Abstract
A hydrogen peroxide (H2O2)-soaked gauze has been used in combination with radiotherapy in anticipation of sensitizing tumors exposed to the skin surface. Although used empirically in the clinic, the method is rarely reported in the literature, making its efficacy and tolerability unclear. Here, we report a case of primary metastatic breast cancer whose primary tumor was treated with palliative radiotherapy using an H2O2-soaked gauze. The primary tumor in the right breast regrew after treatment with palbociclib plus letrozole followed by fulvestrant and denosumab. The tumor was exposed to the skin surface, causing exudation, bleeding, pain, and difficulty in raising the right upper limb. Radiotherapy (51 Gy in 17 fractions) using the H2O2-soaked gauze resolved the patient's symptoms and the tumor showed macroscopic complete remission at three months post-treatment. This case indicates that radiotherapy with an H2O2-soaked gauze is an effective and tolerable palliative treatment for superficially exposed tumors. This non-invasive and inexpensive method of radiosensitization warrants validation and optimization in a prospective setting.
Keywords: bleeding, hydrogen peroxide, palliation, breast cancer, radiotherapy
Introduction
Radiotherapy is used to treat tumors in frail or elderly individuals who are not suitable for or refuse surgery. However, it is often difficult for radiotherapy alone to eradicate tumors or even to relieve tumor-related symptoms such as bleeding and pain. Thus, there is an unmet clinical need for novel methods of radiosensitization.
Preclinical evidence suggests that hydrogen peroxide (H2O2) sensitizes cancer cells to X-rays [1,2]. A study in a mouse tumor model showed that intratumoral injection of 0.5% H2O2 (in 0.83% sodium hyaluronate gel) combined with X-rays resulted in greater growth suppression than X-rays alone [3]. A recent phase one clinical trial analyzing 12 inoperable patients with breast cancer demonstrated that intratumoral injection of H2O2 plus radiotherapy was tolerable [4]. Based on these data, a phase two clinical trial is underway.
On the other hand, an H2O2-soaked gauze has been used in combination with radiotherapy in anticipation of sensitizing the tumors exposed to the skin surface. Although this method is used empirically in the clinic, the use of an H2O2-soaked gauze is rarely reported in the literature (in contrast to intratumoral injection of H2O2); thus, the efficacy and tolerability of the bolus method are unclear. Here, we report a case of primary metastatic breast cancer whose primary tumor was treated with palliative radiotherapy plus an H2O2-soaked gauze.
Case presentation
A 77-year-old woman was referred to our radiation oncology unit for palliative treatment of a recurrent primary tumor in the right breast. The tumor caused exudation, bleeding, pain, and difficulty in raising the right upper limb, thereby reducing the patient's quality of life (QOL).
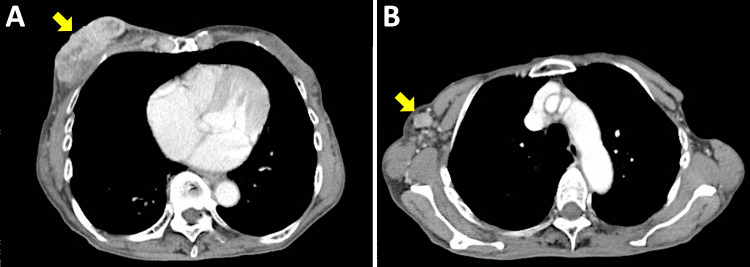
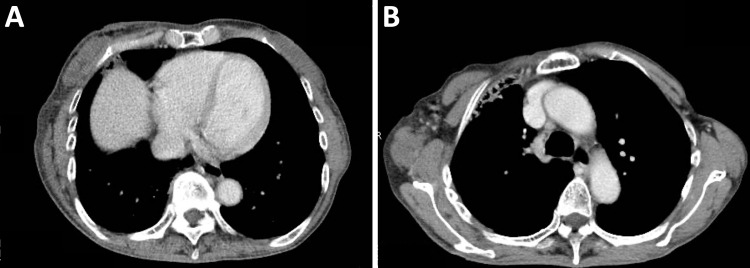
Nineteen months before the referral, the patient visited the surgical oncology unit and was newly diagnosed with stage IV (T2N0M1) breast cancer with lung metastases. The primary tumor occupied the lateral half of the right breast. The tumor was 47 mm in diameter, as assessed by computed tomography (CT), and was estrogen and progesterone receptor-positive, and human epidermal growth factor receptor-2 (HER2)-negative. The patient developed bone metastasis after four courses of palbociclib plus letrozole as first-line treatment. After 13 courses of fulvestrant as second-line treatment combined with monthly denosumab, the stable primary tumor became progressive, with axially lymph node metastasis (Figure 1). The primary tumor was considered inoperable, resulting in a referral to the radiation oncology unit.
Figure 1. Contrast-enhanced computed tomography image obtained at the time of referral to the radiation oncology unit.
(A) The primary tumor in the right breast (70 x 24 mm). (B) Metastasis to a right axial lymph node (15 x 11 mm). Arrows indicate the tumors.
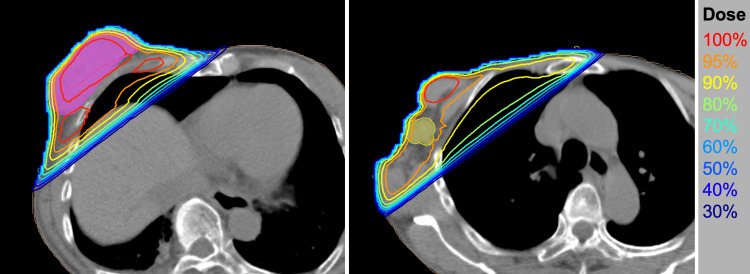
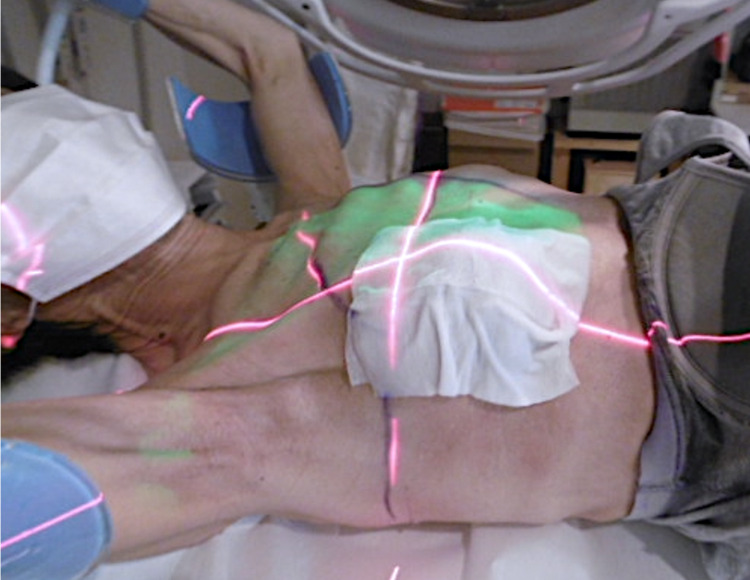
Employing standard tangentially opposed irradiation techniques, we performed conventional three-dimensional conformal radiotherapy with 6-MV photons [4]. The gross tumor, metastatic node, and tumor bed received 51 Gy delivered in 17 fractions (five fractions per week) (Figure 2). At each radiotherapy session, an H2O2-soaked gauze (approximately 5 mm thick) was freshly prepared and placed on the right breast to cover the tumor (Figure 3). Practically, when the patient entered the treatment room, a nurse poured oxydol (i.e., 2.5-3.5 w/v% H2O2; KENEI Pharmaceutical Co. Ltd., Osaka, Japan) in a plastic bag and a gauze was soaked in the bag. As the treatment progressed, the tumor showed a fair amount of shrinkage. Exudation and bleeding from the tumor, as well as the pain and difficulty in raising the right upper limb, resolved upon completion of the treatment. The patient experienced grade one radiation dermatitis (assessed by the Common Terminology Criteria for Adverse Events 4.0) but did not experience pain from the application of the H2O2 gauze. The dermatitis was resolved post-radiotherapy without topical medication.
Figure 2. Treatment plan for radiotherapy.
(A) A plane showing the primary tumor (solid magenta). (B) A plane showing the axial lymph node metastasis (solid yellow). Isodose lines are shown on computed tomography images.
Figure 3. Application of the hydrogen peroxide-soaked gauze during the radiotherapy session.
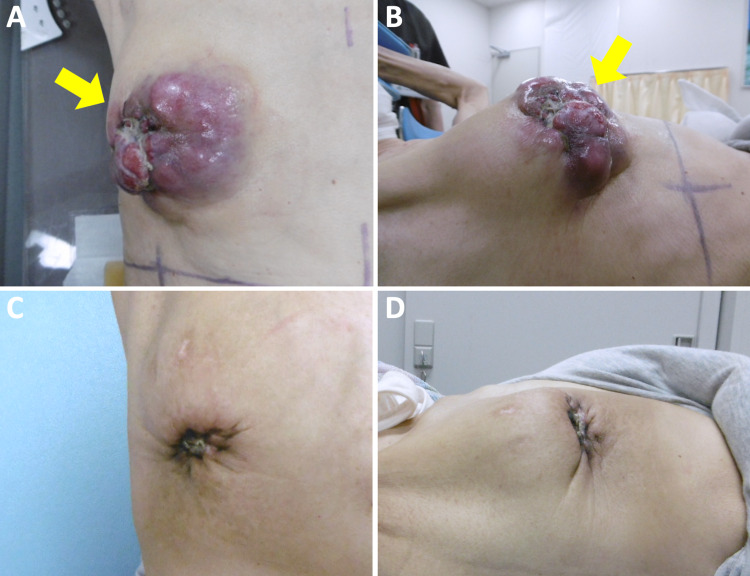
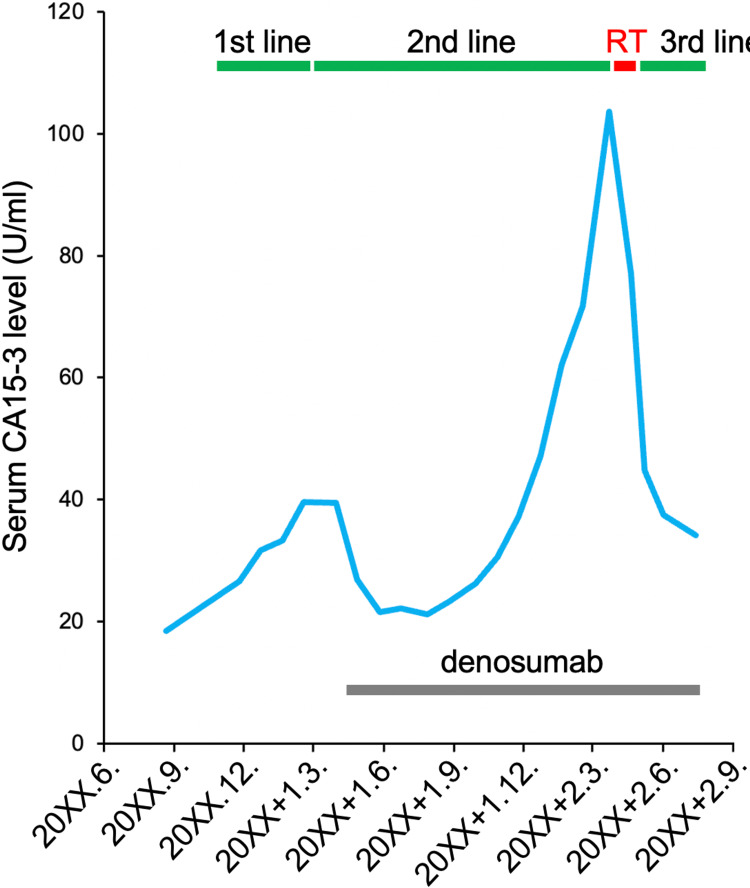
Upon completion of radiotherapy, tegafur was started as a third-line treatment. At three months post-completion of radiotherapy, the tumor showed macroscopic complete remission (Figure 4). The irradiated tumor was almost unidentifiable in CT images obtained at four months post-completion of radiotherapy (Figure 5). Serum cancer antigen 15-3 (CA15-3), a marker reflecting disease activity, also showed a substantial decrease after radiotherapy (Figure 6). The patient has resumed her daily life without symptoms and with peace of mind, although the progressive disease is present in other organs.
Figure 4. Pictures of the patient's right breast.
(A, B) Radiotherapy day one. Arrows indicate the primary tumor. (C, D) Three months post-completion of radiotherapy.
Figure 5. Contrast-enhanced computed tomography image obtained at four months post-treatment.
(A) Primary tumor site. (B) Metastatic lymph node site.
Figure 6. Serum CA15-3 kinetics.
1st line, palbociclib plus letrozole; 2nd line, fulvestrant; 3rd line, tegafur; RT, radiotherapy with the hydrogen peroxide bolus; CA15-3, cancer antigen 15-3.
Discussion
Breast cancer exposed to the skin surface threatens QOL severely by causing various symptoms including bleeding and pain. Such cases are often medically inoperable. In addition, clinical evidence suggests that, at least for breast cancer, approximately half of the tumor volume is hypoxic; hypoxia-enriched tumors are radioresistant [5]. Therefore, radiotherapy alone may be insufficient to resolve the symptoms, especially in the case of bulky tumors. In our case, radiotherapy with an H2O2-soaked gauze led to temporal complete remission of a bulky breast tumor exposed to the skin surface, with minimal toxicity; these findings suggest the potential efficacy and tolerability of this treatment for palliation.
The radiosensitization strategy using an H2O2-soaked gauze was first described by Ogawa and colleagues in 2008 [6]. In that paper, the authors reported five cases of locally advanced and/or recurrent tumors exposed to the skin surface, which were treated with electrons plus an H2O2-soaked gauze (Table 1). A few other cases treated with the same strategy have been reported in a Japanese book (Table 1). In the previously reported cases, the equivalent dose in 2 Gy per fraction with an α/β ratio of 10 (EQD2α/β10) ranged from 54 Gy to 64 Gy. All cases showed a partial or complete response. A total of 85% (6/7) of the cases treated using EQD2α/β10 equal to or less than 56 Gy experienced grade one radiation dermatitis, whereas one patient with penile cancer treated using 64 Gy in 32 fractions experienced grade three radiation dermatitis. In our case, the EQD2α/β10 was 55.2 Gy and the treatment resulted in temporal complete tumor remission, with grade one radiation dermatitis. These data indicate that the use of an H2O2-soaked gauze is tolerable when administered alongside an EQD2α/β10 of approximately <60 Gy. Nevertheless, solid evidence of tolerable and optimal dose fractionation for this treatment is still lacking, thereby warranting further research in a prospective setting.
Table 1. Summary of previously reported cases of superficial tumors treated with radiotherapy using a hydrogen peroxide bolus.
fr., fraction; EQD2α/β10, the equivalent dose in 2 Gy per fraction with α/β of 10; M, months; Refs, references; NA, not assessed; PR, partial response; CR, complete response. Adverse effect was assessed by the Common Terminology Criteria for Adverse Events 4.0, where G indicates grade. *Japanese book (ISBN: 978-4-86705-804-6).
| Cancer type | n | Radiation | Dose/fr. | EQD2α/β10 | Follow-up (M) | Outcome | Adverse effect | Refs |
| Breast cancer | 1 | Electrons | 48 Gy/12 fr. | 56 Gy | 3 | PR | Dermatitis (G1) | [6] |
| Melanoma | 1 | Electrons | 48 Gy/12 fr. | 56 Gy | 12 | CR | Dermatitis (G1) | [6] |
| Malignant fibrous histiocytoma | 1 | Electrons | 48 Gy/12 fr. | 56 Gy | 3 | PR | Dermatitis (G1) | [6] |
| Skin squamous cell carcinoma | 1 | Electrons | 48 Gy/12 fr. | 56 Gy | 1 | PR | Dermatitis (G1) | [6] |
| Extramammary Paget's disease | 1 | Electrons | 48 Gy/12 fr. | 56 Gy | 12 | CR | Dermatitis (G1) | [6] |
| Angiosarcoma | 2 | Electrons | 52.5 Gy/21 fr. | 54.7 Gy | NA | NA | Dermatitis (G2, 50%) | * |
| Malignant trichilemmoma | 1 | X-rays | 54 Gy/27 fr. | 54 Gy | 3 | CR | NA | * |
| Penile cancer | 1 | X-rays | 64 Gy/32 fr. | 64 Gy | NA | CR | Dermatitis (G3) | * |
| Breast cancer | 1 | X-rays | 51 Gy/17 fr. | 55.2 Gy | 4 | CR | Dermatitis (G1) | Present case |
Although the detailed mechanisms underlying the radiosensitizing effect of H2O2 are currently being investigated in the laboratory setting, the putative mechanism is as follows: first, two molecules of H2O2 break down to yield one molecule of oxygen and two molecules of water. This process can contribute to the reoxygenation of the radioresistant hypoxic tumor microenvironment. Second, H2O2 in combination with ionizing radiation induces intracellular production of reactive oxygen species (ROS). This can increase the induction of DNA double-strand breaks (i.e., the indirect effect). In addition, it is possible that ROS activate inflammatory signals that may upregulate antitumor immune responses [7]. Third, H2O2 influences a number of radioresistance-associated signaling pathways, including extracellular signal-regulated kinase (ERK), mitogen-activated protein kinase (MAPK), and nuclear factor kappa B (NFkB) [8-10]. Further research is needed to fully elucidate the underlying mechanisms and the optimal method of radiosensitization by H2O2.
As the limitation of this case report, first, there is a possibility that the H2O2-soaked gauze just functioned to improve dose distribution as same as water-soaked gauze. In fact, a water-density 5-mm thick structure improved the dose distribution near the skin in this case (data not shown). To address this issue, a randomized study, that employs water-soaked gauze as control, is mandatory. Second, we used a commercially available oxydol (i.e., 2.53.5 w/v% H2O2) according to the original report [6]; nevertheless, there is a possibility that the concentration of H2O2 can be further optimized so that it achieves maximal radiosensitizing effect with tolerable adverse effect, warranting further research. Third, in our case, we cannot exclude the possibility that tegafur contributed to the fair tumor response post-radiotherapy. Lastly, the possibility that the present case was an exceptional responder to ionizing radiations cannot be excluded, warranting validation by a prospective study.
Conclusions
An H2O2-soaked gauze has been used in combination with radiotherapy in anticipation of sensitizing the tumor exposed to the skin surface. Although this method is used empirically in the clinic, the use of an H2O2-soaked gauze is rarely reported in the literature (in contrast to intratumoral injection of H2O2); thus, the efficacy and tolerability of the gauze method are unclear. Here, we report a case of primary metastatic breast cancer whose primary tumor was treated with palliative radiotherapy plus an H2O2-soaked gauze. Exudation, bleeding, pain, and difficulty in raising the right upper limb were resolved and the tumor showed macroscopic complete remission at three months post-treatment. This case indicates that radiotherapy with an H2O2-soaked gauze is both effective and tolerable as a palliative treatment for superficially exposed tumors.
Acknowledgments
We thank Mr. Nobuyuki Sawai, Mr. Michiyuki Wada, Mr. Naonori Haraguchi, Ms. Takami Sugimoto, Ms. Yuki Suzuki, Ms. Erina Ishikawa, and Ms. Mariko Kaneko of Sano Kousei General Hospital for clinical assistance. This work was supported by Gunma University Heavy Ion Medical Center.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Ethical Review Committee of Sano Kousei General Hospital issued approval Exempt
References
- 1.Mechanism of hydrogen peroxide-induced apoptosis of the human osteosarcoma cell line HS-Os-1. Ogawa Y, Takahashi T, Kobayashi T, et al. Int J Mol Med. 2003;12:459–463. [PubMed] [Google Scholar]
- 2.Combination treatment of hydrogen peroxide and X-rays induces apoptosis in human prostate cancer PC-3 cells. Kariya S, Sawada K, Kobayashi T, Karashima T, Shuin T, Nishioka A, Ogawa Y. Int J Radiat Oncol Biol Phys. 2009;75:449–454. doi: 10.1016/j.ijrobp.2009.04.092. [DOI] [PubMed] [Google Scholar]
- 3.New enzyme-targeting radiosensitizer (KORTUC) containing hydrogen peroxide & sodium hyaluronate for intra-tumoral injection using mice transplanted with SCC VII tumor. Akima R, Ogawa Y, Morita-Tokuhiro S, et al. Int J Cancer Clin Res. 2016;3:1–6. [Google Scholar]
- 4.Intratumoral hydrogen peroxide with radiation therapy in locally advanced breast cancer: results from a phase 1 clinical trial. Nimalasena S, Gothard L, Anbalagan S, et al. Int J Radiat Oncol Biol Phys. 2020;108:1019–1029. doi: 10.1016/j.ijrobp.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Hypoxia in breast cancer: pathogenesis, characterization and biological/therapeutic implications. Vaupel P, Briest S, Höckel M. Wien Med Wochenschr. 2002;152:334–342. doi: 10.1046/j.1563-258x.2002.02032.x. [DOI] [PubMed] [Google Scholar]
- 6.New radiosensitization treatment (KORTUC I) using hydrogen peroxide solution-soaked gauze bolus for unresectable and superficially exposed neoplasms. Ogawa Y, Ue H, Tsuzuki K, et al. Oncol Rep. 2008;19:1389–1394. [PubMed] [Google Scholar]
- 7.Immunogenic cell death in cancer and infectious disease. Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 8.Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Gough DR, Cotter TG. Cell Death Dis. 2011;2:0. doi: 10.1038/cddis.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hydrogen peroxide sensing and signaling. Veal EA, Day AM, Morgan BA. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Sies H. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]