Abstract
This systematic review and meta-analysis aimed to analyse negative effects of smoking in orthopaedic and trauma patients.
A PubMed search was carried out for studies published until July 2020 regarding effects of smoking on fracture risk, nonunion, infection after orthopaedic surgery, and persisting nonunion after scaphoid nonunion surgery. Random effects models calculated for outcome parameters, and relative risks (RR) with 95% confidence intervals are provided. No adjustments for covariates were made. Heterogeneity was assessed with Higgins’ I2, publication bias with Harbord’s p (Hp), sensitivity analysis performed on funnel plots and quality of studies was analysed using the Newcastle-Ottawa Scale.
Of 3362 retrieved entries, 69 were included in the final analysis. Unadjusted RR for smokers to develop vertebral (six studies, seven entries; RR: 1.61; p = 0.008; I2 = 89.4%), hip (11 studies, 15 entries; RR: 1.28; p = 0.007; I2 = 84.1%), and other fractures (eight studies, 10 entries; RR: 1.75; p = 0.019; I2 = 89.3%) was significantly higher. Postoperative infection risk was generally higher for smokers (21 studies; RR: 2.20; p < 0.001; I2 = 58.9%), and remained upon subgroup analysis for elective spinal (two studies; RR: 4.38; p < 0.001; I2 = 0.0%) and fracture surgery (19 studies; RR: 2.10; p < 0.001; I2 = 58.5%). Nonunion risk after orthopaedic (eight studies; RR: 2.15; p < 0.001; I2 = 35.9%) and fracture surgery (11 studies; RR: 1.85; p < 0.001; I2 = 39.9%) was significantly higher for smokers, as was persisting nonunion risk after surgery for scaphoid nonunion (five studies; RR: 3.52; p < 0.001; I2 = 0.0%). Sensitivity analysis for each model reduced heterogeneity whilst maintaining significance (all I2 < 20.0%).
Smoking has a deleterious impact on fracture incidence, and (subsequent) development of nonunions and postoperative infections.
Cite this article: EFORT Open Rev 2021;6:1006-1019. DOI: 10.1302/2058-5241.6.210058
Keywords: fracture risk, nonunion risk, smoking
Introduction
Since the 1960s, tobacco smoking has been a well-known risk factor for development of malignancies and cardiovascular disease, and has been associated with increased mortality rates.1–3 According to the World Health Organization (WHO), the prevalence of tobacco smoking in 2018 was 13.0% in Norway, 19.2% in the United Kingdom, 25.1% in the United States, and 29.1% in Austria.4 As further detrimental effects of smoking were recognized, the negative effects of tobacco smoking on the musculoskeletal system, including bone healing, became likewise apparent.5–8 In active smokers, among other factors, bone metabolism is reduced by tissue hypoxia, reduced blood supply, altered activity of osteoblasts and osteoclasts resulting in overall impaired bone turnover, and decreased calcium absorption.8–13 More than 4000 toxins released by cigarettes have been identified that could have a negative effect on bone metabolism.14
Previous meta-analyses have reported on a negative impact of smoking regarding various outcome parameters in orthopaedics and trauma, including incidence of fracture, risk for lateral epicondylitis, fracture nonunion and postoperative complication rate.15–18
The aim of the current systematic review and meta-analysis was to comprehensively analyse the potential negative effect of smoking on fracture risk, nonunion risk after elective orthopaedic procedures and fracture surgery in general, postoperative infection risk after trauma and orthopaedic surgery, and persisting nonunion after surgery for scaphoid nonunion.
Methods
Search strategy and selection criteria
For this systematic review and meta-analysis, the review process was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A literature search in PubMed was performed, using the search terms: (smoking) AND (fracture), (smoking) AND (traumatology), and (smoking) AND (bone healing). Any study published until July 2020 and being available in PubMed was potentially eligible, with no retrospective time limit determined. Full search codes are listed in Supplemental Table 1 (14.8KB, docx) .
All English or German studies dealing with the effect of smoking on bone quality, fracture incidence, fracture healing, fracture treatment outcome and prognosis, as well as incidence, complications and outcome of elective orthopaedic and traumatological procedures were included.
Studies not analysing the effect of smoking on bone quality, elective orthopaedic or traumatological procedures, fracture healing, incidence, outcome of fracture treatment, or prognosis of patients with fractures, bone healing, studies with maxillofacial surgery topics, preclinical studies, reviews, editorials, case reports, insufficient data regarding the effect of smoking on fractures, surveys as well as articles not written in English or German were excluded. Searches and data extraction were performed by one of the co-authors (MAS), and the underlying data subsequently re-evaluated by another author (LL).
Data analysis
The following information was collected from each study finally included: main topic (i.e. orthopaedics, traumatology), outcome measure (e.g. fracture incidence, union rate), type of study (e.g. retrospective cohort study, randomized controlled trial), gender (i.e. male, female, or both), number of patients analysed, number of patients exposed and not exposed to the outcome parameter, and level of evidence as defined by the Oxford Centre for Evidence-Based Medicine (OCEBM).19
Studies were grouped into four categories according to their main outcome parameter, i.e. fracture incidence by localization (subdivided into hip fracture, vertebral fracture, and fracture at other sites), postoperative infection risk, nonunion after fracture surgery or elective orthopaedic procedures in general, and persistent nonunion after scaphoid nonunion. Crude values rather than variable-adjusted results were used in order not to skew the meta-analysis based on different multivariate models demonstrated in individual studies.
Quality of the studies finally included in the meta-analysis was assessed using the Newcastle-Ottawa Scale (NOS) for non-randomized cohort and case-control studies.20 This tool allows assessment of studies based on three items, i.e. selection (maximum 4 points), comparability (maximum 2 points), and exposure (for case-control studies; maximum 3 points) or outcome (for cohort studies; maximum 3 points). By adding the points of each category, a total score ranging from 0 to 9 can be obtained, with higher scores being indicative of studies of higher quality.20 Of note, level III and level II studies were also analysed using the NOS, as randomization had been performed for smoking in one study only.21
Random effects models using the restricted maximum likelihood method were calculated for each outcome parameter of interest. No adjustment for potential confounders such as age or gender were made. Higgins’ I2 was calculated for each model to assess heterogeneity between studies.22 Small, medium and large heterogeneity was defined as I2 being ≤ 25%, ≤ 50%, and ≤ 75%, respectively.22 Presence of publication bias was assessed with Harbord’s test.23 Sensitivity analyses were performed to assess the robustness of findings, based on funnel plots identifying outlier studies. All statistical analyses were performed with Stata Version 16.1 (StataCorp, College Station, TX, USA).
Results
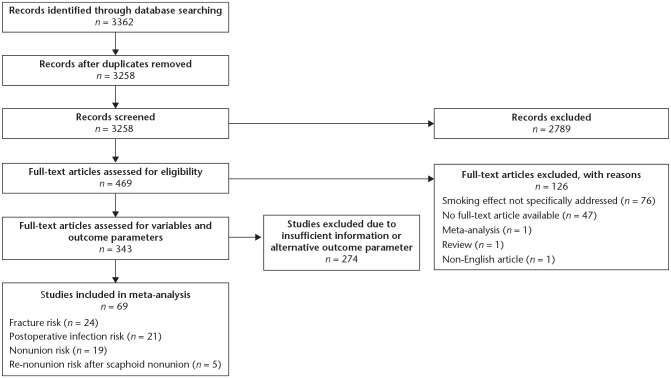
The search retrieved 3362 studies, of which 3258 were screened after exclusion of 104 duplicates. Subsequently, 2789 studies not meeting the inclusion criteria according to the title and abstract were excluded. The full-text articles of the remaining 469 studies were further screened, with 343 studies being thoroughly analysed for outcome parameters and variables of interest. Of these, 274 had to be excluded due to insufficient information or outcome parameters other than the ones defined. Thus, 69 articles could be included in the quantitative analysis (Fig. 1). Mean NOS score over all 69 studies was 6.5 ± 1.3 points (Supplemental Table 2 (23.8KB, docx) ). Fifty articles comprised level IV studies (72.5%), 16 level III studies (23.2%), and three level II studies (4.3%). Twenty-four (34.8%), 21 (30.4%), 19 (27.5%), and five (7.2%) studies reported on fracture risk, postoperative infection risk, nonunion risk, and persisting scaphoid nonunion risk, respectively.
Fig. 1.
Flow chart of studies included.
General fracture risk
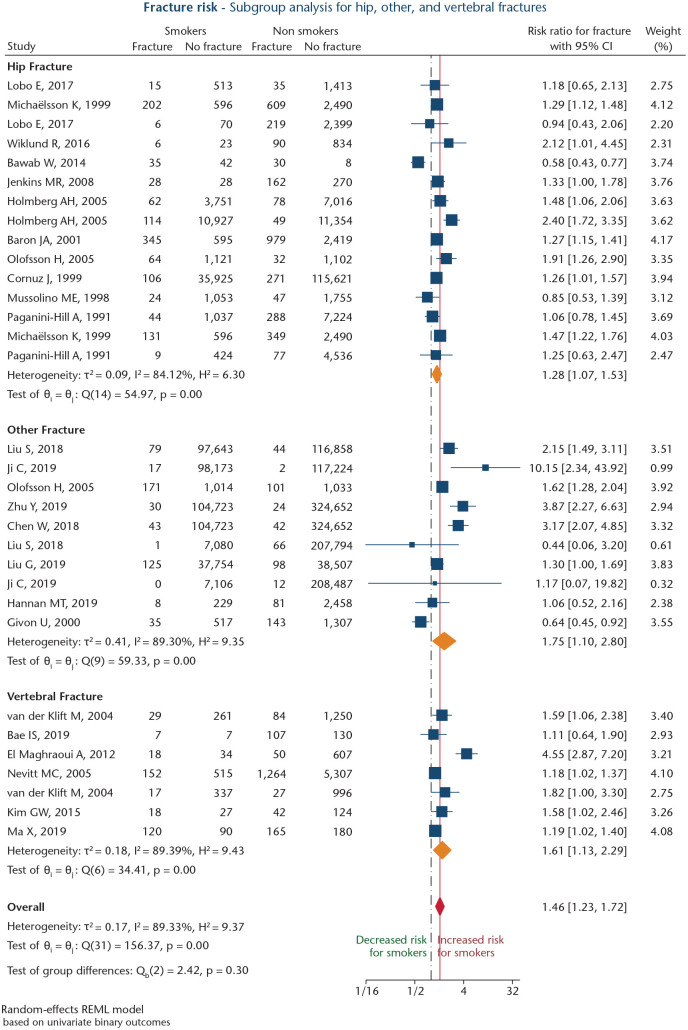
Twenty-four studies were analysed regarding impact of smoking on fracture risk.24–47 Mean NOS score for these studies was 6.9 ± 1.5 points. Six of these studies reported separate results for males and females,26,28,30,31,46,47 one provided individual results for fractures at the trochanteric region and femoral neck,32 and one study presented different results for fractures at any location and hip fractures.33 The resulting meta-analysis thus comprised 32 study entries, involving 2,037,159 patients, of whom 518,995 (25.5%) were active smokers. Notably, these numbers also included three study entries reporting on identical patient cohorts (Table 1).25,28,30 Of the 24 studies analysed, 11 were retrospective studies (45.8%; level IV),25,27–30,34,36,39,40,42,43 11 were longitudinal population-based cohort studies (45.8%; level III),31–33,35,37,38,41,44–47 one a prospective study (4.2%; level III),24 and one a large retrospective study (4.2%; level III).26 Overall relative risk (RR) for smokers (regardless of fracture site) was 1.46 (95% confidence interval [CI]: 1.23–1.72; p < 0.001; Fig. 2), with high heterogeneity between studies (I2 = 89.3%), and no significant publication bias (Harbord’s p = 0.267). Subsequent subgroup analyses were performed for studies focusing on vertebral fractures, hip fractures, and other fracture sites.
Table 1.
Summary of studies included in meta-analysis
| Fracture risk | ||||||
| Author | Study type | Gender | Smokers total | Non-smokers total | Outcome factor | Evidence level |
| Ji et al (2019)28 | Retrospective study | Female | 7106 | 208499 | Humerus fracture risk | IV |
| Male | 98190 | 117226 | ||||
| Hannan et al (2019)41 | Longitudinal population-based cohort study | Female | 237 | 2539 | Nonvertebral fracture risk | III |
| Givon et al (2000)4 | Retrospective study | Both | 552 | 1450 | Stress fracture risk | IV |
| Olofsson et al (2005)33 | Longitudinal population-based cohort study | Male | 1185 | 1134 | Any fracture risk | III |
| 1185 | 1134 | Hip fracture risk | ||||
| Zhu et al (2019)35 | Longitudinal population-based cohort study | Both | 104753 | 324676 | Calcaneal fracture risk | III |
| Michaëlsson et al (1999)32 | Longitudinal population-based cohort study | Female | 798 | 3099 | Cervical hip fracture risk | III |
| 727 | 2849 | Trochanteric hip fracture risk | ||||
| Chen et al (2018)25 | Retrospective study | Both | 104766 | 324694 | Clavicula fracture risk | IV |
| Liu et al (2018)30 | Retrospective study | Female | 7081 | 207860 | Foot fracture risk | IV |
| Male | 97722 | 116902 | ||||
| Liu et al (2019)29 | Retrospective study | Male | 37879 | 38605 | Fracture risk (general) | IV |
| Lobo et al (2017)31 | Longitudinal population-based cohort study | Female | 76 | 2618 | Hip fracture risk | III |
| Male | 528 | 1448 | ||||
| Wiklund et al (2016)34 | Retrospective study | Both | 29 | 924 | Hip fracture risk | IV |
| Bawab et al (2014)24 | Prospective cohort study | Both | 77 | 118 | Hip fracture risk | III |
| Jenkins et al (2008)27 | Retrospective study | Female | 56 | 432 | Hip fracture risk | IV |
| Holmberg et al (2005)26 | Retrospective study (large) | Female | 3808 | 7094 | Hip fracture risk | III |
| Male | 11041 | 11403 | ||||
| Baron et al (2001)37 | Longitudinal population-based cohort study | Female | 940 | 3470 | Hip fracture risk | III |
| Cornuz et al (1999)38 | Longitudinal population-based cohort study | Female | 36031 | 115892 | Hip fracture risk | III |
| Mussolino et al (1998)44 | Longitudinal population-based cohort study | Male | 1077 | 1802 | Hip fracture risk | III |
| Paganini-Hill et al (1991)46 | Longitudinal population-based cohort study | Female | 1081 | 7512 | Hip fracture risk | III |
| Male | 433 | 4614 | ||||
| Bae et al (2019)36 | Retrospective study | Both | 14 | 232 | Vertebral fracture risk | IV |
| El Maghraoui et al (2012)39 | Retrospective study | Male | 52 | 657 | Vertebral fracture risk | IV |
| Nevitt et al (2005)45 | Longitudinal population-based cohort study | Female | 667 | 6571 | Vertebral fracture risk | III |
| van der Klift et al (2004)47 | Longitudinal population-based cohort study | Female | 290 | 1334 | Vertebral fracture risk | III |
| Male | 354 | 1023 | ||||
| Kim et al (2015)42 | Retrospective study | Both | 45 | 166 | Vertebral fracture risk | IV |
| Ma et al (2019)43 | Retrospective study | Both | 210 | 345 | Vertebral re-fracture risk (following vertebral fracture) | IV |
| Postoperative infection risk | ||||||
| Author | Study type | Gender | Smokers total | Non-smokers total | Outcome factor | Evidence level |
| Nåsell et al (2011)21 | Retrospective study | Both | 185 | 721 | Postoperative infection following ORIF for ankle fractures | IV |
| Lack et al (2015)50 | Retrospective study | Both | 39 | 98 | Postoperative infection following ORIF for open tibial fracture | IV |
| Li et al (2020)51 | Retrospective study | Both | 84 | 131 | Postoperative infection following ORIF for open tibial fracture | IV |
| Khan et al (2019)48 | Retrospective study | Both | 10 | 14 | Postoperative infection after spinal surgery | IV |
| Xu et al (2019)52 | Retrospective study | Both | 130 | 304 | Postoperative infection after ORIF for distal femoral fracture | IV |
| Bai et al (2019)53 | Retrospective study | Both | 148 | 517 | Postoperative infection after ORIF for distal femoral fracture | IV |
| Lu et al (2019)54 | Retrospective study | Both | 135 | 589 | Postoperative infection after ORIF for distal femoral fracture | IV |
| Meng et al (2018)55 | Retrospective study | Both | 554 | 2063 | Postoperative infection following ORIF for ankle fractures | IV |
| Sun et al (2018)56 | Retrospective study | Both | 355 | 1155 | Postoperative infection following ORIF for ankle fractures | IV |
| Ma et al (2018)58 | Retrospective study | Both | 73 | 603 | Postoperative infection following ORIF for tibial plateau fracture | IV |
| Su et al (2017)59 | Retrospective study | Both | 114 | 204 | Postoperative infection after ORIF for calcaneal fracture | IV |
| Iqbal et al (2017)60 | Retrospective study | Both | 63 | 187 | Postoperative infection after ORIF for acetabular fracture | IV |
| Olsen et al (2017)61 | Retrospective study | Both | 283 | 760 | Postoperative infection following ORIF for ankle fractures | IV |
| Saeedinia et al (2015)49 | Prospective non-randomized study | Both | 132 | 846 | Postoperative infection after spinal surgery | III |
| Claessen et al (2016)62 | Retrospective study | Both | 343 | 977 | Postoperative infection after ORIF for elbow fracture | IV |
| Lin et al (2014)63 | Retrospective study | Both | 105 | 151 | Postoperative infection following ORIF for tibial plateau fracture | IV |
| Morris et al (2013)64 | Retrospective study | Both | 137 | 165 | Postoperative infection following ORIF for tibial plateau fracture | IV |
| Kamath et al (2005)65 | Prospective non-randomized study | Both | 31 | 61 | Postoperative infection after surgery for hip fracture | III |
| Zhu et al (2017)66 | Prospective non-randomized study | Both | 22 | 213 | Postoperative infection following ORIF for tibial plateau fracture | III |
| Singh et al (2018)67 | Retrospective study | Both | 26 | 77 | Postoperative infection following ORIF for open tibial fracture | IV |
| Nonunion risk | ||||||
| Author | Study type | Gender | Smokers total | Non-smokers total | Outcome factor | Evidence level |
| McKee et al (2003)74 | Retrospective study | Both | 47 | 39 | Nonunion after Ilizarov reconstruction | IV |
| Dailey et al (2018)80 | Retrospective study | Both | 244 | 261 | Nonunion after tibial fracture | IV |
| Murray et al (2013)81 | Retrospective study | Both | 219 | 722 | Nonunion after mid-clavicular fractures | IV |
| Kim et al (2005)68 | Retrospective study | Both | 15 | 81 | Nonunion after spinal fusion | IV |
| Glassman et al (2000)69 | Retrospective study | Both | 188 | 169 | Nonunion after spinal fusion | IV |
| Tay et al (2014)82 | Retrospective study | Both | 161 | 262 | Nonunion after diaphyseal femoral and tibial fracture | IV |
| Rodriguez et al (2014)83 | Retrospective study | Both | 34 | 249 | Nonunion after distal femoral fracture | IV |
| Özbek et al (2017)84 | RCT | Both | 19 | 56 | Nonunion after thoracolumbar fractures | II |
| Bydon et al (2014)75 | Retrospective study | Both | 50 | 231 | Nonunion after lumbar fusion | IV |
| Krause et al (2016)70 | RCT | Both | 44 | 326 | Nonunion after hindfoot and ankle fusion | II |
| Nappo et al (2019)85 | Retrospective study | Both | 34 | 42 | Nonunion after open forearm fracture | IV |
| Giuseffi et al (2015)71 | Retrospective study | Both | 17 | 72 | Nonunion after high tibial osteotomy | IV |
| Meidinger et al (2011)72 | Retrospective study | Both | 46 | 140 | Nonunion after high tibial osteotomy | IV |
| Hoffmann et al (2019)86 | Retrospective study | Both | 32 | 161 | Nonunion after intertrochanteric femoral fracture | IV |
| Gaspar et al (2016)73 | Retrospective study | Both | 17 | 55 | Nonunion after ulnar shortening osteotomy | IV |
| Neuhaus et al (2014)76 | Retrospective study | Both | 19 | 60 | Nonunion after mid-diaphyseal humeral fractures | IV |
| Ding et al (2014)77 | Retrospective study | Both | 165 | 494 | Nonunion after diaphyseal humeral fractures | IV |
| Liu et al (2015)78 | Retrospective study | Both | 155 | 649 | Nonunion after mid-clavicular fractures | IV |
| Giannoudis et al (2000)79 | Retrospective study | Both | 31 | 68 | Nonunion after diaphyseal femoral fractures | IV |
| Persisting nonunion risk in scaphoid nonunion | ||||||
| Author | Study type | Gender | Smokers total | Non-smokers total | Outcome factor | Evidence level |
| Dinah et al (2007)87 | Retrospective study | Both | 20 | 17 | Re-nonunion after surgery for scaphoid nonunion | IV |
| Little et al (2006)88 | Retrospective study | Both | 30 | 34 | Re-nonunion after surgery for scaphoid nonunion | IV |
| Rahimnia et al (2018)89 | Retrospective study | Both | 19 | 22 | Re-nonunion after vascularized bone graft for scaphoid nonunion | IV |
| Hirche et al (2014)90 | Retrospective study | Both | 13 | 15 | Re-nonunion after vascularized bone graft for scaphoid nonunion | IV |
| Chang et al (2006)91 | Retrospective study | Both | 13 | 35 | Re-nonunion after vascularized bone graft for scaphoid nonunion | IV |
Note. RCT, randomized controlled trial; ORIF, open reduction and internal fixation.
Fig. 2.
Forest plot of studies analysing risk of smoking on fracture incidence, divided into hip fractures, vertebral fractures, and other fracture sites. Orange diamonds depict effect sizes for subgroups, and the red diamond shows overall effect size. The dashed black line depicts the no-effects line. The solid red line marks overall effect size value.
Vertebral fracture risk
Meta-analysis of the six studies (seven study entries)36,39,42,43,45,47 with vertebral fractures as the primary endpoint showed a significant association for smoking (RR: 1.61; 95% CI: 1.13–2.29; p = 0.008; Fig. 2). Heterogeneity between studies was high (I2 = 89.4%), and a significant publication bias was present (Harbord’s p = 0.016). Sensitivity analysis was performed after excluding one study reporting on presence of vertebral fractures and abdominal aortic calcification,39 one study assessing vertebral re-fracture risk after already having sustained a vertebral fracture,43 and one study analysing vertebral fracture risk in women > 65 years of age.45 This analysis revealed a significant association for smoking (RR: 1.51; 95% CI: 1.19–1.92; p < 0.001), whilst heterogeneity could be diminished (I2 = 0.0%).
Hip fracture risk
For 11 studies (15 study entries)24,26,27,31–34,37,38,44,46 reporting on hip fracture risk, meta-analysis showed a strong association between active smoking and risk for hip fracture (RR: 1.28; 95%CI: 1.07–1.53; p = 0.007; Fig. 2). Heterogeneity between studies was large (I2 = 84.1%). No significant publication bias was present (Harbord’s p = 0.894).
Sensitivity analysis was performed after excluding two retrospective studies (i.e. three study entries).24,26 This analysis revealed a significant association between smoking and increased hip fracture risk (RR: 1.32; 95% CI: 1.24–1.40; p < 0.001). Heterogeneity could be diminished (I2 = 0.0%).
Other fracture risk
Meta-analysis of eight studies (10 study entries)25,28–30,33,35,40,41 reporting on fracture risk at any anatomical site revealed a significant association between smoking and risk for any fracture (RR: 1.75; 95% CI: 1.10–2.80; p = 0.019; Fig. 2). Between-study heterogeneity was high (I2 = 89.3%). No significant publication bias was found (Harbord’s p = 0.505). After excluding four studies investigating fracture incidences in a large Chinese population (> 400,000 patients),25,28,30,35 and one study with stress fracture as the endpoint,40 sensitivity analysis revealed a significant association between smoking and increased risk for fractures at other sites than vertebrae or hip (RR: 1.50; 95% CI: 1.25–1.80; p < 0.001), and low heterogeneity (I2 = 16.9%).
Postoperative infection risk
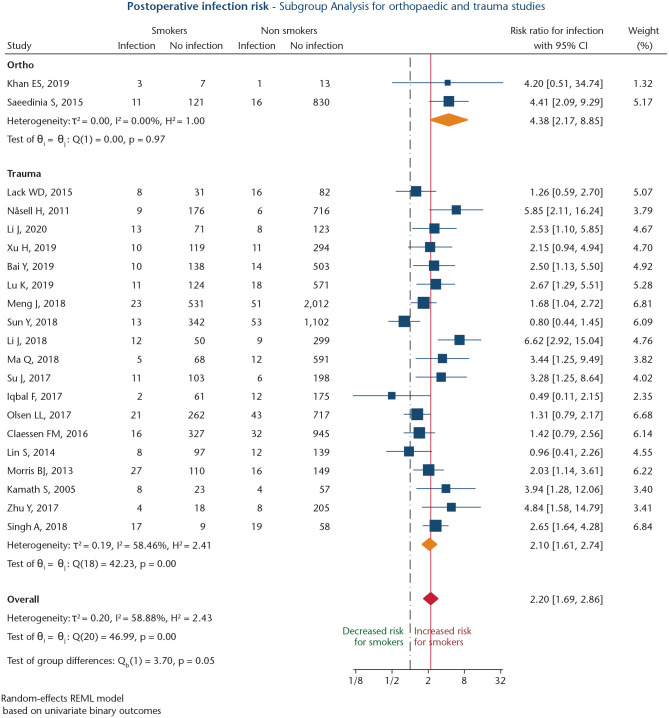
Altogether, 21 studies analysed risk for postoperative infection after fracture surgery (n = 19), or elective orthopaedic surgery (n = 2),48,49 involving 13176 patients of whom 3030 were smokers (23.0%; Table 1).21,48–67 Mean NOS score was 6.6 ± 1.2 points. Three studies were prospective non-randomized cohort studies (14.3%; level III),49,65,66 and 18 were retrospective studies (85.7%; level IV).21,48,50–64,67 The overall RR for smokers to develop postoperative infections was 2.20 (95% CI: 1.69–2.86; p < 0.001; Fig. 3). Heterogeneity was high (I2 = 58.9%), and a significant publication bias was found (Harbord’s p = 0.001). Subgroup meta-analysis of two studies analysing infection risk after elective spinal surgery48,49 revealed a significant association between smoking and risk for postoperative infection (RR: 4.38; 95% CI: 2.17–8.85; p < 0.001; Fig. 3). Heterogeneity between studies was low (I2 = 0.0%).
Fig. 3.
Forest plot for studies investigating association between smoking status and postoperative infection risk, separated by elective orthopaedic procedures and trauma surgeries. Orange diamonds depict effect sizes for subgroups, and the red diamond shows overall effect size. The dashed black line depicts the no-effects line. The solid red line marks overall effect size value.
Nineteen studies assessed infection risk following surgery for fractures,21,50–67 including five studies on tibial plateau fractures,57,58,63,64,66 four studies on ankle fractures,21,55,56,61 three studies on open tibial fractures,50,51,67 three studies on distal femoral fractures,52–54 and one study each on elbow fractures,62 hip fractures,65 acetabular fractures60 and calcaneal fractures.59 The subgroup meta-analysis for this cohort revealed a significant association between smoking and elevated risk for postoperative infections (RR: 2.10; 95% CI: 1.61–2.74; p < 0.001; Fig. 3). Heterogeneity was high (I2 = 58.5%), and a significant publication bias was observed (Harbord’s p = 0.001). Sensitivity analysis for this subgroup, after excluding four studies,21,56,57,60 revealed a significant association between smoking and infection risk (RR: 2.01; 95% CI: 1.65–2.45; p < 0.001), and heterogeneity could be reduced (I2 = 15.2%).
General nonunion risk
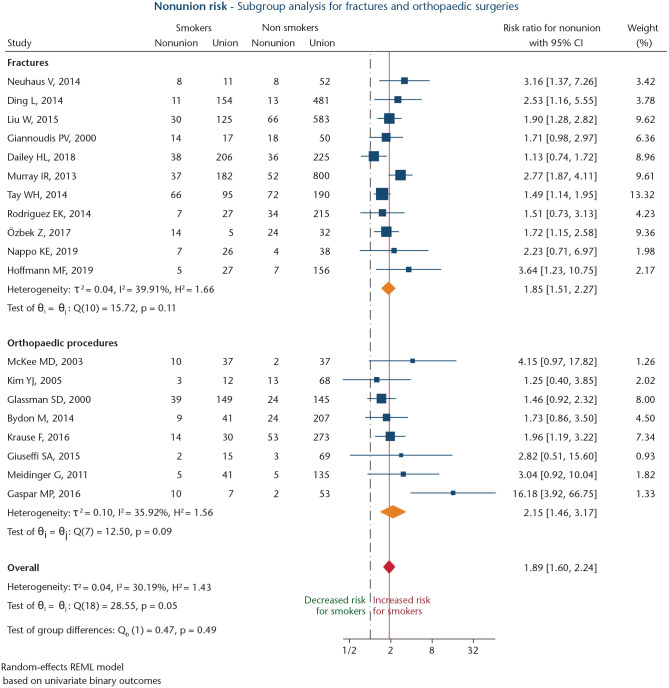
Nineteen studies, involving 5805 patients of whom 26.5% were smokers, reported on nonunion risk following either elective orthopaedic procedures (n = 8),68–75 or fractures (n = 11; Table 1).76–86 Of these, 17 were retrospective studies (89.5%; level IV),68,69,71–83,85,86 and two were randomized controlled trials (10.5%; level II).70,84 Mean NOS score was 6.3 ± 1.1 points. Meta-analysis of the 19 studies68–86 revealed an overall RR of 1.89 (95% CI: 1.60–2.24; p < 0.001) for smokers to develop nonunion (Fig. 4). Moderate heterogeneity was present (I2 = 58.5%). A significant publication bias was found (Harbord’s p = 0.003).
Fig. 4.
Forest plot of studies analysing the effect of smoking on nonunion risk following elective orthopaedic surgical procedures or traumatic fracture surgery. Orange diamonds depict effect sizes for subgroups, and the red diamond shows overall effect size. The dashed black line depicts the no-effects line. The solid red line marks overall effect size value.
Nonunion risk after orthopaedic procedures
Subgroup meta-analysis of the eight studies evaluating nonunion risk following elective orthopaedic procedures68–75 revealed a significant association for smoking (RR: 2.15; 95% CI: 1.46–3.17; p < 0.001; Fig. 4), with moderate heterogeneity (I2 = 35.9%), and no significant publication bias (Harbord’s p = 0.237). Sensitivity analysis, after exclusion of one study,73 showed a significant association between smoking and increased nonunion risk (RR: 1.79; 95% CI: 1.36–2.36; p < 0.001), with low heterogeneity (I2 = 0.0%).
Nonunion risk after fractures
Subgroup meta-analysis of 11 studies analysing nonunion risk after fractures76–86 also showed a significant positive association for smoking (RR: 1.85; 95% CI: 1.51–2.27; p < 0.001; Fig. 4). Heterogeneity was moderate (I2 = 39.9%). A significant publication bias was found (Harbord’s p = 0.047). After exclusion of two studies,80,81 sensitivity analysis revealed a significant association between smoking and nonunion risk (RR: 1.76; 95% CI: 1.49–2.08; p < 0.001). Heterogeneity could be diminished (I2 = 0.0%).
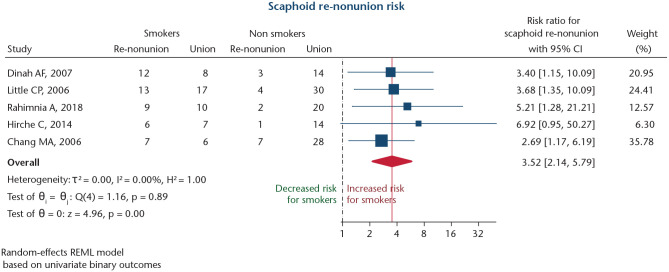
Persisting nonunion risk after scaphoid nonunion
Five studies investigated the risk for persisting nonunion after surgical revision for scaphoid nonunion (Table 1).87–91 Mean NOS score for these studies was 4.8 ± 0.4 points. Altogether, 218 patients were included, of whom 43.6% were smokers. All studies were retrospective studies (level IV).87–91 Meta-analysis revealed a significant association between smoking and an increased risk for persistent nonunion after surgical revision for scaphoid nonunion (RR: 3.52; 95% CI: 2.14–5.79; p < 0.001; Fig. 5). Heterogeneity was low (I2 = 0.0%), and no significant publication bias was detected (Harbord’s p = 0.472). Therefore, no sensitivity analysis was performed.
Fig. 5.
Forest plot for studies investigating influence of smoking on development of persistent nonunions after surgery for scaphoid nonunions. The dashed black line depicts the no-effects line. The solid red line marks overall effect size value.
Discussion
According to the present meta-analysis, smoking is not only significantly associated with an overall higher fracture risk, hip fracture risk and vertebral fracture risk, but also with increased risk for nonunion following fracture surgery, postoperative infection risk, and persistent nonunion after surgery for scaphoid nonunions.
One limitation of the current study is the partially vague description of variables in individual studies, wherefore crude values of smoking and non-smoking patients, as well as those with or without an outcome event, could not be ascertained. This was true for 274 of 343 studies thoroughly analysed for variables of interest and outcome parameters. Consequently, only 69 studies were finally eligible for the meta-analysis. Of these, merely 27.5% were level III or level II studies, and only one study had randomized for smoking. Another limitation of the study has to be seen in the overall moderate NOS score, being 6.5 points on average. Therefore, the mostly retrospective studies may be prone to bias regarding cohort definition, covariate adjustment, and outcome assessment. In order not to further skew results, the authors thus decided to perform meta-analyses on unadjusted crude values of each study. Considering that a large proportion of studies had to be excluded due to lack of disaggregated data, the provision of basic results in observational studies and clinical trials should be emphasized in the future. This would enable researchers to eventually draw further conclusions regarding the role of smoking and other risk factors in orthopaedic patients.
Due to the negative effect of tobacco products on bone metabolism, smokers are known to have an overall lower bone mineral density (BMD), wherefore their susceptibility to fractures may be increased.92 However, reduced BMD alone does not seem to explain the overall higher fracture risk of smokers in comparison to non-smokers.16 Indeed, according the present meta-analysis, smokers had a 1.46 times higher risk of sustaining any fracture in comparison to non-smokers. Our findings are comparable to those reported in the meta-analysis by Kanis et al back in 2005.16 However, we did not account for potential differences in BMD depending on smoking status. Furthermore, factors such as long-term prescription of corticosteroids, (family) history of fractures and secondary osteoporosis are known to significantly increase fracture risk.16,93–95 As the simultaneous occurrence of these risk factors and smoking cannot be ruled out in this meta-analysis, the negative effect of smoking alone has to be interpreted bearing this limitation in mind.
Surgical site infections are regarded as the most common type of hospital-acquired infection.96 Any surgical site infection may be associated with increased patient morbidity, reduced subjective quality of life, prolonged hospitalization time, and increased costs to the healthcare system.97,98 Smoking leads to decreased microperfusion and tissue hypoxia. Moreover, the direct effect of carbon monoxide on oxyhaemoglobin leads to a leftwards shift of the oxygen dissociation curve.7,8,99 Consequently, wound healing may be impaired in smokers. In line with this, we discovered an RR of 2.20 for smokers to develop postoperative infections after elective orthopaedic and trauma surgeries in comparison to non-smokers. More specifically, the risk of smokers developing infections after fracture surgery was 2.1 times higher than for non-smokers, which is higher than the RR of 1.29 described in a previous meta-analysis including patients with open fractures.100
Depending on fracture site and treatment, risk for nonunion after bone fractures ranges between 5% and 10%.101–103 Development of nonunion is not only associated with prolonged and often complex treatment, but also a significant financial burden and negative impact on patients’ quality of life.104,105 Bearing in mind the non-modifiable risk factors eventually contributing to development of nonunion, such as type of fracture and site, bone morphology, and associated infection, potentially modifiable factors including treatment approach, (postoperative) mobilization protocols, steroid intake, and smoking should be particularly addressed by treating healthcare professionals.106,107 Notably, in the present meta-analysis, the RR of active smokers to develop nonunions following fractures or elective orthopaedic procedures was 1.89, being comparable to the RR reported by Pearson et al in a meta-analysis involving 40 studies.18 Likewise, Scolaro et al discovered a significantly higher risk for smokers to develop nonunions after open fractures (odds ratio: 1.95) and fractures in general (odds ratio: 2.32).108
Scaphoid fractures in particular are at high risk of nonunion, occurring in 10–15% of both operatively and conservatively treated patients, owing to the limited blood supply via blood vessels entering the bone distally.109,110 Revision surgery of scaphoid nonunions is challenging and usually requires harvesting of autologous bone grafts.111,112 it is noteworthy that the present meta-analysis of five studies investigating persisting nonunion risk after surgery for scaphoid nonunions revealed an RR of 3.52 for smokers in comparison to non-smokers, in line with a previous meta-analysis comparing surgical flaps for scaphoid nonunion and healing depending on smoking status.113
According to the results of this meta-analysis, smoking is associated with significant increases in fracture incidences, postoperative complications, and nonunions. Yet, whilst diabetes is known as a significant risk factor for these outcomes, and special attention is usually paid to these patients perioperatively, the awareness among orthopaedic and trauma surgeons towards the risk of smokers likewise developing nonunions and infections is less well pronounced. However, according to Zura et al, past or current smoking seems (multivariate OR: 1.20) to increase nonunion risk to a greater extent than diabetes mellitus type 2 (multivariate OR: 1.15). Also, postoperative infection risk in open fractures is likewise increased in diabetic (RR: 1.72) and smoking patients (RR: 1.29).101
Conclusions
Considering the deleterious effects of smoking on risk of developing fractures, subsequent nonunion and postoperative infection risk, any orthopaedic or trauma surgeon should strongly advise patients to quit smoking and encourage their participation in smoking cessation programmes.
Footnotes
ICMJE Conflict of interest statement: None of the authors has any conflicts of interest related to the current study to declare.
OA licence text: This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Supplemental Material: Supplemental material is available for this paper at https://online.boneandjoint.org.uk/doi/suppl/10.1302/2058-5241.6.210058
Funding statement
Although none of the authors has received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article, benefits have been or will be received but will be directed solely to a research fund, foundation, educational institution, or other non-profit organization with which one or more of the authors are associated.
References
- 1. Royal College of Physicians, London Smoking and health: summary and report of the Royal College of Physicians of London on smoking in relation to cancer of the lung and other diseases. London: Pitman Medical Publishing Co, 1962. [PubMed] [Google Scholar]
- 2. Bayne-Jones S, Burdette WJ, Cochran WG, et al. Smoking and health: report of the Advisory Committee to the Surgeon-General of the Public Health Service. Washington, DC: Department of Health, Education and Welfare, 1964. [Google Scholar]
- 3. Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med 2014;370:60–68. [DOI] [PubMed] [Google Scholar]
- 4. WHO. Age-standardized estimates of current tobacco use, tobacco smoking and cigarette smoking (Tobacco control: Monitor), 2018. https://www.who.int/data/gho/data/indicators/indicator-details/GHO/gho-tobacco-control-monitor-current-tobaccouse-tobaccosmoking-cigarrettesmoking-agestd-tobagestdcurr (date last accessed 29 March 2021).
- 5. Nelson HD, Nevitt MC, Scott JC, Stone KL, Cummings SR; Study of Osteoporotic Fractures Research Group. Smoking, alcohol, and neuromuscular and physical function of older women. JAMA 1994;272:1825–1831. [DOI] [PubMed] [Google Scholar]
- 6. Raikin SM, Landsman JC, Alexander VA, Froimson MI, Plaxton NA. Effect of nicotine on the rate and strength of long bone fracture healing. Clin Orthop Relat Res 1998;353:231–237. [DOI] [PubMed] [Google Scholar]
- 7. Mosely LH, Finseth F, Goody M. Nicotine and its effect on wound healing. Plast Reconstr Surg 1978;61:570–575. [DOI] [PubMed] [Google Scholar]
- 8. Lee JJ, Patel R, Biermann JS, Dougherty PJ. The musculoskeletal effects of cigarette smoking. J Bone Joint Surg [Am] 2013;95-A:850–859. [DOI] [PubMed] [Google Scholar]
- 9. Krall EA, Dawson-Hughes B. Smoking and bone loss among postmenopausal women. J Bone Miner Res 1991;6-A:331–338. [DOI] [PubMed] [Google Scholar]
- 10. Naruse M, Ishihara Y, Miyagawa-Tomita S, Koyama A, Hagiwara H. 3-Methylcholanthrene, which binds to the arylhydrocarbon receptor, inhibits proliferation and differentiation of osteoblasts in vitro and ossification in vivo. Endocrinology 2002;143:3575–3581. [DOI] [PubMed] [Google Scholar]
- 11. Porter SE, Hanley EN, Jr. The musculoskeletal effects of smoking. J Am Acad Orthop Surg 2001;9:9–17. [DOI] [PubMed] [Google Scholar]
- 12. Gaston MS, Simpson AH. Inhibition of fracture healing. J Bone Joint Surg [Br] 2007;89-B:1553–1560. [DOI] [PubMed] [Google Scholar]
- 13. Tanaka H, Tanabe N, Shoji M, et al. Nicotine and lipopolysaccharide stimulate the formation of osteoclast-like cells by increasing macrophage colony-stimulating factor and prostaglandin E2 production by osteoblasts. Life Sci 2006;78:1733–1740. [DOI] [PubMed] [Google Scholar]
- 14. Whiteford L. Nicotine, CO and HCN: the detrimental effects of smoking on wound healing. Br J Community Nurs 2003;8:S22–S26. [DOI] [PubMed] [Google Scholar]
- 15. Vestergaard P, Mosekilde L. Fracture risk associated with smoking: a meta-analysis. J Intern Med 2003;254:572–583. [DOI] [PubMed] [Google Scholar]
- 16. Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int 2005;16:155–162. [DOI] [PubMed] [Google Scholar]
- 17. Sayampanathan AA, Basha M, Mitra AK. Risk factors of lateral epicondylitis: a meta-analysis. Surgeon 2020;18:122–128. [DOI] [PubMed] [Google Scholar]
- 18. Pearson RG, Clement RG, Edwards KL, Scammell BE. Do smokers have greater risk of delayed and non-union after fracture, osteotomy and arthrodesis? A systematic review with meta-analysis. BMJ Open 2016;6:e010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Group OLoEW. The Oxford 2011 levels of evidence. Oxford: Oxford Centre for Evidence-Based Medicine, 2011. [Google Scholar]
- 20. Wells GA. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (date last accessed 5 January 2021).
- 21. Nåsell H, Ottosson C, Törnqvist H, Lindé J, Ponzer S. The impact of smoking on complications after operatively treated ankle fractures: a follow-up study of 906 patients. J Orthop Trauma 2011;25:748–755. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006;25:3443–3457. [DOI] [PubMed] [Google Scholar]
- 24. Bawab W, Saad M, Hajjar N, et al. Evaluation of hip fracture risk factors in older adults in the Lebanese population. J Res Health Sci 2014;14:193–197. [PubMed] [Google Scholar]
- 25. Chen W, Zhu Y, Liu S, et al. Demographic and socioeconomic factors influencing the incidence of clavicle fractures, a national population-based survey of five hundred and twelve thousand, one hundred and eighty seven individuals. Int Orthop 2018;42:651–658. [DOI] [PubMed] [Google Scholar]
- 26. Holmberg AH, Johnell O, Nilsson PM, Nilsson JA, Berglund G, Akesson K. Risk factors for hip fractures in a middle-aged population: a study of 33,000 men and women. Osteoporos Int 2005;16:2185–2194. [DOI] [PubMed] [Google Scholar]
- 27. Jenkins MR, Denison AV. Smoking status as a predictor of hip fracture risk in postmenopausal women of northwest Texas. Prev Chronic Dis 2008;5:A09. [PMC free article] [PubMed] [Google Scholar]
- 28. Ji C, Li J, Zhu Y, et al. Assessment of incidence and various demographic risk factors of traumatic humeral shaft fractures in China. Sci Rep 2019;9:1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu G, Li Y, Zhu Y, et al. Unhealthy lifestyles are associated with the increased risk of low-energy fracture in Chinese men ≥ 50 years, a population-based survey. Arch Osteoporos 2019;14:57. [DOI] [PubMed] [Google Scholar]
- 30. Liu S, Zhu Y, Wang L, Chen W, Zhang X, Zhang Y. Incidence and risk factors for foot fractures in China: a retrospective population-based survey. PLoS One 2018;13:e0209740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lobo E, Marcos G, Santabárbara J, et al. ; ZARADEMP Workgroup. Gender differences in the incidence of and risk factors for hip fracture: a 16-year longitudinal study in a southern European population. Maturitas 2017;97:38–43. [DOI] [PubMed] [Google Scholar]
- 32. Michaëlsson K, Weiderpass E, Farahmand BY, et al. ; Swedish Hip Fracture Study Group. Differences in risk factor patterns between cervical and trochanteric hip fractures. Osteoporos Int 1999;10:487–494. [DOI] [PubMed] [Google Scholar]
- 33. Olofsson H, Byberg L, Mohsen R, Melhus H, Lithell H, Michaëlsson K. Smoking and the risk of fracture in older men. J Bone Miner Res 2005;20:1208–1215. [DOI] [PubMed] [Google Scholar]
- 34. Wiklund R, Toots A, Conradsson M, et al. Risk factors for hip fracture in very old people: a population-based study. Osteoporos Int 2016;27:923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu Y, Li J, Liu S, et al. Socioeconomic factors and lifestyles influencing the incidence of calcaneal fractures, a national population-based survey in China. J Orthop Surg Res 2019;14:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bae IS, Kim JM, Cheong JH, Han MH, Ryu JI. Association between cerebral atrophy and osteoporotic vertebral compression fractures. PLoS One 2019;14:e0224439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baron JA, Farahmand BY, Weiderpass E, et al. Cigarette smoking, alcohol consumption, and risk of hip fracture in women. Arch Intern Med 2001;161:983–988. [DOI] [PubMed] [Google Scholar]
- 38. Cornuz J, Feskanich D, Willett WC, Colditz GA. Smoking, smoking cessation, and risk of hip fracture in women. Am J Med 1999;106:311–314. [DOI] [PubMed] [Google Scholar]
- 39. El Maghraoui A, Rezqi A, Mounach A, Achemlal L, Bezza A, Ghozlani I. Relationship between vertebral fracture prevalence and abdominal aortic calcification in men. Rheumatology (Oxford) 2012;51:1714–1720. [DOI] [PubMed] [Google Scholar]
- 40. Givon U, Friedman E, Reiner A, Vered I, Finestone A, Shemer J. Stress fractures in the Israeli defense forces from 1995 to 1996. Clin Orthop Relat Res 2000;373:227–232. [DOI] [PubMed] [Google Scholar]
- 41. Hannan MT, Weycker D, McLean RR, et al. Predictors of imminent risk of nonvertebral fracture in older, high-risk women: the Framingham Osteoporosis Study. JBMR Plus 2019;3:e10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim GW, Joo HJ, Park TS, et al. Vertebral compression fractures may increase mortality in male patients with chronic obstructive pulmonary disease. Int J Tuberc Lung Dis 2015;19:603–609. [DOI] [PubMed] [Google Scholar]
- 43. Ma X, Xia H, Wang J, et al. Re-fracture and correlated risk factors in patients with osteoporotic vertebral fractures. J Bone Miner Metab 2019;37:722–728. [DOI] [PubMed] [Google Scholar]
- 44. Mussolino ME, Looker AC, Madans JH, Langlois JA, Orwoll ES. Risk factors for hip fracture in white men: the NHANES I epidemiologic follow-up study. J Bone Miner Res 1998;13:918–924. [DOI] [PubMed] [Google Scholar]
- 45. Nevitt MC, Cummings SR, Stone KL, et al. Risk factors for a first-incident radiographic vertebral fracture in women > or = 65 years of age: the study of osteoporotic fractures. J Bone Miner Res 2005;20:131–140. [DOI] [PubMed] [Google Scholar]
- 46. Paganini-Hill A, Chao A, Ross RK, Henderson BE. Exercise and other factors in the prevention of hip fracture: the Leisure World study. Epidemiology 1991;2:16–25. [DOI] [PubMed] [Google Scholar]
- 47. van der Klift M, de Laet CE, McCloskey EV, et al. Risk factors for incident vertebral fractures in men and women: the Rotterdam Study. J Bone Miner Res 2004;19:1172–1180. [DOI] [PubMed] [Google Scholar]
- 48. Khan ES, Kow RY, Arifin KBBM, Komahen C, Low CL, Lim BC. Factors associated with deep surgical site infection following spinal surgery: a pilot study. Cureus 2019;11:e4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saeedinia S, Nouri M, Azarhomayoun A, et al. The incidence and risk factors for surgical site infection after clean spinal operations: a prospective cohort study and review of the literature. Surg Neurol Int 2015;6:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lack WD, Karunakar MA, Angerame MR, et al. Type III open tibia fractures: immediate antibiotic prophylaxis minimizes infection. J Orthop Trauma 2015;29:1–6. [DOI] [PubMed] [Google Scholar]
- 51. Li J, Wang Q, Lu Y, et al. Relationship between time to surgical debridement and the incidence of infection in patients with open tibial fractures. Orthop Surg 2020;12:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xu H, Yu L, Li Y, Gong Z. Prolonged surgical duration, higher body mass index and current smoking increases risk of surgical site infection after intra-articular fracture of distal femur. ANZ J Surg 2019;89:723–728. [DOI] [PubMed] [Google Scholar]
- 53. Bai Y, Zhang X, Tian Y, Tian D, Zhang B. Incidence of surgical-site infection following open reduction and internal fixation of a distal femur fracture: an observational case-control study. Medicine (Baltimore) 2019;98:e14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lu K, Zhang J, Cheng J, et al. Incidence and risk factors for surgical site infection after open reduction and internal fixation of intra-articular fractures of distal femur: a multicentre study. Int Wound J 2019;16:473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meng J, Sun T, Zhang F, Qin S, Li Y, Zhao H. Deep surgical site infection after ankle fractures treated by open reduction and internal fixation in adults: a retrospective case-control study. Int Wound J 2018;15:971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sun Y, Wang H, Tang Y, et al. Incidence and risk factors for surgical site infection after open reduction and internal fixation of ankle fracture: a retrospective multicenter study. Medicine (Baltimore) 2018;97:e9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li J, Zhu Y, Liu B, Dong T, Chen W, Zhang Y. Incidence and risk factors for surgical site infection following open reduction and internal fixation of adult tibial plateau fractures. Int Orthop 2018;42:1397–1403. [DOI] [PubMed] [Google Scholar]
- 58. Ma Q, Aierxiding A, Wang G, Wang C, Yu L, Shen Z. Incidence and risk factors for deep surgical site infection after open reduction and internal fixation of closed tibial plateau fractures in adults. Int Wound J 2018;15:237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Su J, Cao X. Risk factors of wound infection after open reduction and internal fixation of calcaneal fractures. Medicine (Baltimore) 2017;96:e8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iqbal F, Younus S, Asmatullah, Zia OB, Khan N. Surgical site infection following fixation of acetabular fractures. Hip Pelvis 2017;29:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Olsen LL, Møller AM, Brorson S, Hasselager RB, Sort R. The impact of lifestyle risk factors on the rate of infection after surgery for a fracture of the ankle. J Bone Joint Surg [Br] 2017;99-B:225–230. [DOI] [PubMed] [Google Scholar]
- 62. Claessen FM, Braun Y, van Leeuwen WF, Dyer GS, van den Bekerom MP, Ring D. What factors are associated with a surgical site infection after operative treatment of an elbow fracture? Clin Orthop Relat Res 2016;474:562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lin S, Mauffrey C, Hammerberg EM, Stahel PF, Hak DJ. Surgical site infection after open reduction and internal fixation of tibial plateau fractures. Eur J Orthop Surg Traumatol 2014;24:797–803. [DOI] [PubMed] [Google Scholar]
- 64. Morris BJ, Unger RZ, Archer KR, Mathis SL, Perdue AM, Obremskey WT. Risk factors of infection after ORIF of bicondylar tibial plateau fractures. J Orthop Trauma 2013;27:e196–e200. [DOI] [PubMed] [Google Scholar]
- 65. Kamath S, Sinha S, Shaari E, Young D, Campbell AC. Role of topical antibiotics in hip surgery: a prospective randomised study. Injury 2005;36:783–787. [DOI] [PubMed] [Google Scholar]
- 66. Zhu Y, Liu S, Zhang X, Chen W, Zhang Y. Incidence and risks for surgical site infection after adult tibial plateau fractures treated by ORIF: a prospective multicentre study. Int Wound J 2017;14:982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Singh A, Jiong Hao JT, Wei DT, et al. Gustilo IIIB open tibial fractures: an analysis of infection and nonunion rates. Indian J Orthop 2018;52:406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim YJ, Bridwell KH, Lenke LG, Rinella AS, Edwards C, II. Pseudarthrosis in primary fusions for adult idiopathic scoliosis: incidence, risk factors, and outcome analysis. Spine 2005;30:468–474. [DOI] [PubMed] [Google Scholar]
- 69. Glassman SD, Anagnost SC, Parker A, Burke D, Johnson JR, Dimar JR. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine 2000;25:2608–2615. [DOI] [PubMed] [Google Scholar]
- 70. Krause F, Younger AS, Baumhauer JF, et al. Clinical outcomes of nonunions of hindfoot and ankle fusions. J Bone Joint Surg [Am] 2016;98-A:2006–2016. [DOI] [PubMed] [Google Scholar]
- 71. Giuseffi SA, Replogle WH, Shelton WR. Opening-wedge high tibial osteotomy: review of 100 consecutive cases. Arthroscopy 2015;31:2128–2137. [DOI] [PubMed] [Google Scholar]
- 72. Meidinger G, Imhoff AB, Paul J, Kirchhoff C, Sauerschnig M, Hinterwimmer S. May smokers and overweight patients be treated with a medial open-wedge HTO? Risk factors for non-union. Knee Surg Sports Traumatol Arthrosc 2011;19:333–339. [DOI] [PubMed] [Google Scholar]
- 73. Gaspar MP, Kane PM, Zohn RC, Buckley T, Jacoby SM, Shin EK. Variables prognostic for delayed union and nonunion following ulnar shortening fixed with a dedicated osteotomy plate. J Hand Surg [Am] 2016;41:237–243. [DOI] [PubMed] [Google Scholar]
- 74. McKee MD, DiPasquale DJ, Wild LM, Stephen DJ, Kreder HJ, Schemitsch EH. The effect of smoking on clinical outcome and complication rates following Ilizarov reconstruction. J Orthop Trauma 2003;17:663–667. [DOI] [PubMed] [Google Scholar]
- 75. Bydon M, De la Garza-Ramos R, Abt NB, et al. Impact of smoking on complication and pseudarthrosis rates after single- and 2-level posterolateral fusion of the lumbar spine. Spine 2014;39:1765–1770. [DOI] [PubMed] [Google Scholar]
- 76. Neuhaus V, Menendez M, Kurylo JC, Dyer GS, Jawa A, Ring D. Risk factors for fracture mobility six weeks after initiation of brace treatment of mid-diaphyseal humeral fractures. J Bone Joint Surg [Am] 2014;96-A:403–407. [DOI] [PubMed] [Google Scholar]
- 77. Ding L, He Z, Xiao H, Chai L, Xue F. Factors affecting the incidence of aseptic nonunion after surgical fixation of humeral diaphyseal fracture. J Orthop Sci 2014;19:973–977. [DOI] [PubMed] [Google Scholar]
- 78. Liu W, Xiao J, Ji F, Xie Y, Hao Y. Intrinsic and extrinsic risk factors for nonunion after nonoperative treatment of midshaft clavicle fractures. Orthop Traumatol Surg Res 2015;101:197–200. [DOI] [PubMed] [Google Scholar]
- 79. Giannoudis PV, MacDonald DA, Matthews SJ, Smith RM, Furlong AJ, De Boer P. Nonunion of the femoral diaphysis: the influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg [Br] 2000;82-B:655–658. [DOI] [PubMed] [Google Scholar]
- 80. Dailey HL, Wu KA, Wu PS, McQueen MM, Court-Brown CM. Tibial fracture nonunion and time to healing after reamed intramedullary nailing: risk factors based on a single-center review of 1003 patients. J Orthop Trauma 2018;32:e263–e269. [DOI] [PubMed] [Google Scholar]
- 81. Murray IR, Foster CJ, Eros A, Robinson CM. Risk factors for nonunion after nonoperative treatment of displaced midshaft fractures of the clavicle. J Bone Joint Surg [Am] 2013;95-A:1153–1158. [DOI] [PubMed] [Google Scholar]
- 82. Tay WH, de Steiger R, Richardson M, Gruen R, Balogh ZJ. Health outcomes of delayed union and nonunion of femoral and tibial shaft fractures. Injury 2014;45:1653–1658. [DOI] [PubMed] [Google Scholar]
- 83. Rodriguez EK, Boulton C, Weaver MJ, et al. Predictive factors of distal femoral fracture nonunion after lateral locked plating: a retrospective multicenter case-control study of 283 fractures. Injury 2014;45:554–559. [DOI] [PubMed] [Google Scholar]
- 84. Özbek Z, Özkara E, Önner H, et al. Treatment of unstable thoracolumbar fractures: does fracture-level fixation accelerate the bone healing? World Neurosurg 2017;107:362–370. [DOI] [PubMed] [Google Scholar]
- 85. Nappo KE, Hoyt BW, Balazs GC, et al. Union rates and reported range of motion are acceptable after open forearm fractures in military combatants. Clin Orthop Relat Res 2019;477:813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hoffmann MF, Khoriaty JD, Sietsema DL, Jones CB. Outcome of intramedullary nailing treatment for intertrochanteric femoral fractures. J Orthop Surg Res 2019;14:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dinah AF, Vickers RH. Smoking increases failure rate of operation for established non-union of the scaphoid bone. Int Orthop 2007;31:503–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Little CP, Burston BJ, Hopkinson-Woolley J, Burge P. Failure of surgery for scaphoid non-union is associated with smoking. J Hand Surg [Br] 2006;31-B:252–255. [DOI] [PubMed] [Google Scholar]
- 89. Rahimnia A, Rahimnia AH, Mobasher-Jannat A. Clinical and functional outcomes of vascularized bone graft in the treatment of scaphoid non-union. PLoS One 2018;13:e0197768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hirche C, Heffinger C, Xiong L, et al. The 1,2-intercompartmental supraretinacular artery vascularized bone graft for scaphoid nonunion: management and clinical outcome. J Hand Surg [Am] 2014;39:423–429. [DOI] [PubMed] [Google Scholar]
- 91. Chang MA, Bishop AT, Moran SL, Shin AY. The outcomes and complications of 1,2-intercompartmental supraretinacular artery pedicled vascularized bone grafting of scaphoid nonunions. J Hand Surg [Am] 2006;31:387–396. [DOI] [PubMed] [Google Scholar]
- 92. Nelson HD, Morris CD, Kraemer DF, et al. Osteoporosis in postmenopausal women: diagnosis and monitoring. Evid Rep Technol Assess (Summ) 2001;28:1–2. [PMC free article] [PubMed] [Google Scholar]
- 93. Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002;359:1929–1936. [DOI] [PubMed] [Google Scholar]
- 94. van Staa TP, Leufkens HG, Cooper C. Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos Int 2002;13:624–629. [DOI] [PubMed] [Google Scholar]
- 95. Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, III, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 2000;15:721–739. [DOI] [PubMed] [Google Scholar]
- 96. Suetens C, Hopkins S, Kolman J, Diaz-Hoegberg L. Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals 2011–2012. Stockholm: European Centre for Disease Prevention and Control, 2013. [Google Scholar]
- 97. Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect 2017;96:1–15. [DOI] [PubMed] [Google Scholar]
- 98. Sanger PC, Hartzler A, Han SM, et al. Patient perspectives on post-discharge surgical site infections: towards a patient-centered mobile health solution. PLoS One 2014;9:e114016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jensen JA, Goodson WH, Hopf HW, Hunt TK. Cigarette smoking decreases tissue oxygen. Arch Surg 1991;126:1131–1134. [DOI] [PubMed] [Google Scholar]
- 100. Kortram K, Bezstarosti H, Metsemakers WJ, Raschke MJ, Van Lieshout EMM, Verhofstad MHJ. Risk factors for infectious complications after open fractures: a systematic review and meta-analysis. Int Orthop 2017;41:1965–1982. [DOI] [PubMed] [Google Scholar]
- 101. Zura R, Xiong Z, Einhorn T, et al. Epidemiology of fracture nonunion in 18 human bones. JAMA Surg 2016;151:e162775. [DOI] [PubMed] [Google Scholar]
- 102. Tzioupis C, Giannoudis PV. Prevalence of long-bone non-unions. Injury 2007;38:S3–S9. [DOI] [PubMed] [Google Scholar]
- 103. Calori GM, Mazza E, Colombo M, Ripamonti C, Tagliabue L. Treatment of long bone non-unions with polytherapy: indications and clinical results. Injury 2011;42:587–590. [DOI] [PubMed] [Google Scholar]
- 104. Dahabreh Z, Dimitriou R, Giannoudis PV. Health economics: a cost analysis of treatment of persistent fracture non-unions using bone morphogenetic protein-7. Injury 2007;38:371–377. [DOI] [PubMed] [Google Scholar]
- 105. Brinker MR, Trivedi A, O’Connor DP. Debilitating effects of femoral nonunion on health-related quality of life. J Orthop Trauma 2017;31:e37–e42. [DOI] [PubMed] [Google Scholar]
- 106. Bishop JA, Palanca AA, Bellino MJ, Lowenberg DW. Assessment of compromised fracture healing. J Am Acad Orthop Surg 2012;20:273–282. [DOI] [PubMed] [Google Scholar]
- 107. Copuroglu C, Calori GM, Giannoudis PV. Fracture non-union: who is at risk? Injury 2013;44:1379–1382. [DOI] [PubMed] [Google Scholar]
- 108. Scolaro JA, Schenker ML, Yannascoli S, Baldwin K, Mehta S, Ahn J. Cigarette smoking increases complications following fracture: a systematic review. J Bone Joint Surg [Am] 2014;96-A:674–681. [DOI] [PubMed] [Google Scholar]
- 109. Steinmann SP, Adams JE. Scaphoid fractures and nonunions: diagnosis and treatment. J Orthop Sci 2006;11:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kawamura K, Chung KC. Treatment of scaphoid fractures and nonunions. J Hand Surg [Am] 2008;33:988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bindra R, Bednar M, Light T. Volar wedge grafting for scaphoid nonunion with collapse. J Hand Surg [Am] 2008;33:974–979. [DOI] [PubMed] [Google Scholar]
- 112. Cooney WP, III, Dobyns JH, Linscheid RL. Nonunion of the scaphoid: analysis of the results from bone grafting. J Hand Surg [Am] 1980;5:343–354. [DOI] [PubMed] [Google Scholar]
- 113. Ditsios K, Konstantinidis I, Agas K, Christodoulou A. Comparative meta-analysis on the various vascularized bone flaps used for the treatment of scaphoid nonunion. J Orthop Res 2017;35:1076–1085. [DOI] [PubMed] [Google Scholar]