To the Editor: Nodular fasciitis (NF) has been described as a rapidly growing benign soft tissue tumor with fibroblastic/myofibroblastic proliferation.[1] It commonly involves the upper extremities of adults aged 20 to 40 years.[1] Due to its rapid growth rate, high cellularity, and brisk mitosis, these lesions are easily confused with soft tissue sarcomas in the diagnosis-making process. Recent findings indicated that recurrent gene rearrangement of ubiquitin-specific protease 6 (USP6), located at 17p13.2, favors the clonally proliferative nature of NF.[1]USP6 rearrangements have been discovered in approximately 90% of NF cases and > 65% of NFs harbor myosin heavy chain 9 (MYH9)-USP6 fusions with a type I (exon 1–exon 1) or type II (exon 1–exon 2) pattern.[1,2] Thus, USP6 rearrangements have been adopted as a valuable diagnostic biomarker for distinguishing challenging cases from their histologic mimics. NF is frequently seen in adulthood but extremely rare in infants. Here, we evaluated the clinical, pathological, and genetic features of infantile NF to understand the tumorigenesis mechanism underlying this entity.
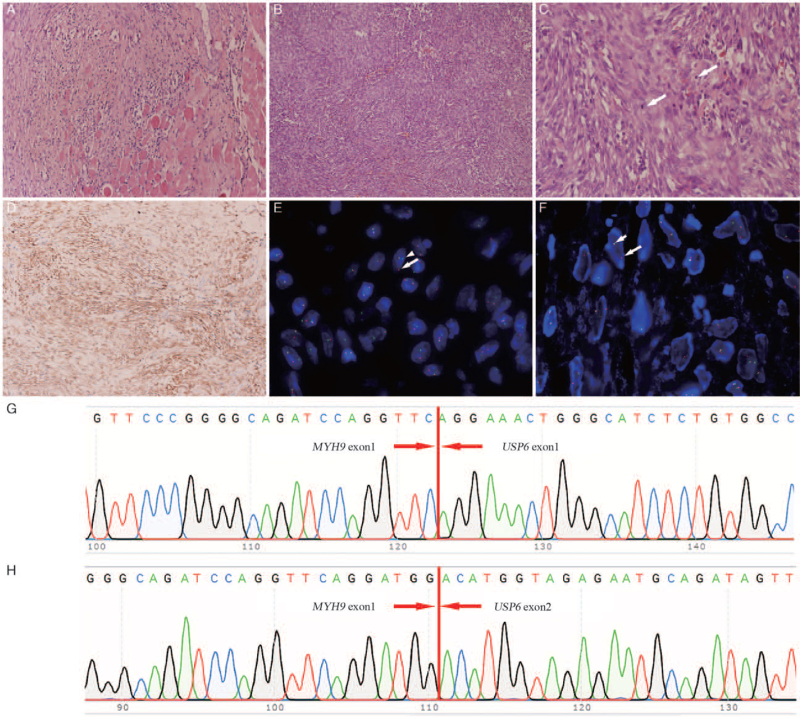
This study was approved by the West China Hospital Institutional Review Board. A systematized nomenclature of medicine search of the hospital surgical pathology files from January 2008 to August 2020 identified 604 NFs, among which 12 infantile cases were identified. Eleven cases (<2 years old) were enrolled in our study, while another case was previously published and not included. [3] Of the 11 cases included, seven patients were male and four were female. Patients commonly (10/11) presented with firm, round, and solid soft tissue masses. Duration data were available in eight cases with durations of 0.5 to 12.0 months (median 3 months). Spontaneous resolution was not identified in the duration. All of these cases denied trauma history. Birth histories were available for nine patients, out of which three were delivered by cesarean section, while the other six were delivered naturally. Six cases (54.5%) were identified in the head and neck, including the neck (n = 3), ear (n = 2), and parotid gland (n = 1); four cases (36.4%) were located at the trunk; and one case (9.1%) was in the extremities. The diagnosed age ranged from 4 to 23 months (median 11 months). Imaging data (including ultrasound and computed tomography) were available in four cases and unclear boundaries were shown in three of these cases (75%). All patients underwent surgical excision and the resected tumors were 1.4 to 4.0 cm (median 1.5 cm) in the largest dimension. Totally, five lesions were located in the subcutis, four in the muscle, one was identified in the parotid gland connective tissue, and one case lacked in-depth information. The resection of most specimens showed an ill-defined, firm nodule with a gray-white appearance. Histologically, muscular invasion was observed in five of the 11 patients [Figure 1A]. All tumors were mainly composed of spindle cells arranged in a fascicular pattern [Figure 1B]. Most cases (7/11) presented abundant fat spindle cells with a small amount of lymphocyte infiltration, while some lesions showed spindle cells with medium density and apparent inflammatory cell infiltration. Most cases (7/11) presented with microcystic changes. Red blood cell extravasation was not obvious in most cases (9/11). Scattered osteoclastic giant cells were observed in five of the 11 (45.5%) patients. The mitotic figures ranged from 1 to 13 per 10 high-power fields (HPFs) in these cases [Figure 1C]. Immunohistochemical staining was conducted, and all the cases with available data were positive for smooth muscle actin (SMA) [Figure 1D] and negative for desmin. The MIB-1 index was available in nine cases and ranged from 8% to 25%. The details of the clinicopathological data are shown in Supplementary Table 1.
Figure 1.
Pathological and molecular findings. (A) Surrounding muscle invasion was observed (hematoxylin and eosin, original magnification ×200). (B) Spindle cells were arranged in a fascicular pattern (hematoxylin and eosin, original magnification ×100). (C) Brisk mitotic figures (arrow) in HPFs (hematoxylin and eosin, original magnification ×400) were seen. (D) The tumor cells were diffusely positive for SMA (IHC, original magnification ×100). (E) Flourescene in situ hybridization (FISH) showed a positive balanced arrangement of USP6 with a 1F + 1G (arrowhead) + 1R (arrow) pattern. (F) FISH showed a positive unbalanced arrangement of USP6 with a 1F (short arrow) + 1R (arrow) pattern. (G, H) Sanger sequencing confirmed MYH9-USP6 fusion with type I and type II patterns. HPFs: High-power fields; MYH9: Myosin heavy chain 9; SMA: Smooth muscle actin; USP6: Ubiquitin-specific protease 6.
Fluorescence in situ hybridization was performed to detect USP6 gene rearrangement (ZytoVision, Bremerhaven, Germany).[2,4] Nine of the 11 cases (81.8%) showed positivity for USP6 arrangement, and the percentage of split red-green signals ranged from 15% to 78%; among these cases, eight showed balanced rearrangement with one-fusion (1F), one-green (1G), and one-red (1G) (1F + 1G + 1R) signal patterns [Figure 1E]. In contrast, one case showed unbalanced rearrangement, of which 55% of cells showed a one-fusion and one-red (1F + 1R) [Figure 1F] signal pattern.[3] Two cases were negative for USP6 rearrangement. Notably, the tissues of seven cases with USP6 rearrangement were retrievable for reverse transcription-polymerase chain reaction (RT-PCR) and Sanger sequencing. The RT-PCR primers for detecting common fusion types of USP6 are listed in Supplementary Table 2. The results showed that only one case was positive for MYH9-USP6 with a type I [Figure 1G] and type II [Figure 1H] pattern, while six were negative for MYH9-USP6 in either type I or type II pattern [Supplementary Table 3].
No other treatment was provided after the simple surgical excisions. Follow-up information was available for 81.8% (9/11) of the patients who underwent tumor excision, with a median follow-up duration of 51 months (range: 5–121). Among these nine patients, there were two patients who developed local recurrence in 5 months after surgery; among which one was performed with re-excision and has been alive without evidence of recurrence for 19 months after the second operation, while another one has been alive with relapsed tumor for 3 months. All the other seven patients were disease-free after surgery.
This report presented 11 infantile NFs and reviewed the published literature to deeply understand this entity's characteristics. Although a few previous studies have reported NF in childhood and summarized the clinical and pathological features, none of them focused on infantile patient groups.[5,6] After carefully reviewing the previous literature, it was found that patients < 2 years old have been sporadically reported in previous studies by other researchers [Supplementary Table 4]. In the current study, the lesions were most frequently found in the head and neck, which may differ from those in adults or even in older children. Although NF has been suggested to be a trauma-related lesion, no patients in this study were reported to have a trauma history before diagnosis. Histologically, in our series of cases, most of the patients showed many hypercellular spindle cells, while myxoid changes, extravasation, and lymphocytes were not obvious when compared with those of conventional NFs. Mitotic figures (1–13/10 HPFs) were shown in ten cases of the current study, but no atypia was observed, which were similar to the previous findings.[1] As reported, more myxoid changes are commonly seen in the early stage of the lesion, while more fibrous components are shown in the late stage.[1] However, in our cases, despite a highly prolonged observation period of up to 12 months (median 3 months), no apparent fibrous changes were identified.
Cytogenetic studies of NF patients < 2 years old are extremely rare in previous studies. To our knowledge, we are the first group to investigate genetic changes in a series of infantile cases. In our study, 81.8% of cases demonstrated USP6 rearrangement, similar to the findings of adult patients. RT-PCR was the most commonly used method for identifying MYH9-USP6 formation in previous studies.[1,2] In total, 65% to 75% of patients with USP6 rearrangements were tested and found to be positive for MYH9-USP6 fusion in type I or/and type II patterns via RT-PCT.[1,2] In our study, the MYH9-USP6 transcript was identified in only 14.2% (1/7) of patients via RT-PCR, which is a significantly lower percentage than that in previous studies of NF cases.[1,2] Considering the limitation of the traditional RT-PCR method, such as limitations of reported primers and relative low detection sensitivity, further molecular studies such as next generation sequencing based technology are needed in discovering other occult or novel alterations. Notably, one of the nine patients with USP6 rearrangement showed an unbalanced type. This case showed much higher cellularity and brisk mitoses (13/10 HPF). To further understand the role of an unbalanced rearrangement of USP6, we performed a thorough review of the English literature. Unbalanced rearrangements were identified in three patients with NFs.[3,7,8] However, more larger cohort are needed to clarify the associations between atypical USP6 rearrangement and clinicopathological features.
In clinical work, accurate histopathologic diagnosis is crucial, as misdiagnosis may lead to aggressive or excessive treatment. In our series of cases, most cases showed high cellularity, rapid growth rate, and brisk mitosis, and none of them presented with self-regression, even in those with quite a long duration time. NF is extremely prone to be confused with some non-self-limited benign lesions and soft tissue malignancies. The differential diagnoses for infantile NFs mainly include cellular fibrous histiocytoma, fibromatosis, inflammatory myofibroblastic tumor, and some spindle cell sarcomas, such as infantile fibrosarcoma, spindle cell rhabdomyosarcoma, and myxofibrosarcoma.[1]USP6 rearrangements have been identified in infantile NFs but not in any of the abovementioned histologic mimics. Therefore, USP6 status, combined with the clinicopathologic, molecular, and genetic characteristics of infantile NF histologic mimics, can be useful in the diagnosis-making process.[1]
Surgical resection is a common treatment for NF and recurrences are rare.[1] However, in our cohort, the longest duration period was up to 12 months, and no signs of regression were observed. Two of the nine patients in the present study showed recurrence in 5 months postoperatively. Both these two lesions of the two patients were revealed as masses in the auricle of the ear. Local resection was performed to remove these masses. The recurrence may have resulted from the positive margin because of the difficulty of achieving complete surgical excision around the ear.[1] Therefore, we suggest that complete surgery is the optimal treatment for infantile NF, if possible.
In conclusion, we have summarized the clinicopathological features and identified USP6 rearrangements and MYH9-USP6 fusion in a series of infantile NFs, which increases our knowledge in this field. We argue that this entity may have some special characteristics, but further studies with large cohorts are still needed to obtain a more comprehensive characterization in the future.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81972520 and 81472510), Key R&D (Major Science and Technology Project) Project of Sichuan Science and Technology Department (No. 2020YFS0270), and the 135 Project for Disciplines of Excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (No. 2018HXFH011).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Qiu Y, Hu X, He X, Zeng WJ, Zhang HY. Clinicopathological and genetic findings of infantile nodular fasciitis. Chin Med J 2021;134:2768–2770. doi: 10.1097/CM9.0000000000001727
Supplemental digital content is available for this article.
References
- 1.Olivera AM, Wang J, Wang WL. Nodular Fasciitis. Soft Tissue and Bone Tumours. WHO Classification of Tumours 5th ed.2020; Lyon: International Agency for Research on Cancer, 49–50. [Google Scholar]
- 2.Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest 2011; 91:1427–1433. doi: 10.1038/labinvest.2011.118. [DOI] [PubMed] [Google Scholar]
- 3.Qiu Y, Peng R, Chen H, Zhuang H, He X, Zhang H. Atypical nodular fasciitis with a novel PAFAH1B1-USP6 fusion in a 22-month-old boy. Virchows Arch 2020; doi: 10.1007/s00428-020-02961-y. [DOI] [PubMed] [Google Scholar]
- 4.Lu Y, He X, Qiu Y, Chen H, Zhuang H, Yao J, et al. Novel CTNNB1-USP6 fusion in intravascular fasciitis of the large vein identified by next-generation sequencing. Virchows Arch 2020; 477:455–459. doi: 10.1007/s00428-020-02792-x. [DOI] [PubMed] [Google Scholar]
- 5.Pandian TK, Zeidan MM, Ibrahim KA, Moir CR, Ishitani MB, Zarroug AE. Nodular fasciitis in the pediatric population: a single center experience. J Pediatr Surg 2013; 48:1486–1489. doi: 10.1016/j.jpedsurg.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 6.Bemrich-Stolz CJ, Kelly DR, Muensterer OJ, Pressey JG. Single institution series of nodular fasciitis in children. J Pediatr Hematol Oncol 2010; 32:354–357. doi: 10.1097/MPH.0b013e3181df6305. [DOI] [PubMed] [Google Scholar]
- 7.Guo R, Wang X, Chou MM, Asmann Y, Wenger DE, Al-Ibraheemi A, et al. PPP6R3-USP6 amplification: novel oncogenic mechanism in malignant nodular fasciitis. Genes Chromosomes Cancer 2016; 55:640–649. doi: 10.1002/gcc.22366. [DOI] [PubMed] [Google Scholar]
- 8.Teramura Y, Yamazaki Y, Tanaka M, Sugiura Y, Takazawa Y, Takeuchi K, et al. Case of mesenchymal tumor with the PPP6R3-USP6 fusion, possible nodular fasciitis with malignant transformation. Pathol Int 2019; 69:706–709. doi: 10.1111/pin.12851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.