Abstract
The initial reverse shoulder arthroplasty (RSA), designed by Paul Grammont, was intended to treat rotator cuff tear arthropathy in elderly patients. In the early experience, high complication rates (up to 24%) and revision rates (up to 50%) were reported.
The most common complications reported were scapular notching, whereas clinically more relevant complications such as instability and acromial fractures were less commonly described.
Zumstein et al defined a ‘complication’ following RSA as any intraoperative or postoperative event that was likely to have a negative influence on the patient’s final outcome.
High rates of complications related to the Grammont RSA design led to development of non-Grammont designs, with 135 or 145 degrees of humeral inclination, multiple options for glenosphere size and eccentricity, improved baseplate fixation which facilitated glenoid-sided lateralization, and the option of humeral-sided lateralization.
Improved implant characteristics combined with surgeon experience led to a dramatic fall in the majority of complications. However, we still lack a suitable solution for several complications, such as acromial stress fracture.
Cite this article: EFORT Open Rev 2021;6:1097-1108. DOI: 10.1302/2058-5241.6.210039
Keywords: acromion, dislocation, fracture, impingement, neck-shaft angle, PROMs, prosthesis design, results, scapular notching, spine
Introduction
The annual number of shoulder joint replacements has been steadily increasingly worldwide for the last two decades,1 in great part due to the annual increases in reverse shoulder arthroplasty (RSA) performed since its introduction in Europe in 1987 and in the United States in 2004.2 The Grammont-style RSA has a 155 degree neck-shaft angle with a medialized glenoid and a medialized inlay humerus component. Initially, it was developed to treat rotator cuff arthropathy3 but its indications have been expanded to treat numerous other pathologies. Indications include irreparable rotator cuff tears without arthritic changes,4 primary glenohumeral arthritis with associated instability (i.e. B2 or D glenoids),5 revision shoulder arthroplasty,6 inflammatory arthritis,7 post-infectious residue,8 tumour resection,9 chronic dislocations,10 fracture sequela11 and displaced proximal humeral fractures in the elderly.12 Because of the wide range of indications for RSA, including in very demanding cases where RSA is often used as a salvage procedure, it is not surprising that a relatively high incidence of complications has been reported. However, information is mostly reported by heterogeneous studies as they differ in indications, prosthetic design utilized, and in the definition of a complication.13 Accumulated experience resulted in further technological advancements in implant design and surgical technique in order to decrease existing complications and to optimize RSA performance through altered biomechanics for improved and durable functional outcome.14 Modern RSA non-Grammontdesigns offer multiple options for glenosphere offset and eccentricity, 135 or 145 degree neck-shaft angles, improved baseplate stability, and humerus-based lateralization (onlay humeral design).15–17 As it is expected that the increased use of RSA will continue in the next decade18 accurate knowledge of the likelihood and implications of all possible complications is mandatory. The goal of this review article is to present a review of reported mechanical complications and fractures of RSA and to analyse their occurrence based on the various prosthetic designs used.
Definition
Zumstein et al defined a ‘complication’ following RSA as any intraoperative or postoperative event that was likely to have a negative influence on the patient’s final outcome. These included fractures, infections, dislocations, nerve palsies, aseptic loosening of humeral or glenoid components, modular stem or polyethylene disassociations, or glenoid screw problems.19 Meanwhile, they defined a ‘problem’ as an intraoperative or postoperative event that was not likely to affect the patient’s final outcome. They considered these to include scapular notching, haematomas, heterotopic ossification, complex regional pain syndrome, phlebitis, intraoperative dislocations, intraoperative cement extravasation, or radiographic lucent lines of the glenoid.19
Prevalence and risk factors
Reported complication rates after primary RSA range between 3% and 24%,19–21 whereas complication rates after revision RSA have reached as high as 50%.22 The reported rate varies as it might be affected by the mixture of primary and revision cases included in each study.23 Surgeon’s experience seems to play a significant role. Walch et al reported their results with a Grammont-style RSA and analysed complication rates between 240 RSAs performed during May 1995 and June 2003 and 240 RSAs performed during July 2003 and March 2007. They noticed a fall in percentage of complications from 19% to 10%, mainly because of a lower rate of infection and instability.21 The complication-related learning curve diminishes with an increase in the surgeon’s experience, with reports showing this range to be between 10 to 40 cases.24–26 Additional factors such as body mass index,27 diabetes,28 Parkinson disease,29 and preoperative American Society of Anesthesiologists (ASA) score30 have been associated with an increase in complication rate. Most of the studies published on the subject of RSA have described either a Grammont-style RSA with a medialized glenoid or an inlay humeral component with medialized humerus. Non-Grammont designs appear to have led to a decrease in the rate of certain complications. Stephens et al reported in their study statistically significant declines in baseplate failure, humeral dissociation and glenosphere dissociation after implant modifications.31 Similarly, Gorman et al reported statistically significant reductions in the incidence of radiographic loosening and the need for revision with the use of second-generation monolithic humeral stem implant, which primarily utilizes press fit fixation, compared to the first-generation cemented modular humeral stem implant in RSA.32 At the other end of the spectrum, new designs may also be incriminated in the increased rate of other complications or problems.33–35
Mechanical failure
Mechanical failure may occur at the humeral or glenoid side (Fig. 1). Most early reports were cautious of the outcome of these implants, due to the forces occurring at the glenoid. Bacle et al have shown a 93% prosthetic survival rate of Grammont-style prosthesis at ten years using revision as the end point.36
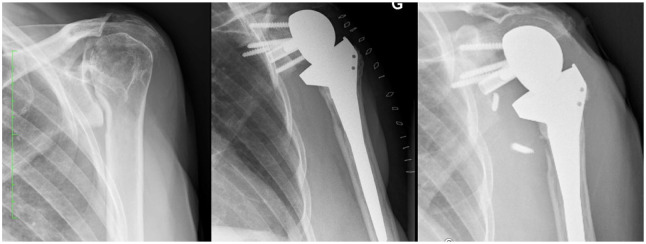
Fig. 1.
(a) Transthoracic view of a patient with bilateral reverse shoulder arthroplasty (RSA) glenoid migration and prosthetic dislocation. (b) Antero-posterior view of right shoulder showing loosening and superior migration of glenoid component after RSA.
Source: (b) From wiki.beemed.com, with permission.
Impingements
Scapular notching was initially described as the result of an impingement of the prosthetic metaphysis against the scapular neck with the arm in adduction consequent to humerus medialization.37 A study by Lädermann et al revealed that two types of impingement interactions coexist, the frank abutment-type impingement (between greater tuberosity and the acromion) and friction-type impingement (anterior, posterior notching and inferior scapular notching) (Fig. 2). Abutment-type impingement seems to restrict movement in abduction and flexion with contact located on the lateral acromion or the coracoid process.38 Friction impingements have been shown to be anterior, posterior, and inferior and are due to a combination of many motions, especially of extension and internal or external rotation with the arm at the side.38 Inferior scapular notching is the most common scapular notching, thus we refer to it mainly as scapular notching.
Fig. 2.
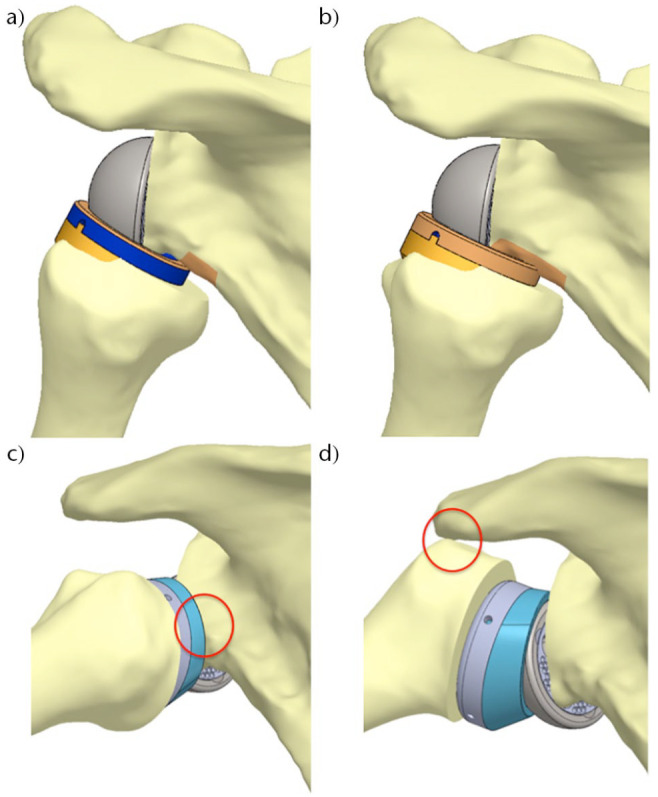
Two types of impingement interactions coexist; it could correspond to a friction of the polyethylene against the bone (A and B). These repetitive frictions might lead with time to progressive bony abrasion. These phenomena are probably the cause of a rapid apparition of scapular notching. They are the results of multiple motions (adduction, rotations, extension) and not the consequence of a simple contact with the pillar in adduction with the arm at the side as previously believed. Contrarily, some impingements are related to an abutment with no possibilities for either component to continue the movement (C and D).
Source: From Lädermann et al,15 with permission.
Inferior impingement: scapular notching
Scapular notching (Fig. 3) is a complication unique to RSA and is often described as its most commonly observed radiological finding, having been reported in up to 88% and 96% of cases.39,40 A recent systematic review by Shah et al reported the global rate of scapular notching at 29.4%, which were classified as low-grade scapular notching (grade I or II) in 79.9%, with a mean follow-up of 3.5 years.41 They reported a decrease in its incidence in recent years, as the pooled averages of cases published in between 2010–2015 and 2016–2018 were 36.2% and 23.9%, respectively. Repetitive contact between polyethylene and bone may result in polyethylene wear debris, chronic inflammation and osteolysis,42 radiolucency around the glenoid component,40 presence of an inferior bone spur and ossification in the glenohumeral space.39 In some cases it is also associated with loosening of the glenoid component.43 But this is not always so, as Nyffeler et al showed in their study that the baseplate remained stable even when the inferior half of the glenoid was absorbed.44 Roche et al conducted a biomechanical study where they assessed the effect of scapular notching on RSA glenoid baseplate fixation. They concluded that severe notching may play a role in initial glenoid baseplate stability.45 However, the effect of less severe scapular notching on clinical outcomes remains unclear. Lévigne et al, who assessed 461 shoulders after Grammont-type RSA implantation, found no relationship between scapular notching and Constant score or pain.46 However, Mollon et al have shown in their study of 476 shoulders that scapular notching is consistent with significantly lower postoperative Shoulder Pain and Disability Index (SPADI) and Constant scores compared to patients without any notching. Additionally, patients who had scapular notching also had significantly lower range of motion (active abduction or forward flexion), less strength and significantly higher complication rate.47
Fig. 3.
Left glenohumeral arthritis (left). The severe superior glenoid bone loss has not been corrected intraoperatively (middle), resulting in high position of the baseplate which has led to a severe scapular notching of grade 4 according to Sirveaux (right).
Source: From wiki.beemed.com, with permission.
Risk factors for scapular notching are: duration of follow-up,46 patient anatomy,38, 48 anterosuperior approach,49,50 a high position of the glenoid component, a superior component tilt,50 a medialized component (glenoidal or humeral)41,50 and a small glenosphere (38 mm).51,52 Preventing factors from scapular notching are: varus neck-shaft angled stem,50,53,54 a large glenosphere (42 mm),51 an eccentric glenosphere,50,55 a lateralized glenoid (bony or metallic)50,54 and > 3.5 mm overhang of the inferior glenosphere.56,57
In a recent review article, Shah et al41 reported that the combination of medialized glenoid and lateralized humerus design had the lowest rate of scapular notching, which was even lower compared to lateralized glenoid and medialized humerus design. A significant decrease of notching rates, particularly those of non-Grammont modern designs, was observed when compared to the findings of Zumstein et al.19
Anterior impingement
Anterior impingement can also occur in the setting of RSA.58 Anterior impingement may specifically jeopardize the clinical outcome and implant survivorship, ranging from limitation in internal rotation, to dislocation by decoaptation, or failure.59 As the conformation of the joint changes from spinning to hinging in RSA, implant version of the humeral stem seems to be the most predictive factor for the occurrence of anterior scapular notching. Grammont initially warned that excessive retroversion led to decreased internal rotation and hence recommended 0 degrees of humeral component version.60 Others favour a balance between anterior and posterior notching with 20 to 40 degrees of humeral retroversion.61 On the other hand, Favre et al have shown that glenoid component version does not seem to influence notching in the axial plane.62 Although Keener et al recommended a 5 degree retroversion of the glenoid according to their computer simulation study.63
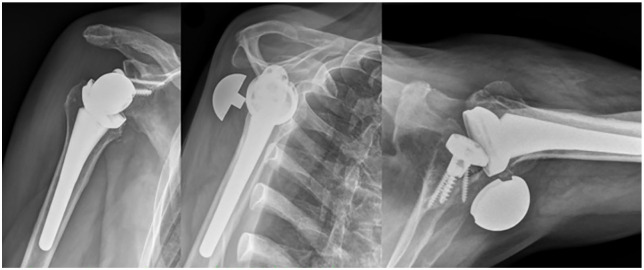
Acromial fracture
Acromial and scapular spine fracture are uncommon but significant complications after RSA (Fig. 4). In 2011, Zumstein et al presented postoperative scapular or acromion fractures as a rare occurrence with the incidence of 1.5%.19 In 2020, Lau and Large64 and Shah et al41 published in their systematic reviews a higher overall incidence of 5% and 2.6%, respectively. However, due to the tendency towards under-diagnosis, the true incidence, especially of stress or fatigue fractures, might be significantly higher. Patient risk factors proposed include osteoporosis,65 acromial thickness,65 and inflammatory arthritis.66 Cases of incidental, fatigue or stress fracture were statistically more common compared to cases as a result of direct trauma like a fall.64 Crosby et al hypothesized an arthritic or stiff acromioclavicular joint to be a risk factor for acromial fracture after RSA.67 However, there is little proof to support their claims as Dubrow et al68 and Walch et al69 did not find it to be predictive of acromial fracture. Currently, no literature supports this correlation.
Fig. 4.
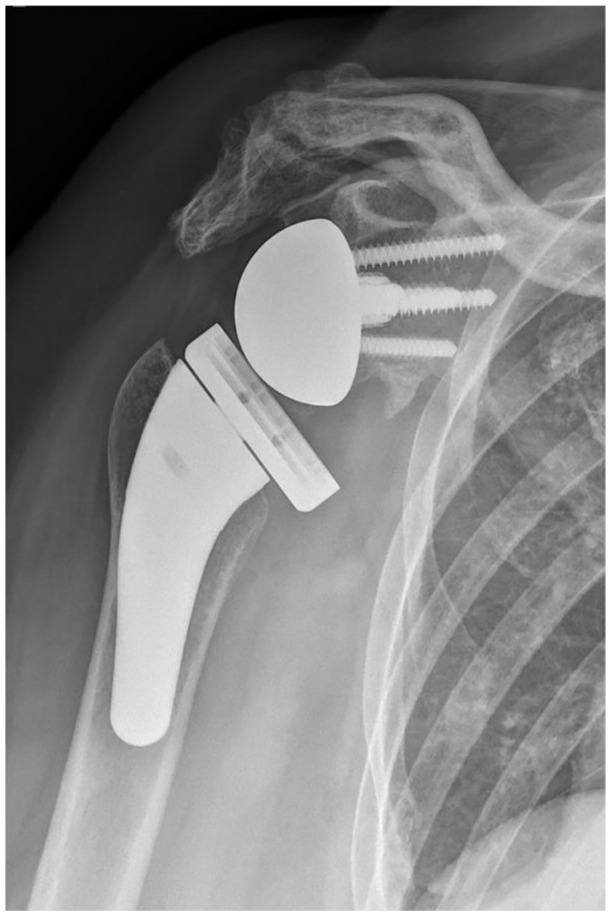
Postoperative anteroposterior X-ray demonstrating an acromial fracture.
Source: From wiki.beemed.com, with permission.
Technical risk factors include screw placement and implant position. Otto et al noted that 11 of 16 scapular spine fractures (Crosby type III fracture) occurred directly from the tip of the most posterior or superior screw, while a further three occurred from the tip of the centre screw but failed to find a statistically significant association between screw placement and stress fracture.65 Kennon et al investigated clinical and biomechanical stresses of superior screw constructs on the scapular spine. In the clinical part of the study, they retrospectively analysed 318 patients of whom nine had Crosby type III scapular fractures which were shown to be in relation to a superior screw, whereas there were none associated with an inferiorly placed screw. In the biomechanical part of the study, the group with all four screws demonstrated significantly lower load to failure by 45% and also had a decreased construct stiffness by 43% compared to the group with inferior screws alone. They concluded that placement of superior screws acted as a significant stress riser in their biomechanical study and recommended the use of inferior screws positioned below the central glenoid axis, except when it would be necessary to stabilize the baseplate construct.70 Biomechanical studies have shown that scapular regions with the highest cortical thickness, and consequently potential bony corridors for superiorly positioned baseplate screws, are located at the scapular spine and base of the coracoid process.71 Positioning of the superior screw towards the spine of the scapula might predispose it to Crosby type III fracture post RSA, whereas positioning of the screw towards the base of the coracoid process might not carry the same risk.
Excessive tensioning of the deltoid is thought to place the acromion at risk of fracture after the implantation of an RSA. Deltoid over-tensioning by a lateralized implant has been cited as a risk factor for acromial or scapular spine stress fracture. In a finite element analysis, Wong et al found that acromial stress significantly increased in glenosphere lateralization (17.2%) compared to glenosphere inferiorization (2.6%).72 However, clinically this has not been observed. Haidamous et al performed a multi-centre retrospective study where they assessed the effect of lateralization and distalization on scapular spine fracture after RSA at a minimum one-year follow-up. They found no relationship between lateralization and stress fracture. A significant association was seen between distalization, with an onlay stem resulting in a 10 mm increase in distalization and a 2.5 times increased risk of scapular spine fracture compared to an inlay stem.33 Other authors have also concluded that the risk of stress fracture increased with an onlay humeral design.73,74 These findings suggest that distalization with an onlay humeral implant design increases the risk of stress fracture. However, the causes are likely multi-factorial and further studies are required.
Non-operative management is most common after acromial or scapular spine stress fractures. The majority of literature reports inferior outcomes after the occurrence of a fracture. Yet, surgical treatment is associated with variable results and a high rate of complications including revision.64
Instability
Dislocation (Fig. 5) is one of the most common complications after RSA, with rates as high as 31% which account for almost half of the complications in some series.75 Shah et al41 reported in their systematic review the average global instability rate at 3.3% at an average follow-up of 3.2 years. Instability rates, especially for modern non-Grammont designs (1.3%), have significantly decreased compared to Zumstein et al (4.7%).19 Most dislocations require revision surgery and occur within the first 90 days after operation as a result of a technical error. Primary RSA instability rates (2.5%) are significantly lower compared to revision RSA (5.7%) or RSA for failed osteosynthesis of a proximal humeral fracture (5.3%). The medialized Grammont-style RSA had a significantly higher instability rate (4.0%) compared to all other designs combined (1.3%). They also found a significantly lower rate with the medial glenoid/lateral humerus design (0.9%) compared to lateral glenoid/medial humerus design (2.0%).41 When a 155 degree neck-shaft angle is used, subscapularis repair proves to be important for lowering the risk of instability.76,77 Erickson et al published a systematic review on the topic of influence of humeral head inclination in RSA. They analysed 2222 shoulders from 38 studies and found no difference in dislocation rates between a 135 and 155 degree humeral inclination.78 The various risk factors having an effect on instability after RSA are summarized in Table 1.
Fig. 5.
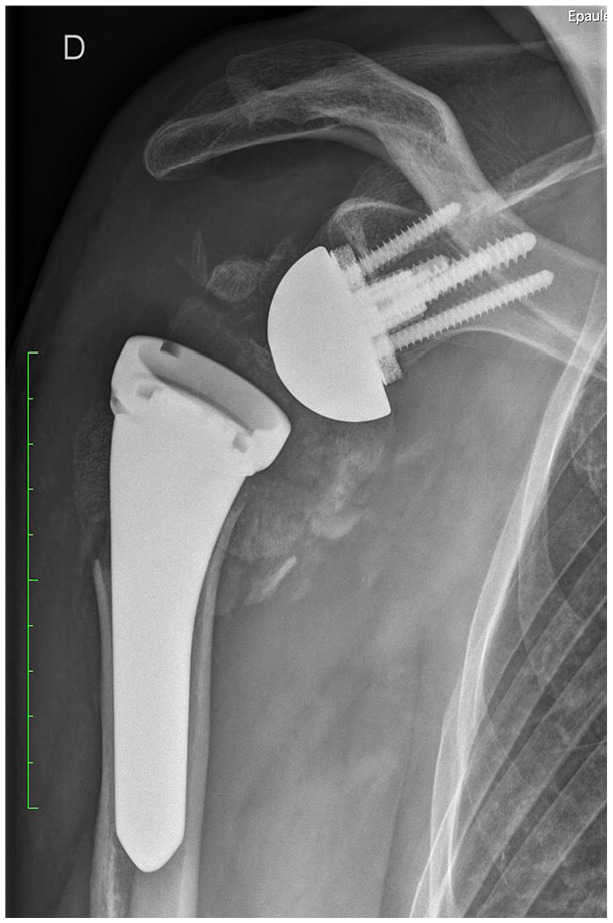
One month after implantation of a reverse shoulder arthroplasty (RSA) for a proximal humeral fracture. The X-ray revealed a prosthetic dislocation. Electroneuromyography (ENMG) confirmed a severe axonotmesis of the axillary nerve.
Source: From wiki.beemed.com, with permission.
Table 1.
Various risk factors having an effect on instability after reverse shoulder arthroplasty (RSA)
| Author | Risk factor |
|---|---|
| Comorbidities and demographic factors | |
| Cheung et al82 | Male gender |
| Padegimas et al104 | Body mass index > 30 |
| Diagnosis | |
| Cheung et al82 | Previous open procedures, preoperative nonunion of proximal humerus or tuberosity |
| Intraoperative factors | |
| Tashjian et al83 | Superior inclination of baseplate |
| Ohl et al105 | Resection of tuberosities |
| Lädermann et al106 | Deltoid insufficiency, intraoperative neurological palsy |
| Cheung et al and Edwards et al82,107 | Lack of anterior restraints including subscapularis insufficiency (controversial, depend on prosthetic design) |
| Lädermann et al and Gallo et al81,108 | Inability to restore humeral length |
| Edwards et al107 | Conjoint tendon weakness and pectoralis major insufficiency |
| Favre et al62 | Malpositioning of the components |
| Johnson et al109 | Impingement |
| Postoperative factors | |
| Gallo et al108 | Infection |
| Lädermann et al106 | Deltoid insufficiency resulting from acromial fracture, polyethylene wear, stem subsidence |
Instability of RSA is a difficult complication to manage and its treatment is controversial.79 Closed reduction has limited success and very commonly (42%) leads to revision surgery or bad outcome with no additional procedures.80 Appropriate restoration of deltoid length (and with that tension) and lateralization is crucial in obtaining stability of the RSA. This can be achieved with a higher polyethylene insert or by adding an additional metallic spacer, which allows up to 15 mm of correction of the length of the upper arm. A greater degree of correction can be obtained by changing the implanted humeral component with a prouder humeral implant.81 Recurrent instability is also a possibility after revision, and even when the shoulder remains stable, a lower functional result is expected.82 It is important to note that instability is differently defined by different authors and consequently more subtle forms of instability, which have a negative effect on ASES score, might have been left out.83
Glenoid or humeral component disassembly
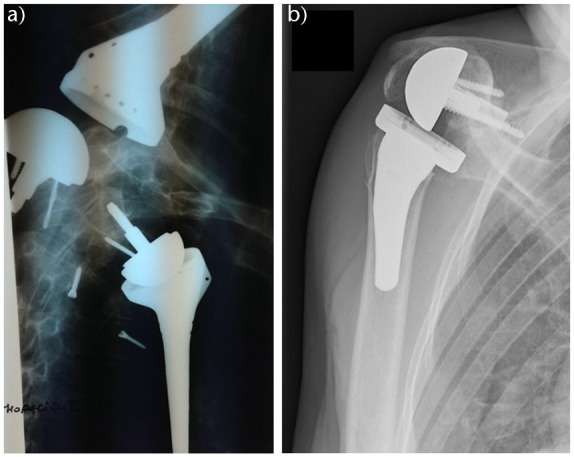
Glenoid and humeral component dissociations (Fig. 6) have been very rarely described in the literature, mostly in case reports and series. Stephens et al31 retrospectively analysed complications of 1418 patients who underwent RSA from 2000 to 2012. The incidences of humeral component and glenosphere dissociation were 0.7% and 0.6%, respectively. They found a decrease in revision rates after modification of the prosthesis design. Newer metal metaphyseal shell and polyethylene insert (0.2%) had a significantly lower rate of humeral component dissociation compared to the polyethylene humeral sockets (2.1%). Glenosphere dissociation rate also declined from 1.3% to 0.3% after the introduction of the Morse taper central fixation and a new central screw fixation, as also found by Zumstein et al.19 Stephens et al31 reported that the most frequent manner of failure in patients after RSA reimplantation was humeral dissociation (40%), followed by glenohumeral dislocation (20%). They found that larger glenospheres (40 mm and 44 mm) had a greater incidence of dissociations compared to smaller glenospheres (32 mm and 26 mm); the same finding was reported by Cusick et al.84 The majority of patients after the glenosphere dissociation had no specific event before dissociation, whereas a few reported traumatic aetiology.31 Besides the obvious clinical findings of immediate loss of range of motion, milder symptoms are also a possibility. It is important to recognize key imaging findings, which consist of an appearance of subluxation with significantly less than 100% displacement.85 Although there are descriptions of successful closed reductions after RSA dislocation in the literature, Paynter et al85 propose the use of open reduction, which has been reported to be more successful (85%) compared to closed reduction (60%).86 Treatment of glenosphere dissociation consists of isolated glenosphere exchange with the same or smaller size or with an exchange of the glenosphere and modular humeral socket.31 Cusick et al84 analysed glenosphere dissociation and reported that taper changes were limited to fretting wear with minimal proof of taper corrosion. They assigned glenosphere dissociation to improper taper engagement during trauma and surgery. Stephens et al reported that humeral component dissociations in the non-Grammont designs happened between the metaphyseal shell and humeral stem and were treated with an exchange to a new humeral socket. Dissociations of polyethylene sockets which were implanted before 2005 were exchanged to the metal modular socket. They hypothesized that the decline of humeral dissociation after socket prosthesis modification was due to increased ability of the metal metaphyseal shell to withstand stress. Of humeral dissociations, 80% happened in patients with some degree of preoperative proximal humeral bone loss.31 Cuff et al found significantly more micromotion in the humeral stem when proximal humeral support was lacking, and an increased risk of mechanical failure for modular components. Consequently, they recommend implantation of monobloc humeral stem in cases lacking proximal humeral support.87
Fig. 6.
Six weeks postoperative anteroposterior, Neer and axial imaging revealing a disassembly of the glenosphere on the baseplate.
Source: From wiki.beemed.com, with permission.
Periprosthetic fractures
Periprosthetic humeral fractures are relatively rare and there is limited information in the literature regarding such injuries (Fig. 7 and Fig. 8). In their systematic review, Shah et al41 noted intraoperative humerus fracture in 1.8% of cases, intraoperative glenoid fracture in 0.3%, postoperative humerus fracture in 1.2% and postoperative glenoid fracture in 0.1%. Most of the humerus and glenoid intraoperative fractures were treated without any additional interventions. Intraoperative glenoid fractures (0.1%) and intraoperative humerus fractures (0%) rates using modern non-Grammont designs have significantly decreased compared to Zumstein et al,19 where the intraoperative glenoid fracture rate was 1.3% and intraoperative humerus fracture rate was 3.0%. Multiple factors affect the incidence of periprosthetic fractures. Risk factors for periprosthetic fracture can be divided into patient-related and implant-related. The main patient-related risk factors are advanced age, higher chance of falling to the ground, osteoporosis, cardiac and neurology pathologies that increase the risk of instability during walking and/or of falling, female sex, chronic intake of osteopenia-inducing drugs such as corticosteroids, any medical condition affecting bone quality, diabetes and rheumatoid arthritis.88,89 Implant-related risk factors are revision RSA cases, prior hemiarthroplasty, over-reaming or using an oversized broach in humeral component preparation, excessive soft tissue tightness arising due to errors in bone cuts or component size and humeral deformity.89,90 Diagnosis of periprosthetic fractures is straightforward regarding the clinical presentation and its association to trauma. Radiographic evaluation is important in identifying potential prosthesis loosening, whereas a computed tomography (CT) scan is useful for diagnosis in cases of doubt and as a preoperative tool.88 Glenoid fractures during surgery are scarce, and usually associated with the reaming or fixation procedure. Proposals on lowering the rate of intraoperative glenoid fractures consist of starting power reaming before placing the reamer in contact with the glenoid face and avoiding over-reaming. Over-reaming might happen in patients with proximal humeral fractures because of less sclerotic bone due to the absence of glenoid arthrosis. Thus, special care needs to be taken when reaming the glenoid in such cases.91 Significant glenoid fractures might make glenoid component fixation impossible and result in intraoperative conversion to hemiarthroplasty.92 Frequently, glenoid fractures can be treated by fixation or redirection of the baseplate. However, when glenoid fractures are substantial, implementation of a two-stage bone grafting and reimplantation process might be needed. Intraoperative humeral fracture can occur during exposure in patients with either advanced osteopenia or significant fibrosis, which is typical for revision cases.89 Even though the majority of early RSA were at first designed for cemented fixation of the humeral component, cementless fixation has become very frequent. Avoidance of excessive uncontrolled reaming for cementless fixation is important as it may led to a stress riser at the end of the reaming zone and consequently increase the risk of periprosthetic fractures.93 Intraoperative humeral diaphyseal fractures may occur in case of an incorrect sizing of the component or excessive external rotation during preparation of the glenoid and soft tissue release. They usually require the use of a longer implant to bypass the fracture line or an open reduction and internal fixation. Early on, implantation of the short-stem prosthesis94 and stemless implants95 led to many intraoperative fractures. The technical complexity of the stemless implants resulted in high predisposition to intraoperative and postoperative fractures, particularly fracture of the humeral metaphysis because of excessive bone impaction in softened bone.95 An increased risk of postoperative humeral fractures has been noted in patients who received treatment with RSA in combination with allograft-prosthetic composite96 and cement-within-cement fixation of the humeral component in revision RSA.89 Postoperative humeral fractures, which can lead to a poor outcome, are most frequently associated with traumatic aetiology and occur more commonly in elderly patients, females, and when a trans-deltoid approach is used.97 Treatment decision making depends on the general health status and functional needs of the patient, location of the fracture and morphology, bone quality, and prosthesis stability. Surgical experience of the treating surgeon should also be considered.98 Treatment options for periprosthetic shoulder fractures span from conservative treatment to open reduction and internal fixation and revision arthroplasty. Osteosynthesis may be performed using various approaches: deltopectoral, posterior or lateral. Fixation can be performed with plates and screws, plates and cerclages, and plates and screws associated with cerclages. Postoperatively, fractures can be treated either conservatively, if the component is stable, or with revision in cases of unstable components.99–101 Lateral humeral split has been proposed as the least invasive means of removing the humeral implant in order to avoid intraoperative humeral fracture during revision. Periprosthetic humeral fractures healed at a mean time of 18 weeks (range, 16 to 20) with a nonunion rate of about 13%. The overall complication rate of surgically treated periprosthetic humeral fractures is reported to be between 20% and 40%. Nonunion or malunion are particularly found with non-surgical treatment, which may be successful in special cases.102,103
Fig. 7.
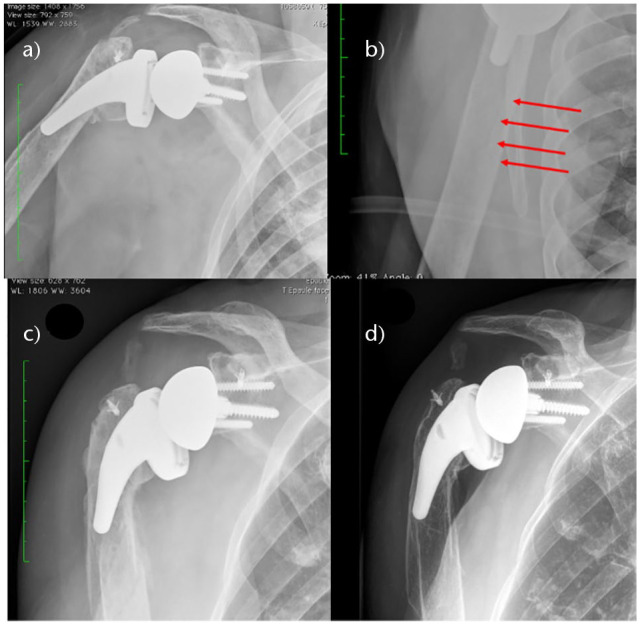
(A) Immediate anteroposterior postoperative X-ray of a right reverse shoulder arthroplasty (RSA). (B) Enlargement on Neer view revealed a subtle fracture under the stem of the prosthesis. (C) After a minor trauma one month postoperatively, X-rays demonstrated a slightly displaced fracture that has been treated conservatively. (D) One year postoperatively the fracture healed perfectly.
Source: From wiki.beemed.com, with permission.
Fig. 8.
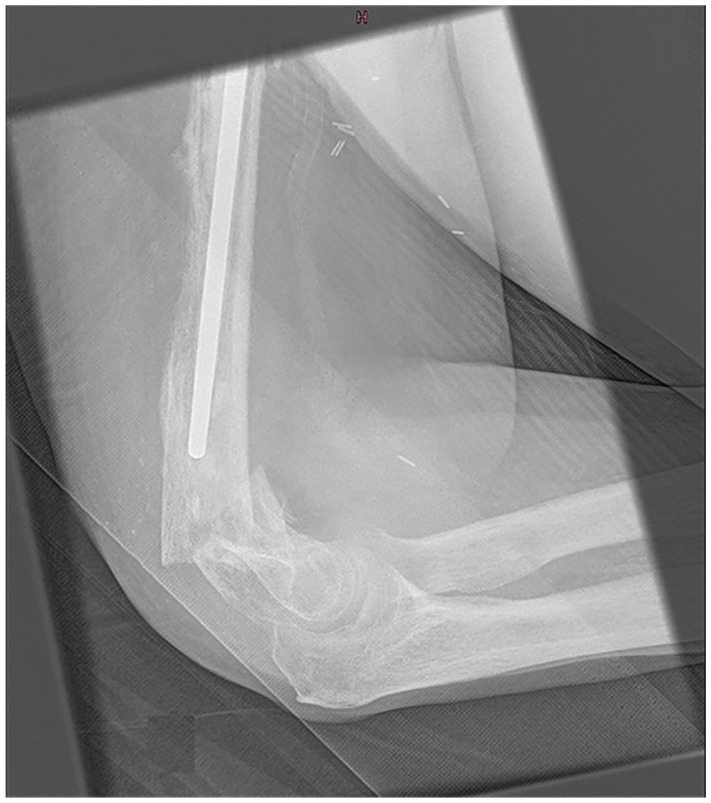
Patient known for a right reverse shoulder arthroplasty (RSA) who sustained a fall on the ipsilateral elbow. A transverse supracondylar fracture of the distal humerus is noted on lateral view.
Source: From wiki.beemed.com, with permission.
Difference in complication rates and types depending on RSA design
The comparison between the Grammont design and non-Grammont design shows a considerable decrease in several complications: scapular notching (42.5% vs. 12.3%) instability (4.0% vs. 1,3%), intraoperative humerus fracture (2.0% vs. 0.0%) and intraoperative glenoid fracture (0.9% vs. 0.1 %) with the exception of scapular or acromial fractures which increased (1.5% vs. 2.5%).19,41 The impact of specific RSA designs on different complications is described in Table 2.
Table 2.
Difference in complication rates depending on specific RSA design
| Implant design type | Effect on the complication rate | |
|---|---|---|
| Glenoid | Lateral offset | Lateralization of glenosphere with metal or bone augment decreases scapular notching,50 increases ROM,17 increases soft tissue tensioning and acromial stress.72
Eccentric glenosphere might decrease scapular notching,54 or at least decrease its severity.55 Glenoid lateralization may be a risk factor for acromial or scapular spine fractures.66,72 |
| Inferior overhang | Inferior overhang of more than > 3.5 mm decreases/minimizes scapular notching56,57 and improves range of motion.17 | |
| Inferior tilt | Placing the glenoid baseplate in 10 degrees of inferior inclination in order to avoid superior inclination might increase shear forces on the glenosphere,110 decrease the likelihood of instability83 scapular notching50,111 and loosening.43,112 | |
| Large diameter | Larger glenospheres have greater incidence of dissociation,31,84 decrease scapular notching51,52 and improve range of motion especially in external rotation and adduction.17 | |
| Humerus | Varus neck-shaft angle | The use of 135 degree neck-shaft angle decreases scapular notching50 compared to 155 degrees. Grammont-style RSA. No difference in dislocation rates between a 135 and 155 degree humeral inclination.78 Use of the 135 or 145 degree humeral neck inclination could contribute to increase in ROM.17 |
| Grammont-style 155 degree neck-shaft angle | Subscapularis integrity proves to be most effective.76,77 | |
| Retrotorsion | Excessive retrotorsion leads to decreased internal and increased external rotation.60
The best compromise between anterior and posterior notching to favour a functional arc of motion seems to be 20 to 40 degrees of humeral retrotorsion.61 |
|
| Short stem | Risk factor for intraoperative fractures.94 | |
| Stemless | Risk factor for intraoperative and postoperative fractures.95 | |
| Onlay stem vs. inlay stem | Onlay stem increases distalization, which leads to increased risk of scapular spine fracture compared to inlay stem.33,73,74 | |
| Different combinations | Medial glenoid/medial humerus | Increases risk of scapular notching.41 |
| Medial glenoid/lateral humerus | Decreases risk of scapular notching and instability significantly more than combination of medial glenoid/medial humerus or lateral glenoid/medial humerus.41 | |
| Lateral glenoid/medial humerus | Decreases risk for scapular notching and instability compared to combination of medial glenoid/medial humerus.41 | |
Note. RSA, reverse shoulder arthroplasty; ROM, range of motion; M, medialized; L lateralized.
Conclusion
Our review of the recent literature on the topic of RSA shows that there has been a considerable decrease in the majority of complications over the years, probably as a result of modifications in the design, materials, biomechanics of the prosthesis, recommendations related to positioning and the experience in RSA implantation acquired by the surgeons. The complications for which we currently still do not have an answer are acromial or scapular spine fractures and loss of internal rotation. With further changes in indications and designs for RSA, it is crucial to accurately track the rates and types of complications to justify new designs and increased indications.
Footnotes
ICMJE Conflict of interest statement: AL reports a grant from FORE 2021–36 and consulting fees from Stryker, Medacta and Arthrex, pertinent to this publication. PJD reports support for travel to meetings for the study or other purposes from Oregon Shoulder Institute, and monies for relevant financial activities outside the submitted work for consultancy, grants, lectures and royalties from Arthrex Inc. PC reports that he is a paid consultant for Wright and Arthrex and receives royalties from Wright. All other authors declare no conflicts of interest relevant to this work.
OA licence text: This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Funding statement
The author or one or more of the authors have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Rasmussen JV, Amundsen A, Sørensen AKB, et al. Increased use of total shoulder arthroplasty for osteoarthritis and improved patient-reported outcome in Denmark, 2006–2015: a nationwide cohort study from the Danish Shoulder Arthroplasty Registry. Acta Orthop 2019;90:489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grammont PM, Trouilloud P, Latfay J, Deries X. Etude et réalisation d’une nouvelle prothèse d’épaule. Rhumatologie 1987;39:407–418. [Google Scholar]
- 3. Baulot E, Sirveaux F, Boileau P. Grammont’s idea: the story of Paul Grammont’s functional surgery concept and the development of the reverse principle. Clin Orthop Relat Res 2011;469:2425–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg 2013;22:1199–1208. [DOI] [PubMed] [Google Scholar]
- 5. Collin P, Hervé A, Walch G, Boileau P, Muniandy M, Chelli M. Mid-term results of reverse shoulder arthroplasty for glenohumeral osteoarthritis with posterior glenoid deficiency and humeral subluxation. J Shoulder Elbow Surg 2019;28:2023–2030. [DOI] [PubMed] [Google Scholar]
- 6. Gauci MO, Cavalier M, Gonzalez JF, et al. Revision of failed shoulder arthroplasty: epidemiology, etiology, and surgical options. J Shoulder Elbow Surg 2020;29:541–549. [DOI] [PubMed] [Google Scholar]
- 7. Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg 2010;19:1076–1084. [DOI] [PubMed] [Google Scholar]
- 8. Cuff DJ, Virani NA, Levy J, et al. The treatment of deep shoulder infection and glenohumeral instability with debridement, reverse shoulder arthroplasty and postoperative antibiotics. J Bone Joint Surg [Br] 2008;90-B:336–342. [DOI] [PubMed] [Google Scholar]
- 9. De Wilde L, Boileau P, Van Der Bracht H, eds. Does reverse shoulder arthroplasty for tumors of the proximal humerus reduce impairment? New York, NY: Springer, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raiss P, Edwards TB, Bruckner T, Loew M, Zeifang F, Walch G. Reverse arthroplasty for patients with chronic locked dislocation of the shoulder (type 2 fracture sequela). J Shoulder Elbow Surg 2017;26:279–287. [DOI] [PubMed] [Google Scholar]
- 11. Grubhofer F, Wieser K, Meyer DC, Catanzaro S, Schürholz K, Gerber C. Reverse total shoulder arthroplasty for failed open reduction and internal fixation of fractures of the proximal humerus. J Shoulder Elbow Surg 2017;26:92–100. [DOI] [PubMed] [Google Scholar]
- 12. Lädermann A, Chiu J, Collin P, Piotton S, Nover L, Scheibel M. Hemi- vs reverse shoulder arthroplasty for acute proximal humeral fractures: a systematic review of level I and II studies. Obere Extrem 2019;14:127–135. [Google Scholar]
- 13. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg 2011;19:439–449. [PubMed] [Google Scholar]
- 14. Wright MA, Murthi AM. Offset in reverse shoulder arthroplasty: where, when, and how much. J Am Acad Orthop Surg 2021;29:89–99. [DOI] [PubMed] [Google Scholar]
- 15. Lädermann A, Denard PJ, Boileau P, et al. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int Orthop 2015;39:2205–2213. [DOI] [PubMed] [Google Scholar]
- 16. Lädermann A, Denard PJ, Boileau P, Farron A, Deransart P, Walch G. What is the best glenoid configuration in onlay reverse shoulder arthroplasty? Int Orthop 2018;42:1339–1346. [DOI] [PubMed] [Google Scholar]
- 17. Lädermann A, Denard PJ, Collin P, et al. Effect of humeral stem and glenosphere designs on range of motion and muscle length in reverse shoulder arthroplasty. Int Orthop 2020;44:519–530. [DOI] [PubMed] [Google Scholar]
- 18. Padegimas EM, Maltenfort M, Lazarus MD, Ramsey ML, Williams GR, Namdari S. Future patient demand for shoulder arthroplasty by younger patients: national projections. Clin Orthop Relat Res 2015;473:1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2011;20:146–157. [DOI] [PubMed] [Google Scholar]
- 20. Lädermann A, Denard PJ, Tirefort J, Collin P, Nowak A, Schwitzguebel AJ. Subscapularis- and deltoid-sparing vs traditional deltopectoral approach in reverse shoulder arthroplasty: a prospective case-control study. J Orthop Surg Res 2017;12:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg 2012;21:1470–1477. [DOI] [PubMed] [Google Scholar]
- 22. Tashjian RZ, Granger E, Broschinsky K, Kawakami J, Chalmers PN. Effect of complications on outcomes after revision reverse total shoulder arthroplasty. JSES Int 2020;4:662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2014;23:1647–1654. [DOI] [PubMed] [Google Scholar]
- 24. Kempton LB, Ankerson E, Wiater JM. A complication-based learning curve from 200 reverse shoulder arthroplasties. Clin Orthop Relat Res 2011;469:2496–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg 2015;24:460–467. [DOI] [PubMed] [Google Scholar]
- 26. Riedel BB, Mildren ME, Jobe CM, Wongworawat MD, Phipatanakul WP. Evaluation of the learning curve for reverse shoulder arthroplasty. Orthopedics 2010;33:01477447-20100225-09. [DOI] [PubMed] [Google Scholar]
- 27. Anakwenze O, Fokin A, Chocas M, et al. Complications in total shoulder and reverse total shoulder arthroplasty by body mass index. J Shoulder Elbow Surg 2017;26:1230–1237. [DOI] [PubMed] [Google Scholar]
- 28. Mahure S, Mollon B, Quien M, Karia R, Zuckerman J, Kwon Y. Impact of diabetes on perioperative complications in patients undergoing elective total shoulder arthroplasty. Bull Hosp Jt Dis 2017;75:173–179. [PubMed] [Google Scholar]
- 29. Burrus MT, Werner BC, Cancienne JM, Gwathmey FW, Brockmeier SF. Shoulder arthroplasty in patients with Parkinson’s disease is associated with increased complications. J Shoulder Elbow Surg 2015;24:1881–1887. [DOI] [PubMed] [Google Scholar]
- 30. Johnson CC, Sodha S, Garzon-Muvdi J, Petersen SA, McFarland EG. Does preoperative American Society of Anesthesiologists score relate to complications after total shoulder arthroplasty? Clin Orthop Relat Res 2014;472:1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stephens BC, Simon P, Clark RE, et al. Revision for a failed reverse: a 12-year review of a lateralized implant. J Shoulder Elbow Surg 2016;25:e115–e124. [DOI] [PubMed] [Google Scholar]
- 32. Gorman RA, II, Christmas KN, Simon P, Mighell MA, Frankle MA. A cohort comparison of humeral implant designs in reverse shoulder arthroplasty: does implant design lead to lower rates of complications and revision? J Shoulder Elbow Surg 2021;30:850–857. [DOI] [PubMed] [Google Scholar]
- 33. Haidamous G, Lädermann A, Frankle MA, Gorman RA, II, Denard PJ. The risk of postoperative scapular spine fracture following reverse shoulder arthroplasty is increased with an onlay humeral stem. J Shoulder Elbow Surg 2020;29:2556–2563. [DOI] [PubMed] [Google Scholar]
- 34. Lädermann A, Chiu JC, Cunningham G, et al. Do short stems influence the cervico-diaphyseal angle and the medullary filling after reverse shoulder arthroplasties? Orthop Traumatol Surg Res 2020;106:241–246. [DOI] [PubMed] [Google Scholar]
- 35. Tross AK, Lädermann A, Wittmann T, et al. Subsidence of uncemented short stems in reverse shoulder arthroplasty: a multicenter study. J Clin Med 2020;9:3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bacle G, Nové-Josserand L, Garaud P, Walch G. Long-term outcomes of reverse total shoulder arthroplasty: a follow-up of a previous study. J Bone Joint Surg [Am] 2017;99-A:454–461. [DOI] [PubMed] [Google Scholar]
- 37. Sirveaux F, Favard L, Oudet D, Huguet D, Lautman S. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive and non repairable cuff rupture. In: Walch G, Boileau P, Molé D, eds. 2000 shoulder prostheses: two to ten year follow-up. Montpellier: Sauramps Medical, 2001:247–252. [Google Scholar]
- 38. Lädermann A, Gueorguiev B, Charbonnier C, et al. Scapular notching on kinematic simulated range of motion after reverse shoulder arthroplasty is not the result of impingement in adduction. Medicine (Baltimore) 2015;94:e1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mélis B, DeFranco M, Lädermann A, et al. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg [Br] 2011;93-B:1240–1246. [DOI] [PubMed] [Google Scholar]
- 40. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg [Am] 2005;87-A:1476–1486. [DOI] [PubMed] [Google Scholar]
- 41. Shah SS, Gaal BT, Roche AM, et al. The modern reverse shoulder arthroplasty and an updated systematic review for each complication: part I. JSES Int 2020;4:929–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg 2005;14:524–528. [DOI] [PubMed] [Google Scholar]
- 43. Lädermann A, Schwitzguebel AJ, Edwards TB, et al. Glenoid loosening and migration in reverse shoulder arthroplasty. J Bone Joint Surg [Br] 2019;101-B:461–469. [DOI] [PubMed] [Google Scholar]
- 44. Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved delta III total shoulder prosthesis. J Bone Joint Surg [Br] 2004;86-B:1187–1191. [DOI] [PubMed] [Google Scholar]
- 45. Roche CP, Stroud NJ, Martin BL, et al. The impact of scapular notching on reverse shoulder glenoid fixation. J Shoulder Elbow Surg 2013;22:963–970. [DOI] [PubMed] [Google Scholar]
- 46. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res 2011;469:2512–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mollon B, Mahure SA, Roche CP, Zuckerman JD. Impact of scapular notching on clinical outcomes after reverse total shoulder arthroplasty: an analysis of 476 shoulders. J Shoulder Elbow Surg 2017;26:1253–1261. [DOI] [PubMed] [Google Scholar]
- 48. Paisley KC, Kraeutler MJ, Lazarus MD, Ramsey ML, Williams GR, Smith MJ. Relationship of scapular neck length to scapular notching after reverse total shoulder arthroplasty by use of plain radiographs. J Shoulder Elbow Surg 2014;23:882–887. [DOI] [PubMed] [Google Scholar]
- 49. Aibinder WR, Schoch BS, Cofield RH, Sperling JW, Sánchez-Sotelo J. Reverse shoulder arthroplasty in patients with os acromiale. J Shoulder Elbow Surg 2017;26:1598–1602. [DOI] [PubMed] [Google Scholar]
- 50. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2008;17:925–935. [DOI] [PubMed] [Google Scholar]
- 51. Torrens C, Guirro P, Miquel J, Santana F. Influence of glenosphere size on the development of scapular notching: a prospective randomized study. J Shoulder Elbow Surg 2016;25:1735–1741. [DOI] [PubMed] [Google Scholar]
- 52. Verhofste B, Decock T, Van Tongel A, De Wilde L. Heterotopic ossification after reverse total shoulder arthroplasty. J Bone Joint Surg [Br] 2016;98-B:1215–1221. [DOI] [PubMed] [Google Scholar]
- 53. Gutiérrez S, Comiskey CA, IV, Luo ZP, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty: hierarchy of surgical and implant-design-related factors. J Bone Joint Surg [Am] 2008;90-A:2606–2615. [DOI] [PubMed] [Google Scholar]
- 54. Li X, Dines JS, Warren RF, Craig EV, Dines DM. Inferior glenosphere placement reduces scapular notching in reverse total shoulder arthroplasty. Orthopedics 2015;38:e88–e93. [DOI] [PubMed] [Google Scholar]
- 55. Mizuno N, Denard PJ, Raiss P, Walch G. The clinical and radiographical results of reverse total shoulder arthroplasty with eccentric glenosphere. Int Orthop 2012;36:1647–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Wilde LF, Poncet D, Middernacht B, Ekelund A. Prosthetic overhang is the most effective way to prevent scapular conflict in a reverse total shoulder prosthesis. Acta Orthop 2010;81:719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Poon PC, Chou J, Young SW, Astley T. A comparison of concentric and eccentric glenospheres in reverse shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am 2014;96:e138. [DOI] [PubMed] [Google Scholar]
- 58. Cunningham G, Lädermann A. Redefining anterior shoulder impingement: a literature review. Int Orthop 2018;42:359–366. [DOI] [PubMed] [Google Scholar]
- 59. De Wilde L, Walch G. Humeral prosthetic failure of reversed total shoulder arthroplasty: a report of three cases. J Shoulder Elbow Surg 2006;15:260–264. [DOI] [PubMed] [Google Scholar]
- 60. Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics 1993;16:65–68. [DOI] [PubMed] [Google Scholar]
- 61. Stephenson DR, Oh JH, McGarry MH, Rick Hatch GF, III, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2011;20:652–658. [DOI] [PubMed] [Google Scholar]
- 62. Favre P, Sussmann PS, Gerber C. The effect of component positioning on intrinsic stability of the reverse shoulder arthroplasty. J Shoulder Elbow Surg 2010;19:550–556. [DOI] [PubMed] [Google Scholar]
- 63. Keener JD, Patterson BM, Orvets N, Aleem AW, Chamberlain AM. Optimizing reverse shoulder arthroplasty component position in the setting of advanced arthritis with posterior glenoid erosion: a computer-enhanced range of motion analysis. J Shoulder Elbow Surg 2018;27:339–349. [DOI] [PubMed] [Google Scholar]
- 64. Lau SC, Large R. Acromial fracture after reverse total shoulder arthroplasty: a systematic review. Shoulder Elbow 2020;12:375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Otto RJ, Virani NA, Levy JC, Nigro PT, Cuff DJ, Frankle MA. Scapular fractures after reverse shoulder arthroplasty: evaluation of risk factors and the reliability of a proposed classification. J Shoulder Elbow Surg 2013;22:1514–1521. [DOI] [PubMed] [Google Scholar]
- 66. King JJ, Dalton SS, Gulotta LV, Wright TW, Schoch BS. How common are acromial and scapular spine fractures after reverse shoulder arthroplasty? A systematic review. J Bone Joint Surg [Br] 2019;101-B:627–634. [DOI] [PubMed] [Google Scholar]
- 67. Crosby LA, Hamilton A, Twiss T. Scapula fractures after reverse total shoulder arthroplasty: classification and treatment. Clin Orthop Relat Res 2011;469:2544–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dubrow S, Streit JJ, Muh S, Shishani Y, Gobezie R. Acromial stress fractures: correlation with acromioclavicular osteoarthritis and acromiohumeral distance. Orthopedics 2014;37:e1074–e1079. [DOI] [PubMed] [Google Scholar]
- 69. Walch G, Mottier F, Wall B, Boileau P, Molé D, Favard L. Acromial insufficiency in reverse shoulder arthroplasties. J Shoulder Elbow Surg 2009;18:495–502. [DOI] [PubMed] [Google Scholar]
- 70. Kennon JC, Lu C, McGee-Lawrence ME, Crosby LA. Scapula fracture incidence in reverse total shoulder arthroplasty using screws above or below metaglene central cage: clinical and biomechanical outcomes. J Shoulder Elbow Surg 2017;26:1023–1030. [DOI] [PubMed] [Google Scholar]
- 71. DiStefano JG, Park AY, Nguyen TQ, Diederichs G, Buckley JM, Montgomery WH, III. Optimal screw placement for base plate fixation in reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2011;20:467–476. [DOI] [PubMed] [Google Scholar]
- 72. Wong MT, Langohr GDG, Athwal GS, Johnson JA. Implant positioning in reverse shoulder arthroplasty has an impact on acromial stresses. J Shoulder Elbow Surg 2016;25:1889–1895. [DOI] [PubMed] [Google Scholar]
- 73. LeDuc R, Salazar D, Garbis N. Incidence of post-operative acromial fractures with onlay vs inlay reverse shoulder arthroplasty. J Shoulder Elbow Surg 2019;28:e206. [Google Scholar]
- 74. Merolla G, Walch G, Ascione F, et al. Grammont humeral design versus onlay curved-stem reverse shoulder arthroplasty: comparison of clinical and radiographic outcomes with minimum 2-year follow-up. J Shoulder Elbow Surg 2018;27:701–710. [DOI] [PubMed] [Google Scholar]
- 75. Kohan EM, Chalmers PN, Salazar D, Keener JD, Yamaguchi K, Chamberlain AM. Dislocation following reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:1238–1245. [DOI] [PubMed] [Google Scholar]
- 76. Lädermann A, Lübbeke A, Mélis B, et al. Prevalence of neurologic lesions after total shoulder arthroplasty. J Bone Joint Surg [Am] 2011;93-A:1288–1293. [DOI] [PubMed] [Google Scholar]
- 77. Lowe JT, Lawler SM, Testa EJ, Jawa A. Lateralization of the glenosphere in reverse shoulder arthroplasty decreases arm lengthening and demonstrates comparable risk of nerve injury compared with anatomic arthroplasty: a prospective cohort study. J Shoulder Elbow Surg 2018;27:1845–1851. [DOI] [PubMed] [Google Scholar]
- 78. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2015;24:988–993. [DOI] [PubMed] [Google Scholar]
- 79. Teusink MJ, Pappou IP, Schwartz DG, Cottrell BJ, Frankle MA. Results of closed management of acute dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:621–627. [DOI] [PubMed] [Google Scholar]
- 80. Raiss P, Edwards TB, da Silva MR, Bruckner T, Loew M, Walch G. Reverse shoulder arthroplasty for the treatment of nonunions of the surgical neck of the proximal part of the humerus (type-3 fracture sequelae). J Bone Joint Surg [Am] 2014;96-A:2070–2076. [DOI] [PubMed] [Google Scholar]
- 81. Lädermann A, Williams MD, Mélis B, Hoffmeyer P, Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009;18:588–595. [DOI] [PubMed] [Google Scholar]
- 82. Cheung EV, Sarkissian EJ, Sox-Harris A, et al. Instability after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2018;27:1946–1952. [DOI] [PubMed] [Google Scholar]
- 83. Tashjian RZ, Martin BI, Ricketts CA, Henninger HB, Granger EK, Chalmers PN. Superior baseplate inclination is associated with instability after reverse total shoulder arthroplasty. Clin Orthop Relat Res 2018;476:1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cusick MC, Hussey MM, Steen BM, et al. Glenosphere dissociation after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:1061–1068. [DOI] [PubMed] [Google Scholar]
- 85. Paynter JW, Griswold BG, DeFoor MT, Crosby LA, Parada SA. Polyethylene liner dissociation after reverse shoulder arthroplasty dislocation: a case series. J Radiol Case Rep 2020;14:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chalmers BP, Wagner ER, Sperling JW, Cofield RH, Sanchez-Sotelo J. Treatment and outcomes of reverse shoulder arthroplasty dislocations. J Shoulder Elbow Arthroplasty 2017;1:2471549217695260. [Google Scholar]
- 87. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg 2011;20:646–651. [DOI] [PubMed] [Google Scholar]
- 88. Canton G, Fazzari F, Fattori R, Ratti C, Murena L. Post-operative periprosthetic humeral fractures after reverse shoulder arthroplasty: a review of the literature. Acta Biomed 2019;90:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wagner ER, Houdek MT, Elhassan BT, Sanchez-Sotelo J, Cofield RH, Sperling JW. What are risk factors for intraoperative humerus fractures during revision reverse shoulder arthroplasty and do they influence outcomes? Clin Orthop Relat Res 2015;473:3228–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Athwal GS, Sperling JW, Rispoli DM, Cofield RH. Periprosthetic humeral fractures during shoulder arthroplasty. J Bone Joint Surg [Am] 2009;91-A:594–603. [DOI] [PubMed] [Google Scholar]
- 91. Russo R, Della Rotonda G, Cautiero F, Ciccarelli M. Reverse shoulder prosthesis to treat complex proximal humeral fractures in the elderly patients: results after 10-year experience. Musculoskelet Surg 2015;99:S17–S23. [DOI] [PubMed] [Google Scholar]
- 92. Barco R, Savvidou OD, Sperling JW, Sanchez-Sotelo J, Cofield RH. Complications in reverse shoulder arthroplasty. EFORT Open Rev 2017;1:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lee M, Chebli C, Mounce D, Bertelsen A, Richardson M, Matsen F, III. Intramedullary reaming for press-fit fixation of a humeral component removes cortical bone asymmetrically. J Shoulder Elbow Surg 2008;17:150–155. [DOI] [PubMed] [Google Scholar]
- 94. Atoun E, Van Tongel A, Hous N, et al. Reverse shoulder arthroplasty with a short metaphyseal humeral stem. Int Orthop 2014;38:1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Levy O, Narvani A, Hous N, et al. Reverse shoulder arthroplasty with a cementless short metaphyseal humeral implant without a stem: clinical and radiologic outcomes in prospective 2- to 7-year follow-up study. J Shoulder Elbow Surg 2016;25:1362–1370. [DOI] [PubMed] [Google Scholar]
- 96. Cox JL, McLendon PB, Christmas KN, Simon P, Mighell MA, Frankle MA. Clinical outcomes following reverse shoulder arthroplasty-allograft composite for revision of failed arthroplasty associated with proximal humeral bone deficiency: 2- to 15-year follow-up. J Shoulder Elbow Surg 2019;28:900–907. [DOI] [PubMed] [Google Scholar]
- 97. Ascione F, Domos P, Guarrella V, Chelli M, Boileau P, Walch G. Long-term humeral complications after Grammont-style reverse shoulder arthroplasty. J Shoulder Elbow Surg 2018;27:1065–1071. [DOI] [PubMed] [Google Scholar]
- 98. Cho CH, Song KS, Koo TW. Clinical outcomes and complications during the learning curve for reverse total shoulder arthroplasty: an analysis of the first 40 cases. Clin Orthop Surg 2017;9:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. King JJ, Farmer KW, Struk AM, Wright TW. Uncemented versus cemented humeral stem fixation in reverse shoulder arthroplasty. Int Orthop 2015;39:291–298. [DOI] [PubMed] [Google Scholar]
- 100. Sanchez-Sotelo J, Wright TW, O’Driscoll SW, Cofield RH, Rowland CM. Radiographic assessment of uncemented humeral components in total shoulder arthroplasty. J Arthroplasty 2001;16:180–187. [DOI] [PubMed] [Google Scholar]
- 101. Thés A, Klouche S, de Tienda M, Bauer T, Hardy P. Cortical onlay strut allograft with cerclage wiring of periprosthetic fractures of the humerus without stem loosening: technique and preliminary results. Eur J Orthop Surg Traumatol 2017;27:553–557. [DOI] [PubMed] [Google Scholar]
- 102. Ragusa PS, Vadhera A, Jang JM, Ali I, McFarland EG, Srikumaran U. Nonoperative treatment of periprosthetic humeral shaft fractures after reverse total shoulder arthroplasty. Orthopedics 2020;43:e553–e560. [DOI] [PubMed] [Google Scholar]
- 103. Wierks C, Skolasky RL, Ji JH, McFarland EG. Reverse total shoulder replacement: intraoperative and early postoperative complications. Clin Orthop Relat Res 2009;467:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Padegimas EM, Zmistowski BM, Restrepo C, et al. Instability after reverse total shoulder arthroplasty: which patients dislocate? Am J Orthop 2016;45:E444–E450. [PubMed] [Google Scholar]
- 105. Ohl X, Bonnevialle N, Gallinet D, et al. ; SOFCOT. How the greater tuberosity affects clinical outcomes after reverse shoulder arthroplasty for proximal humeral fractures. J Shoulder Elbow Surg 2018;27:2139–2144. [DOI] [PubMed] [Google Scholar]
- 106. Lädermann A, Walch G, Denard PJ, et al. Reverse shoulder arthroplasty in patients with pre-operative impairment of the deltoid muscle. J Bone Joint Surg [Br] 2013;95-B:1106–1113. [DOI] [PubMed] [Google Scholar]
- 107. Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009;18:892–896. [DOI] [PubMed] [Google Scholar]
- 108. Gallo RA, Gamradt SC, Mattern CJ, et al. ; Sports Medicine and Shoulder Service at the Hospital for Special Surgery, New York, NY. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg 2011;20:584–590. [DOI] [PubMed] [Google Scholar]
- 109. Johnson JE, Caceres AP, Anderson DD, Patterson BM. Postimpingement instability following reverse shoulder arthroplasty: a parametric finite element analysis. Seminars in Arthroplasty: JSES 2020;31. [Google Scholar]
- 110. Gutiérrez S, Walker M, Willis M, Pupello DR, Frankle MA. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg 2011;20:732–739. [DOI] [PubMed] [Google Scholar]
- 111. Laver L, Garrigues GE. Avoiding superior tilt in reverse shoulder arthroplasty: a review of the literature and technical recommendations. J Shoulder Elbow Surg 2014;23:1582–1590. [DOI] [PubMed] [Google Scholar]
- 112. Lignel A, Berhouet J, Loirat MA, et al. Reverse shoulder arthroplasty for proximal humerus fractures: is the glenoid implant problematic? Orthop Traumatol Surg Res 2018;104:773–777. [DOI] [PubMed] [Google Scholar]