Abstract
Background:
Breast cancer patients with ipsilateral supraclavicular lymph node metastasis (ISLNM) but without distant metastasis are considered to have a poor prognosis. This study aimed to develop a nomogram to predict the overall survival (OS) of breast cancer patients with ISLNM but without distant metastasis.
Methods:
Medical records of breast cancer patients who received surgical treatment at the Affiliated Cancer Hospital of Zhengzhou University, Jiyuan People's Hospital and Huaxian People's Hospital between December 21, 2012 and June 30, 2020 were reviewed retrospectively. Overall, 345 patients with pathologically confirmed ISLNM and without evidence of distant metastasis were identified. They were further randomized 2:1 and divided into training (n = 231) and validation (n = 114) cohorts. A nomogram to predict the probability of OS was constructed based on clinicopathologic variables identified by the univariable and multivariable analyses. The predictive accuracy and discriminative ability were measured by calibration plots, concordance index (C-index), and risk group stratification.
Results:
Univariable analysis showed that estrogen receptor-positive (ER+), progesterone receptor-positive (PR+), human epidermal growth factor receptor 2-positive (HER2+) with Herceptin treatment, and a low axillary lymph node ratio (ALNR) were prognostic factors for better OS. PR+, HER2+ with Herceptin treatment, and a low ALNR remained independent prognostic factors for better OS on multivariable analysis. These variables were incorporated into a nomogram to predict the 1-, 3-, and 5-year OS of breast cancer patients with ISLNM. The C-indexes of the nomogram were 0.737 (95% confidence interval [CI]: 0.660–0.813) and 0.759 (95% CI: 0.636–0.881) for the training and the validation cohorts, respectively. The calibration plots presented excellent agreement between the nomogram prediction and actual observation for 3 and 5 years, but not 1 year, OS in both the cohorts. The nomogram was also able to stratify patients into different risk groups.
Conclusions:
In this study, we established and validated a novel nomogram for predicting survival of patients with ISLNM. This nomogram may, to some extent, allow clinicians to more accurately estimate prognosis and to make personalized therapeutic decisions for individual patients with ISLNM.
Keywords: Breast cancer, Ipsilateral supraclavicular lymph node metastasis, Nomogram, Prognosis
Introduction
With 1.7 million new patients diagnosed each year, breast cancer represents a serious threat to the health of women worldwide.[1] Breast cancer patients with ipsilateral supraclavicular lymph node metastasis (ISLNM) are considered to have a poor prognosis.[2] The incidence of breast cancer patients presenting with ISLNM but without distant metastasis at the time of diagnosis is low, comprising approximately 1% to 4% of all cases of breast cancer.[3] In the 1997 American Joint Committee on Cancer (AJCC) staging system, ISLNM was classified as M1, even without any evidence of further distant metastasis. However, Brito et al[4] reported that the prognosis of patients with ISLNM at initial diagnosis was more similar to that of patients with stage-IIIB locally advanced breast cancer and was significantly better than that of patients with distant metastasis. Accordingly, in 2003, ISLNM was re-categorized as N3c in the 6th edition of the AJCC-Tumor Node Metastasis (TNM) staging system.[5]
Advances in systemic treatment have improved survival in patients with ISLNM, with the 5-year overall survival (OS) rate ranging from 41.4% to 54.8%.[6,7] The prognosis of breast cancer patients with ISLNM is influenced by many factors, including molecular sub-types, ISLNM size, local treatment strategies, and multidisciplinary therapies.[4,6–8] However, under the current systemic treatment protocols, accurate prediction of the prognosis of breast cancer patients with ISLNM allows for the adoption of individualized treatment plans, thereby avoiding undertreatment or overtreatment. For early-stage breast cancer, several prognostic prediction models have been constructed and are widely accepted.[9–13] However, to our knowledge, no prognostic model predicting the survival of breast cancer patients with ISLNM have been established yet.
Therefore, in this study, we aimed to explore the risk factors related to survival in breast cancer patients with ISLNM. We then used these factors to establish a nomogram to predict the prognosis of this patient population and verify the predictive efficiency of this model.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Data were obtained after this study had been approved by the Ethical Review Committee of the Affiliated Cancer Hospital of Zhengzhou University (No. 2019188). As this study was retrospectively designed, informed consent was waived by the Affiliated Cancer Hospital of Zhengzhou University.
Study population
Medical records of breast cancer patients who received surgical treatment at the Affiliated Cancer Hospital of Zhengzhou University, Jiyuan People's Hospital, and Huaxian People's Hospital, between December 21, 2012, and June 30, 2020, were retrospectively and consecutively reviewed. The inclusion criteria were as follows: (1) Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; (2) histopathological confirmation of invasive breast cancer before neoadjuvant chemotherapy (NAC); (3) ISLNM confirmed by histopathology or cytopathology; (4) intact clinicopathological and follow-up data; and (5) received at least two cycles of NAC and completed surgical treatment. The exclusion criteria were as follows: (1) distant metastasis; (2) presence of other malignant tumors; or (3) bilateral breast cancer.
Clinical data
The following data were collected for each patient: age at diagnosis of breast cancer with ISLNM, ECOG score, family history, menopausal status, clinical T staging, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status, Ki67 index, NAC regimen, NAC cycle, chemotherapy regimen (whether including Herceptin or not), palpability of ipsilateral supraclavicular lymph node (ISLN) before NAC, size of ISLN after NAC, Response Evaluation Criteria in Solid Tumors (RECIST)-based treatment response, breast surgery strategies, whether ipsilateral supraclavicular lymph node dissection (ISLND) was performed or not, breast pathologic complete response (pCR), axillary pCR, dose of radiotherapy (RT), endocrine therapy, and axillary lymph node ratio (ALNR), which was defined as the ratio of the number of positive lymph nodes (LNs) to the total number of LNs removed. An ER- and PR-positive (PR+) status were defined as >1% of tumor cells with nuclear staining. A HER2-positive status was defined as 3+ by immunohistochemistry staining or as 2+ in addition to a positive fluorescence in situ hybridization result. Five tumor sub-types were determined, according to the ER, PR, HER2, and Ki-67 status: luminal A (estrogen receptor-positive [ER+], PR > 20%, HER2−, and Ki-67 ≤ 14%), luminal B (ER+ and/or progesterone receptor-positive [PR+], HER2−, and Ki-67 > 14%), luminal-HER2 (ER+ and/or PR+, human epidermal growth factor receptor 2-positive [HER2+], and any Ki-67), HER2-positive (ER−, PR−, and HER2+), and triple-negative (ER−, PR−, and HER2−). The clinical stages were classified based on the 8th AJCC-TNM staging system. Tumor responses were categorized as complete response, partial response, stable disease, and progressive disease, based on the RECIST criteria. Breast pCR was defined as the complete disappearance of all invasive tumor cells from breast tissue, regardless of the presence of residual ductal carcinoma in situ (ypT0/is). Nodal pCR was defined as no evidence of residual tumor in the axillary or ipsilateral supraclavicular LNs.
Statistical analysis
OS was defined as the time from surgery to the date of death from any cause or to the follow-up cutoff (June 30, 2020). Accurate rates of OS were calculated according to the Kaplan-Meier method from the date of surgery, and survival curves were compared using the log-rank test. By random stratified sampling according to the ratio of 2:1, the 345 patients were divided into a training cohort (n = 231) and a validation cohort (n = 114). The training set samples were used to establish a Cox regression model to determine the risk factors and to establish a nomogram. Variables that achieved significance at P < 0.05 in the univariable analysis were incorporated into the Cox multivariable regression analysis. In the Cox multivariable regression analysis, a nomogram was selected using a backward step-down process, which used the Akaike information criterion as a stopping rule. To evaluate the discriminative power of the nomogram, we used the Harrell concordance index (C-index) with a 95% confidence interval (CI). To assess the accuracy of the nomogram, we used calibration plots to visualize the agreement between the predicted and actual OS in both the training and validation cohorts. By calculating the total risk scores (from highest to lowest), we divided the training and validation cohorts into low-risk and high-risk groups according to the median method. Statistical analyses were performed using R version 3.2.0 software (http://www.r-project.org, R Foundation for Statistical Computing, Vienna, Austria). A two-sided P value of < 0.005 was considered statistically significant.
Results
Patient characteristics
Overall, 345 breast cancer patients with ISLNM but without distant metastases were enrolled in the final analysis. The clinicopathological features of the patients in the training (n = 231) and validation cohorts (n = 114) are reported in Table 1. There were no significant differences between the training and validation cohorts. All of the patients were female, and the mean age was 50 years (range: 22–77 years). The rates of ER-, PR-, and HER2-positivity were 58.6% (202/345), 51.0% (176/345), and 44.3% (153/345), respectively. NAC regimens were as follows: anthracycline plus taxane (241), anthracycline-based (38), and taxane-based (66). Among HER2-positive patients, 58.2% (89/153) received Herceptin therapy. For the local treatment of ISLNM, 300 patients received ISLND combined with RT, while the remaining 45 patients received RT only. The breast and axillary pCR rates for the entire cohort were 31.3% (108/345), and 30.1% (104/345), respectively. The median follow-up was 26.8 months. The 1-, 3-, and 5-year OS rates were 93.7%, 76.3%, and 65.6%, respectively.
Table 1.
Clinicopathological characteristics of breast cancer patients with ipsilateral supraclavicular lymph node metastasis in the training and validation cohorts.
| Characteristics | Training cohort, N (%) | Validation cohort, N (%) |
| Total | 231 (100.0) | 114 (100.0) |
| Age | ||
| ≤40 years | 43 (18.6) | 18 (15.8) |
| >40 years | 188 (81.4) | 96 (84.2) |
| Family history of cancer | ||
| Yes | 32 (13.9) | 14 (12.3) |
| No | 199 (86.1) | 100 (87.7) |
| Clinical T staging | ||
| T1+T2 | 173 (74.9) | 86 (75.4) |
| T3+T4 | 58 (25.1) | 28 (24.6) |
| ER status | ||
| Positive | 140 (60.6) | 62 (54.4) |
| Negative | 91 (39.4) | 52 (45.6) |
| PR status | ||
| Positive | 116 (50.2) | 60 (52.6) |
| Negative | 115 (49.8) | 54 (47.4) |
| HER2 status and Herceptin usage | ||
| HER2 negative | 125 (54.1) | 67 (58.8) |
| HER2 positive but not using Herceptin | 43 (18.6) | 21 (18.4) |
| HER2 positive and using Herceptin | 63 (27.3) | 26 (22.8) |
| Ki67 index | ||
| ≥30 | 201 (87.0) | 96 (84.2) |
| <30 | 30 (13.0) | 18 (15.8) |
| Palpability of ISLN before NAC | ||
| Yes | 50 (21.6) | 28 (24.6) |
| No | 181 (78.4) | 86 (75.4) |
| NAC regimens | ||
| Anthracycline plus taxane | 157 (68.0) | 84 (73.7) |
| Anthracycline based | 27 (11.7) | 11 (9.6) |
| Taxane based | 47 (20.3) | 19 (16.7) |
| Cycles of NAC | ||
| <5 | 54 (23.4) | 24 (21.1) |
| ≥5 | 177 (76.6) | 90 (78.9) |
| RECIST-based treatment response | ||
| CR+PR | 198 (85.7) | 97 (85.1) |
| SD+PD | 33 (14.3) | 17 (14.9) |
| Size of ISLN after NAC (mm) | ||
| <10 | 196 (84.8) | 87 (76.3) |
| ≥10 | 35 (15.2) | 27 (23.7) |
| Breast surgery strategies | ||
| Mastectomy | 226 (97.8) | 108 (94.7) |
| BCS+reconstruction | 5 (2.2) | 6 (5.3) |
| ISLND or not | ||
| Yes | 205 (88.7) | 95 (83.3) |
| No | 26 (11.3) | 19 (16.7) |
| Breast pCR | ||
| Yes | 77 (33.3) | 31 (27.2) |
| No | 154 (66.7) | 83 (72.8) |
| Axillary pCR | ||
| Yes | 77 (33.3) | 27 (23.7) |
| No | 154 (66.7) | 87 (76.3) |
| ALNR (%) | ||
| <35 | 135 (58.4) | 57 (50.0) |
| ≥35 | 96 (41.6) | 57 (50.0) |
| Radiation dose | ||
| Normal | 171 (74.0) | 84 (73.7) |
| High | 60 (26.0) | 30 (26.3) |
ALNR: Axillary lymph node ratio; BCS: Breast-conserving surgery; CR: Complete response; ER: Estrogen receptor; HER2: Human epidermal growth factor receptor 2; ISLN: Ipsilateral supraclavicular lymph node; ISLND: Ipsilateral supraclavicular lymph node dissection; NAC: Neoadjuvant chemotherapy; pCR: Pathological complete response; PD: Progressive disease; PR: Progesterone receptor; RECIST: Response evaluation criteria in solid tumors; SD: Stable disease.
Screening for prognostic factors in the training cohort
In the training cohort, all clinicopathological factors that potentially affected OS were included in the Cox regression model for univariable and multivariable analyses [Table 2]. The univariable analysis showed that ER positivity, PR positivity, HER2 positivity with Herceptin treatment, and a low ALNR, were prognostic factors for better OS. All significant factors in the univariable analysis were entered into the multivariable analysis based on the Cox regression. In the multivariable analysis, PR positivity, HER2 positivity with Herceptin treatment, and a low ALNR remained independent prognostic factors for better OS.
Table 2.
Univariable and multivariable analyses for predictive factors of OS in the training cohort of patients with breast cancer patients with ipsilateral supraclavicular lymph node metastasis.
| Univariable analysis | Multivariable analysis | |||||
| Variables | HR | 95% CI | P | HR | 95% CI | P |
| Age (years): >40 vs. ≤40 | 0.579 | 0.307–1.091 | 0.0910 | |||
| Family history of cancer: yes vs. no | 1.755 | 0.822–3.746 | 0.1460 | |||
| Clinical T staging: T3 + T4 vs. T1 + T2 | 0.900 | 0.470–1.723 | 0.7510 | |||
| ER status: positive vs. negative | 0.396 | 0.225–0.698 | 0.0010 | 0.585 | 0.277–1.232 | 0.1580 |
| PR status: positive vs. negative | 0.348 | 0.187–0.645 | <0.0010 | 0.413 | 0.185–0.924 | 0.0310 |
| HER2 status and Herceptin usage | ||||||
| HER2 negative | Reference | Reference | ||||
| HER2 positive but not using Herceptin | 0.889 | 0.475–1.664 | 0.7130 | 0.698 | 0.367–1.331 | 0.2750 |
| HER2 positive and using Herceptin | 0.219 | 0.067–0.717 | 0.0120 | 0.240 | 0.072–0.797 | 0.0200 |
| Ki67 index (%): ≥30 vs. <30 | 1.193 | 0.508–2.802 | 0.6850 | |||
| Palpable of ISLN before NAC: yes vs. no | 1.594 | 0.887–2.862 | 0.1190 | |||
| NAC regimens | ||||||
| Anthracycline plus taxane | Reference | |||||
| Anthracycline based | 0.731 | 0.257–2.074 | 0.5550 | |||
| Taxane based | 0.893 | 0.427–1.868 | 0.7650 | |||
| NAC cycles: ≥5 vs. <5 | 0.788 | 0.434–1.431 | 0.4340 | |||
| RECIST-based treatment response: SD+PD vs. CR + PR | 1.366 | 0.612–3.047 | 0.4470 | |||
| size of ISLN after NAC (mm): ≥10 vs. <10 | 1.603 | 0.820–3.133 | 0.1680 | |||
| Breast surgery strategies: BCS + reconstruction vs. Mastectomy | 1.664 | 0.229–12.111 | 0.6150 | |||
| ISLND or not: yes vs. no | 0.974 | 0.386–2.457 | 0.9560 | |||
| Breast pCR: yes vs. no | 0.900 | 0.497–1.632 | 0.7290 | |||
| Axillary pCR: yes vs. no | 0.526 | 0.263–1.052 | 0.0690 | |||
| ALNR (%): <35 vs. ≥35 | 1.950 | 1.111–3.420 | 0.0200 | 2.030 | 1.139–3.617 | 0.0160 |
| Radiation dose: normal vs. high | 0.818 | 0.418–1.599 | 0.5560 | |||
ALNR: Axillary lymph node ratio; BCS: Breast-conserving surgery; CI: Confidence interval; CR: Complete response; ER: Estrogen receptor; HER2: Human epidermal growth factor receptor 2; HR: hormone receptor; ISLN: Ipsilateral supraclavicular lymph node; ISLND: Ipsilateral supraclavicular lymph node dissection; NAC: Neoadjuvant chemotherapy; OS: Overall survival; pCR: Pathological complete response; PD: Progressive disease; PR: Progesterone receptor; RECIST: Response evaluation criteria in solid tumors; SD: Stable disease.
Prognostic nomogram for OS
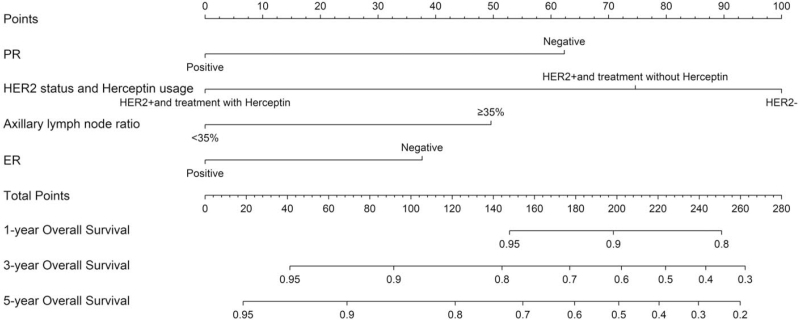
A nomogram was constructed to predict the probability of OS using the following four factors: PR status, HER2 status with Herceptin use, ALNR, and ER status [Figure 1]. According to the Cox regression model, the following predictive probability formula was obtained:
Figure 1.
Nomogram for predicting the 1-, 3-, and 5-year OS of breast cancer patients with ISLNM. ER: Estrogen receptor; HER2: Human epidermal growth factor receptor 2; HER2+: Human epidermal growth factor receptor 2-positive; ISLNM: Ipsilateral supraclavicular lymph node metastasis; OS: Overall survival; PR: Progesterone receptor.
Risk score = exp (−0.884139487 × PR-positive – 0.358941253 × HER2 status and Herceptin use [HER2-positive but not using Herceptin] – 1.426178179 × HER2 status and Herceptin use [HER2-positive and using Herceptin] + 0.708094615 × ALNR (≥35%) – 0.536911728 × ER-positive – 0.930885).
A vertical line for each variable was drawn to the top axis of the figure, and the score derived from the “points” axis represented the score for each individual variable. The individual scores were then added together to obtain the total score. A vertical line was then drawn from the “total points” axis down to the axis termed “survival probability,” and the predicted probability of OS was thus obtained.
Calibration and validation of the nomogram
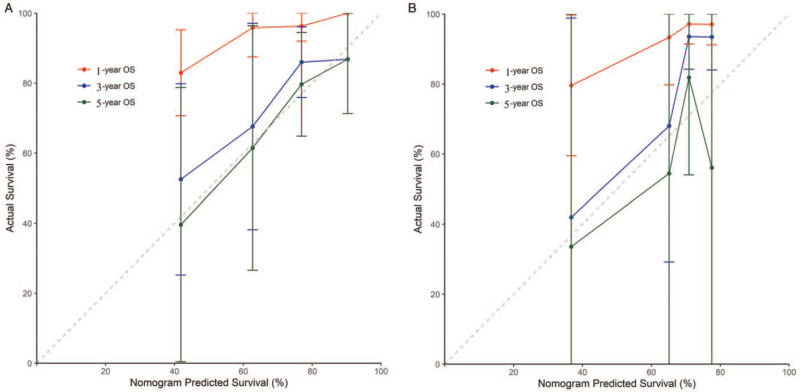
The C-indexes of the nomogram were 0.737 (95% CI: 0.660–0.813) and 0.759 (95% CI: 0.636–0.881) for the training and the validation cohorts, respectively. The calibration plots presented excellent agreement in both cohorts between the nomogram prediction and actual observation for 3- and 5-year OS; however, they demonstrated poor matching for 1-year OS [Figure 2].
Figure 2.
The calibration curves for predicting the 1-, 3-, and 5-year OS of breast cancer patients with ISLNM in the (A) training and (B) validation cohorts. Nomogram-predicted OS is plotted on the x-axis; actual OS is plotted on the y-axis. ISLNM: Ipsilateral supraclavicular lymph node metastasis; OS: Overall survival.
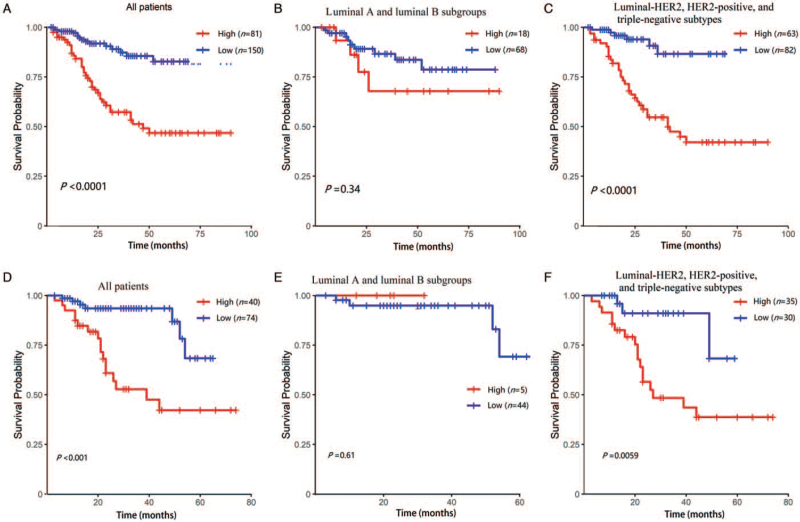
Risk stratifications using the new nomogram
We assigned the patients in the training cohort to low-risk and high-risk subgroups based on the median of the total risk scores (from highest to lowest) [Figure 3A–C]. Among the entire population, the 1-year OS of the low-risk and high-risk subgroups was 98.0% and 86.9%, respectively; the 3-year OS was 87.3% and 57.3%, respectively; the 5-year OS was 82.8% and 46.8%, respectively [Figure 3A] (P < 0.0001). In the luminal A and luminal B sub-types, the 1-year OS of the low-risk and high-risk subgroups was 97.0% and 93.3%, respectively; the 3-year OS was 86.6% and 67.8%, respectively; the 5-year OS was 78.7% and 67.8%, respectively [Figure 3B] (P = 0.3400). In the luminal-HER2, HER2-positive, and triple-negative sub-types, the 1-year OS of the low-risk and high-risk subgroups was 98.8% and 85.3%, respectively; the 3-year OS was 86.6% and 54.7%, respectively; and the 5-year OS was 86.6% and 42.1%, respectively [Figure 3C] (P < 0.0001). In the validation cohort, the low-risk and high-risk subgroups of the whole population demonstrated a significant difference between the Kaplan-Meier curves [Figure 3D]. In the validation cohort, the Kaplan-Meier curves for the low-risk and high-risk subgroups of the luminal A and luminal B sub-types did not show statistical differences [Figure 3E]. In the validation cohort, the low-risk and high-risk subgroups of the luminal-HER2, HER2-positive, and triple-negative sub-types demonstrated a significant difference between the Kaplan-Meier curves [Figure 3F].
Figure 3.
Survival probability of nomogram-based stratification of different population. (A) All patients with breast cancer patients with ISLNM in the training cohort; (B) luminal A and luminal B subgroups in the training cohort; (C) luminal-HER2, HER2-positive, and triple-negative sub-types in the training cohort; (D) all patients in the validation cohort; (E) luminal A and luminal B subgroups in the validation cohort; (F) luminal-HER2, HER2-positive, and triple-negative sub-types in the validation cohort. HER2: Human epidermal growth factor receptor 2; HER2-positive: Human epidermal growth factor receptor 2-positive; ISLNM: Ipsilateral supraclavicular lymph node metastasis.
Discussion
In this study, we developed a postoperative nomogram to predict OS in breast cancer patients with ISLNM. Through univariable analysis and subsequent multivariable analysis, we identified PR status, HER2 status along with Herceptin use, and the ALNR as independent prognostic factors for OS.
Interestingly, our study found that the ER status was predictive of prognosis in the univariable analysis but was not an independent predictor of prognosis in the multivariable analysis. In contrast, other studies have suggested that both ER and PR are independent prognostic factors.[14,15] ER positivity has previously been shown to be a strong indicator of response to endocrine therapy.[16] According to the long-term prevailing theory, PR is located downstream of ER, and the amount of PR in tumors potentially reflects a functional ER pathway, thus predicting the effect of endocrine therapy. Although the prognostic value of PR has been recognized, there is not yet a consensus on whether it can be used as an independent predictor of adjuvant endocrine therapy.[16–18] Several studies have suggested that the level of hormone receptor content is of importance, and some have suggested that the PR is a better predictor of the benefits of adjuvant endocrine treatment than ER.[18–21] Some studies have suggested that when the PR is absent, the tumor biology of breast cancer is more aggressive and the prognosis is worse.[20,22,23] Our study also confirmed an association between PR negativity and worse OS in breast cancer initially diagnosed with ISLNM; however, due to small sample size and retrospective bias, we did not find that ER was an independent predictor of OS.
Breast cancers with very high expression of HER2 are characterized by a more aggressive phenotype, resulting in worse disease prognosis.[24] However, in this study we found that patients with HER2-positive cancer who were treated with Herceptin had a better prognosis than that of HER2-negative patients. Surprisingly, Li et al[14] also found that in a cohort of patients with stage-IV breast cancer, the prognosis of HER2-positive patients was better than that of HER2-negative patients, but this phenomenon was not explained in their article. The clinical stage of all patients in this study was IIIC, which indicates a poor prognosis and an urgent need for effective systemic treatment. Trastuzumab, a humanized monoclonal antibody that specifically targets HER2, significantly improves the prognosis of HER2-positive breast cancer.[25–27] In contrast, patients with HER2-negative cancer, especially those who are both HER2- and ER-negative, have no effective new drugs except chemotherapy, and their prognosis remains poor. Compared with other studies, we have established a model that takes into account the HER2 status and Herceptin use, which is more in line with the real-world scenario.
Traditionally, the TNM staging system distinguishes patients by counting the absolute number of positive LNs, regardless of the potential impact of the total number of LNs retrieved. Many studies have shown that the ALNR, the ratio of the number of positive LNs to the total number of resected LNs, may be a superior prognostic factor than the (pN) stage in patients without NAC,[28–32] but very few studies have examined the efficacy of the ALNR in a NAC setting. This study is rarely explore the prognostic significance of ALNR in breast cancer patients who are clinically stage-IIIC and receiving NAC. Our study found that a single cutoff ratio of ALNR (0.35) helped to distinguish between favorable and unfavorable OS among stage-IIIC patients who received NAC, which is similar to the findings of previous studies.[33,34] Keam et al[33] found that an ALNR > 0.25 was associated with poor survival among 205 stage-II/III patients who received NAC. According to Tsai et al,[34] an ALNR ≤ 0.15 was found to discriminate between favorable and unfavorable outcomes among patients with hormone receptor-positive and triple negative breast cancer cancers who received NAC. Of the 345 patients with stage-IIIC in our study, the axillary pCR rate was as low as 30.1%, but for the remaining 69.9% of non-pCR patients, a better method for distinguishing the disease burden in the axilla, predicting prognosis, and tailoring postoperative treatment strategies is required. It has been reported that the total number of LNs removed during axillary dissection are reduced in most cases treated with NAC compared to that in patients treated without NAC,[35–37] and that traditional pN staging may underestimate true residual nodal disease in these patients treated with NAC. Therefore, ALNR might be a complementary or alternative method to traditional pN staging in evaluating disease burden after NAC and tailoring postoperative treatment strategies in patients with ISLNM.
Based on the stratification analysis, the nomogram developed in this study was able to identify patients with different risks. In the luminal A/B subgroups, most patients were classified into the low-risk population, as calculated by our nomogram, and therefore had a good prognosis. For those patients classified as high-risk according to the nomogram calculations, the prognosis remained relatively poor and required more aggressive systemic treatment. In general, the prognosis for advanced breast cancer patients with triple-negative or HER2-positive disease remained poor. Our study found that among luminal-HER2, HER2-positive, and triple-negative sub-types, patients determined by the nomogram to be low-risk had a relatively better prognosis and therefore should be given intensive treatment with curative intent; the prognosis of patients determined by the nomogram to be high-risk was particularly poor, and the current conventional treatment regimen might not be very effective. For this high-risk patient population, treatment should be highly individualized, and a balance should be struck between efficacy, tolerance, and quality of life.
We acknowledge the limitations of our nomogram due to the retrospective nature and the relatively small sample size. First, the calibration plots did not present an acceptable level of agreement between the nomogram prediction and actual observation for 1-year OS in either the training or the validation cohorts. Some patients in our study, especially triple-negative and HER2-positive patients, developed distant metastases without any signs, such as brain metastasis, shortly after surgery, resulting in eventual death. These events were frequently unable to be accurately predicted, resulting in the poor accuracy of our nomogram in predicting 1-year OS. Second, we did not have another database to externally verify our nomogram. Finally, the short duration of follow-up might have impacted on the discriminatory and predictive ability of our nomogram. Further studies with larger sample sizes and better methodology are warranted.
In this study, we established and validated a novel nomogram for predicting the survival of patients with ISLNM. The new nomogram can stratify patients into different risk subgroups. This nomogram may, to some extent, allow clinicians to more accurately estimate prognosis and in making personalized therapeutic decisions for individual patients with ISLNM. Further prospective studies are warranted on a larger scale to validate the new nomogram.
Funding
This study was supported by a grant from the Science and Technology development plan of He’nan (No. 202102310428).
Conflicts of interest
None.
Footnotes
How to cite this article: Lyu MH, Ma YZ, Tian PQ, Guo HH, Wang C, Liu ZZ, Chen XC. Development and validation of a nomogram for predicting survival of breast cancer patients with ipsilateral supraclavicular lymph node metastasis. Chin Med J 2021;134:2692–2699. doi: 10.1097/CM9.0000000000001755
References
- 1.Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res 2017; 50:33.doi: 10.1186/s40659-017-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellapasqua S, Bagnardi V, Balduzzi A, Iorfida M, Rotmensz N, Santillo B, et al. Outcomes of patients with breast cancer who present with ipsilateral supraclavicular or internal mammary lymph node metastases. Clin Breast Cancer 2014; 14:53–60. doi: 10.1016/j.clbc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Chen SC, Chen MF, Hwang TL, Chao TC, Lo YF, Hsueh S, et al. Prediction of supraclavicular lymph node metastasis in breast carcinoma. Int J Radiat Oncol Biol Phys 2002; 52:614–619. doi: 10.1016/s0360-3016(01)02680-3. [DOI] [PubMed] [Google Scholar]
- 4.Brito RA, Valero V, Buzdar AU, Booser DJ, Ames F, Strom E, et al. Long-term results of combined-modality therapy for locally advanced breast cancer with ipsilateral supraclavicular metastases: the University of Texas M.D. Anderson Cancer Center experience. J Clin Oncol 2001; 19:628–633. doi: 10.1200/JCO.2001.19.3.628. [DOI] [PubMed] [Google Scholar]
- 5.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol 2002; 20:3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Jin R, Hu X, Luo J. Clinical characteristics and prognostic analysis of ipsilateral supraclavicular lymph node metastases in breast cancer patients: a retrospective study. Int J Clin Exp Pathol 2019; 12:3526–3534. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen QT, Zeng LY, Ouyang DJ, Zhao P, Zou QY, Pei L, et al. Surgery of the primary tumor offers survival benefits of breast cancer with synchronous ipsilateral supraclavicular lymph node metastasis. World J Surg 2020; 44:1163–1172. doi: 10.1007/s00268-019-05293-4. [DOI] [PubMed] [Google Scholar]
- 8.Lv M, Li J, Guo H, Wang C, Tian P, Ma Y, et al. Impact of ipsilateral supraclavicular lymph node dissection (ISLND) for breast cancer patients and a nomogram for predicting ipsilateral supraclavicular pathological complete response (ispCR). Ann Surg Oncol 2021; 28:5098–5109. [DOI] [PubMed] [Google Scholar]
- 9.Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010; 11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelhardt EG, Garvelink MM, de Haes JHCJM, van der Hoeven JJM, Smets EMA, Pieterse AH, et al. Predicting and communicating the risk of recurrence and death in women with early-stage breast cancer: a systematic review of risk prediction models. J Clin Oncol 2014; 32:238–250. doi: 10.1200/JCO.2013.50.3417. [DOI] [PubMed] [Google Scholar]
- 11.Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol 2001; 19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 12.van’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AAM, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002; 415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 13.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Zhao J, Zhu L, Su F, Chen K. Development and validation of a nomogram predicting the overall survival of stage IV breast cancer patients. Cancer Med 2017; 6:2586–2594. doi: 10.1002/cam4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun W, Jiang YZ, Liu YR, Ma D, Shao ZM. Nomograms to estimate long-term overall survival and breast cancer-specific survival of patients with luminal breast cancer. Oncotarget 2016; 7:20496–20506. doi: 10.18632/oncotarget.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartlett JMS, Brookes CL, Robson T, van de Velde CJH, Billingham LJ, Campbell FM, et al. Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: a prospectively powered pathology study in the Tamoxifen and Exemestane Adjuvant Multinational trial. J Clin Oncol 2011; 29:1531–1538. doi: 10.1200/JCO.2010.30.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colozza M, Larsimont D, Piccart MJ. Progesterone receptor testing: not the right time to be buried. J Clin Oncol 2005; 23:3867–3868. doi: 10.1200/JCO.2005.05.167. [DOI] [PubMed] [Google Scholar]
- 18.Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol 2003; 21:1973–1979. doi: 10.1200/JCO.2003.09.099. [DOI] [PubMed] [Google Scholar]
- 19.Ferno M, Stal O, Baldetorp B, Hatschek T, Kallstrom AC, Malmstrom P, et al. Results of two or five years of adjuvant tamoxifen correlated to steroid receptor and S-phase levels. South Sweden Breast Cancer Group, and South-East Sweden Breast Cancer Group. Breast Cancer Res Treat 2000; 59:69–76. doi: 10.1023/a:1006332423620. [DOI] [PubMed] [Google Scholar]
- 20.Lamy PJ, Pujol P, Thezenas S, Kramar A, Rouanet P, Guilleux F, et al. Progesterone receptor quantification as a strong prognostic determinant in postmenopausal breast cancer women under tamoxifen therapy. Breast Cancer Res Treat 2002; 76:65–71. doi: 10.1023/a:1020228620173. [DOI] [PubMed] [Google Scholar]
- 21.Dowsett M, Cuzick J, Wale C, Howell T, Houghton J, Baum M. Retrospective analysis of time to recurrence in the ATAC trial according to hormone receptor status: an hypothesis-generating study. J Clin Oncol 2005; 23:7512–7517. doi: 10.1200/JCO.2005.01.4829. [DOI] [PubMed] [Google Scholar]
- 22.Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst 2005; 97:1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 23.Punglia RS, Kuntz KM, Winer EP, Weeks JC, Burstein HJ. The impact of tumor progesterone receptor status on optimal adjuvant endocrine therapy for postmenopausal patients with early-stage breast cancer: a decision analysis. Cancer 2006; 106:2576–2582. doi: 10.1002/cncr.21919. [DOI] [PubMed] [Google Scholar]
- 24.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 2009; 14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 25.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 26.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 27.Lv M, Guo H, Wang C, Tian P, Ma Y, Chen X, et al. Neoadjuvant docetaxel with or without carboplatin plus dual HER2 blockade for HER2-positive breast cancer: a retrospective multi-center Chinese study. Gland Surg 2020; 9:2079–2090. doi: 10.21037/gs-20-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodward WA, Vinh-Hung V, Ueno NT, Cheng YC, Royce M, Tai P, et al. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol 2006; 24:2910–2916. doi: 10.1200/JCO.2005.03.1526. [DOI] [PubMed] [Google Scholar]
- 29.Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol 2009; 27:1062–1068. doi: 10.1200/JCO.2008.18.6965. [DOI] [PubMed] [Google Scholar]
- 30.Danko ME, Bennett KM, Zhai J, Marks JR, Olson JA, Jr. Improved staging in node-positive breast cancer patients using lymph node ratio: results in 1788 patients with long-term follow-up. J Am Coll Surg 2010; 210:797–805.e1, 805-7.doi: 10.1016/j.jamcollsurg.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 31.Ahn SH, Kim HJ, Lee JW, Gong GY, Noh DY, Yang JH, et al. Lymph node ratio and pN staging in patients with node-positive breast cancer: a report from the Korean breast cancer society. Breast Cancer Res Treat 2011; 130:507–515. doi: 10.1007/s10549-011-1730-9. [DOI] [PubMed] [Google Scholar]
- 32.Dings PJM, Elferink MAG, Strobbe LJA, de Wilt JHW. The prognostic value of lymph node ratio in node-positive breast cancer: a Dutch nationwide population-based study. Ann Surg Oncol 2013; 20:2607–2614. doi: 10.1245/s10434-013-2932-7. [DOI] [PubMed] [Google Scholar]
- 33.Keam B, Im SA, Kim HJ, Oh DY, Kim JH, Lee SH, et al. Clinical significance of axillary nodal ratio in stage II/III breast cancer treated with neoadjuvant chemotherapy. Breast Cancer Res Treat 2009; 116:153–160. doi: 10.1007/s10549-008-0160-9. [DOI] [PubMed] [Google Scholar]
- 34.Tsai J, Bertoni D, Hernandez-Boussard T, Telli ML, Wapnir IL. Lymph node ratio analysis after neoadjuvant chemotherapy is prognostic in hormone receptor-positive and triple-negative breast cancer. Ann Surg Oncol 2016; 23:3310–3316. doi: 10.1245/s10434-016-5319-8. [DOI] [PubMed] [Google Scholar]
- 35.van der Wal BCH, Butzelaar RMJM, van der Meij S, Boermeester MA. Axillary lymph node ratio and total number of removed lymph nodes: predictors of survival in stage I and II breast cancer. Eur J Surg Oncol 2002; 28:481–489. doi: 10.1053/ejso.2002.1239. [DOI] [PubMed] [Google Scholar]
- 36.Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Oswald MJ, Outlaw ED, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol 2004; 22:2303–2312. doi: 10.1200/JCO.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 37.Neuman H, Carey LA, Ollila DW, Livasy C, Calvo BF, Meyer AA, et al. Axillary lymph node count is lower after neoadjuvant chemotherapy. Am J Surg 2006; 191:827–829. doi: 10.1016/j.amjsurg.2005.08.041. [DOI] [PubMed] [Google Scholar]