Abstract
Cell death occurs in various tissues and organs in the body. It is a physiological or pathological process that has different effects. It is of great significance in maintaining the morphological function of cells and clearing abnormal cells. Pyroptosis, apoptosis, and necrosis are all modes of cell death that have been studied extensively by many experts and scholars, including studies on their effects on the liver, kidney, the heart, other organs, and even the whole body. The heart, as the most important organ of the body, should be a particular focus. This review summarizes the mechanisms underlying the various cell death modes and the relationship between the various mechanisms and heart diseases. The current research status for heart therapy is discussed from the perspective of pathogenesis.
Keywords: Cell death, Apoptosis, Necrosis, Pyroptosis, Autophagy, Pathogenesis, Treatment
Introduction
Cell death is the key starting point for organ and even body death. Some forms of cell death are physiological, which is conducive for the development of the body, while other forms lead to organ dysfunction and more severe outcomes.
At present, known modes of cell death include, but are not limited to, apoptosis, necrosis, pyroptosis, and autophagy. These modes occur independently but interact with each other. In other words, dead cells can simultaneously show features of autophagy, apoptosis, pyroptosis, and necrosis. Recent reports have confirmed interactions between these mechanisms, including several common cell stress-induced signaling pathways. For example, reactive oxygen species (ROS) and calcium (Ca2+) can regulate autophagy and apoptosis simultaneously.[1–3] Cardiovascular diseases are responsible for the highest incidence rate and mortality rate among all diseases worldwide.[4] Cardiac insufficiency is a common complication of sepsis and other serious diseases and is an important cause of death. Cardiac dysfunction-related diseases caused by myocardial cell death impose substantial burdens on society and individuals.
The composition of the heart is 70% of non-cardiomyocytes and 30% of cardiomyocytes.[5] Death of cardiomyocytes and non-cardiomyocytes induced by stimuli such as ROS, β1-adrenergic receptor agonists, angiotensin II, and proinflammatory cytokines is also mediated by various cell death modes. A previous study of 62 cases of heart failure with dilated cardiomyopathy showed autophagy, apoptosis, and morphological features of necrosis.
This paper summarizes the mechanisms underlying several major cell death modes and focuses on their impact on the heart. Additionally, according to mechanism and impact, we summarize their significance for treatment, promotion, and guidance on their use in the clinical setting. Comparison and features of the different forms of cell death are summarized in Table 1.
Table 1.
Comparison of different forms of cell death and their biological features.
| Mode | Object of study | Intervention mode | Conclusion | Ref |
| A | Mice | Melatonin | Melatonin protects against sepsis-induced cardiac dysfunction by regulating apoptosis and autophagy via activation of SIRT1 in mice. | [49] |
| A | Septic mice | TMZ | TMZ protected against LPS-induced myocardial dysfunction and apoptosis, accompanied by inhibition of macrophage pro-inflammatory responses. | [50] |
| P | Mice | TMZ | TMZ as a potential therapeutic agent for septic or endotoxemia-associated cardiac dysfunction in mice. | [45] |
| A | Rats | miR-25 | miR-25 inhibits sepsis-induced cardiomyocyte apoptosis by targeting PTEN. | [51] |
| A | Septic rats | AC2-26 | AC2-26 may alleviate the sepsis-induced cardiomyocyte apoptosis in vivo and in vivo through the LXA4/PI3K/AKT signaling pathway. | [52] |
| A | Septic rats | Calpain inhibitors | Akt/eNOS/NO pathway might lead to a novel pharmacological therapy for cardiomyocytes apoptosis in sepsis. | [53] |
| A | Septic rats | Esmolol | Esmolol reduces apoptosis and inflammation in early sepsis rats with abdominal infection. | [54] |
| P and A | Mice | Stimulator of interferon genes | STING-IRF3 contributes to LPS-induced cardiac dysfunction, inflammation, apoptosis, and pyroptosis by activating NLRP3. | [55] |
| P | HCM cells | Angiotensin II | miR-133a-3p upregulation may be a promising strategy for cardiac hypertrophy treatment. | [56] |
| P | H9C2 rat cardiomyoblast cell | LPS | LPS aggravates high glucose- and hypoxia/reoxygenation-induced injury through activating ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2 cardiomyocytes. | [57] |
| P | Septic BALB/c mice | Thymoquinone | Role of thymoquinone in cardiac damage caused by sepsis from BALB/c mice. | [58] |
| P | LPS-treated mice | Geniposide | Geniposide protects against sepsis-induced myocardial dysfunction through the AMPKα-dependent pathway. | [59] |
| P | A mouse hyperuricemia model | IR (MI/R) | UA aggravates MI/R-induced activation of the NLRP3 inflammatory cascade and pyroptosis by promoting ROS generation, while inflammasome inhibitors and ROS scavengers partly reverse the injury. | [60] |
| P | LPS-induced mice/CORM-3-treated mice | CO-releasing molecule-3 | Carbon monoxide releasing molecule-3 improves myocardial function in mice with sepsis by inhibiting NLRP3 inflammasome activation in cardiac fibroblasts. | [61] |
| N | Mice lacking CypD | CypD-deficient mice by gene targeting | The CypD-dependent mPT regulates some forms of necrotic death, but not apoptotic death. | [10] |
| N | Mouse | CypD or RIP3 knockout | RIP3–CaMKII–MPTP myocardial necroptosis pathway, a promising target for the treatment of ischemia- and oxidative stress-induced myocardial damage and heart failure. | [62] |
| N | Heart failure patients | Detection of the level of death receptor | Necrosis-related factor and miR-873 can regulate programmed necrosis in the heart. | [63] |
| Au | Domestic swine | IR (MI/R) | Autophagy, triggered by ischemia, could be a homeostatic mechanism, by which apoptosis is inhibited and the deleterious effects of chronic ischemia are limited. | [35] |
| Au | Sepsis-induced rats | Melatonin | The study sheds light on the important role of Beclin-1 acetylation in regulating autophagy in sepsis and suggests that melatonin is a potential candidate drug for the treatment of sepsis. | [64] |
| Au | H9C2 rat cardiomyoblast cell | Hypoxia/reoxygenation (H/R) | miR-542-5p/autophagy pathway might be a potential target for the treatment of H/R-related heart diseases. | [65] |
A: Apoptosis; Au: Autophagy; CypD: Cyclophilin D; IR: Ischemia-reperfusion; LPS: Lipopolysaccharides; MPTP: Mitochondrial permeability transition pore; N: Necrosis; NLRP3: Nucleotide-binding oligomerization domain-like receptor protein 3; P: Pyroptosis; ROS: Reactive oxygen species; TMZ: Trimetazidine; AC2-26: an active N-terminal peptide of AnxA1(Annexin A1); MI/R: Myocardial ischemia reperfusion; CO: Carbon monoxide; mPT: Mitochondrial permeability transition; RIP3:Receptor-interacting serine-threonine kinase 3.
Mechanisms of the Modes of Cell Death
Necrosis
Necrosis, which was proposed around 1960, is a form of cell death characterized by organelle and cell membrane destruction, often accompanied by inflammatory reactions.[6,7] In the past, necrosis was considered to be an unregulated, accidental, and passive process. However, increasing evidence has shown that necrosis is an active process regulated by cells destined for death. For example, a study in Caenorhabditis elegans showed that all forms of cell death are actively mediated.[8] The study showed that necrosis is mediated by genes encoding plasma membrane and endoplasmic reticulum Ca2+ channels. After activation of these genes, intracellular Ca2+ concentration increases.[9,10] Studies have shown that necrosis caused by mitochondrial events triggered by the mitochondrial permeability transition pore (MPTP) is regulated by cyclophilin D (CypD), which exists in the mitochondrial matrix.[11–13] These studies demonstrated that gene regulation is involved in necrosis.
Mitochondrial pathway of necrosis
The MPTP, composed of the voltage-dependent anion channel, adenine nuclear translocator, cyclin D, and other molecules, plays an important role in the mitochondrial pathway of necrosis. Stimulation by increased intracellular Ca2+, inorganic phosphate, alkaline pH, and ROS can lead to the opening of MPTP, which in turn leads to mitochondrial membrane permeability, a process also known as mitochondrial depolarization. Oxidative phosphorylation results in loss of the proton gradient and the cessation of adenosine trihosphate (ATP) production, leading to mitochondrial swelling, outer membrane rupture, and simultaneous destruction of the cell membrane and organelles, which typify the mitochondria-mediated pathway of necrosis.[14]
Death receptor pathway of necrosis
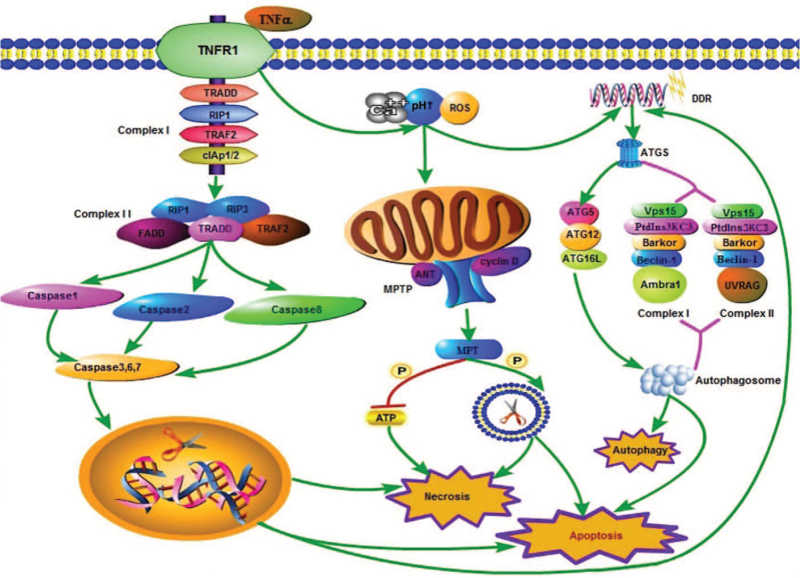
The death receptor pathway is divided into the initiation of death receptor-mediated necrosis and execution of death receptor-mediated necrosis. It can be stimulated by apoptosis-activating ligands, such as tumor necrosis factor (TNF), Fas ligand, and TNF-related apoptosis-inducing ligand. Receptor-interacting proteins (RIPs) play a key role in the death receptor pathway. When the body is subjected to certain stimuli, the binding of TNF-α to TNF receptor 1 (TNFR1) leads to exposure of the death domain of TNFR1 in the cytoplasm, which subsequently leads to recruitment of TNF-receptor-associated death domain (TRADD). This provides a scaffold for the assembly of complex I (composed of TNFR1, TRADD, receptor-interacting proteins 1 (RIP1), TNF-related apoptosis-inducing ligand receptors [TRAF2], and cellular inhibitors of apoptosis 1/2 [cIAP1/2]) on the plasma membrane. The complex can activate nuclear factor-k-gene binding (NF-κB), and activate the cell self-rescue pathway by inhibiting activation of downstream caspases. It can also promote the formation of complex II (composed of Fas-associated via death domain [FADD], TRADD, RIP1/3, TRAF2, and caspase-8) under certain conditions. FADD can mediate the recruitment and activation of procaspase-8, and trigger apoptosis. Phosphorylation of the necrotic bodies formed by RIP1 and RIP3 can activate certain downstream catabolic enzymes, increase oxidative phosphorylation, and produce ROS, leading to necrosis. Phosphorylated RIP1/RIP3 can also activate other pathways leading to necrosis. Activated RIP3 also binds its substrate, mixed-lineage kinase domain-like protein (MLKL).[15,16] MLKL can destroy the complete structure of cell and organelle membranes, alter cell permeability, and induce necrosis.[17]
Some metabolic enzymes, such as glycogen phosphorylase and glutamate dehydrogenase 1, have been found to be downstream targets of the necrotic bodies formed by RIP1 and RIP3.[18] That is, they are involved in necrosis mediated by metabolic pathways. Furthermore, studies found that mitochondrial phosphatase PGAM5 is a key molecule involved in the formation of the necrosome.[19,20]
Apoptosis
Apoptosis is a process involving changes in organelles, the cell membrane, and the nucleus. The morphological changes include chromatin condensation and fragmentation of the nucleus, cell contraction, and apoptotic body formation.[21] Simultaneously, macrophages or their adjacent cells phagocytize apoptotic bodies to achieve their elimination. This process is extremely concealed, and often occurs in a single cell to avoid the occurrence of inflammation.
The triggering process includes the intrinsic and extrinsic pathways, which are also known as the mitochondrial pathway and death receptor pathway, respectively. When stimulated by growth factors, hypoxia, oxidative stress, or DNA damage, the intrinsic pathway can play a significant role. It can activate downstream “B-cell lymphoma (BCL)” family proteins, including BCL-2, BCL-8, BCL-9, and MCL-1, which inhibit apoptosis, and Bax and Bak, which promote apoptosis. The ultimate effect is to promote apoptosis by inhibiting the formation of anti-apoptotic factors.[22,23]
Many studies have shown that the death receptor pathway is related to apoptosis and other cell death modes.[24–26] When Fas ligand or TNF-α binds the homologous receptor on the plasma membrane, caspase-8 is activated downstream, which in turn activates caspase-3.[27,28] Caspases play a central role in apoptosis. Under certain conditions, procaspases (inactive proenzymes) are synthesized. They are composed of a prodomain and P20 subunit. The prodomain is involved in the process of binding procaspases with other proteins. The P20 subunit has specific catalytic functions, while the P10 subunit has substrate specificity. After they are activated, downstream molecules are subsequently activated to promote apoptosis. In other words, various factors lead to the dissolution of the cell-matrix, nuclear condensation, DNA fragmentation, plasma membrane contraction, and formation of a large number of plasma membrane vesicles and apoptotic bodies. This process does not involve inflammation. Notably, the caspase family is also involved in the occurrence of other cell death modes [Figure 1].
Figure 1.
The mechanisms underlying apoptosis, necrosis, and autophagy. In the mitochondrial pathway of necrosis, intracellular and extracellular Ca2+, alkaline pH, and ROS can stimulate the opening of the MPTP (composed of the voltage-dependent anion channel, adenine nuclear translocator [ANT], and cyclin D). Oxidative phosphorylation leads to the cessation of ATP production and destruction of the cell membrane, leading to necrosis. This process can also lead to apoptosis. When the death receptor pathway is triggered, the binding of TNF to TNFR1 leads to exposure of the cytoplasmic death domain of TNFR1, which in turn leads to the recruitment of TRADD. It provides a scaffold for the assembly of complex I (TNFR1, TRADD, RIP1, TRAF2, and cIAP1/2) on the plasma membrane. The complex can promote the formation of complex II (FADD, TRADD, RIP1/3, and TRAF2) under certain conditions. Complex II can mediate the recruitment and activation of caspase-1/2/8, and then activate the downstream caspase-3/6/7. These caspase proteins can destroy DNA in the nucleus, and lead to the destruction of a large number of plasma and cell membranes, thereby leading to necrosis. The activation of caspase proteins, especially caspase-3, is the key factor for triggering apoptosis. DDR induced by various factors leads to the formation of ATGs, such as ATG5 and Beclin-1. ATG5 mediates the formation of the autophagosome through the formation of ATG5–ATG1–ATG16L, while Beclin-1 mediates the formation of the autophagosome through the formation of complex I (composed of Beclin-1, PtdIns3KC3, Vps15, Bakor, and Ambra1) and complex II (composed of Beclin-1, PtdIns3KC3, Vps15, Bakor, and UVRAG), which mediate the formation of autophagosomes, eventually leading to autophagy. The process of apoptosis also depends on the formation of ATGs. ATGs: Autophagy-related proteins; cIAP1/2: Cellular inhibitors of apoptosis 1/2; DDR: DNA damage response; FADD: Fas-associated via death domain; MPTP: Mitochondrial permeability transition pore; PtdIns3KC3: Phosphatidylinositol 3-kinase; ROS: Reactive oxygen species; TNF: Tumor necrosis factor; TNFR1: TNF receptor1; TRADD: TNF-receptor-associated death domain; TRAF2: TNF-related apoptosis-inducing ligand receptors.
Pyroptosis
There are similarities and differences between pyroptosis and apoptosis. They both involve the caspase family. The differences lie with which subtypes of caspases are involved in cell death.[29] Pyroptosis refers to the spontaneous death of cells under genetic control.[12] It can be blocked by signal transduction inhibitors. At present, it occurs in monocytes, dendritic cells, vascular endothelial cells, and other cells.[30]
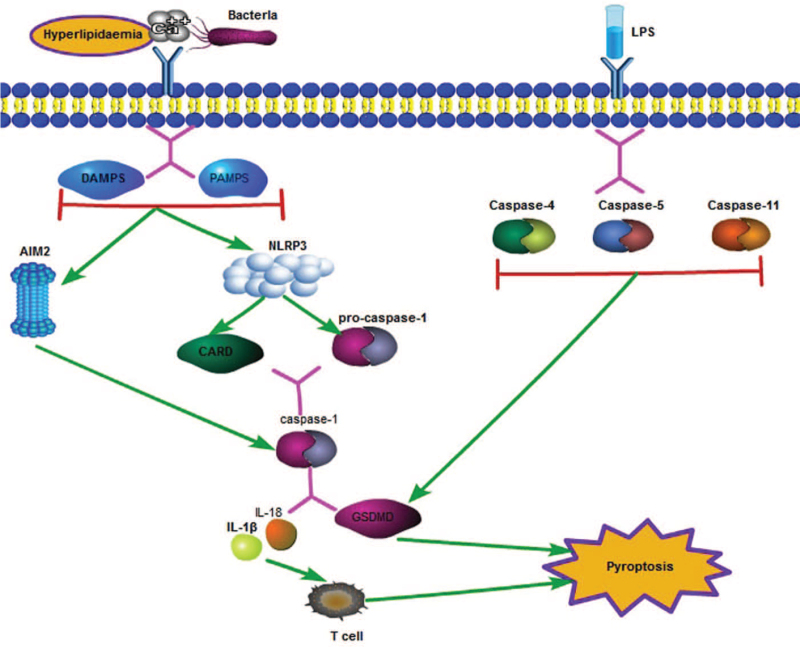
The mechanism underlying pyroptosis involves many molecules and substances. It is considered to be divided into two major pathways. In the classical pathway, its occurrence depends on the participation of caspase-1. In the non-classical pathway, which is independent of caspase-1, it is mediated by other caspase family molecules. When subjected to certain stimuli, such as hyperlipidemia and inflammation, pathogen-associated molecular pattern and danger-associated molecular pattern signaling is activated, resulting in activation of the downstream nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) and absent in melanoma 2 (AIM2) inflammasome. The N-terminal pyrin domain (PYD) of NLRP3, as a scaffold-like substance, mediates the formation of apoptosis-associated speck-like protein (ASC), which can activate caspase recruitment domains, and also functions as a PYD and caspase activation and recruitment domain (CARD). CARDs combine with procaspase-1, which promotes the latter to undergo self-cleavage to form mature caspase-1. The AIM2 inflammasome leads directly to the binding of double-stranded DNA through its HIN200 domain, resulting in AIM2 oligomerization and ASC recruitment. The activation of these inflammatory bodies can trigger the expression of caspase-1. Caspase-1 can further activate downstream molecules. For example, caspase-1 participates in the cleavage process of Gasdermin D (GSDMD, a member of the Gasdermin protein family, consisting of >500 amino acids), which causes the formation of a 31-kDa N-terminal product (GSDMD-N) in the membrane pore, resulting in the formation of cell membrane pores, which leads to the outflow of cellular content and expands the scope of the damage.[31] It is also involved in the recognition and recruitment of inflammatory factors such as interleukin-1β (IL-1β) and IL-18, which induce inflammation. Pro-IL-1β is an inactive precursor molecule that is recognized by caspase-1 and cleaved into mature IL-1β (116 amino acids in length). The latter promotes inflammation by recruiting innate immune cells to the site of infection and regulating adaptive immune cells (T helper 1 [Th1], Th17).[32]
The caspase proteins involved in the non-classical pathway are caspase-4 and caspase-5 in humans and caspase-11 in mice. This process can be induced by lipopolysaccharides (LPS). These caspase family members can directly cleave GSDMD, leading to pyroptosis.
The process of NLRP3-mediated pyroptosis can also be accomplished via other mechanisms. When cell membrane pores are formed, extracellular NLRP3 agonists enter the cytoplasm and directly activate NLRP3, thereby mediating a series of reactions involving downstream molecules. Lysosomes can also mediate cell rupture and eventually leads to the occurrence of pyroptosis.
Another caspase-1-independent pathway is activated by LPS and can cause activation of downstream caspase-4/5 and caspase-11. These caspase proteins are also involved in the activation of inflammatory factors such as GSDMD and IL-18, which mediate the occurrence of pyroptosis [Figure 2].
Figure 2.
Caspase-1-dependent and independent signaling pathways of pyroptosis. In the classical pathway, when the body is stimulated by hyperlipidemia, abnormal Ca2+ levels, and bacteria, infection cells activate downstream inflammatory bodies, including NLRP3 and AIM2 through PAMP and DAMP pathways. NLRP3 can establish a link with CARD and procaspase-1. Procaspase-1 bound to CARD can self-cleave into mature caspase-1, and AIM2 can directly activate caspase-1. Mature caspase-1 can cleave the downstream GSDMD and can induce large production of IL-18 and other inflammatory factors. The N-terminal of GSDMD, which is cleaved, will combine with phospholipids and protein on the cell membrane to form pores, leading to the outflow of inflammatory factors and cell contents. Inflammatory factors can activate T cell immunity in the body, further aggravate body damage, and eventually lead to cell death. The non-classical pathway of pyroptosis can be induced by LPS, which can activate caspase-4/5/11, and also lead to GSDMD cleavage. AIM2: Absent in melanoma 2; CARD: Caspase activation and recruitment domain; DAMP: Danger-associated molecular patterns; GSDMD: Gasdermin D; IL: Interleukin; LPS: Lipopolysaccharides; NLRP3: Nucleotide-binding oligomerization domain-like receptor protein 3; PAMP: Pathogen-associated molecular pattern.
Autophagy
Autophagy, a cell death mode mediated by double-membrane autophagosomes, serves to degrade malformed proteins and excess or defective organelles. Similar to other modes of cell death, it is involved in physiological and pathological processes. Autophagy is usually non-selective and is a method of survival for cells under poor conditions. It can retain long-lived proteins and organelles.[33,34] As a protein on the lysozyme membrane, lysosomal-associated membrane protein 2 (LAMP2) plays an important role in autophagy initiated by Beclin-1. Autophagy depends on a series of autophagy-related proteins (ATGs), such as ATG5 and Beclin-1 (yeast atg6 homolog), which not only promote the formation of the autophagosome but also induce apoptosis. ATG5 mediates the formation of autophagosomes by forming the ubiquitination system, ATG5–ATG12–ATG16L, while Beclin-1 induces autophagy by forming phosphatidylinositol 3-kinase (PtdIns3KC3). There are two types of PtdIns3KC3 complex: Complex I is composed of Beclin-1, PtdIns3KC3, Vps15, Barkor, and Ambra1; Complex II is composed of Beclin-1, PtdIns3KC3, Vps15, Barkor, and ultraviolet radiation resistance-associated gene (UVRAG). Chaperone-mediated autophagy is responsible for the recognition of chaperone proteins by heat shock-like proteins (Hsc70). These proteins are bound to LAMP2 and transported to lysosomes. Autolysosomes degrade these specific proteins to complete the process of autophagy.
The process of autophagy generally includes the isolation of cytoplasmic proteins and organelles and their degradation in lysosomes. Autophagy has a certain significance, such as in the adaptation to nutritional deficiency, intracellular clearance of proteins and organelles, development, anti-aging, elimination of microorganisms, and tumor inhibition.[35]
Cardiac Dysfunction Caused by Multiple Modes of Cell Death
Necrosis
Historically, necrosis was largely ignored because it was considered unregulated. Myocardial cell necrosis, considered to be the main pathological injury in acute myocardial infarction, has not been formally evaluated until recently. Myocardial cell necrosis caused by oxidative stress and ischemia-reperfusion (IR) is one of the pathophysiological mechanisms of heart failure. Oxidative stress-induced necrosis is thought to be caused by MPTP opening and ATP depletion.[36] As mentioned above, CypD is a component of the MPTP. Studies have shown that following IR, myocardial injury is significantly lower in CypD-deficient mice compared with controls. Lactate dehydrogenase content was also greatly reduced.
Apoptosis
Apoptosis is very rare in the normal myocardium. The incidence rate is approximately 1/10,000, although increased cardiomyocyte apoptosis has important pathophysiological effects. The proportion of apoptotic cells observed in heart failure patients was approximately 0.8%.[37–39]
As recently as 20 years ago, a large number of studies revealed the role of myocardial cell death in myocardial infarction and heart failure. Apoptosis, as a form of cell death that could be regulated at that time, became a research focus by numerous scholars. These studies indicate that apoptosis is an important part of the pathogenesis of myocardial infarction and heart failure. Previous studies have shown that the ROS-apoptosis signal-regulated kinase 1 (ASK1)-c-Jun N-terminal kinase (JNK) pathway plays an important role in cardiomyocyte apoptosis. ASK1 is a ROS-sensitive mitogen-activated protein kinase, which leads to apoptosis by activating downstream MAP kinase kinases, followed by activation of the p38 and JNK pathways.[40] Studies have repeatedly shown that in the heart of ASK1-deficient mice, the increase in JNK phosphorylation is significantly reduced, and cardiomyocytes are resistant to oxidative stress-induced apoptosis, which is conducive to the prevention of heart failure.[41]
Pyroptosis
Pyroptosis is closely related to the occurrence of many heart diseases and involves several cell types including endothelial cells and vascular smooth muscle cells. For example, the occurrence of atherosclerosis involves local inflammatory reactions mediated by monocytes, macrophages, and neutrophils. T and B lymphocytes are also involved in the formation and development of atherosclerosis by promoting the release of inflammatory factors described above and are closely related to plaque stability. As a key protein, NLRP3 is activated by oxidized low-density lipoprotein (oxLDL), which leads to inflammation in the pyroptosis pathway. Moreover, lipid accumulation occurs after vascular endothelial injury. Previous studies have also detected large amounts of caspase-1 — the key molecule of the pyroptosis pathway — in accumulated plaques. oxLDL can also activate macrophages to promote the occurrence of pyroptosis.
In the process of diabetic heart disease, high glucose can stimulate excessive production of ROS, promote the activation of the NLRP3 inflammasome through NF-κB, and indirectly lead to the occurrence of pyroptosis. Furthermore, studies found that IL-1β is an important proinflammatory cytokine in the development of dilated cardiomyopathy.[42] Long non-coding RNAs (lncRNAs) have also been reported to be closely related to the occurrence of pyroptosis. Among them, a lncRNA located on human chromosome 11p15.5 is considered to be involved in various cardiovascular diseases, including cardiac dysfunction and arrhythmia.[43] Silencing lncRNA can improve cardiac function and fibrosis in mice. Previous studies have also found that the levels of caspase-1 and IL-1β are significantly upregulated in hypertrophic cardiomyocytes. Furthermore, inhibiting the expression of these factors can reduce myocardial hypertrophy.[44]
Autophagy
Autophagy is observable in heart failure caused by various heart diseases, such as dilated heart disease and ischemic myocardial disease.[45,46] Whether autophagy plays a positive or negative role in the heart has always been controversial. Most scholars believe the key molecular substances in the regulation of autophagy can affect the function of cardiomyocytes, and the positive role is dominant. For example, a previous study showed that after enhancing Beclin-1, a key autophagy protein regulatory complex, the activity of Bax can be reduced, thereby alleviating myocardial injury to a certain degree.[47] LAMP2-deficient mice have blunted autophagy and aggravated cardiomyocyte injury.[48] Deletion of ATG5 (the key protein involved in the extension of the phagocyte membrane in autophagic vesicles) leads to the accumulation of abnormal proteins and organelles, especially the accumulation of mitochondria, which can directly lead to the occurrence of cardiac dysfunction.[49] All these observations indicate a role for autophagy in cardiac protection.
Recent Advances in the Treatment of Heart Disease Based on Various Cell Death Methods
To explore the improvement of cardiac function in heart disease, necrosis can be divided into the death receptor pathway and mitochondrial pathway. Certain molecules or substances, as inhibitors or blockers of these pathways, can significantly improve myocardial cell necrosis, thereby improving cardiac function. For example, a prior study showed that RIP3 knockout can reduce inflammation of the ischemic heart and improve ejection fraction.[50] Nec-1 can bind RIP1 and antagonize its activity, and necrosulfonamide can be used as an MLKL inhibitor. These inhibitors also have certain effects on other cell death modes.
As mentioned above, ASK1, a key molecule in the apoptosis signaling pathway, may represent a new therapeutic target for patients with heart failure. Furthermore, its downstream molecule — p38 — may serve as an entry point for the prevention and improvement of cardiomyocyte apoptosis.[40] Trimetazidine (TMZ) can significantly reduce intracellular acidosis and apoptosis, thereby protecting myocardial function.[51]
Interfering with molecules associated with the pyroptosis pathway (such as NLRP3, AIM2, caspase-1, and IL-1β) can affect the occurrence of pyroptosis, thereby affecting the occurrence and development of cardiovascular disease, and may represent potential therapeutic targets for cardiovascular disease. This has been verified in many previous studies. For example, resveratrol — a natural polyphenol — has been shown to have anti-inflammatory, anti-oxidation, and anti-cancer properties, which can protect heart tissue from damage.[52] Resveratrol can reduce heart injury by inhibiting phosphorylation of the mitogen-activated protein kinase signaling pathway and inhibiting activation of the NLRP3 inflammasome. Additionally, after silencing NLRP3, cardiac dysfunction was significantly improved.[53] A low dose (≤50 mg/kg) of erucic acid can inhibit activation of the NLRP3 inflammasome by downregulating the expression of lncRNA, and reducing the levels of endothelin-1 and IL-1β, thereby reducing cell damage.[54] TMZ has also been reported to reduce LPS-induced cardiomyocyte death and cardiac dysfunction by regulating the expression of chemokine receptors to promote neutrophil recruitment to heart tissue. In other words, TMZ can be used to treat septic heart failure. More studies are definitely needed to confirm this hypothesis.[55] Melatonin (also known as N-acetyl-5-methoxytryptamine), as an effective antioxidant, can reduce the expression of oncosis-related genes in endothelial cells, including NLRP3, ASC, caspase-1, GSDMD, IL-1β, and IL-18, thereby improving heart function.[56] Recent studies have also shown that astragaloside IV can significantly downregulate the expression of NLRP3, caspase-1, and IL-18 in isoproterenol-induced mice, thereby inhibiting myocardial fibrosis.[57] Liraglutide (a glucagon-like peptide-1 analog) can inhibit activation of the NLRP3 inflammasome by upregulating and reducing the production of ROS, thereby playing a protective role in myocardial ischemia.[58]
Conclusion and Perspectives
Cell death has important effects on the body. Proper cell death is beneficial. However, cell death activated by stimulating factors imposes burdens on the body, which are sometimes irreversible. The relationship between the many modes of cell death is very complex. Necrosis and apoptosis are two different modes of cell death. They are often compared with each other, and they share common features. For example, both of them occur via a mitochondrial pathway and necrosis pathway. When the body is damaged, they often occur simultaneously. There are also common activators between apoptosis and autophagy, and each form of cell necrosis neither exists nor occurs independently.
The heart plays a very important role in the body. Myocardial cell death also has a significant impact on cardiac function. When the heart is stimulated by ischemic blood oxygen, oxidative stress, or direct injury, myocardial cell death through apoptosis, necrosis, and autophagy leads to cardiac dysfunction. Many questions remain to be answered regarding the study of the various modes of cardiac cell death. For example, the process of necrosis can be considered subversive because it is a regulated process, and more research is required to clarify its specific mechanisms, and whether its regulation is influenced by cell type. Furthermore, whether there is diversity in cell death modes among cardiac cells still requires substantial experimentation to demonstrate. Only by clarifying the mechanism can the pathway be blocked to exert a cardioprotective effect. As another example, we know that there are both connections and differences between the several modes of cell death described herein. They can be activated at the same time after being subjected to the same stimulus or can be activated before or after the stimulus. It remains to be determined if there are any differences in cell death modes between the process of injury of cardiac cells and cardiac function. The impact of this difference on the survival rate of patients must be explored. Moreover, it is established that autophagy is a mode of cell death that differs from necrosis. Apoptosis is a mild and non-inflammatory mode of cell death. Autophagy and apoptosis are closely related (many factors leading to apoptosis can cause autophagy), and blockage of the apoptosis and autophagy pathways has been studied by many scholars. Therefore, following a stimulus, if the inevitable process of necrosis in cardiomyocytes can be transformed into apoptosis or autophagy by appropriate intervention, this will slow or even reduce damage to the heart. Inhibiting the expression of molecules in related pathways can significantly improve the degree of cardiac dysfunction. These are potential targets for the treatment of cardiac dysfunction-related myocardial diseases. Naturally, the use of some of the drugs targeting these molecules is quite mature in clinical practice. However, many potential clinical or animal research objectives remain to be explored shortly, to realize the transformation from basic research to clinical application, so as to serve clinical needs.
Funding
This work was supported by a grant from the Project Fund of the Lanzhou University of Second Hospital (No. CY-2018-MS03).
Conflicts of interest
None.
Footnotes
How to cite this article: Li P, Dong XR, Zhang B, Zhang XT, Liu JZ, Ma DS, Ma L. Molecular mechanism and therapeutic targeting of necrosis, apoptosis, pyroptosis, and autophagy in cardiovascular disease. Chin Med J 2021;134:2647–2655. doi: 10.1097/CM9.0000000000001772
References
- 1.Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, et al. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci U S A 2003; 100:15883–15888. doi: 10.1073/pnas.2136717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev 2006; 86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017; 135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nag AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios 1980; 28:41–61. [PubMed] [Google Scholar]
- 5.Bulluck H, Rosmini S, Abdel-Gadir A, White SK, Bhuva AN, Treibel TA, et al. Residual myocardial iron following intramyocardial hemorrhage during the convalescent phase of reperfused ST-segment-elevation myocardial infarction and adverse left ventricular remodeling. Circ Cardiovasc Imaging 2016; 9:e004940.doi: 10.1161/CIRCIMAGING.116.004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev 2012; 249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaham S, Horvitz HR. Developing Caenorhabditis elegans neurons may contain both cell-death protective and killer activities. Genes Dev 1996; 10:578–591. doi: 10.1101/gad.10.5.578. [DOI] [PubMed] [Google Scholar]
- 8.Syntichaki P, Xu K, Driscoll M, Tavernarakis N. Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature 2002; 419:939–944. doi: 10.1038/nature01108. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi L, Gerstbrein B, Frøkjaer-Jensen C, Royal DC, Mukherjee G, Royal MA, et al. The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: implications for necrosis initiation. Nat Neurosci 2004; 7:1337–1344. doi: 10.1038/nn1347. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005; 434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 11.Kitsis RN, Molkentin JD. Apoptotic cell death “Nixed” by an ER-mitochondrial necrotic pathway. Proc Natl Acad Sci U S A 2010; 107:9031–9032. doi: 10.1073/pnas.1003827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res 2003; 93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell 2014; 54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol 2015; 15:362–374. doi: 10.1038/nri3834. [DOI] [PubMed] [Google Scholar]
- 15.Declercq W, Berghe TV, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell 2009; 138:229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 2012; 148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Karch J, Kwong JQ, Burr AR, Sargent MA, Elrod JW, Peixoto PM, et al. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. Elife 2013; 2:e00772.doi: 10.7554/eLife.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 19.Galluzzi L, Vitale I, Aaronson S, Abrams J, Adam D, Agostinis P. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 2018; 25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shan B, Pan H, Najafov A, Yuan J. Necroptosis in development and diseases. Genes Dev 2018; 32:327–340. doi: 10.1101/gad.312561.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM. FADD a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 1995; 81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 22.Jeng MJ, Soong WJ, Lee YS, Tsao PC, Yang CF, Chiu SY, et al. Meconium exposure dependent cell death and apoptosis in human alveolar epithelial cells. Pediatr Pulmonol 2010; 45:816–823. doi: 10.1002/ppul.21262. [DOI] [PubMed] [Google Scholar]
- 23.Hu Q, Zhang T, Yi L, Zhou X, Mi M. Dihydromyricetin inhibits NLRP3 inflammasome-dependent pyroptosis by activating the Nrf2 signaling pathway in vascular endothelial cells. Biofactors 2018; 44:123–136. doi: 10.1002/biof.1395. [DOI] [PubMed] [Google Scholar]
- 24.Reisetter AC, Stebounova LV, Baltrusaitis J, Powers L, Gupta A, Grassian VH, et al. Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles. J Biol Chem 2011; 286:21844–21852. doi: 10.1074/jbc.M111.238519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afonina IS, Zhong Z, Karin M, Beyaert R. Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nat Immunol 2017; 18:861–869. doi: 10.1038/ni.3772. [DOI] [PubMed] [Google Scholar]
- 26.Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 2017; 277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He X, Qian Y, Li Z, Fan EK, Li Y, Wu L, et al. TLR4-upregulated IL-1β and IL-1RI promote alveolar macrophage pyroptosis and lung inflammation through an autocrine mechanism. Sci Rep 2016; 6:31663.doi: 10.1038/srep31663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 2007; 8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 29.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest 2005; 115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Empel VP, Bertrand AT, Hofstra L, Crijns HJ, Doevendans PA, De Windt LJ. Myocyte apoptosis in heart failure. Cardiovasc Res 2005; 67:21–29. doi: 10.1016/j.cardiores.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Hikoso S, Ikeda Y, Yamaguchi O, Takeda T, Higuchi Y, Hirotani S, et al. Progression of heart failure was suppressed by inhibition of apoptosis signal-regulating kinase 1 via transcoronary gene transfer. J Am Coll Cardiol 2007; 50:453–462. doi: 10.1016/j.jacc.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 32.Chen K, Zhang J, Zhang W, Zhang J, Yang J, Li K, et al. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol 2013; 45:932–943. doi: 10.1016/j.biocel.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Dai Y, Yan S, Shi Y, Han B, Li J, et al. Down-regulation of lncRNA KCNQ1OT1 protects against myocardial ischemia/reperfusion injury following acute myocardial infarction. Biochem Biophys Res Commun 2017; 491:1026–1033. doi: 10.1016/j.bbrc.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Bai Y, Sun X, Chu Q, Li A, Qin Y, Li Y, et al. Caspase-1 regulates Ang II-induced cardiomyocyte hypertrophy via up-regulation of IL-1β. Biosci Rep 2018; 38:BSR20171438.doi: 10.1042/BSR20171438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, et al. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A 2005; 102:13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem 2006; 281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 37.Nishino I, Fu J, Tanji K, Yamada T, Shimojo S, Koori T, et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature 2000; 406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 38.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 2007; 13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 39.Luedde M, Lutz M, Carter N, Sosna J, Jacoby C, Vucur M, et al. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res 2014; 103:206–216. doi: 10.1093/cvr/cvu146. [DOI] [PubMed] [Google Scholar]
- 40.Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, et al. p38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol 2004; 24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci B, et al. Beclin-1-dependent autophagy protects the heart during sepsis. Circulation 2018; 138:2247–2262. doi: 10.1161/CIRCULATIONAHA.117.032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng ZL, Lin XL, Tan LL, Liu YM, Qu K, Wang Z. MicroRNAs: important regulators of induced pluripotent stem cell generation and differentiation. Stem Cell Rev Rep 2018; 14:71–81. doi: 10.1007/s12015-017-9785-6. [DOI] [PubMed] [Google Scholar]
- 43.Luo B, Li B, Wang W, Liu X, Liu X, Xia Y, et al. Rosuvastatin alleviates diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK pathways in a type 2 diabetes rat model. Cardiovasc Drugs Ther 2014; 28:33–43. doi: 10.1007/s10557-013-6498-1. [DOI] [PubMed] [Google Scholar]
- 44.Han Y, Qiu H, Pei X, Fan Y, Tian H, Geng J. Low-dose sinapic acid abates the pyroptosis of macrophages by downregulation of lncRNA-MALAT1 in rats with diabetic atherosclerosis. J Cardiovasc Pharmacol 2018; 71:104–112. doi: 10.1097/FJC.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Wang B, Lai J, Braunstein Z, He M, Ruan G, et al. Trimetazidine attenuates cardiac dysfunction in endotoxemia and sepsis by promoting neutrophil migration. Front Immunol 2018; 9:2015.doi: 10.3389/fimmu.2018.02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lochner A, Marais E, Huisamen B. Melatonin and cardioprotection against ischaemia/reperfusion injury: what's new? A review. J Pineal Res 2018; 65:e12490.doi: 10.1111/jpi.12490. [DOI] [PubMed] [Google Scholar]
- 47.Wan Y, Xu L, Wang Y, Tuerdi N, Ye M, Qi R. Preventive effects of astragaloside IV and its active sapogenin cycloastragenol on cardiac fibrosis of mice by inhibiting the NLRP3 inflammasome. Eur J Pharmacol 2018; 833:545–554. doi: 10.1016/j.ejphar.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 48.Chen A, Chen Z, Xia Y, Lu D, Yang X, Sun A, et al. Liraglutide attenuates NLRP3 inflammasome-dependent pyroptosis via regulating SIRT1/NOX4/ROS pathway in H9c2 cells. Biochem Biophys Res Commun 2018; 499:267–272. doi: 10.1016/j.bbrc.2018.03.142. [DOI] [PubMed] [Google Scholar]
- 49.Zhang WX, He BM, Wu Y, Qiao JF, Peng ZY. Melatonin protects against sepsis-induced cardiac dysfunction by regulating apoptosis and autophagy via activation of SIRT1 in mice. Life Sci 2019; 217:8–15. doi: 10.1016/j.lfs.2018.11.055. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Lai J, Yang L, Ruan G, Chaugai S, Ning Q, et al. Trimetazidine prevents macrophage-mediated septic myocardial dysfunction via activation of the histone deacetylase sirtuin 1. Br J Pharmacol 2016; 173:545–561. doi: 10.1111/bph.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao Y, Sun F, Lei M. miR-25 inhibits sepsis-induced cardiomyocyte apoptosis by targetting PTEN. Biosci Rep 2018; 38:BSR20171511.doi: 10.1042/BSR20171511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Zheng YL, Hu RH, Zhu L, Hu CC, Cheng F, et al. Annexin A1 mimetic peptide AC2-26 inhibits sepsis-induced cardiomyocyte apoptosis through LXA4/PI3K/AKT signaling pathway. Curr Med Sci 2018; 38:997–1004. doi: 10.1007/s11596-018-1975-1. [DOI] [PubMed] [Google Scholar]
- 53.Luo R, Chen X, Ma H, Yao C, Liu M, Tao J, et al. Myocardial caspase-3 and NF-κB activation promotes calpain-induced septic apoptosis: the role of Akt/eNOS/NO pathway. Life Sci 2019; 222:195–202. doi: 10.1016/j.lfs.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 54.Lu Y, Yang Y, He X, Dong S, Wang W, Wang D, et al. Esmolol reduces apoptosis and inflammation in early sepsis rats with abdominal infection. Am J Emerg Med 2017; 35:1480–1484. doi: 10.1016/j.ajem.2017.04.056. [DOI] [PubMed] [Google Scholar]
- 55.Li N, Zhou H, Wu H, Wu Q, Duan M, Deng W, et al. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol 2019; 24:101215.doi: 10.1016/j.redox.2019.101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu YF, Wang R, Chen W, Cao YD, Li LP, Chen X. MiR-133a-3p attenuates cardiomyocyte hypertrophy through inhibiting pyroptosis activation by targeting IKKε. Acta Histochem 2021; 123:151653.doi: 10.1016/j.acthis.2020.151653. [DOI] [PubMed] [Google Scholar]
- 57.Qiu Z, He Y, Ming H, Lei S, Leng Y, Xia ZY. Lipopolysaccharide (LPS) aggravates high glucose- and hypoxia/reoxygenation-induced injury through activating ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2 cardiomyocytes. J Diabetes Res 2019; 2019:8151836.doi: 10.1155/2019/8151836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu H, Sun Y, Zhang Y, Yang G, Guo L, Zhao Y, et al. Role of thymoquinone in cardiac damage caused by sepsis from BALB/c mice. Inflammation 2019; 42:516–525. doi: 10.1007/s10753-018-0909-1. [DOI] [PubMed] [Google Scholar]
- 59.Song P, Shen DF, Meng YY, Kong CY, Zhang X, Yuan YP, et al. Geniposide protects against sepsis-induced myocardial dysfunction through AMPKα-dependent pathway. Free Radic Biol Med 2020; 152:186–196. doi: 10.1016/j.freeradbiomed.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 60.Shen S, He F, Cheng C, Xu B, Sheng J. Uric acid aggravates myocardial ischemia-reperfusion injury via ROS/NLRP3 pyroptosis pathway. Biomed Pharmacother 2021; 133:110990.doi: 10.1016/j.biopha.2020.110990. [DOI] [PubMed] [Google Scholar]
- 61.Zhang W, Tao A, Lan T, Cepinskas G, Kao R, Martin CM, et al. Carbon monoxide releasing molecule-3 improves myocardial function in mice with sepsis by inhibiting NLRP3 inflammasome activation in cardiac fibroblasts. Basic Res Cardiol 2017; 112:16.doi: 10.1007/s00395-017-0603-8. [DOI] [PubMed] [Google Scholar]
- 62.Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med 2016; 22:175–182. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- 63.Wang K, Liu F, Liu CY, An T, Zhang J, Zhou LY, et al. The long noncoding RNA NRF regulates programmed necrosis and myocardial injury during ischemia and reperfusion by targeting miR-873. Cell Death Differ 2016; 23:1394–1405. doi: 10.1038/cdd.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pi QZ, Wang XW, Jian ZL, Chen D, Zhang C, Wu QC. Melatonin alleviates cardiac dysfunction via increasing sirt1-mediated beclin-1 deacetylation and autophagy during sepsis. Inflammation 2021; 44:1184–1193. doi: 10.1007/s10753-021-01413-2. [DOI] [PubMed] [Google Scholar]
- 65.Wang F, Min X, Hu SY, You DL, Jiang TT, Wang L, et al. Hypoxia/reoxygenation-induced upregulation of miRNA-542-5p aggravated cardiomyocyte injury by repressing autophagy. Hum Cell 2021; 34:349–359. doi: 10.1007/s13577-020-00466-z. [DOI] [PubMed] [Google Scholar]