Abstract
Background:
Oropharyngeal cancers associated with high-risk human papillomavirus (HR-HPV) infection are increasing in the U.S., especially among men. We evaluated prevalence and predictors of concurrent (genital and oral) and concordant (same-type) HR-HPV infections in U.S.
Methods:
We used National Health and Nutrition Examination Survey from 2009–2016. Predictors were assessed via multivariable logistic regression.
Results:
Among 10,334 respondents, 172 (2.1%) had concurrent infections [109 (3.5%) men and 63 (0.76%) women]. Ninety-three (1.0%) had concordant infections [54 (1.6%) men and 39 (0.5%) women]. Predictors of concurrence in men were: no longer married vs. married [2.3 (OR); 1.3–4.9 (95% CI)], living with a partner vs. married [3.0; 1.2–7.5], and having 2–5 lifetime oral sex partners [3.0; 1.2–7.5]. In women they were: no longer married vs. married [3.6; 1.3–10.3], ≥2 recent sex partners [4.6; 1.4–15.6 for 2–5 partners and 3.9; 1.1–14.3 for 6+ partners], and marijuana use [2.2; 1.0–4.5]. The predictor of concordance in men and women was no longer married vs. married [3.5; 1.2–9.9 in men and 3.2; 1.1–9.4 in women].
Conclusions:
Concurrent and concordant HR-HPV infections occur at a high rate, especially among men, and are associated with behavioral factors. This underscores the importance of HPV vaccination, screening, and education in men.
Keywords: Concurrence, concordance, high-risk HPV infection, NHANES
Summary:
This study found a high prevalence rates of concurrent and concordant high risk-HPV infections, especially among men, adding to the knowledge base for developing new screening and vaccination guidelines for high risk-HPV infections, specifically targeting the U.S. male population.
Introduction
Human papillomavirus (HPV) is one of the most common sexually transmitted infections in the United States (U.S.) [1]. The most common anatomical sites for HPV infection are the anogenital and upper aerodigestive tracts. There are over 100 types of HPV categorized either into high-risk (HR) types with oncogenic potential or low-risk types. Persistent HR-HPV infection is known to cause cancer at several anatomic sites such as cervix, vulva, vagina, oropharynx, anus, and penis [2]. The causal effect of HPV on cervical cancer is well-known; 90–99% of cervical cancer cases are attributable to HPV [3, 4]. Between 2004–2012 there has been a decrease in yearly cervical cancer rates in the U.S., likely due to improved screening, vaccination, and testing in women; however, the overall rate of HPV-associated cancers has increased during the same time period [1].
The increase in HPV-associated cancers is partly attributed to the increasing incidence of HPV-positive oropharyngeal cancers (OPC). The OPCs include “cancers of the base of the tongue, pharyngeal tonsils, anterior and posterior tonsillar pillars, and glosstonsillar sulci; anterior surface of soft palate and uvula; and lateral and posterior pharyngeal walls” [1]. Between 1988 and 2004, the incidence of HPV-positive OPCs increased by 225%, while the incidence of HPV-negative OPCs declined by 50% [5]. Approximately 70% of OPCs are associated with HPV [3, 5]. It was estimated that the annual number of HPV-associated OPCs would surpass the annual number of HPV-associated cervical cancers by 2020 if these trends continued; however, this happened earlier than expected, by 2012 [1, 5]. Studies from other countries have also reported increasing incidence of OPCs [6].
While oral HPV infections are relatively rare, the rate of HPV-associated OPCs in men is about four times higher than that of women [1, 2, 6, 7]. Viens et al. reported the rates as 7.6 in men and 1.7 in women per 100,000 persons during 2008–2012 in the U.S. [1]. These figures are concerning given a lack of Food and Drug Administration approved screening tools for oral HR-HPV infection, which disproportionately affects men. Additionally, HPV up-to-date vaccination coverage was lower among men (37.5%) as compared to women (49.5%) in 2016 [8]. While no HPV vaccine trial (to our knowledge) has used oral HPV as an endpoint, some researchers have inferred a link between the vaccine and potential protection against oral HPV in addition to genital HPV [9, 10]. Thus, it is possible that the higher rate of OPCs in men could be due to a lower vaccination rate among men.
There have been only few studies to date focusing on both concurrent and concordant infections. Concurrent HPV infection is defined as simultaneous detection of any HR-HPV type in both the oral cavity and the cervix/penis of a person. Concordant HPV infection is defined as one or more of the same HR-HPV types detected in both the oral cavity and the cervix/penis of a person. Separate studies on men and women have estimated prevalence of concurrence from earlier cycles of the National Health and Nutrition Examination Survey (NHANES) [11, 12]. Limitations of these studies included small sample sizes and lack of sufficient power to analyze the HR-HPV subgroups responsible for the majority of cancers described above (e.g., subgroup analyses of HPV 16 and 18). A study of men in rural China estimated prevalence of concurrence and concordance but included low-risk subtypes. Results from that study are not generalizable to the U.S. population [13]. Another study estimated prevalence of concurrent infections in the U.S. using NHANES, but its focus was comparative and it did not break down infections by high-risk and low-risk [14]. We aimed to fill the gap in knowledge of concurrent and concordant HR-HPV infections by evaluating the prevalence and predictors of concurrent and concordant HR-HPV infections among U.S. men and women using all available NHANES cycles, including the most recent to date.
Methods
Study Design and Participants
We used data from NHANES, a nationally representative survey of the non-institutionalized U.S. population, collected by the National Center for Health Statistics and the Centers for Disease Control and Prevention (CDC) [15]. During the informed consent process, survey participants were assured that collected data will be used only for study purposes and will not be released to others without consent of the individual or the establishment [16]. Participant characteristics included 18–59 years old men who completed the survey from 2013–2016 and women from 2009–2016 for whom oral and genital HPV test results were available.
Specimen Collection and Laboratory Methods
The NHANES procedure for sample collection and laboratory methods are outlined in detail elsewhere [17]. Briefly, oral samples were collected by having participants rinse and gargle with Scope mouthwash [17]. Genital samples were collected using self-collected vaginal and penile swabs. These were analyzed for 37 types of HPV using a multiplex polymerase chain-reaction (PCR) assay [17]. Out of 37 types, 18 are classified as HR-HPV: 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82 [18].
Statistical Methods
First, we estimated the prevalence of HR-HPV concurrent and concordant infections for four clinically important sub-groups: 1.) All high-risk types, 2.) The high-risk types covered in the 9-valent vaccine (HPV 16, 18, 31, 33, 45, 52, and 58), 3.) The most common types found in cervical cancers (HPV 16, 18, or 45), and 4.) The most common type found in HPV-associated cancers (HPV 16) [19, 20].
To estimate the prevalence of concurrent infections, we identified participants with simultaneous HR-HPV oral and genital infections. In the same manner, to estimate the prevalence of concordant infections, we identified participants with oral and genital infections who had at least one of the same HR-HPV types. The survey adjusted prevalence was calculated for total population, men and women.
We computed new NHANES sample weights by dividing CDC weights by the number of cycles [15]. Thus, for men we used the four-year sample weights by dividing by 2 (2013–2016). For women, we used eight-year sample weighs by dividing by 4 (2009–2016).
We also conducted Monte Carlo simulations for the four above-mentioned HR-HPV groups to test whether concurrent and concordant HR-HPV infections occur more than expected by the population marginal prevalence of oral and genital infections. Ten thousand individuals were sampled with HR-HPV infection status based on the actual marginal oral and genital prevalence assuming a binomial distribution. The p-value was calculated as 2 × the proportion of times the simulated HR-HPV concurrent/concordant prevalence estimate was smaller than that observed in NHANES. The 95% confidence interval (CI) was 2.5 and 97.5 percentiles of the simulated proportions.
Finally, we evaluated the predictors of concurrent and concordant HR-HPV infections using univariate and multivariable survey weighted logistic regression models in men and women separately. We computed survey-adjusted chi-square statistics for categorical predictors of concurrence and concordance. Variables included: ethnicity (non-Hispanic white, non-Hispanic black, and other), age groups (18 to 24, 25 to 39, and 40 to 59), marital status (married, no longer married [widowed, divorced, or separated], never married, living with partner, and missing), lifetime and recent number of sex (vaginal, oral or anal) partners (0–1, 2–5, 6–10, 11 or more, and missing), and lifetime and recent number of oral sex partners (0–1, 2–5, 6–10, 11 or more, and missing), HPV vaccination (yes, no), smoking and marijuana use (never, ever, and missing), and sexual orientation (heterosexual, homosexual/bisexual, and other). Multivariable models included any predictor with p<0.15 on univariate analysis [14]. Statistical significance was set at a two-sided type-1 error rate (α) of 0.05. We flagged all parameter estimates with a relative standard error greater than 30% as these are considered unstable and should be interpreted with caution. All the statistical analysis was done using R software.
Results
Prevalence of concurrent and concordant HR-HPV infections in the U.S.
Of 10,334 individuals (3241 men and 7093 women) tested for both oral and genital HPV infections, 172 (2.1%) had concurrent HR-HPV infection, while 93 (1.0%) had concordant HR-HPV infection (Tables 1 and 2). The prevalence of concurrent HR-HPV infection in the total population, men, and women was 2.1% (n=172), 3.5% (n=109), and 0.76% (n=63), respectively. For the 9-valent vaccine types, it was 0.56% (n=46), 0.86% (n=27), and 0.27% (n=19), respectively; for HPV 16, 18, or 45, it was 0.34% (n=32), 0.50% (n=18), and 0.20% (n=14), respectively; and for HPV 16, it was 0.15% (n=18), 0.17% (n=10), and 0.13% (n=8), respectively (Table 1). The odds of having oral HR-HPV infection for those with vs. without an HPV genital infection for the total population, men and women were as follows, for any HR-HPV: 3.44, 3.6, and 2.59, respectively; for the HR 9-valent vaccine: 3.42, 2.96, and 5.33, respectively; for HPV 16, 18 or 45: 4.77, 3.71, and 10.62, respectively; for HPV 16: 5.4, 3.26, and 18.76, respectively. The chi-square p-value for each of the odds ratios (OR) was <0.005 (Table 1).
Table 1.
Prevalence of Concurrent Oral and Genital High-Risk Human Papillomavirus (HR-HPV) Infections among Four Groups of HR-HPV for Total Population, Men, and Women (NHANES: 2013–2016 for Men, 2009–2016 for Women).
| HR-HPV Groups | Oral HR-HPV Infection Given Genital Infection | |||||
|---|---|---|---|---|---|---|
| Yes |
No |
Total |
||||
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
|
| ||||||
| Total Population | ||||||
| HR-HPVa | OR = 3.44; Chi2 = 162.81, p-value < .005 | |||||
| Yes | 172 | 2.1 (1.7, 2.7) | 2649 | 24.3 (22.8, 25.9) | 2821 | 26.4 (24.8, 28.2) |
| No | 158 | 1.8 (1.4, 2.3) | 7355 | 71.8 (69.9, 73.5) | 7513 | 73.6 (71.8, 75.2) |
| Total | 330 | 3.9 (3.3, 4.6) | 10004 | 96.1 (95.4, 96.7) | 10334 | |
| HR 9V Typesb | OR = 3.42; Chi2 = 65.51, p-value < .005 | |||||
| Yes | 46 | 0.56 (0.37, 0.84) | 1231 | 11.2 (10.3, 12.1) | 1277 | 11.7 (10.8, 12.7) |
| No | 85 | 1.3 (0.93, 1.7) | 8972 | 87.0 (85.9, 88.0) | 9057 | 88.3 (87.3, 89.2) |
| Total | 131 | 1.8 (1.5, 2.2) | 10203 | 98.2 (97.8, 98.5) | 10334 | |
| HPV 16, 18, or 45c | OR = 4.77; Chi2 = 75.60, p-value < .005 | |||||
| Yes | 32 | 0.34 (0.22, 0.55) | 676 | 6.5 (5.8, 7.2) | 708 | 6.8 (6.2, 7.6) |
| No | 64 | 1.0 (0.78, 1.3) | 9562 | 92.1 (91.4, 92.8) | 9626 | 93.2 (92.4, 93.8) |
| Total | 96 | 1.4 (1.1, 1.7) | 10238 | 98.6 (98.3, 98.9) | 10334 | |
| HPV 16d | OR = 5.4; Chi2 = 44.70, p-value < .005 | |||||
| Yes | 18 | 0.15 (0.09, 0.25) | 336 | 3.6 (3.1, 4.2) | 354 | 3.7 (3.2, 4.3) |
| No | 46 | 0.75 (0.55, 1.0) | 9934 | 95.5 (94.9, 96.1) | 9980 | 96.3(95.7, 96.8) |
| Total | 64 | 0.90 (0.70, 1.1) | 10270 | 99.1 (98.9, 99.3) | 10334 | |
| Men | ||||||
| HR-HPVa | OR = 3.6; Chi2 = 84.16, p-value < .005 | |||||
| Yes | 109 | 3.5 (2.7, 4.6) | 808 | 24.6 (22.4, 26.9) | 917 | 28.1 (25.5, 30.8) |
| No | 92 | 2.7 (2.0, 3.7) | 2232 | 69.2 (66.2, 72.0) | 2324 | 71.9 (69.2, 74.5) |
| Total | 201 | 6.3 (5.2, 7.6) | 3040 | 93.7 (92.4, 94.8) | 3241 | |
| HR 9V Typesb | OR = 2.96; Chi2 = 24.21, p-value < .005 | |||||
| Yes | 27 | 0.86 (0.52, 1.4) | 378 | 11.5 (10.0, 13.0) | 405 | 12.3 (10.8, 14.0) |
| No | 53 | 2.2 (1.5, 3.1) | 2783 | 85.5 (83.6, 87.2) | 2836 | 87.7(86.0, 89.2) |
| Total | 80 | 3.0 (2.3, 3.9) | 3161 | 97.0 (96.1, 97.7) | 3241 | |
| HPV 16, 18, or 45c | OR = 3.71; Chi2 = 23.36, p-value < .005 | |||||
| Yes | 18 | 0.50 (0.27, 0.91) | 210 | 6.8 (5.7, 8.0) | 228 | 7.3 (6.3, 8.4) |
| No | 42 | 1.8 (1.3, 2.4) | 2971 | 90.9 (89.7, 92.0) | 3013 | 92.7 (91.6, 93.7) |
| Total | 60 | 2.3 (1.8, 3.0) | 3181 | 97.7 (97.0, 98.2) | 3241 | |
| HPV 16d | OR = 3.26; Chi2 = 7.41, p-value < .05 | |||||
| Yes | 10 | 0.17 (0.09, 0.34)^ | 109 | 3.8 (3.0, 4.8) | 119 | 4.0 (3.2, 4.9) |
| No | 32 | 1.3 (0.93, 1.9) | 3090 | 94.7 (93.6, 95.6) | 3122 | 96.0 (95.1, 96.8) |
| Total | 42 | 1.5 (1.1, 2.0) | 3199 | 98.5 (98.0, 98.9) | 3241 | |
| Women | ||||||
| HR-HPVa | OR = 2.59; Chi2 = 27.68, p-value < .005 | |||||
| Yes | 63 | 0.76 (0.56, 1.0) | 1841 | 24.1 (22.6, 25.7) | 1904 | 24.8 (23.3, 26.5) |
| No | 66 | 0.90 (0.65, 1.2) | 5123 | 74.3 (72.7, 75.8) | 5189 | 75.2 (73.5, 76.7) |
| Total | 129 | 1.7 (1.3, 2.1) | 6964 | 98.3 (97.9, 98.7) | 7093 | |
| HR 9V Typesb | OR = 5.33; Chi2 = 39.15, p-value < .005 | |||||
| Yes | 19 | 0.27 (0.15, 0.46) | 853 | 10.9 (9.9, 11.9) | 872 | 11.1 (10.2, 12.2) |
| No | 32 | 0.41 (0.26, 0.64) | 6189 | 88.5 (87.4, 89.5) | 6221 | 88.9 (87.8, 89.8) |
| Total | 51 | 0.67 (0.45, 0.99) | 7042 | 99.3 (99.0, 99.5) | 7093 | |
| HPV 16, 18, or 45c | OR = 10.62; Chi2 = 69.37, p-value < .005 | |||||
| Yes | 14 | 0.20 (0.10, 0.40)^ | 466 | 6.2 (5.5, 7.1) | 480 | 6.4 (5.7, 7.3) |
| No | 22 | 0.28 (0.16, 0.49) | 6591 | 93.3 (92.4, 94.1) | 6613 | 93.6 (92.7, 94.3) |
| Total | 36 | 0.48 (0.28, 0.80) | 7057 | 99.5 (99.2, 99.7) | 7093 | |
| HPV 16d | OR = 18.76; Chi2 = 90.90, p-value < .005 | |||||
| Yes | 8 | 0.13 (0.06, 0.30)^ | 227 | 3.4 (2.8, 4.0) | 235 | 3.5 (2.9, 4.2) |
| No | 14 | 0.20 (0.10, 0.40)^ | 6844 | 96.3 (95.6, 96.9) | 6858 | 96.5 (95.8, 97.1) |
| Total | 22 | 0.33 (0.19, 0.59) | 7071 | 99.7 (99.4, 99.8) | 7093 | |
NHANES: National Health and Nutrition Examination Survey
CI: Confidence Interval
OR: Odds Ratio
HR-HPV: All 18 high-risk types
HPV 9V Types: HR-HPV types covered in the 9-valent vaccine (types 16, 18, 31, 33, 45, 52, and 58)
HPV 16, 18, 45: The most common types found in cervical cancers
HPV 16: The most common type found in HPV-associated cancers
The relative standard error of the weighted prevalence estimate was > 30%
Table 2.
Prevalence of Concordant Oral and Genital High-Risk Human Papillomavirus (HR-HPV) Infections for Total Population, Men, and Women. (NHANES: 2013–2016 for Men, 2009–2016 for Women).
| Total Population | Men | Women | ||||
|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
| Total Population | 10334 | 3241 | 7093 | |||
| Concordant | 93 | 1.0 (0.77, 1.4) | 54 | 1.6 (1.1, 2.2) | 39 | 0.51 (0.36, 0.72) |
| Non-Concordant | 79 | 1.1 (0.79, 1.5) | 55 | 2.0 (1.4, 2.8) | 24 | 0.25 (0.16, 0.39) |
| No Concurrent | 10162 | 97.9 (97.3, 98.3) | 3132 | 96.5 (95.4, 97.3) | 7030 | 99.2 (99.0, 99.4) |
| Individuals with Concurrent Infections | 172 | 109 | 63 | |||
| Concordant | 93 | 48.5 (38.9, 58.3) | 54 | 44.4 (33.1, 56.3) | 39 | 67.1 (53.8, 78.1) |
| Non-Concordant | 79 | 51.5 (41.7, 61.1) | 55 | 55.6 (43.7, 66.9) | 24 | 32.9 (21.9, 46.2) |
NHANES: National Health and Nutrition Examination Survey
CI: Confidence Interval
The prevalence of concordant HR-HPV infection in total population, men, and women was 1% (n=93), 1.6% (n=54), and 0.51% (n=39), respectively. However, the prevalence of concordant HR-HPV infection in those who had concurrent infection was 48.5% (n=93), 44.4% (n=54), and 67.1% (n=39), respectively (Table 2). HPV 16 was the most common concordant type in our study (Supplementary Table 1 that enumerates the complete and partial type of HR-HPV concordances). This is important since HPV 16 is responsible for the majority of oral and cervical cancers [19, 20].
Monte Carlo simulations demonstrated that concurrence and concordance occurred significantly more than expected given the population marginal prevalence of oral and genital infections of the total population (Figure 1 for concurrence), men, and women (Supplementary Figures 1, 2, 3, 4 and 5, all p-values for the test of independence were p<0.0001).
Figure 1.
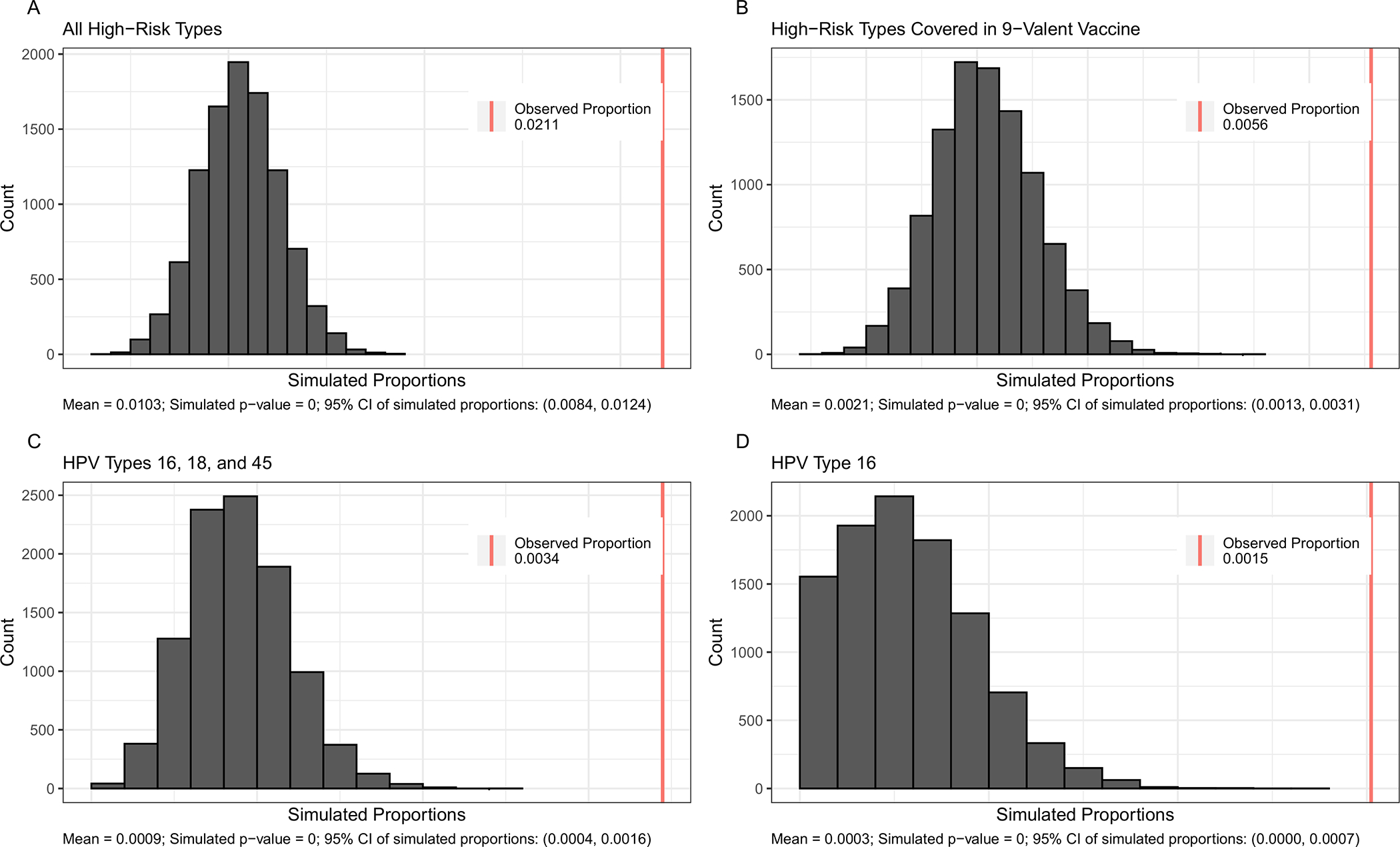
Histograms of Simulated Proportions of Concurrent High–Risk Human Papillomavirus (HR–HPV) Infection for Total Population by Four HR-HPV Groups. (NHANES: 2013–2016 for Men, 2009–2016 for Women).
NHANES: National Health and Nutrition Examination Survey
Panels:
A. All High–Risk Types
B. High–Risk Types Covered in 9–Valent Vaccine
C. HPV Types 16, 18, and 45
D. HPV Type 16
Demographic and behavioral predictors of concurrent HR-HPV infections in men and women
Tables 3 and 4 display the prevalence by demographic and behavioral predictors (left), and logistic regression results (right) for concurrent and concordant infection among men and women, respectively. In univariate analysis for men; ethnicity, marital status, number of lifetime and recent sex partners, number of lifetime and recent oral sex partners, cigarette use, marijuana use, and sexual orientation were associated with concurrence. In multivariable analysis, men who were no longer married had higher odds of having a concurrent HR-HPV infection compared to married men (OR=2.5, 95% CI: 1.2–5.2). Men who were living with a partner had higher odds of a concurrent infection compared to married men (OR=3.0, 95% CI: 1.2–7.5). Men with 2–5 lifetime oral sex partners had higher odds of having concurrent infection compared to men with 0 or 1 partner (OR=3.0; 95% CI: 1.2–7.5).
Table 3.
Prevalence and Predictors of the Concurrent and Concordant High-Risk Human Papillomavirus (HR-HPV) Infections among Men. (NHANES: 2013–2016).
| Variables | Concurrent Infection |
Concordant Infection |
||||||
|---|---|---|---|---|---|---|---|---|
| Prevalence | Logistic Regression | Prevalence | Logistic Regression | |||||
| n | % (95% CI) | Univariate OR | Adjusted OR | n | % (95% CI) | Univariate OR | Adjusted OR | |
|
| ||||||||
| Marital Status ‡‡‡ ††† | ||||||||
| Married | 30 | 1.7 (0.83, 2.5) | Ref | Ref | 14 | 0.68 (0.25, 1.1)^ | Ref | Ref |
| No longer married | 21 | 9.5 (5.9, 13.1) | 5.4 (2.9, 10.1)*** | 2.5 (1.2, 5.2)*^ | 10 | 4.1 (1.5, 6.8)^ | 6.9 (2.5, 18.6)*** | 3.5 (1.2, 9.9)*^ |
| Never married | 34 | 4.5 (2.8, 6.2) | 2.9 (1.7, 5.0)*** | 2.3 (1.0, 4.9)^ | 18 | 2.1 (0.99, 3.1) | 3.2 (1.5, 7.0)**^ | 1.4 (0.41, 4.9)^ |
| Living with partner | 21 | 5.8 (2.6, 9.0) | 3.7 (1.6, 8.4)***^ | 3.0 (1.2, 7.5)*^ | 10 | 2.7 (0.79, 4.6)^ | 4.3 (1.5, 12.5)*^ | 2.6 (0.71, 9.7)^ |
| Missing | 3 | 0.94 (−0.28, 2.2)^ | 0.58 (0.13, 2.6) | 1.5 (0.17, 13.5)^ | 2 | 0.77 (−0.39, 1.9)^ | 1.2 (0.21, 7.0) | 1.2 (0.07, 20.9)^ |
| Lifetime Sex Partners ‡‡‡ ††† | ||||||||
| 0–5 | 13 | 0.87 (0.21, 1.5)^ | Ref | Ref | 11 | 0.62 (0.11, 1.1)^ | Ref | Ref |
| 6–10 | 18 | 2.1 (0.90, 3.3) | 2.5 (1.1, 5.7)*^ | 1.1 (0.38, 3.4)^ | 8 | 1.3 (0.30, 2.3)^ | 2.1 (0.71, 6.2)^ | 1.2 (0.31, 5.0)^ |
| 11+ | 71 | 7.0 (4.8, 9.3) | 8.6 (3.7, 20.1)*** | 3.3 (1.0, 10.6)^ | 33 | 2.8 (1.6, 4.0) | 4.7 (1.9, 12.0)***^ | 2.4 (0.60, 9.4)^ |
| Missing | 7 | 4.7 (0.54, 8.8)^ | - | - | 2 | 0.94 (−0.30, 2.2)^ | - | - |
| Recent Sex Partners ‡‡‡ ††† | ||||||||
| 0–1 | 56 | 2.2 (1.4, 2.9) | Ref | Ref | 26 | 0.89 (0.53, 1.2) | Ref | Ref |
| 2–5 | 36 | 9.3 (5.8, 12.8) | 4.6 (2.9, 7.4)*** | 2.7 (1.0, 7.3)^ | 19 | 4.3 (2.2, 6.3) | 5.0 (3.1, 8.1)*** | 1.5 (0.65, 3.6)^ |
| 6+ | 10 | 6.6 (1.7, 11.6)^ | 3.2 (1.3, 8.1)*^ | 1.3 (0.33, 5.2)^ | 7 | 5.1 (0.39, 9.8)^ | 6.0 (2.0, 18.0)***^ | 1.4 (0.27, 6.8)^ |
| Missing | 7 | 4.7 (0.54, 8.8)^ | - | - | 2 | 0.94 (−0.30, 2.2)^ | - | - |
| Lifetime Oral Sex Partners ‡‡‡ ††† | ||||||||
| 0–1 | 10 | 0.53 (0.12, 0.94)^ | Ref | Ref | 8 | 0.44 (0.07, 0.82)^ | Ref | Ref |
| 2–5 | 33 | 3.2 (2.1, 4.2) | 6.1 (2.7, 13.5)*** | 3.0 (1.2, 7.5)*^ | 16 | 1.6 (0.54, 2.6)^ | 3.5 (1.2, 10.1)*^ | 1.6 (0.53, 5.1)^ |
| 6+ | 59 | 6.6 (4.5, 8.8) | 13.2 (5.9, 29.6)*** | 2.7 (1.1, 6.7)^ | 28 | 2.7 (1.4, 4.1) | 6.3 (2.5, 15.7)*** | 1.2 (0.31, 4.3)^ |
| Missing | 7 | 4.7 (0.54, 8.8)^ | - | - | 2 | 0.94 (−0.30, 2.2)^ | - | - |
| Recent Oral Sex Partners ‡‡‡ ††† | ||||||||
| 0–1 | 68 | 2.6 (1.8, 3.5) | Ref | Ref | 30 | 0.99 (0.58, 1.4) | Ref | Ref |
| 2–5 | 27 | 9.1 (5.4, 12.7) | 3.7 (2.3, 6.0)***^ | 0.92 (0.41, 2.1) | 17 | 5.6 (2.6, 8.6) | 5.9 (3.2, 11.1)*** | 2.5 (0.81, 7.5)^ |
| 6+ | 7 | 9.7 (1.4, 17.9)^ | 4.0 (1.5, 11.0)*^ | 1.3 (0.25, 6.5)^ | 5 | 6.7 (0.61, 12.8)^ | 7.2 (2.4, 21.6)*** | 2.1 (0.27, 16.9)^ |
| Missing | 7 | 4.7 (0.54, 8.8)^ | - | - | 2 | 0.94 (−0.30, 2.2)^ | - | - |
| Marijuana Use ‡‡‡ | ||||||||
| Never | 21 | 1.5 (0.72, 2.3) | Ref | Ref | 15 | 1.2 (0.48, 2.0)^ | Ref | - |
| Ever | 81 | 4.5 (3.1, 5.9) | 3.1 (1.6, 5.8)*** | 1.3 (0.63, 2.5)^ | 37 | 1.8 (1.1, 2.5) | 1.4 (0.72, 2.9)^ | - |
| Missing | 7 | 4.5 (0.53, 8.5)^ | - | - | 2 | 0.92 (−0.29, 2.1)^ | - | - |
NHANES: National Health and Nutrition Examination Survey; CI: Confidence Interval
Additional adjusted covariates: ethnicity, age group, HPV vaccine, cigarette use, and sexual orientation
Concurrent Chi-Squared p-values:
p-value < .05
p-value < .01
p-value < .005
Concordant Chi-Squared p-values:
p-value < .05
p-value < .01
p-value < .005.
Logistic regression odds ratio p-values:
p-value < .05
p-value < .01
p-value < .005.
The relative standard error of the weighted prevalence estimate was > 30%.
Table 4.
Prevalence and Predictors of the Concurrent and Concordant High-Risk Human Papillomavirus (HR-HPV) Infections among Women. (NHANES: 2009–2016).
| Variables | Concurrent Infection |
Concordant Infection |
||||||
|---|---|---|---|---|---|---|---|---|
| Prevalence | Logistic Regression | Prevalence | Logistic Regression | |||||
| n | % (95% CI) | Univariate OR | Adjusted OR | n | % (95% CI) | Univariate OR | Adjusted OR | |
|
| ||||||||
| Marital Status ‡‡‡ ††† | ||||||||
| Married | 11 | 0.28 (0.10, 0.46)^ | Ref | Ref | 9 | 0.24 (0.07, 0.42)^ | Ref | Ref |
| No longer married | 16 | 1.7 (0.75, 2.6) | 5.8 (2.2, 15.7)*** | 3.6 (1.3, 10.3)*^ | 11 | 1.3 (0.44, 2.2)^ | 5.3 (1.8, 15.8)***^ | 3.2 (1.1, 9.4)*^ |
| Never married | 21 | 0.95 (0.49, 1.4) | 3.2 (1.4, 7.5)**^ | 2.6 (0.88, 7.9)^ | 9 | 0.45 (0.15, 0.75)^ | 1.8 (0.67, 5.1)^ | 1.5 (0.40, 5.4)^ |
| Living with partner | 6 | 0.86 (0.17, 1.6)^ | 2.7 (0.90, 8.1)^ | 2.3 (0.72, 7.4)^ | 2 | 0.27 (−0.11, 0.64)^ | 1.1 (0.22, 5.5)^ | 0.96 (0.21, 4.5) |
| Missing | 9 | 1.8 (0.38, 3.1)^ | 6.2 (2.2, 17.4)*** | 6.7 (1.4, 32.2)*^ | 8 | 1.6 (0.25, 3.0)^ | 6.6 (2.2, 20.1)***^ | 6.0 (0.99, 35.9)^ |
| Lifetime Sex Partners † | ||||||||
| 0–1 | 8 | 0.28 (0.07, 0.49)^ | Ref | Ref | 7 | 0.21 (0.05, 0.38)^ | Ref | Ref |
| 2–5 | 17 | 0.66 (0.15, 1.2)^ | 2.3 (0.71, 7.3)^ | 1.2 (0.24, 6.0)^ | 10 | 0.41 (0.05, 0.77)^ | 1.9 (0.52, 6.8)^ | 0.77 (0.13, 4.5) |
| 6–10 | 18 | 0.96 (0.45, 1.5) | 3.3 (1.3, 8.7)*^ | 1.0 (0.29, 3.7)^ | 11 | 0.61 (0.19, 1.0)^ | 2.8 (1.0, 7.7)^ | 0.80 (0.19, 3.3) |
| 11+ | 17 | 1.3 (0.56, 2.1) | 4.5 (1.7, 12.0)***^ | 0.92 (0.22, 3.9) | 11 | 1.0 (0.27, 1.8)^ | 4.7 (1.6, 13.7)**^ | 1.0 (0.24, 4.6)^ |
| Missing | 3 | 0.32 (−0.05, 0.70)^ | - | - | 0 | - | - | - |
| Recent Sex Partners ‡‡‡ ††† | ||||||||
| 0–1 | 34 | 0.51 (0.31, 0.71) | Ref | Ref | 24 | 0.35 (0.21, 0.49) | Ref | Ref |
| 2–5 | 23 | 2.4 (1.2, 3.6) | 4.7 (2.4, 9.1)*** | 4.6 (1.4, 15.6)*^ | 13 | 1.6 (0.53, 2.6)^ | 4.5 (2.0, 10.3)*** | 4.2 (0.83, 21.0)^ |
| 6+ | 3 | 4.6 (−2.03, 11.3)^ | 9.4 (1.8, 47.5)**^ | 3.9 (1.1, 14.3)*^ | 2 | 3.8 (−2.74, 10.3)^ | 10.9 (1.7, 69.1)*^ | 3.8 (0.77, 18.4)^ |
| Missing | 3 | 0.32 (−0.05, 0.70)^ | - | - | 0 | - | - | - |
| Lifetime Oral Sex Partners ‡ † | ||||||||
| 0–1 | 17 | 0.46 (0.18, 0.74)^ | Ref | Ref | 11 | 0.24 (0.06, 0.42)^ | Ref | Ref |
| 2–5 | 27 | 0.84 (0.47, 1.2) | 1.8 (0.94, 3.5)^ | 1.1 (0.43, 2.9)^ | 19 | 0.69 (0.35, 1.0) | 2.9 (1.1, 7.6)*^ | 1.8 (0.42, 7.4)^ |
| 6+ | 16 | 1.3 (0.61, 2.1) | 2.9 (1.2, 6.6)*^ | 1.1 (0.28, 4.7)^ | 9 | 0.83 (0.16, 1.5)^ | 3.4 (1.1, 10.6)*^ | 0.94 (0.15, 5.9) |
| Missing | 3 | 0.33 (−0.05, 0.70)^ | - | - | 0 | - | - | - |
| Recent Oral Sex Partners ‡‡‡ ††† | ||||||||
| 0–1 | 45 | 0.67 (0.45, 0.89) | Ref | Ref | 28 | 0.44 (0.27, 0.62) | Ref | Ref |
| 2–5 | 12 | 1.6 (0.54, 2.6)^ | 2.4 (1.2, 4.8)*^ | 0.50 (0.14, 1.8) | 9 | 1.2 (0.35, 2.1)^ | 2.8 (1.2, 6.6)*^ | 0.71 (0.14, 3.5) |
| 6+ | 3 | 7.1 (−3.07, 17.4)^ | 11.1 (2.2, 57.3)**^ | 2.8 (0.65, 12.3)^ | 2 | 5.8 (−4.16, 15.8)^ | 13.4 (2.0, 88.1)**^ | 3.9 (0.69, 22.2)^ |
| Missing | 3 | 0.33 (−0.05, 0.70)^ | - | - | 0 | - | - | - |
| Marijuana Use ‡‡‡ ††† | ||||||||
| Never | 14 | 0.37 (0.14, 0.61)^ | Ref | Ref | 9 | 0.25 (0.04, 0.45)^ | Ref | Ref |
| Ever | 46 | 1.1 (0.77, 1.5) | 3.0 (1.5, 6.0)***^ | 2.2 (1.0, 4.5)*^ | 30 | 0.80 (0.49, 1.1) | 3.2 (1.3, 8.4)*^ | 2.0 (0.70, 5.5)^ |
| Missing | 3 | 0.32 (−0.05, 0.69)^ | - | - | 0 | - | - | - |
NHANES: National Health and Nutrition Examination Survey; CI: Confidence Interval
Additional adjusted covariates: ethnicity, age group, HPV vaccine, cigarette use, and sexual orientation
Concurrent Chi-Squared p-values
p-value < .05
p-value < .01
p-value < .005;
Concordant Chi-Squared p-values
p-value < .05
p-value < .01
p-value < .005.
Logistic regression odds ratio p-values
p-value < .05
p-value < .01
p-value < .005.
The relative standard error of the weighted prevalence estimate was > 30%.
In univariate analysis for women; marital status, number of lifetime and recent sex partners, number of lifetime and recent oral sex partners, cigarette use, and marijuana use were associated with concurrence. In multivariable analysis, only marital status, number of recent sex partners, and marijuana use remained associated. Women who were no longer married had higher odds of having a concurrent infection vs. married women (OR=3.6, 95% CI: 1.3–10.3). Women with 2–5 and 6–10 recent sex partners had higher odds of having a concurrent infection compared to women with 0 or 1 partner (OR=4.6, 95% CI: 1.4–15.6) and (OR=3.9, 95% CI: 1.1–14.3), respectively. Women who ever used marijuana had higher odds of having concurrent infection compared to never-users (OR=2.2, 95% CI: 1.0–4.5).
Demographic and behavioral predictors of concordant HR-HPV infections in men and women
Univariate logistic regression results were similar to that for concurrence. For men, marital status, number of lifetime and recent sex partners, number of lifetime and recent oral sex partners, and sexual orientation were associated with concordance (Table 3). For women, marital status, number of lifetime and recent sex partners, number of lifetime and recent oral sex partners, and marijuana use were associated with concordance (Table 4).
Moreover, for multivariable regression, both men and women who were no longer married had more than three times the odds of having a concordant infection compared to married men and women, after adjusting for other variables (Men OR=3.5, 95% CI: 1.2–9.9, Women OR=3.2, 95% CI: 1.1–9.4) (Tables 3 and 4).
Discussion
The literature on concurrent and concordant high risk HPV infections is limited. We believe ours is the largest and latest nationally representative U.S. study to focus solely on estimating prevalence and determining predictors of concurrent and concordant HR-HPV infections. This study showed that 2.1% of the U.S. population had concurrent and 1% had concordant HR-HPV infection from 2009–2016. Aligning with the 2010 U.S. Census, this roughly equates to 6.4 million Americans living with concurrent and 3.1 million with concordant HR-HPV infections. The burden is particularly large for men, equating to roughly 5.3 million (3.5%) and 2.4 million (1.6%) men as compared to roughly 1.2 million (0.76%) and 0.8 million (0.51%) women with concurrent and concordant HR-HPV infections, respectively.
Our study findings on difference in the burden are consistent with results from other studies, which have typically included both low and high risk HPV types. Kedarisetty et al. showed that for any HPV type, 7% of women with genital HPV infection also had oral HPV infection while only 1% of women with no genital HPV infection had oral HPV infection [11]. Patel et al. found similar results for any HPV in men where 19% of men with penile HPV infection had oral HPV infection while 4% of men with no penile HPV infection had oral HPV infection [13]. Sonawane et al. showed similar associations for oral HPV infections between both men and women with and without genital HPV infections [14]. Moreover, a study for both high and low-risk HPV in a geographically distinct rural Chinese sample also found overall greater concurrence and concordance than expected [12], using the same simulation-based approach as our study.
Another noteworthy finding of our study was the prevalence of HR 9-valent vaccine type concurrent infections among men (0.86%) and women (0.27%) reflecting roughly 1.3 million men and just over 400,000 women who have infections amenable to prevention by the HPV 9-valent vaccine. Such difference is alarming considering low HPV vaccine uptake, especially in men. The Advisory Committee on Immunization Practices has recommended vaccination for girls aged 11 or 12 years since 2006, and for boys since 2011 [21]. HPV 16 and 18 are the two most common types found in HPV-associated cancers causing 63% of cases [1]. All HPV vaccines (bivalent, quadrivalent and nine-valent) protect against types 16 and 18. Vaccination against types 16 and 18 could prevent almost 25,000 cancer cases annually in the U.S. [3]. Of U.S. female aged 13–17 years, 65.1% received one or more doses, 55.0% received two or more doses, and 43.0% received three or more doses in 2016; for males, the rates were 56.0%, 43.6%, and 31.5%, respectively [8]. This is in stark contrast to the Hepatitis-B vaccine for which 94.1% of adolescent males received three or more doses [8]. In general, our study underscores the importance of the HPV vaccine, and for providing support for its continued recommendation for boys and men, who have much lower vaccination rates than girls and women [8]. The role of HPV vaccine in protecting against certain types of HPV that can cause OPCs may contribute in preventing OPCs overall [9, 10]. Moreover, among high risk group of people such as men who have sex with men for whom oral sex is common, it is also important to use condom or dental dam to prevent transmission of infection via oral sex.
Although concurrent HR-HPV infections are relatively rare, the rates of concordant infection among those with concurrent infections were incredibly high, particularly among women (67.1% in women vs. 44.4% in men). In this case, HR-HPV genital infection can flag the potential for HR-HPV oral infection. Conversely, individuals diagnosed with HR-HPV oral infection could be more closely monitored for genital infections and cancer. This is more important for men as they are not screened for genital infections at all. Just as an anal pap test is recommended for high-risk group of men [22], a penile swab could be used in practice to test HPV DNA based on personal medical history and presence of risk factors. Selective screening guidelines should be developed to risk stratify people and recommend additional screening in those known to have a positive HR-HPV infection at one anatomic site, as also suggested by Kedarisetty et al. [11]. Moreover, OPCs are rising among young U.S. men and there is no existing protocol for HPV-positive OPC screening.
In the U.S., the rates of any (low or high risk) HPV oral infections are higher in men than women [11, 13, 14]. Current results are similar with respect to HR-HPV infections. Although the exact reason is unknown, possible explanation include men have more sexual partners than women, transmission of HPV is more efficient when performing oral sex on infected females compared to infected males, and/or women may have partial immunity from cervical infections that protect them against oral HPV infections [2].
One risk factor associated with both concurrence and concordance in men and women was marital status. Our finding of a greater risk among women who are no longer married, is supported by a major population-based study in Italy that found a higher prevalence of HPV among single women [23]. As compared to married people, no longer married people may get involved with a greater number of sex (oral or any sex) partners that can ultimately put them at risk of HR-HPV infections. Additionally, sexual behavior associated with concurrence was 2–5 lifetime oral sex partners in men and ≥2 recent sex partners in women. Association with this predictor is clinically unsurprising and well-supported by the literature [13, 14, 24, 25].
Marijuana use was a risk factor for the concurrence among women. Although it is not related to oral or genital HPV infection natural history [26, 27], it may cause oral infection via sharing smoking apparatus [27]. In addition, marijuana users also indulge into other substance use and risky sexual behavior due to impulsivity [28, 29].
Our study should be considered in light of some limitations. First, some adjusted model estimates had a relative standard error more than 30%; therefore, we suggest the results to be considered for hypothesis generating only. Second, we could not demonstrate temporality due to cross-sectional nature of the study; however, our study is backed by well-established associations that have been vastly reported in the literature. Third, self-reported sexual behaviors can lead to misclassification of the exposure; however, this is the nature of the data collection procedures specific to NHANES. Fourth, NHANES does not collect data on marriage between two men or two women; such data could be helpful in evaluating number of lifetime oral sex partners, especially among men, in order to identify possible differences in transmission being higher from oral sex with a woman or with a man. Also, NHANES data does not differentiate between receptive vs insertive number of oral sex partners for men, which could be important to evaluate the prevalence by specific site (oral vs penile) of HPV infection.
Major strengths of this study are that it is the largest nationally representative survey data, well-known for its comprehensively detailed data on infections. Additionally, our study adds clarity and urgency about high-risk HPV, a greater public health concern than low-risk HPV, which is often lumped in with HR-HPV for the sake of epidemiologic analyses [11–13].
In conclusion, the prevalence of concurrent and concordant HR-HPV infection was relatively higher among men as compared to women in the U.S.; and marital status and certain behaviors were associated with concurrence and concordance in the U.S. population. Therefore, we hypothesize that oral screening of people with genital HR-HPV infection, as well as increasing HPV vaccination uptake, particularly among men, can be central to reducing HR-HPV infections, OPCs and other HPV-associated cancers in the U.S.
Supplementary Material
Prevalence of Genital and Oral Human Papillomavirus (HPV) infection by High-Risk (HR) Type, and Histograms of Simulated Proportions of Concordant HR-HPV Infection for Total Population (NHANES: 2013–2016 for Men, 2009–2016 for Women)
Histograms of Simulated Proportions of Concurrent High-Risk Human Papillomavirus (HR-HPV) Infection for Women (NHANES: 2009–2016).
Histograms of Simulated Proportions of Concurrent High-Risk Human Papillomavirus (HR-HPV) Infection for Men (NHANES: 2013–2016).
High-Risk Human Papillomavirus (HR-HPV) Types in Concordant Infections (NHANES: 2013–2016 for Men, 2009–2016 for Women).
Prevalence of Genital and Oral Human Papillomavirus (HPV) infection by High-Risk (HR) Type, and Histograms of Simulated Proportions of Concordant HR-HPV Infection for Men (NHANES: 2013–2016)
Prevalence of Genital and Oral Human Papillomavirus (HPV) infection by High-Risk (HR) Type, and Histograms of Simulated Proportions of Concordant HR-HPV Infection for Women (NHANES: 2009–2016)
Acknowledgments
The authors received no specific funding for this work.
Footnotes
The authors of this manuscript do not have any conflicts of interest to disclose.
The information has not been presented at any conference.
References
- 1.Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus–associated cancers—United States, 2008–2012. Morbidity and Mortality Weekly Report 2016; 65:661–6. [DOI] [PubMed] [Google Scholar]
- 2.Giuliano AR, Nyitray AG, Kreimer AR, et al. E UROGIN 2014 roadmap: Differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int J Cancer 2015; 136:2752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. JNCI: Journal of the National Cancer Institute 2015; 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of pathology 1999; 189:12–9. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mourad M, Jetmore T, Jategaonkar AA, Moubayed S, Moshier E, Urken ML. Epidemiological trends of head and neck cancer in the United States: a SEER population study. J Oral Maxillofac Surg 2017; 75:2562–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus–positive head and neck squamous cell carcinoma. J Clin Oncol 2015; 33:3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morbidity and mortality weekly report 2017; 66:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J Clin Oncol 2018; 36:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Cancer Institute. HPV Vaccination Linked to Decreased Oral HPV Infections. Available at: https://www.cancer.gov/news-events/cancer-currents-blog/2017/hpv-vaccine-oral-infection. Accessed 24 July, 2020.
- 11.Kedarisetty S, Orosco RK, Hecht AS, Chang DC, Weissbrod PA, Coffey CS. Concordant oral and vaginal human papillomavirus infection in the United States. JAMA otolaryngology–head & neck surgery 2016; 142:457–65. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Hang D, Deng Q, et al. Concurrence of oral and genital human papillomavirus infection in healthy men: a population-based cross-sectional study in rural China. Sci Rep 2015; 5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel EU, Rositch AF, Gravitt PE, Tobian AA. Concordance of penile and Oral human papillomavirus infections among men in the United States. The Journal of infectious diseases 2017; 215:1207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonawane K, Suk R, Chiao EY, et al. Oral human papillomavirus infection: differences in prevalence between sexes and concordance with genital human papillomavirus infection, NHANES 2011 to 2014. Ann Intern Med 2017; 167:714–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available at: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed 18 September 2018.
- 16.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: Informed Consent. Available at: https://www.cdc.gov/nchs/nhanes/genetics/genetic_participants.htm.
- 17.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey - MEC Laboratory Procedures Manual.
- 18.Munoz N, Bosch FX, De Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–27. [DOI] [PubMed] [Google Scholar]
- 19.Arbyn M, De Sanjosé S, Saraiya M, et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int J Cancer 2012; 131:1969–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tjalma WA, Depuydt CE, Stoler MH, Wright TL. Don’t forget HPV-45 in cervical cancer screening. Am J Clin Pathol 2012; 137:161–3. [DOI] [PubMed] [Google Scholar]
- 21.Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report 2016; 65:1405–8. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. HPV and Men - Fact Sheet. Available at: https://www.cdc.gov/std/hpv/stdfact-hpv-and-men.htm. Accessed 24 July 2020.
- 23.Ronco G, Ghisetti V, Segnan N, et al. Prevalence of human papillomavirus infection in women in Turin, Italy. Eur J Cancer 2005; 41:297–305. [DOI] [PubMed] [Google Scholar]
- 24.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813–9. [DOI] [PubMed] [Google Scholar]
- 25.Gargano JW, Unger ER, Liu G, et al. Prevalence of genital human papillomavirus in males, United States, 2013–2014. The Journal of infectious diseases 2017; 215:1070–9. [DOI] [PubMed] [Google Scholar]
- 26.D’Souza G, Palefsky JM, Zhong Y, et al. Marijuana use is not associated with cervical human papillomavirus natural history or cervical neoplasia in HIV-seropositive or HIV-seronegative women. Cancer Epidemiology and Prevention Biomarkers 2010; 19:869–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zwenger S Bogarting that joint might decrease oral HPV among cannabis users. Current Oncology 2009; 16:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrade LF, Carroll KM, Petry NM. Marijuana use is associated with risky sexual behaviors in treatment-seeking polysubstance abusers. The American journal of drug and alcohol abuse 2013; 39:266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoner SA. Marijuana and Sexual Risk Behavior Among Youth and Emerging Adults: What Do We Know? Available at: https://adai.uw.edu/pubs/pdf/2018MarijuanaRSB.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prevalence of Genital and Oral Human Papillomavirus (HPV) infection by High-Risk (HR) Type, and Histograms of Simulated Proportions of Concordant HR-HPV Infection for Total Population (NHANES: 2013–2016 for Men, 2009–2016 for Women)
Histograms of Simulated Proportions of Concurrent High-Risk Human Papillomavirus (HR-HPV) Infection for Women (NHANES: 2009–2016).
Histograms of Simulated Proportions of Concurrent High-Risk Human Papillomavirus (HR-HPV) Infection for Men (NHANES: 2013–2016).
High-Risk Human Papillomavirus (HR-HPV) Types in Concordant Infections (NHANES: 2013–2016 for Men, 2009–2016 for Women).
Prevalence of Genital and Oral Human Papillomavirus (HPV) infection by High-Risk (HR) Type, and Histograms of Simulated Proportions of Concordant HR-HPV Infection for Men (NHANES: 2013–2016)
Prevalence of Genital and Oral Human Papillomavirus (HPV) infection by High-Risk (HR) Type, and Histograms of Simulated Proportions of Concordant HR-HPV Infection for Women (NHANES: 2009–2016)


