, etc.) which may be important contributors to exposure.
Neurodevelopment
The links between organophosphate pesticides and children’s neurodevelopment have hastened the development of experimental models investigating behavioral impacts of OPE exposures. Experimental evidence has focused on OPE exposures and behavioral changes in model organisms (112–116), specifically zebrafish, which are proving to be useful model organisms for neurodevelopmental toxicity screening (117, 118). For example, Oliveri et al. (115) reported that zebrafish exposed to TDCIPP in early life exhibited elevated locomotor activity and reduced predator escape behavior in adulthood, relative to controls. Similarly, Noyes et al. reported neurodevelopmental defects in embryonic zebrafish exposed to several OPEs (114).
Three prospective epidemiologic studies and one cross-sectional study evaluated the neurodevelopmental impacts of OPE levels in the U.S. over the past two decades (119–121) (122). Lipscomb et al. (122) studied the sum of OPE concentrations measured in passive silicone wristband samplers worn by Oregon preschool children in relation to the children’s scores on the Social Skills Improvement Rating Scale, a teacher-rated social behavior assessment (n=72). Higher OPE concentrations in the passive samplers were associated with poorer performance on the Responsibility subscales and Externalizing subscales. However, the cross-sectional nature of this study limits causal inference.
Among the prospective studies, Castorina et al. (121) investigated three OPE metabolites measured in maternal prenatal urine and offspring’s performance on three psychometric assessments administered at 7 years of age to children in California (n’s from 248 to 282). The authors reported that higher concentrations of DPHP (as well as ΣOPEs, including DPHP, BDCIPP, and ip-PPP) in prenatal urine were associated with worse scores on the Wechsler Intelligence Scale for Children (WISC-IV), (particularly the Working Memory scale), and that higher concentrations of ip-PPP were associated with higher scores on the Behavior Assessment System for Children (BASC-2) Hyperactivity scale, suggesting more hyperactive behaviors. While this work has a number of strengths, it is important to note that mothers in this study were enrolled between 1999 and 2000, prior to the PBDE phase-out and subsequent increase in OPE usage; therefore, OPE exposure levels in this cohort may not reflect exposures post-PBDE phase-out, particularly for more recently introduced compounds, which are likely to be higher.
Among participants of the PIN study in North Carolina (2005-2008), concentrations of OPE metabolites in prenatal urine were associated with both cognitive and behavioral development at 2-3 years of age (n from 149 to 227) (119, 120). Specifically, higher ip-PPP concentrations were associated with poorer performance on two cognitive assessments: the Mullen Scales of Early Learning (MSEL) and the MacArthur-Bates Communicative Development Inventories (MB-CDI) (120). Higher BDCIPP concentrations were associated with more withdrawal and attention problems among children and higher DPHP concentrations were associated with greater hyperactivity and attention problems (assessed using the BASC-2) (119).
In summary, the limited body of available epidemiologic evidence suggests early-life exposures to OPEs, like exposure to their PBDE predecessors, may be associated with cognitive and behavioral effects, though these studies are not without their limitations. Reproduction of their results in other study populations, with repeated measures of OPE exposure are necessary to make stronger inference about the potential cognitive and behavioral effects of early-life OPE exposures and to identify periods of developmental susceptibility.
Conclusions and Recommendations
The past decade has seen an explosion of research investigating OPE exposures, with numerous studies demonstrating near ubiquitous detection of OPEs in various microenvironments and in human populations world-wide. In contrast, epidemiologic studies investigating potential health impacts remain limited in number; however, the available evidence suggests that OPEs may be affecting children’s health. Neurodevelopment and asthma are perhaps the most well characterized adverse health outcomes, but the few available studies have notable limitations. Importantly, prior studies have been relatively small and have been limited in their capacity to investigate potential sex-specific impacts, and many have also been limited by cross-sectional designs, which precludes assessment of temporal ordering between exposure and outcome. Though most past studies have evaluated TPHP and TDCIPP metabolites, other OPEs investigated vary between studies making it difficult to assess consistency. In addition, past studies have relied heavily on urinary exposure biomarkers, which may be problematic given the rapid metabolism of OPEs. Although some research suggests moderate reliability of biomarkers over time [e.g. (24, 55, 63, 64, 66)], relying on a single spot urine measurement to capture long term exposure is likely to result in substantial exposure misclassification. External exposure monitoring (e.g. silicone wristbands or hand wipes) may be useful complementary measures of exposure in future studies (35, 71, 123).
Despite recent efforts, there remain important areas of children’s health that have not been evaluated with respect to early-life OPE exposure. Of particular importance, perinatal exposure has not been assessed with respect to metabolic disorders (e.g. the development of obesity, diabetes, etc.). Given that childhood obesity is a risk factor for a multitude of adverse health outcomes throughout the life course and animal models suggest endocrine disrupting properties may impact growth, research in this area is urgently needed. Although experimental and epidemiologic studies of adults demonstrate impacts on endocrine function, there are no data evaluating OPE exposures and children’s endocrine function, to our knowledge. While we find these novel areas of research to be particularly compelling for understanding the potential risks of early OPE exposure, additional studies evaluating all children’s health outcomes are urgently needed. Much of the work that has been done investigating reproductive and children’s health outcomes associated with OPE exposure thus far has been limited to a few cohorts (EARTH and PIN), and replication is essential. Also of importance in moving the field forward, researchers should consider possible patterns of co-exposure to other toxicants and the potential for interaction between contaminants in impacting children’s growth, development and health. Given that OPEs are also used as plasticizers, similar to phthalates, it may be particularly interesting to look at co-exposures to these two groups. For example, we recently reported correlation between biomarkers of exposure to OPEs and phthalates in young children in the U.S. (124). Thus, it may be important to consider exposure to phthalates when evaluating health impacts of OPE exposures.
Changes in regulations surrounding the use of flame retardants in building materials, electronics and furniture could impact the use of flame retardants moving forward, and subsequently result in reductions in the use of these chemicals. However, OPEs are environmentally persistent in indoor environments, and exposure will continue for many years to come. To date, there has been very little research evaluating methods to reduce individual exposure. Several studies have assessed handwashing, which has been related to reduced exposure to other flame retardants and indoor contaminants, but estimated OPE exposure reductions have been small and not statistically significant in children’s cohorts (12, 35). A recent cross-over study demonstrated that house cleaning and hand washing led to decreased OPE urinary metabolites in adult mothers, but similar relationships were not assessed among their children (125). Given the environmental ubiquity of OPE, research evaluating possible interventions to reduce exposure is urgently needed.
Though they have been in commerce for decades, data suggest that the use of OPEs increased in the early 2000s as other brominated flame retardants were phased-out due to toxicity concerns. At that time, OPEs were largely thought to be safer because they are less persistent in the human body than their predecessors. However, as an increasing number of studies suggest ubiquitous detection in human samples and possible health impacts, the safety of OPEs should be rigorously investigated and their potential as a regrettable substitution should be scrutinized.
References
- 1.Dodson RE, Rodgers KM, Carey G, Cedeno Laurent JG, Covaci A, Poma G, et al. Flame Retardant Chemicals in College Dormitories: Flammability Standards Influence Dust Concentrations. Environmental science & technology. 2017;51(9):4860–9. [DOI] [PubMed] [Google Scholar]
- 2.Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, et al. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environmental science & technology. 2012;46(24):13056–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, et al. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environmental science & technology. 2012;46(24):13432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88(10):1119–53. [DOI] [PubMed] [Google Scholar]
- 5.Reemtsma T, Quintana J, Rodil R, Farcia-Lopez M, Rodriguez I. Organophosphorus flame retardants and plasticizers in water and air occurrence and fate. Trends in Analytical Chemistry. 2008;27(9):727–37. [Google Scholar]
- 6.ATSDR. Toxicological Profile for Phosphate Ester Flame Retardants. U.S. DHHS. 2012. [PubMed] [Google Scholar]
- 7.Wei GL, Li DQ, Zhuo MN, Liao YS, Xie ZY, Guo TL, et al. Organophosphorus flame retardants and plasticizers: sources, occurrence, toxicity and human exposure. Environ Pollut. 2015;196:29–46. [DOI] [PubMed] [Google Scholar]
- 8.Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, et al. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environmental science & technology. 2009;43(19):7490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marklund A, Andersson B, Haglund P. Screening of organophosphorus compounds and their distribution in various indoor environments. Chemosphere. 2003;53(9):1137–46. [DOI] [PubMed] [Google Scholar]
- 10.Abbasi G, Saini A, Goosey E, Diamond ML. Product screening for sources of halogenated flame retardants in Canadian house and office dust. The Science of the total environment. 2016;545-546:299–307. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson H, Ulrika N, C O. Video display units: an emission source of the contact allergenic flame retardant triphenyl phosphate in the indoor environment. Environ Sci Technol. 2000;2000(34):3885–9. [Google Scholar]
- 12.Hoffman K, Butt CM, Chen A, Limkakeng AT Jr., Stapleton HM. High Exposure to Organophosphate Flame Retardants in Infants: Associations with Baby Products. Environmental science & technology. 2015;49(24):14554–9. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Zhao L, Letcher RJ, Zhang Y, Jian K, Zhang J, et al. A review on organophosphate Ester (OPE) flame retardants and plasticizers in foodstuffs: Levels, distribution, human dietary exposure, and future directions. Environment international. 2019;127:35–51. [DOI] [PubMed] [Google Scholar]
- 14.Gomes G, Ward P, Lorenzo A, Hoffman K, Stapleton HM. Characterizing Flame Retardant Applications and Potential Human Exposure in Backpacking Tents. Environmental science & technology. 2016;50(10):5338–45. [DOI] [PubMed] [Google Scholar]
- 15.Keller AS, Raju NP, Webster TF, Stapleton HM. Flame Retardant Applications in Camping Tents and Potential Exposure. Environmental science & technology letters. 2014;1(2):152–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendelsohn E, Hagopian A, Hoffman K, Butt CM, Lorenzo A, Congleton J, et al. Nail polish as a source of exposure to triphenyl phosphate. Environment international. 2016;86:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergh C, Torgrip R, Emenius G, Ostman C. Organophosphate and phthalate esters in air and settled dust - a multi-location indoor study. Indoor air. 2011;21(1):67–76. [DOI] [PubMed] [Google Scholar]
- 18.Betts KS. Exposure to TDCPP appears widespread. Environmental health perspectives. 2013;121(5):a150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He R, Li Y, Xiang P, Li C, Zhou C, Zhang S, et al. Organophosphorus flame retardants and phthalate esters in indoor dust from different microenvironments: Bioaccessibility and risk assessment. Chemosphere. 2016;150:528–35. [DOI] [PubMed] [Google Scholar]
- 20.Wu M, Yu G, Cao Z, Wu D, Liu K, Deng S, et al. Characterization and human exposure assessment of organophosphate flame retardants in indoor dust from several microenvironments of Beijing, China. Chemosphere. 2016;150:465–71. [DOI] [PubMed] [Google Scholar]
- 21.Zhou L, Hiltscher M, Gruber D, Puttmann W. Organophosphate flame retardants (OPFRs) in indoor and outdoor air in the Rhine/Main area, Germany: comparison of concentrations and distribution profiles in different microenvironments. Environmental science and pollution research international. 2017;24(12):10992–1005. [DOI] [PubMed] [Google Scholar]
- 22.Castorina R, Butt C, Stapleton HM, Avery D, Harley KG, Holland N, et al. Flame retardants and their metabolites in the homes and urine of pregnant women residing in California (the CHAMACOS cohort). Chemosphere. 2017;179:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JW, Isobe T, Sudaryanto A, Malarvannan G, Chang KH, Muto M, et al. Organophosphorus flame retardants in house dust from the Philippines: occurrence and assessment of human exposure. Environmental science and pollution research international. 2013;20(2):812–22. [DOI] [PubMed] [Google Scholar]
- 24.Meeker JD, Cooper EM, Stapleton HM, Hauser R. Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environmental health perspectives. 2013;121(5):580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meeker JD, Stapleton HM. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environmental health perspectives. 2010;118(3):318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tajima S, Araki A, Kawai T, Tsuboi T, Ait Bamai Y, Yoshioka E, et al. Detection and intake assessment of organophosphate flame retardants in house dust in Japanese dwellings. The Science of the total environment. 2014;478:190–9. [DOI] [PubMed] [Google Scholar]
- 27.Cao Z, Xu F, Covaci A, Wu M, Yu G, Wang B, et al. Differences in the seasonal variation of brominated and phosphorus flame retardants in office dust. Environment international. 2014;65:100–6. [DOI] [PubMed] [Google Scholar]
- 28.Yang F, Ding J, Huang W, Xie W, Liu W. Particle size-specific distributions and preliminary exposure assessments of organophosphate flame retardants in office air particulate matter. Environmental science & technology. 2014;48(1):63–70. [DOI] [PubMed] [Google Scholar]
- 29.Bradman A, Barr DB, Claus Henn BG, Drumheller T, Curry C, Eskenazi B. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: a validation study. Environmental health perspectives. 2003;111(14):1779–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fromme H, Lahrz T, Kraft M, Fembacher L, Mach C, Dietrich S, et al. Organophosphate flame retardants and plasticizers in the air and dust in German daycare centers and human biomonitoring in visiting children (LUPE 3). Environment international. 2014;71:158–63. [DOI] [PubMed] [Google Scholar]
- 31.Carignan CC, McClean MD, Cooper EM, Watkins DJ, Fraser AJ, Heiger-Bernays W, et al. Predictors of tris(1,3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environment international. 2013;55:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cequier E, Sakhi AK, Marce RM, Becher G, Thomsen C. Human exposure pathways to organophosphate triesters - a biomonitoring study of mother-child pairs. Environment international. 2015;75:159–65. [DOI] [PubMed] [Google Scholar]
- 33.Dodson RE, Van den Eede N, Covaci A, Perovich LJ, Brody JG, Rudel RA. Urinary biomonitoring of phosphate flame retardants: levels in California adults and recommendations for future studies. Environmental science & technology. 2014;48(23):13625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou R, Xu Y, Wang Z. Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere. 2016;153:78–90. [DOI] [PubMed] [Google Scholar]
- *35.Phillips AL, Hammel SC, Hoffman K, Lorenzo AM, Chen A, Webster TF, et al. Children’s residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study. Environment international. 2018;116:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work demonstrates that hand-to-mouth contact and dermal absorption may be important pathways of OPE exposure, especially for young children.
- 36.Hughes MF, Edwards BC, Mitchell CT, Bhooshan B. In vitro dermal absorption of flame retardant chemicals. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2001;39(12):1263–70. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Yu G, Cao Z, Wang B, Huang J, Deng S, et al. Occurrence of organophosphorus flame retardants on skin wipes: Insight into human exposure from dermal absorption. Environment international. 2017;98:113–9. [DOI] [PubMed] [Google Scholar]
- 38.Makinen MS, Makinen MR, Koistinen JT, Pasanen AL, Pasanen PO, Kalliokoski PJ, et al. Respiratory and dermal exposure to organophosphorus flame retardants and tetrabromobisphenol A at five work environments. Environmental science & technology. 2009;43(3):941–7. [DOI] [PubMed] [Google Scholar]
- 39.Schreder ED, Uding N, La Guardia MJ. Inhalation a significant exposure route for chlorinated organophosphate flame retardants. Chemosphere. 2016;150:499–504. [DOI] [PubMed] [Google Scholar]
- 40.Sundkvist AM, Olofsson U, Haglund P. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. Journal of environmental monitoring : JEM. 2010;12(4):943–51. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Yu N, Zhang B, Jin L, Li M, Hu M, et al. Occurrence of organophosphate flame retardants in drinking water from China. Water research. 2014;54:53–61. [DOI] [PubMed] [Google Scholar]
- 42.Cooper EM, Covaci A, van Nuijs AL, Webster TF, Stapleton HM. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Analytical and bioanalytical chemistry. 2011;401(7):2123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lynn RK, Wong K, Garvie-Gould C, Kennish JM. Disposition of the flame retardant, tris(1,3-dichloro-2-propyl) phosphate, in the rat. Drug metabolism and disposition: the biological fate of chemicals. 1981;9(5):434–41. [PubMed] [Google Scholar]
- 44.Nomeir AA, Kato S, Matthews HB. The metabolism and disposition of tris(1,3-dichloro-2-propyl) phosphate (Fyrol FR-2) in the rat. Toxicology and applied pharmacology. 1981;57(3):401–13. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki K, Suzuki T, Takeda M, Uchiyama M. Metabolism of phosphoric acid triesters by rat liver homogenate. Bulletin of environmental contamination and toxicology. 1984;33(3):281–8. [DOI] [PubMed] [Google Scholar]
- 46.Van den Eede N, Maho W, Erratico C, Neels H, Covaci A. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicology letters. 2013;223(1):9–15. [DOI] [PubMed] [Google Scholar]
- 47.Wang G, Du Z, Chen H, Su Y, Gao S, Mao L. Tissue-Specific Accumulation, Depuration, and Transformation of Triphenyl Phosphate (TPHP) in Adult Zebrafish (Danio rerio). Environmental science & technology. 2016;50(24):13555–64. [DOI] [PubMed] [Google Scholar]
- 48.Minegishi K, Kurebayashi H, Nambaru S, Morimoto K, Takahashi T, Yamaha T. Comparative studies on absorption, distribution, and excretion of flame retardants halogenated alkyl phosphate in rats. Eisei Kagaku. 1988;34(2):102–14. [Google Scholar]
- 49.Su G, Crump D, Letcher RJ, Kennedy SW. In Vitro Metabolism of the Flame Retardant Triphenyl Phosphate in Chicken Embryonic Hepatocytes and the Importance of the Hydroxylation Pathway. Environmental science & technology. 2015;48(22):13511–9. [DOI] [PubMed] [Google Scholar]
- 50.Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environmental science & technology. 2014;48(17):10432–8. [DOI] [PubMed] [Google Scholar]
- 51.Butt CM, Hoffman K, Chen A, Lorenzo A, Congleton J, Stapleton HM. Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environment international. 2016;94:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding J, Xu Z, Huang W, Feng L, Yang F. Organophosphate ester flame retardants and plasticizers in human placenta in Eastern China. The Science of the total environment. 2016;554-555:211–7. [DOI] [PubMed] [Google Scholar]
- 53.Feng L, Ouyang F, Liu L, Wang X, Wang X, Li YJ, et al. Levels of Urinary Metabolites of Organophosphate Flame Retardants, TDCIPP, and TPHP, in Pregnant Women in Shanghai. Journal of environmental and public health. 2016;2016:9416054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffman K, Butt CM, Webster TF, Preston EV, Hammel SC, Makey C, et al. Temporal Trends in Exposure to Organophosphate Flame Retardants in the United States. Environmental science & technology letters. 2017;4(3):112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoffman K, Daniels JL, Stapleton HM. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environment international. 2014;63:169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffman K, Lorenzo A, Butt CM, Adair L, Herring AH, Stapleton HM, et al. Predictors of urinary flame retardant concentration among pregnant women. Environment international. 2017;98:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu LY, He K, Hites RA, Salamova A. Hair and Nails as Noninvasive Biomarkers of Human Exposure to Brominated and Organophosphate Flame Retardants. Environmental science & technology. 2016;50(6):3065–73. [DOI] [PubMed] [Google Scholar]
- 58.Liu LY, Salamova A, He K, Hites RA. Analysis of polybrominated diphenyl ethers and emerging halogenated and organophosphate flame retardants in human hair and nails. Journal of chromatography A. 2015;1406:251–7. [DOI] [PubMed] [Google Scholar]
- 59.Su G, Letcher RJ, Yu H, Gooden DM, Stapleton HM. Determination of glucuronide conjugates of hydroxyl triphenyl phosphate (OH-TPHP) metabolites in human urine and its use as a biomarker of TPHP exposure. Chemosphere. 2016;149:314–9. [DOI] [PubMed] [Google Scholar]
- 60.Van den Eede N, Heffernan AL, Aylward LL, Hobson P, Neels H, Mueller JF, et al. Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environment international. 2015;74:1–8. [DOI] [PubMed] [Google Scholar]
- **61.Ospina M, Jayatilaka NK, Wong LY, Restrepo P, Calafat AM. Exposure to organophosphate flame retardant chemicals in the US general population: Data from the 2013-2014 National Health and Nutrition Examination Survey. Environment international. 2018;110:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provides an assessment of a range of OPEs in a large, representative U.S. population.
- 62.Sasaki K, Takeda M, Uchiyama M. Toxicity, absorption and elimination of phosphoric acid triesters by killifish and goldfish. Bulletin of environmental contamination and toxicology. 1981;27(6):775–82. [DOI] [PubMed] [Google Scholar]
- 63.Romano ME, Hawley NL, Eliot M, Calafat AM, Jayatilaka NK, Kelsey K, et al. Variability and predictors of urinary concentrations of organophosphate flame retardant metabolites among pregnant women in Rhode Island. Environmental health : a global access science source. 2017;16(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM. Monitoring indoor exposure to organophosphate flame retardants: hand wipes and house dust. Environmental health perspectives. 2015;123(2):160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **65.Carignan CC, Minguez-Alarcon L, Butt CM, Williams PL, Meeker JD, Stapleton HM, et al. Urinary Concentrations of Organophosphate Flame Retardant Metabolites and Pregnancy Outcomes among Women Undergoing in Vitro Fertilization. Environmental health perspectives. 2017;125(8):087018. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work evaluates preconception OPEs and associations with pregnancy outcomes. Inverse associations between the sum of three OPE metabolites (BDCIPP, ip-PPP and DPHP) and proportions of fertilization, implantation, clinical pregnancy and live birth are reported.
- 66.Wang Y, Li W, Martinez-Moral MP, Sun H, Kannan K. Metabolites of organophosphate esters in urine from the United States: Concentrations, temporal variability, and exposure assessment. Environment international. 2019;122:213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *67.Zhao F, Chen M, Gao F, Shen H, Hu J. Organophosphorus Flame Retardants in Pregnant Women and Their Transfer to Chorionic Villi. Environmental science & technology. 2017;51(11):6489–97. [DOI] [PubMed] [Google Scholar]; This work measured several OPEs and their metabolites in human chorionic villi and deciduae. Findings indicate potential maternal-fetal transfer in early gestation, prior to the development of a mature placenta.
- 68.Baldwin KR, Phillips AL, Horman B, Arambula SE, Rebuli ME, Stapleton HM, et al. Sex Specific Placental Accumulation and Behavioral Effects of Developmental Firemaster 550 Exposure in Wistar Rats. Scientific reports. 2017;7(1):7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phillips AL, Chen A, Rock KD, Horman B, Patisaul HB, Stapleton HM. Transplacental and Lactational Transfer of Firemaster(R) 550 Components in Dosed Wistar Rats. Toxicological sciences : an official journal of the Society of Toxicology. 2016;153(2):246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He C, Toms LL, Thai P, Van den Eede N, Wang X, Li Y, et al. Urinary metabolites of organophosphate esters: Concentrations and age trends in Australian children. Environment international. 2018;111:124–30. [DOI] [PubMed] [Google Scholar]
- *71.Gibson EA, Stapleton HM, Calero L, Holmes D, Burke K, Martinez R, et al. Differential exposure to organophosphate flame retardants in mother-child pairs. Chemosphere. 2019;219:567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work found that children had higher levels of two of six assessed OPEs measured in urine than their mothers.
- 72.Chen Y, Fang J, Ren L, Fan R, Zhang J, Liu G, et al. Urinary metabolites of organophosphate esters in children in South China: Concentrations, profiles and estimated daily intake. Environ Pollut. 2018;235:358–64. [DOI] [PubMed] [Google Scholar]
- 73.He C, English K, Baduel C, Thai P, Jagals P, Ware RS, et al. Concentrations of organophosphate flame retardants and plasticizers in urine from young children in Queensland, Australia and associations with environmental and behavioural factors. Environ Res. 2018;164:262–70. [DOI] [PubMed] [Google Scholar]
- 74.Cooper EM, Kroeger G, Davis K, Clark CR, Ferguson PL, Stapleton HM. Results from Screening Polyurethane Foam Based Consumer Products for Flame Retardant Chemicals: Assessing Impacts on the Change in the Furniture Flammability Standards. Environmental science & technology. 2016;50(19):10653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Preston EV, McClean MD, Claus Henn B, Stapleton HM, Braverman LE, Pearce EN, et al. Associations between urinary diphenyl phosphate and thyroid function. Environment international. 2017;101:158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu T, Wang Q, Shi Q, Fang Q, Guo Y, Zhou B. Bioconcentration, metabolism and alterations of thyroid hormones of Tris(1,3-dichloro-2-propyl) phosphate (TDCPP) in Zebrafish. Environmental toxicology and pharmacology. 2015;40(2):581–6. [DOI] [PubMed] [Google Scholar]
- 77.Wang Q, Liang K, Liu J, Yang L, Guo Y, Liu C, et al. Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic-pituitary-thyroid axis. Aquatic toxicology (Amsterdam, Netherlands). 2013;126:207–13. [DOI] [PubMed] [Google Scholar]
- 78.Wang Q, Lam JC, Han J, Wang X, Guo Y, Lam PK, et al. Developmental exposure to the organophosphorus flame retardant tris(1,3-dichloro-2-propyl) phosphate: estrogenic activity, endocrine disruption and reproductive effects on zebrafish. Aquatic toxicology (Amsterdam, Netherlands). 2015;160:163–71. [DOI] [PubMed] [Google Scholar]
- 79.Wang Q, Lai NL, Wang X, Guo Y, Lam PK, Lam JC, et al. Bioconcentration and transfer of the organophorous flame retardant 1,3-dichloro-2-propyl phosphate causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish larvae. Environmental science & technology. 2015;49(8):5123–32. [DOI] [PubMed] [Google Scholar]
- 80.McGee SP, Konstantinov A, Stapleton HM, Volz DC. Aryl phosphate esters within a major PentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: potential role of the aryl hydrocarbon receptor. Toxicological sciences : an official journal of the Society of Toxicology. 2013;133(1):144–56. [DOI] [PubMed] [Google Scholar]
- 81.Liu X, Jung D, Jo A, Ji K, Moon HB, Choi K. Long-term exposure to triphenylphosphate alters hormone balance and HPG, HPI, and HPT gene expression in zebrafish (Danio rerio). Environmental toxicology and chemistry. 2016;35(9):2288–96. [DOI] [PubMed] [Google Scholar]
- 82.Liu X, Ji K, Choi K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquatic toxicology (Amsterdam, Netherlands). 2012;114-115:173–81. [DOI] [PubMed] [Google Scholar]
- 83.Kim S, Jung J, Lee I, Jung D, Youn H, Choi K. Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines. Aquatic toxicology (Amsterdam, Netherlands). 2015;160:188–96. [DOI] [PubMed] [Google Scholar]
- 84.Moser VC, Phillips PM, Hedge JM, McDaniel KL. Neurotoxicological and thyroid evaluations of rats developmentally exposed to tris(1,3-dichloro-2-propyl)phosphate (TDCIPP) and tris(2-chloro-2-ethyl)phosphate (TCEP). Neurotoxicology and teratology. 2015;52(Pt B):236–47. [DOI] [PubMed] [Google Scholar]
- 85.Kassotis CD, Kollitz EM, Hoffman K, Sosa JA, Stapleton HM. Thyroid receptor antagonism as a contributory mechanism for adipogenesis induced by environmental mixtures in 3T3-L1 cells. The Science of the total environment. 2019;666:431–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Farhat A, Crump D, Chiu S, Williams KL, Letcher RJ, Gauthier LT, et al. In Ovo effects of two organophosphate flame retardants--TCPP and TDCPP--on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicological sciences : an official journal of the Society of Toxicology. 2013;134(1):92–102. [DOI] [PubMed] [Google Scholar]
- **87.Messerlian C, Williams PL, Minguez-Alarcon L, Carignan CC, Ford JB, Butt CM, et al. Organophosphate flame-retardant metabolite concentrations and pregnancy loss among women conceiving with assisted reproductive technology. Fertil Steril. 2018;110(6):1137–44 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work evaluated preconception OPEs and pregnancy loss. Findings suggest that higher DPHP and summed OPE metabolites are associated with greater risk of pregnancy loss.
- 88.Liu X, Ji K, Jo A, Moon HB, Choi K. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquatic toxicology (Amsterdam, Netherlands). 2013;134-135:104–11. [DOI] [PubMed] [Google Scholar]
- 89.Schang G, Robaire B, Hales BF. Organophosphate Flame Retardants Act as Endocrine-Disrupting Chemicals in MA-10 Mouse Tumor Leydig Cells. Toxicological sciences : an official journal of the Society of Toxicology. 2016;150(2):499–509. [DOI] [PubMed] [Google Scholar]
- 90.Soubry A, Hoyo C, Butt CM, Fieuws S, Price TM, Murphy SK, et al. Human exposure to flame-retardants is associated with aberrant DNA methylation at imprinted genes in sperm. Environ Epigenetics. 2017;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meeker JD. Exposure to environmental endocrine disruptors and child development. Archives of pediatrics & adolescent medicine. 2012;166(10):952–8. [PubMed] [Google Scholar]
- **92.Ingle ME, Minguez-Alarcon L, Carignan CC, Butt CM, Stapleton HM, Williams PL, et al. The association between urinary concentrations of phosphorous-containing flame retardant metabolites and semen parameters among men from a fertility clinic. Int J Hyg Envir Heal. 2018;221(5):809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this work suggest that odds of having a low sperm count decrease with increasing BDCIPP concentrations. Associatiosn with other OPE metabolites were weak and inconsistent.
- **93.Carignan CC, Minguez-Alarcon L, Williams PL, Meeker JD, Stapleton HM, Butt CM, et al. Paternal urinary concentrations of organophosphate flame retardant metabolites, fertility measures, and pregnancy outcomes among couples undergoing in vitro fertilization. Environment international. 2018;111:232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this work indicate that female partner exposure may be more important in pregnancy outcomes than male partner OPE exposures.
- 94.Ely DM, Driscoll AK, Mathews TJ. nfant Mortality by Age at Death in the United States, 2016. NCHS Data Brief No 326. 2018. [PubMed] [Google Scholar]
- 95.Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, et al. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster(R) 550 in rats: an exploratory assessment. Journal of biochemical and molecular toxicology. 2013;27(2):124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crump D, Chiu S, Kennedy SW. Effects of tris(1,3-dichloro-2-propyl) phosphate and tris(1-chloropropyl) phosphate on cytotoxicity and mRNA expression in primary cultures of avian hepatocytes and neuronal cells. Toxicological sciences : an official journal of the Society of Toxicology. 2012;126(1):140–8. [DOI] [PubMed] [Google Scholar]
- 97.McGee SP, Cooper EM, Stapleton HM, Volz DC. Early zebrafish embryogenesis is susceptible to developmental TDCPP exposure. Environmental health perspectives. 2012;120(11):1585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Welsh JJ, Collins TF, Whitby KE, Black TN, Arnold A. Teratogenic potential of triphenyl phosphate in Sprague-Dawley (Spartan) rats. Toxicology and industrial health. 1987;3(3):357–69. [DOI] [PubMed] [Google Scholar]
- 99.Fu J, Han J, Zhou B, Gong Z, Santos EM, Huo X, et al. Toxicogenomic responses of zebrafish embryos/larvae to tris(1,3-dichloro-2-propyl) phosphate (TDCPP) reveal possible molecular mechanisms of developmental toxicity. Environmental science & technology. 2013;47(18):10574–82. [DOI] [PubMed] [Google Scholar]
- **100.Hoffman K, Stapleton HM, Lorenzo A, Butt CM, Adair L, Herring AH, et al. Prenatal exposure to organophosphates and associations with birthweight and gestational length. Environment international. 2018;116:248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work finds associations between several OPE metabolites and gestational duration. The results suggest associations differ based on the infant sex.
- 101.Wade MG, Kawata A, Rigden M, Caldwell D, Holloway AC. Toxicity of Flame Retardant Isopropylated Triphenyl Phosphate: Liver, Adrenal, and Metabolic Effects. Int J Toxicol. 2019:1091581819851502. [DOI] [PubMed] [Google Scholar]
- **102.Boyle M, Buckley JP, Quiros-Alcala L. Associations between urinary organophosphate ester metabolites and measures of adiposity among US children and adults: NHANES 2013-2014. Environment international. 2019;127:754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]; This cross-sectional assessment reports inverse associations between DBUP and the indicators of adiposity and reports that BCEP is associated with increased prevalence odds of being overweight vs. normal weight among children.
- 103.Thomas MB, Stapleton HM, Dills RL, Violette HD, Christakis DA, Sathyanarayana S. Demographic and dietary risk factors in relation to urinary metabolites of organophosphate flame retardants in toddlers. Chemosphere. 2017;185:918–25. [DOI] [PubMed] [Google Scholar]
- 104.Poma G, Sales C, Bruyland B, Christia C, Goscinny S, Van Loco J, et al. Occurrence of Organophosphorus Flame Retardants and Plasticizers (PFRs) in Belgian Foodstuffs and Estimation of the Dietary Exposure of the Adult Population. Environmental science & technology. 2018;52(4):2331–8. [DOI] [PubMed] [Google Scholar]
- 105.Babich MA. Preliminary Risk Assessment of Flame Retardant (FR) Chemicals in Upholstered Furniture Foam Bethesda, MD 20814: U.S. Consumer Product Safety Commission; 2006. [Google Scholar]
- 106.Camarasa JG, Serra-Baldrich E. Allergic contact dermatitis from triphenyl phosphate. Contact Dermatitis. 1992;26(4):264–5. [DOI] [PubMed] [Google Scholar]
- 107.Carlsen L, Andersen KE, Egsgaard H. Triphenyl phosphate allergy from spectacle frames. Contact Dermatitis. 1986;15(5):274–7. [DOI] [PubMed] [Google Scholar]
- **108.Araki A, Bastiaensen M, Bamai YA, Van den Eede N, Kawai T, Tsuboi T, et al. Associations between allergic symptoms and phosphate flame retardants in dust and their urinary metabolites among school children. Environment international. 2018;119:438–46. [DOI] [PubMed] [Google Scholar]; In this work, TDCIPP in house dust, and metabolites of TDCIPP, TBOEP and TCIPP in urine were associated with children’s allergic symptoms.
- 109.Araki A, Saito I, Kanazawa A, Morimoto K, Nakayama K, Shibata E, et al. Phosphorus flame retardants in indoor dust and their relation to asthma and allergies of inhabitants. Indoor air. 2013;24(1):3–15. [DOI] [PubMed] [Google Scholar]
- 110.Canbaz D, van Velzen MJ, Hallner E, Zwinderman AH, Wickman M, Leonards PE, et al. Exposure to organophosphate and polybrominated diphenyl ether flame retardants via indoor dust and childhood asthma. Indoor air. 2016;26(3):403–13. [DOI] [PubMed] [Google Scholar]
- 111.Bi C, Maestre JP, Li H, Zhang G, Givehchi R, Mahdavi A, et al. Phthalates and organophosphates in settled dust and HVAC filter dust of U.S. low-income homes: Association with season, building characteristics, and childhood asthma. Environment international. 2018;121(Pt 1):916–30. [DOI] [PubMed] [Google Scholar]
- 112.Wang Q, Lam JC, Man YC, Lai NL, Kwok KY, Guo Y, et al. Bioconcentration, metabolism and neurotoxicity of the organophorous flame retardant 1,3-dichloro 2-propyl phosphate (TDCPP) to zebrafish. Aquatic toxicology (Amsterdam, Netherlands). 2015;158:108–15. [DOI] [PubMed] [Google Scholar]
- 113.Jarema KA, Hunter DL, Shaffer RM, Behl M, Padilla S. Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicology and teratology. 2015;52(Pt B):194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Noyes PD, Haggard DE, Gonnerman GD, Tanguay RL. Advanced morphological - behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicological sciences : an official journal of the Society of Toxicology. 2015;145(1):177–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oliveri AN, Bailey JM, Levin ED. Developmental exposure to organophosphate flame retardants causes behavioral effects in larval and adult zebrafish. Neurotoxicology and teratology. 2015;52(Pt B):220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dishaw LV, Hunter DL, Padnos B, Padilla S, Stapleton HM. Developmental exposure to organophosphate flame retardants elicits overt toxicity and alters behavior in early life stage zebrafish (Danio rerio). Toxicological sciences : an official journal of the Society of Toxicology. 2014;142(2):445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.de Esch C, Slieker R, Wolterbeek A, Woutersen R, de Groot D. Zebrafish as potential model for developmental neurotoxicity testing: a mini review. Neurotoxicology and teratology. 2012;34(6):545–53. [DOI] [PubMed] [Google Scholar]
- 118.Bailey J, Oliveri A, Levin ED. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res C Embryo Today. 2013;99(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **119.Doherty BT, Hoffman K, Keil AP, Engel SM, Stapleton HM, Goldman BD, et al. Prenatal exposure to organophosphate esters and behavioral development in young children in the Pregnancy, Infection, and Nutrition Study. Neurotoxicology. 2019;73:150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]; Results of this work suggest associations between prenatal exposure to OPEs (i.e., BDCIPP and DPHP) and attention problems, withdrawal, and hyperactivity.
- **120.Doherty BT, Hoffman K, Keil AP, Engel SM, Stapleton HM, Goldman BD, et al. Prenatal exposure to organophosphate esters and cognitive development in young children in the Pregnancy, Infection, and Nutrition Study. Environ Res. 2019;169:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]; Worse cognitive assessment scores were associated with higher prenatal ip-PPP in maternal urine samples in this assessment.
- **121.Castorina R, Bradman A, Stapleton HM, Butt C, Avery D, Harley KG, et al. Current-use flame retardants: Maternal exposure and neurodevelopment in children of the CHAMACOS cohort. Chemosphere. 2017;189:574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]; Prenatal samples of maternal urine were assessed for OPEs and child neurodevelopment was assessed in this work. OPE metabolites were positively associated with worse scores on the WISC-IV (particularly the Working Memory scale), and higher scores on the BASC-2 Hyperactivity scale.
- *122.Lipscomb ST, McClelland MM, MacDonald M, Cardenas A, Anderson KA, Kile ML. Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. Environmental health : a global access science source. 2017;16(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using silicone wristbands to assess exposure cross-sectionally, children with higher ΣOPFR were rated as having less responsible behavior and more externalizing behavior problems.
- 123.Hammel SC, Hoffman K, Webster TF, Anderson KA, Stapleton HM. Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environmental science & technology. 2016;50(8):4483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoffman K, Hammel SC, Phillips AL, Lorenzo AM, Chen A, Calafat AM, et al. Biomarkers of exposure to SVOCs in children and their demographic associations: The TESIE Study. Environment international. 2018;119:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **125.Gibson EA, Stapleton HM, Caler L, Holmes D, Burke K, Martinez R, et al. Flame retardant exposure assessment: findings from a behavioral intervention study. J Expo Sci Env Epid. 2019;29(l):33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]; This case-crossover study suggest that hand washing and cleaning may be effective means of reducing exposure to OPEs.


