Abstract
Background
Preterm infants are at risk of lung atelectasis due to various anatomical and physiological immaturities, placing them at high risk of respiratory failure and associated harms. Nasal continuous positive airway pressure (CPAP) is a positive pressure applied to the airways via the nares. It helps prevent atelectasis and supports adequate gas exchange in spontaneously breathing infants. Nasal CPAP is used in the care of preterm infants around the world. Despite its common use, the appropriate pressure levels to apply during nasal CPAP use remain uncertain.
Objectives
To assess the effects of 'low' (≤ 5 cm H2O) versus 'moderate‐high' (> 5 cm H2O) initial nasal CPAP pressure levels in preterm infants receiving CPAP either: 1) for initial respiratory support after birth and neonatal resuscitation or 2) following mechanical ventilation and endotracheal extubation.
Search methods
We ran a comprehensive search on 6 November 2020 in the following databases: CENTRAL via CRS Web and MEDLINE via Ovid. We also searched clinical trials databases and the reference lists of retrieved articles for randomized controlled trials (RCTs) and quasi‐randomized trials.
Selection criteria
We included RCTs, quasi‐RCTs, cluster‐RCTs and cross‐over RCTs randomizing preterm infants of gestational age < 37 weeks or birth weight < 2500 grams within the first 28 days of life to different nasal CPAP levels.
Data collection and analysis
We used the standard methods of Cochrane Neonatal to collect and analyze data. We used the GRADE approach to assess the certainty of the evidence for the prespecified primary outcomes.
Main results
Eleven trials met inclusion criteria of the review. Four trials were parallel‐group RCTs reporting our prespecified primary or secondary outcomes. Two trials randomized 316 infants to low versus moderate‐high nasal CPAP for initial respiratory support, and two trials randomized 117 infants to low versus moderate‐high nasal CPAP following endotracheal extubation. The remaining seven studies were cross‐over trials reporting short‐term physiological outcomes. The most common potential sources of bias were absent or unclear blinding of personnel and assessors and uncertain selective reporting.
Nasal CPAP for initial respiratory support after birth and neonatal resuscitation
None of the six primary outcomes prespecified for inclusion in the summary of findings was eligible for meta‐analysis. No trials reported on moderate‐severe neurodevelopmental impairment at 18 to 26 months. The remaining five outcomes were reported in a single trial. On the basis of this trial, we are uncertain whether low or moderate‐high nasal CPAP levels improve the outcomes of: death or bronchopulmonary dysplasia (BPD) at 36 weeks' postmenstrual age (PMA) (risk ratio (RR) 1.02, 95% confidence interval (CI) 0.56 to 1.85; 1 trial, 271 participants); mortality by hospital discharge (RR 1.04, 95% CI 0.51 to 2.12; 1 trial, 271 participants); BPD at 28 days of age (RR 1.10, 95% CI 0.56 to 2.17; 1 trial, 271 participants); BPD at 36 weeks' PMA (RR 0.80, 95% CI 0.25 to 2.57; 1 trial, 271 participants), and treatment failure or need for mechanical ventilation (RR 1.00, 95% CI 0.63 to 1.57; 1 trial, 271 participants). We assessed the certainty of the evidence as very low for all five outcomes due to risk of bias, a lack of consistency across multiple studies, and imprecise effect estimates.
Nasal CPAP following mechanical ventilation and endotracheal extubation
One of the six primary outcomes prespecified for inclusion in the summary of findings was eligible for meta‐analysis. On the basis of these data, we are uncertain whether low or moderate‐high nasal CPAP levels improve the outcome of treatment failure or need for mechanical ventilation (RR 1.52, 95% CI 0.92 to 2.50; 2 trials, 117 participants; I2 = 17%; risk difference 0.15, 95% CI −0.02 to 0.32; number needed to treat for an additional beneficial outcome 7, 95% CI −50 to 3). We assessed the certainty of the evidence as very low due to risk of bias, inconsistency across the studies, and imprecise effect estimates. No trials reported on moderate‐severe neurodevelopmental impairment at 18 to 26 months or BPD at 28 days of age. The remaining three outcomes were reported in a single trial. On the basis of this trial, we are uncertain whether low or moderate‐high nasal CPAP levels improve the outcomes of: death or BPD at 36 weeks' PMA (RR 0.87, 95% CI 0.51 to 1.49; 1 trial, 93 participants); mortality by hospital discharge (RR 2.94, 95% CI 0.12 to 70.30; 1 trial, 93 participants), and BPD at 36 weeks' PMA (RR 0.87, 95% CI 0.51 to 1.49; 1 trial, 93 participants). We assessed the certainty of the evidence as very low for all three outcomes due to risk of bias, a lack of consistency across multiple studies, and imprecise effect estimates.
Authors' conclusions
There are insufficient data from randomized trials to guide nasal CPAP level selection in preterm infants, whether provided as initial respiratory support or following extubation from invasive mechanical ventilation. We are uncertain as to whether low or moderate‐high nasal CPAP levels improve morbidity and mortality in preterm infants. Well‐designed trials evaluating this important aspect of a commonly used neonatal therapy are needed.
Plain language summary
Nasal continuous positive airway pressure levels for the prevention of morbidity and mortality in preterm infants
Review question
How does giving preterm infants low versus moderate‐high levels of nasal continuous positive airway pressure (CPAP) impact important health outcomes?
Background
Preterm infants are born earlier than expected. Compared to term infants, preterm infants have immature lungs that are at risk of partial collapse. This collapse makes it difficult for the lungs to bring oxygen into the body and remove carbon dioxide from the body. In response, such infants are commonly treated with nasal CPAP, a therapy that maintains a positive pressure against the airways in an effort to keep the lungs open and to prevent respiratory failure. The pressure is applied through a mask or prongs attached to the nose. Nasal CPAP may be used upon admission to the neonatal intensive care unit as the initial respiratory support and also after a period of mechanical ventilation (use of a breathing machine) and extubation (removal of a breathing tube). Medical providers choose how much pressure to give. The amount of pressure is referred to as the CPAP level, which is measured in units of centimeters of water (cm H2O). Both not enough and too much pressure can be harmful, and it remains uncertain which CPAP pressure levels lead to the best outcomes. We performed a comprehensive search of the medical literature to summarize the impact of using low (≤ 5 cm H2O) versus moderate‐high (> 5 cm H2O) initial CPAP pressure levels in preterm infants.
Study characteristics
The search is up‐to‐date through 6 November 2020. We included 11 studies in the review. Four studies reported health outcomes that we had pre‐selected as being relevant to nasal CPAP levels and to the overall health of preterm infants, while the remaining studies reported short‐term physiological outcomes such as oxygen levels, heart rate, and blood pressure.
Key results
Of the four studies reporting pre‐selected health outcomes, we could only combine data from two studies comparing nasal CPAP levels for initial respiratory support and two studies comparing levels for support following extubation. Based on the data from these studies, we are uncertain as to whether low or moderate‐high nasal CPAP levels improve these outcomes. Future studies are needed to answer our review question.
Certainty of evidence
The overall numbers of studies and participants were small, and some of the included studies had flaws potentially limiting the accuracy of their findings. We therefore judged our findings as of very low certainty.
Summary of findings
Background
Description of the condition
Preterm infants are born with lungs prone to atelectasis (Gregory 1971). This results from several anatomical and physiological factors. Immature neurological control leads to periodic breathing and central apnea (Martin 1977). A compliant chest wall and the relative shape and position of the diaphragm, ribs, and chest wall contribute to inefficient respiratory mechanics, requiring additional work to inflate the lungs and maintain them open (Muller 1979). The supraglottic airway is unstable and prone to obstruction (Miller 1990). The quantity and quality of endogenous surfactant is decreased (Ueda 1994). These factors among others contribute to inadequate pulmonary gas exchange. Respiratory failure is the extreme result of this pathophysiology and is a leading cause of neonatal death (Patel 2015). In turn, the interventions used to treat poor gas exchange, such as supplemental oxygen and mechanical ventilation (MV), can injure the lung and contribute to important neonatal morbidities, such as bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (IVH), and retinopathy of prematurity (ROP).
Description of the intervention
Nasal continuous positive airway pressure (CPAP) is a positive pressure applied to the airways via the nares without an endotracheal tube. This provides a continuous distending airway pressure throughout the respiratory cycle in spontaneously breathing infants. Nasal CPAP is a common mode of non‐invasive respiratory support in preterm infants, applied as either initial respiratory support after birth and neonatal resuscitation or as postextubation support following a period of MV. Providers choose how much CPAP pressure to apply, which is typically measured in centimeters of water (cm H2O). We focused on the impact of different pressure levels in this review.
How the intervention might work
Nasal CPAP provides a continuous distending airway pressure to help offset atelectasis and inadequate gas exchange in preterm infants (Gregory 1971; Martin 1977; Miller 1990). Evidence for CPAP benefit compared to supplemental oxygen alone is established (Davis 2003; Ho 2015). However, it is uncertain how much pressure should be used. While higher levels may increase lung volumes and improve respiratory mechanics and gas exchange in under‐recruited lungs, excessive distending pressures may directly impair gas exchange and worsen respiratory mechanics and cardiovascular performance through overdistension (Abdel‐Hady 2008; Milner 1977). Furthermore, overdistension may contribute to air leak syndromes and facilitate volutrauma, a leading mechanism of lung injury contributing to BPD (Morley 2008; Wheeler 2011). A stratified analysis of the Cochrane Review on nasal CPAP versus headbox oxygen following extubation by pressure levels of < 5 cm H2O or ≥ 5 cm H2O suggests that higher pressures may be more effective in this setting (Davis 2003), but does not focus on studies directly comparing different pressure levels. Large, multicenter trials that compared nasal CPAP to MV used pressure levels as disparate as 4 cm H2O to 8 cm H2O (Göpel 2011; Morley 2008). Approaches to setting a CPAP level include choosing a single consistent pressure level for all preterm infants or an individualized approach. The latter usually selects an initial pressure level with subsequent pressure level titrations guided by clinical observations such as measures of gas exchange, work of breathing, and radiographic lung expansion.
Why it is important to do this review
Systematic reviews comparing the preferential use of non‐invasive respiratory support strategies such as nasal CPAP for initial respiratory support instead of MV in preterm infants favor CPAP for the outcome of survival without BPD (Fischer 2013; Schmölzer 2013). This is likely mediated by avoiding MV and ventilator‐induced lung injury. However, the magnitude of the benefit is modest, with a number needed to treat for an additional beneficial outcome of 25 to 35 and confidence intervals approaching no effect. This may be explained by high CPAP failure rates, observed in 46% to 83% of preterm infants randomized to preferential CPAP (Morley 2008; SUPPORT 2010). Likewise, non‐invasive respiratory support failure is common when used after extubation (Danan 2008). Evidence‐based guidance on specific key aspects of non‐invasive respiratory support may further enhance its benefit. Existing Cochrane Reviews have compared non‐invasive respiratory support modes (nasal CPAP versus nasal intermittent positive pressure ventilation (NIPPV) (Lemyre 2016; Lemyre 2017), as well as differing nasal CPAP devices and pressure sources (De Paoli 2008). This Cochrane Review considers how much pressure should be used by identifying and analyzing trials that compare different nasal CPAP levels.
Objectives
To assess the effects of 'low' (≤ 5 cm H2O) versus 'moderate‐high' (> 5 cm H2O) initial nasal CPAP pressure levels in preterm infants receiving CPAP either: 1) for initial respiratory support after birth and neonatal resuscitation or 2) following MV and endotracheal extubation.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs), quasi‐RCTs, cluster‐RCTs, and cross‐over trials with random nasal continuous positive airway pressure (NCPAP) level sequence allocation. We excluded studies completed prior to 1990 to increase the applicability of findings to modern neonatal practice, recognizing the now common use of antenatal corticosteroids, surfactants, and gentler ventilation strategies.
Types of participants
We included preterm infants of gestational age < 37 weeks or birth weight < 2500 grams randomized within the first 28 days of life. We included studies in which some but not all infants met these demographic criteria if the central tendency of enrolled infants (e.g. mean gestational age, median age at study) satisfied our inclusion criteria.
Types of interventions
We included trials comparing:
two or more different nasal CPAP levels for initial respiratory support after birth and neonatal resuscitation; or
two or more different nasal CPAP levels following mechanical ventilation and endotracheal extubation.
For the purposes of data synthesis, these two indications were considered separately.
We excluded trials in which the intervention was initiated during neonatal resuscitation. We compared interventions as 'low' (≤ 5 cm H2O) versus 'moderate‐high' (> 5 cm H2O) pressure levels. This dichotomy is arbitrary but acknowledges the common use of a pressure level of 5 cm H2O for initial support. For trials that evaluated more than two randomly allocated nasal CPAP levels, these were collapsed into either 'low' or 'moderate‐high' classifications. For example, in a hypothetical three‐arm trial comparing levels of 4 cm H2O versus 6 cm H2O versus 8 cm H2O, we would combine 6 cm H2O and 8 cm H2O as 'moderate‐high' and compare to 4 cm H2O as 'low'. We also planned a sensitivity analysis replacing 5 cm H2O with 8 cm H2O as the dichotomizing value, thereby comparing 'low‐moderate' (≤ 8 cm H2O) versus 'high' (> 8 cm H2O) pressure levels. We excluded trials that compared CPAP levels inconsistent with either dichotomy. For example, a trial comparing 6 cm H2O versus 8 cm H2O would be classified as 'moderate‐high' versus 'moderate‐high' in the primary comparison and as 'low‐moderate' versus 'low‐moderate' in the sensitivity analysis, and would be excluded.
We used the initial nasal CPAP pressure level following randomization for the comparisons above. This acknowledges that selection of an initial pressure level with subsequent titrations is common in clinical practice and may be applied to clinical research protocols.
Types of outcome measures
We included trials reporting at least one of our prespecified primary or secondary outcomes. The following outcome measures apply to both initial and postextubation respiratory support comparisons.
Primary outcomes
Death or BPD at 36 weeks' postmenstrual age (PMA); we defined BPD as supplemental oxygen or positive pressure support use at 36 weeks' PMA (for infants less than 32 weeks' gestational age) (Jobe 2001; Shennan 1988).
Mortality at 28 days, hospital discharge, and one year.
Moderate‐severe neurodevelopmental impairment at 18 to 26 months and at 3 to 5 years. We defined moderate‐severe neurodevelopmental impairment as: cerebral palsy or gross motor disability (defined as ≥ level 2 according to the Gross Motor Function Classification System (GMFCS)) (Palisano 1997); developmental delay (Bayley or Griffith assessment > 2 standard deviations (SD) below the mean) (Bayley 1993; Bayley 2006; Griffiths 1954) or intellectual impairment (intelligent quotient (IQ) > 2 SD below the mean); blindness (vision < 6/60 in both eyes); or sensorineural deafness requiring amplification. We planned to perform separate analyses for children aged 18 to 26 months and 3 to 5 years (Jacobs 2013).
Bronchopulmonary dysplasia, defined as supplemental oxygen use at 28 days of age (NIH 1979).
Bronchopulmonary dysplasia, defined as supplemental oxygen or positive pressure support use at 36 weeks' PMA (for infants less than 32 weeks' gestational age) (Jobe 2001; Shennan 1988).
Treatment failure as indicated by study author prespecified values of recurrent apnea, hypoxia, hypercarbia, increasing oxygen requirement; or the need for mechanical ventilation. If this outcome was reported for more than one time point (e.g. failure by three days, failure by five days, etc.), we used the latest time point up to seven days from randomization.
Need for mechanical ventilation.
We included the first six outcomes in the summary of findings table; we selected mortality at discharge and neurodevelopmental impairment at 18 to 26 months as the time points for inclusion in the summary of findings table from the three time points prespecified for these outcomes.
Secondary outcomes
-
Components of moderate‐severe neurodevelopmental impairment:
cerebral palsy or gross motor disability (defined as ≥ level 2 according to the GMFCS) (Palisano 1997);
developmental delay (Bayley or Griffith assessment > 2 SD below the mean) (Bayley 1993; Bayley 2006; Griffiths 1954) or intellectual impairment (IQ > 2 SD below the mean);
blindness (vision < 6/60 in both eyes);
sensorineural deafness requiring amplification.
Severe intraventricular hemorrhage (Papile grade III or IV) (Papile 1978).
Periventricular leukomalacia (de Vries 1992).
Severe retinopathy of prematurity (International Classification of Retinopathy of Prematurity (ICROP) stage III to V) (ICROP 2005).
Necrotizing enterocolitis requiring surgery.
Pneumothorax.
Pulmonary air leak (any air leak, including pneumothorax, pneumomediastinum, pulmonary interstitial emphysema, pneumopericardium).
Rate of apnea and bradycardia expressed as events per hour.
Patent ductus arteriosus receiving medical or surgical treatment.
Necrotizing enterocolitis (Bell stage 2 or greater) (Bell 1978).
Intraventricular hemorrhage (any) (Papile grade I to IV) (Papile 1978).
Physiological definition of BPD (Walsh 2004).
Severe BPD, defined as the need for ≥ 30% oxygen or positive pressure at 36 weeks’ PMA, or both (Ehrenkranz 2005; Jobe 2001).
Any retinopathy of prematurity (ICROP stage I to V).
Duration of positive pressure ventilation (days).
Duration of oxygen supplementation (days).
Length of hospital stay (days).
Days to establish full feeding (enteral, oral, breast).
Days to regain birth weight.
Weight gain (gram/day) at discharge.
Weight z score (g/kg/day) at discharge.
Growth failure (weight < 10th percentile for the index population measured) at discharge.
Nasal injury as defined by study including hyperemia, septal injury, septal necrosis, scarring.
Gastrointestinal perforation.
Short‐term physiologic measures
Measures of oxygenation, such as fraction of inspired oxygen needed to maintain oxygen saturation targets or alveolar to arterial gradients.
Carbon dioxide values, including venous, capillary, arterial, or transcutaneous measures.
Direct or surrogate measures of cardiac output, including echocardiography.
Heart rate in beats per minute.
Systolic, diastolic, and mean blood pressure in mmHg.
Lung volume measurements such as tidal volume or functional residual capacity.
We summarized physiologic outcomes in tabular form without meta‐analysis due to substantial heterogeneity in definitions, baseline values, and units of measure. We limited eligible outcomes from cross‐over trials to the physiological measures described above. When studies reported repeated measures over time, we used the measure farthest from the intervention, as long as we judged it to plausibly reflect the impact of the pressure level change.
Search methods for identification of studies
Electronic searches
We conducted a search in November 6, 2020 including: the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 11) in the Cochrane Library and Ovid MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions (1946 to 6 November 2020). We have included the search strategies for each database in Appendix 1. We did not apply any language restrictions.
We searched the following clinical trial registries for ongoing or recently completed trials via CENTRAL: World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/search/en/) and ClinicalTrials.gov (clinicaltrials.gov). We also searched the ISRCTN registry (http://www.isrctn.com/) for any unique trials not found through our search of CENTRAL.
This is the second update of this search strategy. Previous search details are listed in Appendix 2.
Searching other resources
We searched the reference lists of studies included in the review to identify additional relevant articles. We additionally used the review authors' existing knowledge of studies on the review topic to identify potentially relevant studies missed by the search.
Data collection and analysis
Selection of studies
We exported the records of the electronic database searches to Covidence to facilitate title and abstract screening. We ensured the single inclusion of duplicate data from the same study identified in multiple reports. Two review authors (NB, JF or AM) independently performed title and abstract screening of the search results, excluding any irrelevant studies. We obtained the full‐text articles of potentially relevant studies when available. Any disagreements were resolved through discussion between review authors. We listed all studies excluded after full‐text assessment and the reasons for their exclusion in the Characteristics of excluded studies table. We illustrated the study selection process in a PRISMA flow diagram (Figure 1).
1.
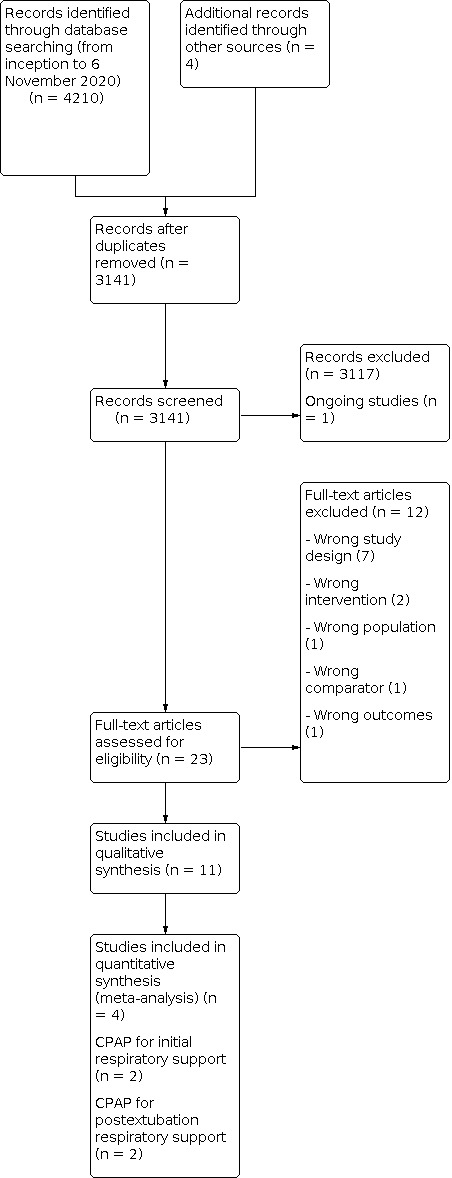
Study flow diagram.
Data extraction and management
We developed a standardized data extraction form to help guide determination of eligibility, study characterization, and data extraction. Two review authors (NB and AM) reviewed the full texts of eligible studies in duplicate. One review author (NB) transcribed data and study details into Review Manager Web (RevMan Web 2019), which a second review author (AM) independently reviewed and verified; any disagreements between review authors were reconciled through discussion or by consulting a third review author (JF) when necessary.
Assessment of risk of bias in included studies
Two review authors (NB and AM) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane risk of bias tool for the following domains (Higgins 2011):
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
any other bias.
We assessed individual trials as having a high overall risk of bias if the number of domains at high risk of bias were greater than those at low risk of bias. This determination was relevant to the sensitivity analyses described below.
Any disagreements were resolved by discussion or by consulting a third review author (JF) when necessary. For a more detailed description of risk of bias for each domain, see Appendix 3.
Measures of treatment effect
For continuous data, we presented treatment effects as the mean difference (MD) or standardized mean difference (SMD) with 95% confidence intervals (CIs) for individual studies and pooled estimates, using an inverse‐variance fixed‐effect approach for meta‐analysis. For outcomes reported as median and interquartile range, we substituted the median for the mean, and derived the median from the interquartile range by dividing the latter by 1.35, as per the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), while noting this approximation within the analysis footnote.
For dichotomous categorical data, we presented treatment effect as risk ratio (RR), risk difference (RD), and number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) with 95% CIs for pooled estimates for all primary outcomes and statistically significant secondary outcomes. We used a Mantel‐Haenszel fixed‐effect approach for meta‐analysis.
For continuous data reflecting short‐term physiologic outcomes, we summarized the data presented in each study in tabular form, using the study's summary measures of central tendency (e.g. mean, median, etc.) and variance (e.g. SD, interquartile range, etc.) We did not pursue meta‐analysis for these outcomes, as discussed above (Secondary outcomes).
Unit of analysis issues
The unit of analysis was the participating infant in individually randomized trials, and an infant was considered only once in each meta‐analysis for all primary and secondary outcomes. Data from cross‐over trials in which an infant contributed multiple observations to an outcome (up to once per cross‐over period) were not restricted to the first cross‐over period, but summarized in aggregate across the study cohort by nasal CPAP level in tabular form and considered in the qualitative synthesis of the review only.
The participating neonatal unit or section of a neonatal unit or hospital was the planned unit of analysis in cluster‐randomized trials; however, none of these have been identified to date. We planned to analyze such trials using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible) or from a similar trial or from a study with a similar population as described in the Cochrane Handbook (Higgins 2021). We planned that if we used ICCs from a similar trial or from a study with a similar population, we would report this and conduct a sensitivity analysis to investigate the effect of variation in the ICC.
In the future, if we identify both cluster‐randomized trials and individually randomized trials, we will only combine the results from both if there is little heterogeneity between the study designs, and interaction between the effect of the intervention and the choice of randomization unit is considered to be unlikely. We will acknowledge any possible heterogeneity in the randomization unit and perform a sensitivity analysis to investigate possible effects of the randomization unit.
Dealing with missing data
When feasible, we carried out analyses on an intention‐to‐treat basis for all outcomes. To the greatest degree possible, we analyzed all participants in the treatment group to which they had been randomized, regardless of the actual treatment received. Assumptions made to deal with missing data were described within the specific analysis. We planned to perform sensitivity analyses to assess the consistency of results to reasonable changes in the underlying assumptions. When applicable, we addressed the potential impact of missing data on the findings in the Discussion.
Assessment of heterogeneity
The Subgroup analysis and investigation of heterogeneity section outlines the steps taken to limit aggregation and meta‐analysis of data at risk of excess heterogeneity and to facilitate assessment of heterogeneity within the overall results. When we pursued meta‐analyses, we performed posterior assessments of heterogeneity through identification of studies contributing ‘outlying’ effect estimates through visual inspection of forest plots. We assessed these studies for unanticipated clinical and methodological characteristics plausibly explaining the observed heterogeneity. Identification of such characteristics did not result in exclusion from the pooled estimates of the primary analysis, but in exclusion from a secondary sensitivity analysis (see Sensitivity analysis). We quantified the degree of heterogeneity by applying the I² statistic together with a Chi² test as a measure of corresponding statistical significance, using a conservative P value of 0.10 for statistical significance. We used the following I² statistic thresholds as a guide to help interpret the degree of heterogeneity:
25%: none;
25% to 49%: low;
50% to 74%: moderate;
75% to 100%: high.
Assessment of reporting biases
We assessed reporting bias by comparing the stated outcomes and the reported outcomes. When study protocols were available, we compared intended and reported outcomes to determine the likelihood of reporting bias. We planned to use funnel plots to screen for publication bias when there were at least 10 studies for an outcome. We considered suggestion of publication bias by significant asymmetry of the funnel plot on visual assessment in our assessment of the certainty of the evidence.
Data synthesis
When we identified multiple studies considered to be sufficiently similar, we performed meta‐analysis using Review Manager Web (RevMan Web 2019), reporting measures of treatment effects as described in Measures of treatment effect. We used fixed‐effect models given our assumption that studies were estimating the same underlying treatment effect. When we judged meta‐analysis to be inappropriate, we analyzed and interpreted trials separately.
Subgroup analysis and investigation of heterogeneity
We used a cautious approach to aggregation and meta‐analysis of data at risk of excess heterogeneity, erring on the side of avoidance when uncertainty existed. Two review authors (NB and JF) independently made this decision, resolving any disagreements through discussion or resolution by a third review author (AM). We planned to further assess heterogeneity by the a priori application of several subgroup analyses stratified on key clinical characteristics. We included study data in the subgroup analysis when all infants met the criteria, unless otherwise specified.
Planned subgroups analyses
For both comparisons (as specified in Types of interventions) we planned subgroup analyses for all primary outcomes stratified on the basis of the following.
-
Gestational age groups:
Late preterm infants, defined as infants 34 to 36 completed weeks' gestational age
Moderate preterm infants, defined as infants born at 32 to 33 completed weeks' gestational age
Very preterm infants, defined as infants born at 28 to 31 completed weeks' gestational age
Extremely preterm infants, defined as infants born at less than 28 completed weeks' gestational age
-
Birth weight groups:
Low birth weight (LBW) infants, defined as infants with birth weight < 2500 grams
Very low birth weight (VLBW) infants, defined as infants with birth weight < 1500 grams
Extremely low birth weight (ELBW) infants, defined as infants with birth weight < 1000 grams
-
Setting:
Low‐ and middle‐income countries, as defined by the World Bank List of Country Economies (World Bank 2021)
High‐income countries
Mixed or not reported
-
Type of CPAP pressure generation:
Underwater bubble CPAP
CPAP delivered by ventilator
CPAP delivered by variable flow device
Mixed or not reported
-
Type of CPAP delivery interface:
Short single prong
Short binasal (double) prongs
Long (nasopharyngeal) prongs
Mask
RAM cannula
Mixed or not reported
-
Antenatal steroid exposure, as baseline characteristic:
Yes, if ≥ 50% of infants were treated with any corticosteroid
No, if < 50% of infants were treated with any corticosteroid
Unknown
-
Methylxanthine exposure, as baseline characteristic:
Yes, if ≥ 50% of infants were treated with any methylxanthine
No, if < 50% of infants were treated with any methylxanthine
Unknown
Sensitivity analysis
For all primary outcomes, we planned the following sensitivity analyses:
excluding studies assessed as at overall high risk of bias;
excluding studies contributing 'outlying effect' estimates, as described in Assessment of heterogeneity;
considering 8 cm H2O as the threshold for CPAP level comparisons, i.e. 'low‐moderate' (≤ 8 cm H2O) versus 'high' (> 8 cm H2O).
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence for the first six primary outcomes listed above (Primary outcomes). Two review authors (NB and JF) independently assessed the certainty of the evidence for each outcome. We considered evidence from RCTs as high certainty, downgrading the certainty of the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used GRADEpro GDT to create summary of findings tables (Table 1; Table 2) to report the certainty of the evidence (GRADEpro GDT).
Summary of findings 1. Summary of Findings Table ‐ Low compared to moderate‐high nasal continuous positive airway pressure levels for preterm infants receiving initial respiratory support.
| Low compared to moderate‐high nasal continuous positive airway pressure levels for preterm infants receiving initial respiratory support | ||||||
| Patient or population: health problem or population Setting: Intervention: Low Comparison: Moderate‐High | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Moderate‐High | Risk with Low | |||||
| Death or BPD at 36 weeks PMA | 135 per 1000 | 138 per 1000 (76 to 250) | RR 1.02 (0.56 to 1.85) | 271 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a | |
| Mortality, by hospital discharge | 98 per 1000 | 102 per 1000 (50 to 207) | RR 1.04 (0.51 to 2.12) | 271 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a | |
| Moderate‐severe neurodevelopmental impairment at 18 to 26 months ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| BPD, defined as supplemental oxygen use at 28 days of age | 105 per 1000 | 116 per 1000 (59 to 228) | RR 1.10 (0.56 to 2.17) | 271 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a | |
| BPD, defined as supplemental oxygen or positive pressure support use at 36 weeks PMA | 45 per 1000 | 36 per 1000 (11 to 116) | RR 0.80 (0.25 to 2.57) | 271 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a | |
| Treatment failure or need for mechanical ventilation | 218 per 1000 | 218 per 1000 (137 to 342) | RR 1.00 (0.63 to 1.57) | 271 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_425006380464097721. | ||||||
a. Downgraded one level for serious limitations based on: risk of bias (high risk of performance bias, unclear risk of detection and reporting bias); one level for consistency across studies (single study); and one level for precision of estimates (single study with 271 participants).
Summary of findings 2. Summary of Findings Table ‐ Low compared to moderate‐high nasal continuous positive airway pressure levels for preterm infants receiving postextubation respiratory support.
| Low compared to moderate‐high nasal continuous positive airway pressure levels for preterm infants receiving postextubation respiratory support | ||||||
| Patient or population: health problem or population Setting: Intervention: Low Comparison: Moderate‐high | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Moderate‐high | Risk with Low | |||||
| Death or BPD at 36 weeks' PMA | 391 per 1000 | 340 per 1000 (200 to 583) | RR 0.87 (0.51 to 1.49) | 93 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a | |
| Mortality, by hospital discharge | 0 per 1000 | 0 per 1000 (0 to 0) | RR 2.94 (0.12 to 70.30) | 93 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a | |
| Moderate‐severe neurodevelopmental impairment at 18 to 26 months ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| BPD, defined as supplemental oxygen use at 28 days of age ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| BPD, defined as supplemental oxygen or positive pressure support use at 36 weeks' PMA | 391 per 1000 | 340 per 1000 (200 to 583) | RR 0.87 (0.51 to 1.49) | 93 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a | |
| Treatment failure or need for mechanical ventilation | 288 per 1000 | 438 per 1000 (265 to 720) | RR 1.52 (0.92 to 2.50) | 117 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW b | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_425006643542116952. | ||||||
a. Downgraded one level for serious limitations based on risk of bias (high risk of performance bias, unclear risk of detection and reporting bias); one level for consistency across studies (single study); and one level for precision of estimates (single study with 93 participants). b. Downgraded one level for serious limitations based on risk of bias (high risk of performance bias, unclear risk of detection and reporting bias); one level for consistency across studies (overall inconsistent estimates); and one level for precision of estimates (two studies with 117 participants).
The GRADE approach results in an assessment of the certainty of a body of evidence into one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
The search results and study identification process are displayed in Figure 1.
Results of the search
From 3141 records retrieved by the search, our screening identified one ongoing study, ACTRN12618001638224, and 23 completed studies for potential inclusion in the review. Following full‐text review, we included 11 studies and excluded 12 studies.
Included studies
See: Characteristics of included studies.
We included 11 trials published between 2001 and 2016. Four trials were two‐arm, parallel‐group RCTs: Murki 2016 and Dhir 2016 compared CPAP levels for initial respiratory support, while Buzzella 2014 and Kitsommart 2013 compared CPAP levels for postextubation support. The remaining seven studies were cross‐over trials with random CPAP level sequence allocation: Lavizzari 2014 enrolled infants on CPAP for initial respiratory support; Magnenant 2004 for postextubation support; and five studies enrolled a mix of the two populations (Beker 2014; Elgellab 2001 Lomp 2010; Miedema 2013; Rehan 2001). Beker 2014, Lomp 2010, and Miedema 2013 predominantly enrolled infants on CPAP for initial respiratory support. Elgellab 2001 and Rehan 2001 predominantly enrolled infants on CPAP following extubation. All of the included studies were performed at a single center. Three studies were conducted in low‐/middle‐income country settings (Dhir 2016, Lomp 2010; Murki 2016), while the remaining eight studies were conducted in high‐income country settings.
Population
The four parallel‐group RCTs enrolled between 24 and 271 infants; only Murki 2016 enrolled over 100 infants. The cross‐over trials contributed 37 infants, in Lomp 2010, or fewer.
Most studies enrolled a broad population of preterm neonates, with all studies including multiple prespecified gestational age or birth weight subgroup categories, except Buzzella 2014, who restricted enrollment to ELBW infants.
In 6 of the 11 studies, half or more infants received antenatal corticosteroids. This information was not provided for the remaining five studies. Only Buzzella 2014 described methylxanthine use in greater than half of infants. Less than 50% of infants were exposed to methylxanthines at baseline in Murki 2016. The remaining nine studies did not describe present or absent methylxanthine use.
Interventions
All four parallel‐group RCTs compared 'low' (≤ 5 cm H2O) versus 'moderate‐high' (> 5 cm H2O) initial nasal CPAP levels. For CPAP as initial respiratory support, both Murki 2016 and Dhir 2016 compared 5 cm H2O versus 7 cm H2O. For postextubation support, both Buzzella 2014 and Kitsommart 2013 compared CPAP levels of 5 cm H2O versus 8 cm H2O. A broader number and range of CPAP levels were evaluated in the included cross‐over trials (Table 3). Four studies evaluated three distinct CPAP levels (Beker 2014; Lavizzari 2014; Miedema 2013; Rehan 2001), while Elgellab 2001, Lomp 2010, and Magnenant 2004 evaluated four levels. The range of CPAP levels evaluated across all included studies was 0 cm H2O, Magnenant 2004, to 8 cm H2O (Beker 2014; Elgellab 2001; Lomp 2010; Rehan 2001). The broadest within‐study ranges were reported by Magnenant 2004 (0 to 6 cm H2O) and Lomp 2010 and Elgellab 2001 (both 2 to 8 cm H2O).
1. Impact of nasal CPAP levels on short‐term physiologic measures.
| Outcome | Study, type | Respiratory support |
Initial CPAP level (cm H2O) |
||||||
| 0 | 2 | 4 | 5 | 6 | 7 | 8 | |||
| Oxygenation measures | |||||||||
| Maximal FiO2 during intervention, median (IQR) | Murki 2016, RCT | Initial | ‐ | ‐ | ‐ |
n = 138 0.40 (40 to 44) |
‐ |
n = 133 0.40 (40 to 40) |
‐ |
| Maximal FiO2 during intervention, mean (SD) | Kitsommart 2013, RCT | Postextubation | ‐ | ‐ | ‐ | n = 11 0.33 (0.12) | ‐ | ‐ | n = 13 0.42 (0.16) |
| Maximal FiO2 during cross‐over period, mean (SD) | Lavizzari 2014, cross‐over RCT | Initial | ‐ |
n = 3 0.22 (1.2) |
n = 2 0.21 (0) |
‐ |
n = 2 0.21 (0) |
‐ | ‐ |
| FiO2 during cross‐over period, mean (SD) | Lomp 2010, cross‐over RCT | Mixed, most initial | ‐ |
n = 37 0.25 (0.7) |
n = 37 0.23 (0.5) |
‐ |
n = 36 0.23 (0.5) |
‐ |
n = 33 0.23 (0.5) |
| Peripheral oxygen saturations, mean (SD) | Rehan 2001, cross‐over RCT | Mixed, most postextubation | ‐ |
n = 12 91 (2.5) |
‐ |
n = 12 95 (2.3) |
‐ | ‐ |
n = 12 98 (2.2) |
| Transcutaneous partial oxygen pressure, in kPa, mean (SD) | Miedema 2013, cross‐over RCT | Mixed, most initial | ‐ |
n = 22 7.2 (1.7) |
n = 22 8.2 (1.9) |
‐ |
n = 22 8.9 (2.0) |
‐ | ‐ |
| Transcutaneous partial oxygen pressure, in mmHg, mean (SD) |
Magnenant 2004, cross‐over RCT |
Postextubation | n = 11 36 (4) |
n = 11 40 (3) |
n = 11 39 (3) |
‐ |
n = 11 42 (4) |
‐ | ‐ |
| Transcutaneous partial oxygen pressure divided by FiO2, in mmHg, mean (SD) |
Magnenant 2004 cross‐over RCT |
Postextubation |
n = 11 134 (15) |
n = 11 154 (14) |
n = 11 163 (14) |
‐ |
n = 11 175 (17) |
‐ | ‐ |
| Carbon dioxide measures | |||||||||
| Transcutaneous partial carbon dioxide pressure, in kPa, mean (SD) |
Miedema 2013, cross‐over RCT |
Mixed, most initial | ‐ |
n = 22 6.4 (0.8) |
n = 22 6.4 (0.8) |
‐ |
n = 22 6.3 (0.8) |
‐ | ‐ |
| Transcutaneous partial carbon dioxide pressures, in mmHg, mean (SD) | Lavizzari 2014, cross‐over RCT | Initial support | ‐ |
n = 3 40.7 (3.5) |
n = 2 45.5 (3.5) |
‐ |
n = 2 37.5 (7.8) |
‐ | ‐ |
| Transcutaneous partial carbon dioxide pressures, in mmHg, mean (SD) | Magnenant 2004, cross‐over RCT | Postextubation |
n = 11 62 (3) |
n = 11 62 (4) |
n = 11 59 (3) |
‐ |
n = 11 62 (5) |
‐ | ‐ |
| Cardiac output measures | |||||||||
| Left ventricular output, as measured on echocardiography, in mL/kg/min, mean (SD) | Beker 2014, cross‐over RCT | Mixed, most initial | ‐ | ‐ |
n = 34 286 (101) |
‐ |
n = 34 283 (83) |
‐ |
n = 282 (88) |
| Right ventricular output, as measured on echocardiography, in mL/kg/min, mean (SD) | Beker 2014, cross‐over RCT | Mixed, most initial | ‐ | ‐ |
n = 34 294 (94) |
‐ |
n = 34 301 (106) |
‐ |
n = 34 294 (92) |
| Heart rate measures | |||||||||
| Heart rate, beats per minute, mean (SD) | Lavizzari 2014, cross‐over RCT | Initial support | ‐ |
n = 3 148 (13.1) |
n = 2 128 (6.4) |
‐ |
n = 2 137 (2.8) |
‐ | ‐ |
| Heart rate, beats per minute, mean (SD) | Rehan 2001, cross‐over RCT | Mixed, most postextubation | ‐ |
n = 12 157 (20) |
‐ |
n = 12 150 (16) |
‐ | ‐ |
n = 12 156 (17) |
| Heart rate, beats per minute, mean (SD) | Beker 2014, cross‐over RCT | Mixed, most initial | ‐ | ‐ |
n = 34 150 (13) |
‐ |
n = 34 148 (13) |
‐ |
n = 34 151 (14) |
| Heart rate, beats per minute, mean (SD) | Lomp 2010, cross‐over RCT | Mixed, most initial | ‐ |
n = 37 149 (19) |
n = 37 149 (17) |
‐ |
n = 37 150 (19) |
‐ |
n = 37 148 (18) |
| Heart rate, beats per minute, mean (SD) | Magnenant 2004 cross‐over RCT | Postextubation |
n = 11 149 (5) |
n = 11 147 (6) |
n = 11 147 (5) |
‐ |
n = 11 150 (5) |
‐ | ‐ |
| Blood pressure measures | |||||||||
| Systolic, via cuff, mmHg, mean (SD) | Beker 20144, cross‐over RCT | Mixed, most initial | ‐ | ‐ |
n = 34 64 (11) |
‐ |
n = 34 63 (10) |
‐ |
n = 34 62 (9) |
| Diastolic, via cuff, mmHg, mean (SD) | Beker 2014 cross‐over RCT | Mixed, most initial | ‐ | ‐ |
n = 34 38 (7) |
‐ |
n = 34 39 (9) |
‐ |
n = 34 39 (8) |
| Mean, via cuff, mmHg, mean (SD) | Beker 2014, cross‐over RCT | Mixed, most initial | ‐ | ‐ |
n = 34 47 (7) |
‐ |
n = 34 47 (9) |
‐ |
n = 34 47 (7) |
| Mean, arterial, mmHg, mean (SD) | Magnenant 2004, cross‐over RCT | Postextubation |
n = 11 46 (2) |
n = 11 44 (3) |
n = 11 47 (2) |
‐ |
n = 11 47 (3) |
‐ | ‐ |
| Lung volume measures | |||||||||
| Tidal volume, measured by respiratory inductance plethysmography, mL, mean (SD) | Lavizzari 2014, cross‐over RCT | Initial support | ‐ |
n = 3 4.3 (1.9) |
n = 2 7.1 (2.1) |
‐ |
n = 2 5.7 (4.6) |
‐ | ‐ |
| Tidal volume, measured by respiratory inductance plethysmography, and reported as % of tidal volume obtained at CPAP 0 cm H2O, i.e. 100% = tidal volume at 0 cm H2O | Elgellab 2001, cross‐over RCT | Mixed, most postextubation | ‐ |
n = 10 104 (10) |
n = 10 110 (18) |
‐ |
n = 10 125 (20) |
‐ |
n = ? 143 (24) |
| Tidal volume, measured by respiratory inductance plethysmography, and reported as % of tidal volume obtained at CPAP 0 cm H2O, i.e. 100% = tidal volume at 0 cm H2O | Magnenant 2004, cross‐over RCT | Postextubation |
n = 11 100 (0) |
n = 11 108 (5) |
n = 11 122 (9) |
‐ |
n = 11 146 (15) |
‐ | ‐ |
| Change in end‐expiratory lung volume, measured by respiratory inductance plethysmography, calculated as the difference in end‐expiratory lung volume compared to CPAP 0 cm H2O and reported as % of tidal volume obtain at CPAP 0 cm H2O, i.e. 100% = change in volume equivalent to tidal volume at 0 cm H2O | Elgellab 2001, cross‐over RCT | Mixed, most postextubation | ‐ |
n = 10 38 (25) |
n = 10 110 (46) |
‐ |
n = 10 135 (49) |
‐ |
n = ? 210 (37) |
CPAP: continuous positive airway pressure;FiO2: fraction of inspired oxygen; IQR: interquartile range;RCT: randomized controlled trial;SD: standard deviation
The following pressure generation devices were used: bubble CPAP (Murki 2016), ventilators (Buzzella 2014; Kitsommart 2013), variable flow devices (Elgellab 2001; Lavizzari 2014; Lomp 2010; Magnenant 2004; Miedema 2013), and either bubble CPAP or ventilators (Beker 2014). Two studies did not detail pressure generation (Dhir 2016; Rehan 2001). All studies describing CPAP interfaces used short binasal prongs, but the interface was not described in 4 of the 11 studies (Dhir 2016; Elgellab 2001; Magnenant 2004; Miedema 2013).
All cross‐over trials reported the duration over which each pressure level was maintained prior to obtaining measures, with a range of 10, Lomp 2010; Miedema 2013; Rehan 2001, to 30 minutes (Elgellab 2001; Magnenant 2004). No study described a wash‐out period in which CPAP levels were returned to a baseline or reference value for a period of time between assessed CPAP levels.
Outcomes
In the two parallel‐group RCTs comparing CPAP levels for initial respiratory support, review primary outcomes reported by the studies were: death or BPD at 36 weeks' PMA (Murki 2016), mortality by hospital discharge (Murki 2016), BPD at 28 days (Murki 2016), BPD at 36 weeks' PMA (Murki 2016), treatment failure or need for mechanical ventilation (Murki 2016), and need for mechanical ventilation (Dhir 2016; Murki 2016). Secondary outcomes reported by Murki 2016 were: severe intraventricular hemorrhage, severe retinopathy of prematurity, pneumothorax, necrotizing enterocolitis, duration of positive pressure ventilation, and duration of oxygen supplementation. No additional review‐specified outcomes were reported by Dhir 2016.
In the two parallel‐group RCTs comparing CPAP levels for postextubation support, review primary outcomes reported by the studies were: death or BPD at 36 weeks' PMA (Buzzella 2014), mortality by hospital discharge (Buzzella 2014), BPD at 36 weeks' PMA (Buzzella 2014), treatment failure or need for mechanical ventilation (Buzzella 2014; Kitsommart 2013), and need for mechanical ventilation (Buzzella 2014; Kitsommart 2013). Severe intraventricular hemorrhage was the only secondary outcome reported by both studies. Additional secondary outcomes reported by Buzzella 2014 were: severe retinopathy of prematurity, pneumothorax, necrotizing enterocolitis, severe BPD, duration of positive pressure ventilation, and duration of oxygen supplementation. Additional secondary outcomes reported by Kitsommart 2013 were: any intraventricular hemorrhage, pulmonary air leak, and nasal injury.
Qualifying short‐term physiologic measures reported by parallel‐group or cross‐over trials are displayed in Table 3. Seven studies reported eight oxygenation measures (Kitsommart 2013; Lavizzari 2014; Lomp 2010; Magnenant 2004; Miedema 2013; Murki 2016; Rehan 2001); three studies reported carbon dioxide measures (Lavizzari 2014; Magnenant 2004; Miedema 2013); one study reported two cardiac output measures (Beker 2014); five studies reported heart rate measures (Beker 2014; Lavizzari 2014; Lomp 2010; Magnenant 2004; Rehan 2001); two studies reported four blood pressure measures (Beker 2014; Magnenant 2004); and three studies reported four lung volume measures (Elgellab 2001; Lavizzari 2014; Magnenant 2004).
Excluded studies
We excluded 12 studies (see Characteristics of excluded studies). The most common reason for exclusion was wrong study design, with seven of the 12 studies identified as cross‐over trials without random CPAP level sequence allocation (Courtney 2003; Courtney 2011; Mukerji 2019; Pandit 2001; Pickerd 2014; Veneroni 2014; Zhou 2020).
Risk of bias in included studies
Methodologic quality varied amongst the included trials (Figure 2). All included studies had unclear or high risk of bias in at least one domain. No trials contributing primary outcomes were judged as having overall high risk of bias and in need of exclusion for planned sensitivity analyses.
2.

Risk of bias summary: review authors' judgements for each risk of bias domain for included studies.
Allocation
We judged all four parallel‐group RCTs to have low risk of selection bias. Random sequence was generated through computer or web‐based programs, and allocation was concealed through the use of sealed, opaque envelopes. In contrast, only two of seven cross‐over trials described low risk of bias for random sequence generation (Beker 2014; Lomp 2010), and only Beker 2014 described allocation concealment through the use of sealed, opaque envelopes.
Blinding
We did not identify any studies in which all participants and personnel were described as masked to the allocated intervention. In Beker 2014 and Rehan 2001, the investigator obtaining or recording the outcome measures was masked to CPAP level. We judged both of these studies as having a low risk of detection bias.
Incomplete outcome data
We judged attrition bias to be low for all trials except Dhir 2016, Elgellab 2001, and Lomp 2010. We assessed Dhir 2016 as at unclear risk of attrition bias on the basis of unclear reporting. Elgellab 2001 described conditional assessments at a CPAP level of 8 cm H2O, without enumerating final sample size at that pressure level. Lomp 2010 reported incomplete analysis in 19 of 56 infants, meeting the prespecified 20% threshold for high risk of bias for this review (Appendix 3).
Selective reporting
We identified trial registrations in 3 of the 11 studies (Beker 2014; Kitsommart 2013; Murki 2016). We judged two studies as having a low risk of reporting bias (Beker 2014; Kitsommart 2013). We noted incomplete outcome reporting for Murki 2016, but only for a few secondary outcomes, while all prespecified primary outcomes were reported, resulting in a judgement of unclear risk of reporting bias.
Other potential sources of bias
We judged there to be other potential sources of bias for Dhir 2016, as the trial was reported as a “Scientific Letter” with sparse methodologic details, and for Lomp 2010, as the study did not undergo peer review. Study details were obtained from a thesis published online.
Effects of interventions
Primary and secondary outcomes are described below; we judged meta‐analysis to be appropriate in all instances where more than one parallel‐group RCT contributed data.
We did not pursue subgroup analyses, as no single outcome included data from more than two studies. We did not perform sensitivity analyses, as outlier effects are not applicable to meta‐analyses of two trials; no studies contributing data for primary outcomes were judged as having a high overall risk of bias; and no studies to date have allocated participants to CPAP levels > 8 cm H2O.
All short‐term physiologic outcomes are summarized in Table 3 and below.
Comparison 1. Low (≤ 5 cm H2O) versus moderate‐high (> 5 cm H2O) CPAP levels for initial respiratory support
Primary outcomes
Death or BPD at 36 weeks' PMA
Data from one trial (271 infants) showed an uncertain effect (Analysis 1.1).
1.1. Analysis.

Comparison 1: Low versus moderate‐high NCPAP level, as initial respiratory support, Outcome 1: Death or BPD at 36 weeks' PMA
Risk ratio (RR) 1.02, 95% confidence interval (CI) 0.56 to 1.85.
Mortality at 28 days
No trials reported this outcome.
Mortality by hospital discharge
Data from one trial (271 infants) showed an uncertain effect (Analysis 1.2).
1.2. Analysis.

Comparison 1: Low versus moderate‐high NCPAP level, as initial respiratory support, Outcome 2: Mortality, by hospital discharge
RR 1.04, 95% CI 0.51 to 2.12.
Mortality at one year
No trials reported this outcome.
Moderate‐severe neurodevelopmental impairment at 18 to 26 months
No trials reported this outcome.
Moderate‐severe neurodevelopmental impairment at 3 to 5 years
No trials reported this outcome.
BPD at 28 to 30 days of age
Data from one trial (271 infants) showed an uncertain effect (Analysis 1.3).
1.3. Analysis.

Comparison 1: Low versus moderate‐high NCPAP level, as initial respiratory support, Outcome 3: BPD, defined as supplemental oxygen use at 28 days of age
RR 1.10, 95% CI 0.56 to 2.17.
BPD, defined as supplemental oxygen or positive pressure support at 36 weeks' PMA
Data from one trial (271 infants) showed an uncertain effect (Analysis 1.4).
1.4. Analysis.

Comparison 1: Low versus moderate‐high NCPAP level, as initial respiratory support, Outcome 4: BPD, defined as supplemental oxygen or positive pressure support use at 36 weeks' PMA
RR 0.80, 95% CI 0.25 to 2.57.
Treatment failure or need for mechanical ventilation
Data from one trial (271 infants) showed an uncertain effect (Analysis 1.5).
1.5. Analysis.

Comparison 1: Low versus moderate‐high NCPAP level, as initial respiratory support, Outcome 5: Treatment failure or need for mechanical ventilation
RR 1.00, 95% CI 0.63 to 1.57.
Need for mechanical ventilation
Meta‐analysis of data from two trials (316 infants) showed an uncertain effect (Analysis 1.6).
1.6. Analysis.

Comparison 1: Low versus moderate‐high NCPAP level, as initial respiratory support, Outcome 6: Need for mechanical ventilation
RR 1.15, 95% CI 0.78 to 1.70 (I2 = 50%); risk difference (RD) 0.03, 95% CI −0.06 to 0.13; number needed to treat for an additional beneficial outcome (NNTB) 33, 95% CI −17 to 8.
Secondary outcomes
Cerebral palsy or gross motor disability
No trials reported this outcome.
Developmental delay or intellectual impairment
No trials reported this outcome.
Blindness
No trials reported this outcome.
Sensorineural deafness requiring amplification
No trials reported this outcome.
Severe intraventricular hemorrhage
Data from one trial (271 infants) showed an uncertain effect (Analysis 1.7).
1.7. Analysis.

Comparison 1: Low versus moderate‐high NCPAP level, as initial respiratory support, Outcome 7: Severe intraventricular hemorrhage
RR 0.77, 95% CI 0.21 to 2.81.
Periventricular leukomalacia
No trials reported this outcome.
Severe retinopathy of prematurity
Data from one trial (271 infants) showed an uncertain effect (Analysis 1.8).
1.8. Analysis.

Comparison 1: Low versus moderate‐high NCPAP level, as initial respiratory support, Outcome 8: Severe retinopathy of prematurity
RR 0.64, 95% CI 0.11 to 3.78.
Necrotizing enterocolitis requiring surgery
No trials reported this outcome.
Pneumothorax
Data from one trial (271 infants) showed an uncertain effect (Analysis 1.9).
1.9. Analysis.

Comparison 1: Low versus moderate‐high NCPAP level, as initial respiratory support, Outcome 9: Pneumothorax
RR 2.41, 95% CI 0.48 to 12.20.
Pulmonary air leak, any
No trials reported this outcome.
Rate of apnea and bradycardia
No trials reported this outcome.
Patent ductus arteriosus receiving medical or surgical treatment
No trials reported this outcome.
Necrotizing enterocolitis
Data from one trial (271 infants) showed an uncertain effect (Analysis 1.10).
1.10. Analysis.

Comparison 1: Low versus moderate‐high NCPAP level, as initial respiratory support, Outcome 10: Necrotizing enterocolitis
RR 1.07, 95% CI 0.45 to 2.55.
Intraventricular hemorrhage, any
No trials reported this outcome.
Physiological definition of BPD
No trials reported this outcome.
Severe bronchopulmonary dysplasia
No trials reported this outcome.
Retinopathy of prematurity, any
No trials reported this outcome.
Duration of positive pressure ventilation
Data from one trial (271 infants) showed an uncertain effect (Analysis 1.11).
1.11. Analysis.

Comparison 1: Low versus moderate‐high NCPAP level, as initial respiratory support, Outcome 11: Duration of positive pressure ventilation, days
Mean difference (MD) (days) 0.30, 95% CI −0.61 to 1.21.
Duration of oxygen supplementation
Data from one trial (271 infants) showed an uncertain effect (Analysis 1.12).
1.12. Analysis.

Comparison 1: Low versus moderate‐high NCPAP level, as initial respiratory support, Outcome 12: Duration of oxygen supplementation, days
MD (days) 1.20, 95% CI −2.13 to 4.53.
Length of hospital stay
No trials reported this outcome.
Days to establish full feeding
No trials reported this outcome.
Days to regain birth weight
No trials reported this outcome.
Weight gain at discharge
No trials reported this outcome.
Weight z‐score at discharge
No trials reported this outcome.
Growth failure at discharge
No trials reported this outcome.
Nasal injury
No trials reported this outcome.
Gastrointestinal perforation
No trials reported this outcome.
Short‐term physiologic outcomes
See also Table 3.
Oxygenation measures
Two of four studies reported improved oxygenation with increasing CPAP levels (Lomp 2010; Miedema 2013); no certain differences were noted in Lavizzari 2014 and Murki 2016.
Carbon dioxide measures
No certain differences were noted in Lavizzari 2014 and Miedema 2013.
Cardiac output measures
No certain differences were noted in Beker 2014.
Heart rate measures
No certain differences were noted in Beker 2014 and Lomp 2010.
Blood pressure measures
No certain differences were noted in Beker 2014.
Lung volume measures
No certain differences were noted in Lavizzari 2014.
Comparison 2. Low (≤ 5 cm H2O) versus moderate‐high (> 5 cm H2O) CPAP levels for postextubation respiratory support
Primary outcomes
Death or BPD at 36 weeks' PMA
Data from one trial (93 infants) showed an uncertain effect (Analysis 2.1).
2.1. Analysis.

Comparison 2: Low versus moderate‐high NCPAP level, following endotracheal extubation, Outcome 1: Death or BPD at 36 weeks' PMA
RR 0.87, 95% CI 0.51 to 1.49.
Mortality at 28 days
No trials reported this outcome.
Mortality by hospital discharge
Data from one trial (93 infants) showed an uncertain effect (Analysis 2.2).
2.2. Analysis.

Comparison 2: Low versus moderate‐high NCPAP level, following endotracheal extubation, Outcome 2: Mortality, by hospital discharge
RR 2.94, 95% CI 0.12 to 70.30.
Mortality at one year
No trials reported this outcome.
Moderate‐severe neurodevelopmental impairment at 18 to 26 months
No trials reported this outcome.
Moderate‐severe neurodevelopmental impairment at 3 to 5 years
No trials reported this outcome.
BPD at 28 to 30 days of age
No trials reported this outcome.
BPD, defined as supplemental oxygen or positive pressure support at 36 weeks' PMA
Data from one trial (93 infants) showed an uncertain effect (Analysis 2.3).
2.3. Analysis.

Comparison 2: Low versus moderate‐high NCPAP level, following endotracheal extubation, Outcome 3: BPD, defined as supplemental oxygen or positive pressure support use at 36 weeks' PMA
RR 0.87, 95% CI 0.51 to 1.49.
Treatment failure or need for mechanical ventilation
Meta‐analysis of data from two trials (117 infants) showed an uncertain effect (Analysis 2.4).
2.4. Analysis.

Comparison 2: Low versus moderate‐high NCPAP level, following endotracheal extubation, Outcome 4: Treatment failure or need for mechanical ventilation
RR 1.52, 95% CI 0.92 to 2.50 (I2 = 17%); RD 0.15, 95% CI −0.02 to 0.32; NNTB 7, 95% CI −50 to 3.
We assessed the certainty of evidence as very low, downgrading one level for risk of bias, one level for inconsistency across studies, and one level for imprecision of effect estimate (Table 2).
Need for mechanical ventilation
Meta‐analysis of data from two trials (117 infants) showed an uncertain effect (Analysis 2.5).
2.5. Analysis.

Comparison 2: Low versus moderate‐high NCPAP level, following endotracheal extubation, Outcome 5: Need for mechanical ventilation
RR 1.26, 95% CI 0.78 to 2.05 (I2 = 78%); RD 0.09, 95% CI −0.08 to 0.26; NNTB 11, 95% CI −13 to 4.
Secondary outcomes
Cerebral palsy or gross motor disability
No trials reported this outcome.
Developmental delay or intellectual impairment
No trials reported this outcome.
Blindness
No trials reported this outcome.
Sensorineural deafness requiring amplification
No trials reported this outcome.
Severe intraventricular hemorrhage
Meta‐analysis of data from two trials (115 infants) showed an uncertain effect (Analysis 2.6).
2.6. Analysis.

Comparison 2: Low versus moderate‐high NCPAP level, following endotracheal extubation, Outcome 6: Severe intraventricular hemorrhage
RR 1.65, 95% CI 0.23 to 11.86 (I2 = 0%).
Periventricular leukomalacia
No trials reported this outcome.
Severe retinopathy of prematurity
Data from one trial (93 infants) showed an uncertain effect (Analysis 2.7).
2.7. Analysis.

Comparison 2: Low versus moderate‐high NCPAP level, following endotracheal extubation, Outcome 7: Severe retinopathy of prematurity
RR 1.96, 95% CI 0.52 to 7.36.
Necrotizing enterocolitis requiring surgery
No trials reported this outcome.
Pneumothorax
Data from one trial (93 infants) showed an uncertain effect (Analysis 2.8).
2.8. Analysis.

Comparison 2: Low versus moderate‐high NCPAP level, following endotracheal extubation, Outcome 8: Pneumothorax
RR 0.14, 95% CI 0.01 to 2.63.
Pulmonary air leak, any
Data from one trial (24 infants) showed an uncertain effect (Analysis 2.9).
2.9. Analysis.

Comparison 2: Low versus moderate‐high NCPAP level, following endotracheal extubation, Outcome 9: Pulmonary air leak
RR 2.36, 95% CI 0.25 to 22.70.
Rate of apnea and bradycardia
No trials reported this outcome.
Patent ductus arteriosus receiving medical or surgical treatment
No trials reported this outcome.
Necrotizing enterocolitis
Data from one trial (93 infants) showed an uncertain effect (Analysis 2.10).
2.10. Analysis.

Comparison 2: Low versus moderate‐high NCPAP level, following endotracheal extubation, Outcome 10: Necrotizing enterocolitis
RR 2.94, 95% CI 0.32 to 27.21.
Intraventricular hemorrhage, any
Data from one trial (24 infants) showed an uncertain effect (Analysis 2.11).
2.11. Analysis.

Comparison 2: Low versus moderate‐high NCPAP level, following endotracheal extubation, Outcome 11: Any intraventricular hemorrhage
RR 0.79, 95% CI 0.41 to 1.51.
Physiological definition of BPD
No trials reported this outcome.
Severe bronchopulmonary dysplasia
Data from one trial (93 infants) showed an uncertain effect (Analysis 2.12).
2.12. Analysis.

Comparison 2: Low versus moderate‐high NCPAP level, following endotracheal extubation, Outcome 12: Severe bronchopulmonary dysplasia
RR 1.22, 95% CI 0.53 to 2.82.
Retinopathy of prematurity, any
No trials reported this outcome.
Duration of positive pressure ventilation
Data from one trial (93 infants) showed an uncertain effect (Analysis 2.13).
2.13. Analysis.

Comparison 2: Low versus moderate‐high NCPAP level, following endotracheal extubation, Outcome 13: Duration of positive pressure ventilation, days
MD (days) 5.00, 95% CI −2.35 to 12.35.
Duration of oxygen supplementation
Data from one trial (93 infants) showed an uncertain effect (Analysis 2.14).
2.14. Analysis.

Comparison 2: Low versus moderate‐high NCPAP level, following endotracheal extubation, Outcome 14: Duration of oxygen supplementation, days
MD (days) −1.00, 95% CI −27.41 to 25.41.
Length of hospital stay
No trials reported this outcome.
Days to establish full feeding
No trials reported this outcome.
Days to regain birth weight
No trials reported this outcome.
Weight gain at discharge
No trials reported this outcome.
Weight z‐score at discharge
No trials reported this outcome.
Growth failure at discharge
No trials reported this outcome.
Nasal injury
Data from one trial (24 infants) showed an uncertain effect (Analysis 2.15).
2.15. Analysis.

Comparison 2: Low versus moderate‐high NCPAP level, following endotracheal extubation, Outcome 15: Nasal injury
RR 1.18, 95% CI 0.08 to 16.78.
Gastrointestinal perforation
No trials reported this outcome.
Short‐term physiologic outcomes
See also: Table 3.
Oxygenation measures
Rehan 2001 reported improved oxygenation with increasing CPAP levels; no certain differences were noted in Kitsommart 2013 and Magnenant 2004.
Carbon dioxide measures
No certain differences were noted in Magnenant 2004.
Cardiac output measures
No trials reported this outcome.
Heart rate measures
No certain differences were noted in Magnenant 2004 and Rehan 2001.
Blood pressure measures
No certain differences were noted in Magnenant 2004.
Lung volume measures
Elgellab 2001 and Magnenant 2004 reported higher tidal volumes with increasing CPAP levels. Elgellab 2001 reported higher end‐expiratory lung volumes with increasing CPAP levels.
Discussion
Summary of main results
There are insufficient data from randomized trials to guide nasal CPAP level selection in preterm infants, whether provided as initial respiratory support or following extubation from invasive mechanical ventilation. For both indications, we identified only two RCTs measuring clinically important outcomes. The outcomes reported by these studies varied, and the overall number of enrolled infants was modest. As a result, comparisons for meta‐analysis were few, and no certain differences between low and moderate‐high nasal CPAP levels were identified. Among outcomes prespecified for inclusion in the summary of findings tables, only treatment failure or need for mechanical ventilation in infants receiving CPAP for postextubation support was meta‐analyzed (Table 2). Though the effect estimate suggests potential benefit with moderate‐high CPAP levels, the estimate was uncertain (Analysis 2.4). The impact of CPAP levels on short‐term physiologic outcomes was unclear based on the available data (Table 3). Three of seven studies reported statistically significant improvements in varying oxygenation measures, but the absolute differences in these were modest and of uncertain clinical relevance. Changing CPAP levels seemed to have minimal impact on carbon dioxide, heart rate, and blood pressure measures. Additional studies are needed to develop evidence‐based recommendations for CPAP level selection in preterm infants.
Overall completeness and applicability of evidence
The overall completeness of the available evidence was very low, and we were unable to make any definitive conclusions regarding the objectives of this review.
We planned to exclude studies published prior to 1990 to increase the applicability of findings to modern neonatal practice, but did not identify any otherwise eligible studies from this time period, therefore none were excluded. The included studies, particularly the four parallel‐groups RCTs contributing data to the primary outcomes, are applicable to modern neonatal practice (see Characteristics of included studies) (Buzzella 2014; Dhir 2016; Kitsommart 2013; Murki 2016). All of these studies were published between 2013 and 2016; enrolled populations that commonly receive nasal CPAP; and compared CPAP pressure levels generated with, and delivered through, currently used pressure sources and nasal interfaces. Both low‐/middle‐ and high‐income countries were represented in these four studies, although both studies comparing CPAP for initial respiratory support were conducted in a low‐/middle‐income country (India) (Dhir 2016; Murki 2016), while both studies comparing CPAP for postextubation support were conducted in high‐income countries (Buzzella 2014, the USA; Kitsommart 2013, Canada). Furthermore, Dhir 2016 and Murki 2016 enrolled a broader and more mature preterm population than Buzzella 2014 and Kitsommart 2013. As such, the applicability of the limited available evidence may be low for less mature preterm infants in high‐income settings when CPAP is given as initial respiratory support, and for more mature infants in lower‐income settings when CPAP is given as postextubation support.
The seven included cross‐over trials are also generally applicable to modern neonatal practice, but span a broader study era (2001 to 2014). All of these studies except Lomp 2010 (South Africa) were conducted in high‐income countries, and the studies enrolled few extremely preterm or extremely low birth weight (< 1000 grams) infants. Though four studies permitted the inclusion of such infants (Elgellab 2001; Lomp 2010; Magnenant 2004; Miedema 2013), the eligible populations were broad, and the central tendencies of enrolled infants suggest these extremely immature infants were few. Furthermore, the cross‐over studies generally enrolled infants that were stable on low levels of pressure support while requiring minimal supplemental oxygen. The overall suggestion that changing CPAP levels has modest impact on physiological outcomes may not be applicable to less mature infants with greater lung disease, precisely the population in whom consideration of higher CPAP levels is most relevant. Lastly, the duration of time required for equilibration of lung volumes following CPAP level changes is uncertain, and may vary as a function of the underlying disease state. Elgellab 2001 maintained CPAP levels for 30 minutes in each cross‐over period, citing preliminary tests that showed that up to 20 minutes are needed to achieve stabilization of end‐expiratory lung volumes after CPAP level changes. Beker 2014, Lavizzari 2014, Lomp 2010, Miedema 2013, and Rehan 2001 all evaluated outcomes within 20 minutes, raising the possibility that the applicability of these findings are limited by insufficient time allowed for equilibration of lung volumes following CPAP level changes.
Quality of the evidence
We graded the overall certainty of the evidence for the outcomes listed in the summary of findings tables to be very low based on the GRADE approach, consistent with very little confidence in the effect estimates (Table 1; Table 2) (Schünemann 2013). For all outcomes in both comparisons, we started with a default of 'high‐certainty' based on study design (RCT), downgrading for risk of bias, lack of consistency across multiple studies, and imprecision.
Regarding bias, we judged the study (Table 1) or studies (Table 2) contributing data to have a low risk of selection bias, but overall high risk of performance bias as participants or personnel were not masked to random CPAP level allocations. Knowledge of the allocation plausibly impacts clinical management decisions such as subsequent pressure level titrations and decisions regarding the need for mechanical ventilation. However, we acknowledge that masking CPAP level is pragmatically challenging and raises safety concerns. We judged there to be an overall unclear risk of detection and reporting bias due to the lack of detail surrounding masking of outcome assessors and inconsistent clinical trial registrations.
All outcomes but one (Analysis 2.4) included in the summary of findings tables reflect data from a single study. We chose to downgrade for a lack of consistency across multiple studies when data from a single study were available. Inconsistency across multiple studies was present in Analysis 2.4, with Buzzella 2014 and Kitsommart 2013 reporting effect estimates in the opposite direction for treatment failure or need for mechanical ventilation among infants randomized to low versus moderate‐high CPAP for postextubation respiratory support. We downgraded for imprecision on the basis of the overall small numbers of included studies and participants.
Potential biases in the review process
Several aspects of our review may have excluded data of arguable relevance to our objectives. Although we included a broad preterm population (gestational age < 37 weeks or birth weight < 2500 grams), we chose to limit inclusion to infants randomized to CPAP levels following neonatal resuscitation and before 28 days of postnatal age. We excluded interventions during neonatal resuscitation because the care delivered in this setting is specialized and often distinct. For example, it often occurs in a distinct physical care setting (e.g. delivery room or dedicated infant resuscitation room rather than the neonatal intensive care unit) and using particular equipment for respiratory support (e.g. face masks rather than dedicated nasal interfaces). Nonetheless, the need for respiratory support and the potential for lung injury begins immediately after preterm birth, and the use of different nasal CPAP levels in this early period would plausibly impact the outcomes considered in this review. We did not identify any completed trials that compared different CPAP levels during neonatal resuscitation, but did exclude two ongoing trials in the screening phase of our search (NCT04372953; Waitz 2020). Future systematic reviews inclusive of or specific to neonatal resuscitation may be a useful adjunct to this review. Our exclusion of infants older than 28 postnatal days at randomization reflects the use of this time point as the earliest at which BPD is ascertained (NIH 1979). Furthermore, the range of CPAP levels considered low versus moderate‐high may be distinct in older infants with evolving or established BPD. We excluded Beker 2015, a cross‐over trial enrolling infants with a median postnatal age of 43 days, on the basis of this decision.
We excluded cross‐over trials without random CPAP level sequence allocation, as these are at an increased risk of systematic bias from the impact of the study procedures or time under study on the measured outcomes. For example, we have observed the physical contact required for non‐invasive procedures such as transthoracic echocardiogram or placement of research equipment to provoke transient changes in hemodynamic and gas exchange parameters in fragile preterm infants. A study sequentially evaluating CPAP level increments may observe worse values at higher pressure levels reflecting the stress provoked by handling and study. Various studies plausibly contributing relevant data were excluded based on this criterion (Courtney 2003; Courtney 2011; Mukerji 2019; Pandit 2001; Pickerd 2014; Veneroni 2014; Zhou 2020). Broader systematic reviews inclusive of non‐randomized studies may be a useful adjunct to this review, particularly with respect to further understanding the impact of CPAP levels on short‐term physiological outcomes.
We initially attempted to correspond with study authors to obtain otherwise unavailable data, or specific data conforming to prespecified eligibility criteria or subgroup analyses. Some of the data included in this review were obtained as such, as noted for various studies in Characteristics of included studies. However, as described in Differences between protocol and review, we revised and updated the in‐progress review in late 2020 at the request of the World Health Organization. At that time, a decision was made to not re‐engage study authors and newly request data conforming to the updated protocol, and to instead predominantly rely on published, publicly available data. Concurrently, we chose to include data from cross‐over trials with random sequence allocation in aggregate, collapsed by CPAP level, as is typical of reporting in these trials. In contrast, the original protocol pursued data restricted to the first random CPAP level allocation through author correspondence. This was motivated by a concern that lung recruitment from CPAP level increments in preceding cross‐over periods could lead to persistent physiological changes despite subsequent pressure level decrements. This is not only plausible, but the rationale behind transient lung recruitment maneuvers with positive end‐expiratory pressure during invasive mechanical ventilation (Fajardo 2014; Wu 2014 ). Although using the aggregate results of cross‐over trials allowed us to be more inclusive of relevant data, these outcomes should be interpreted with this limitation in mind.
Unsuccessful correspondence with study authors during the initial conduct of this review and our subsequent decision to not re‐engage study authors following review revisions contributed to some additional limitations. In a few instances, we noted pertinent data that were either described but not reported in published manuscripts, or that were presented in figures without the text or tabular summaries needed for accurate extraction. For example, Elgellab 2001 described a lack of significant changes in oxygenation, carbon dioxide, heart rate, and blood pressure measures, but did not provide quantitative values. Miedema 2013 reported increasing tidal volumes and end‐expiratory lung volumes at greater CPAP levels, but presented the data graphically. These instances contribute to incomplete data for some of the outcome measures included in this review.
Agreements and disagreements with other studies or reviews
We are unaware of any other reviews, systematic or otherwise, addressing our objectives.
Authors' conclusions
Implications for practice.
There are insufficient data from randomized trials to guide nasal continuous positive airway pressure (CPAP) level selection in preterm infants, whether provided as initial respiratory support or following extubation from invasive mechanical ventilation. Based on the available data, we are uncertain as to whether low or moderate‐high nasal CPAP levels improve morbidity and mortality in preterm infants.
Implications for research.
Well‐designed trials evaluating this important aspect of a commonly used neonatal therapy are needed. Trials reporting clinically important endpoints such as the primary outcomes of this review are needed in particular, while including the review outcomes will facilitate future evidence synthesis. The comparative effects of different nasal CPAP levels are particularly relevant to less mature preterm infants at highest risk of non‐invasive respiratory treatment failure; these infants should be prioritized in future trials. Although masking all personnel to treatment allocation may be challenging, efforts to reduce performance bias through standardized protocols to guide subjective postallocation management, and to reduce detection bias through masking of outcome assessors when applicable, will help strengthen the certainty of future evidence.
History
Protocol first published: Issue 9, 2017
Acknowledgements
We would like to thank Cochrane Neonatal: Jane Cracknell, Managing Editor, Roger Soll, Co‐ordinating Editor, Bill McGuire, Co‐ordinating Editor, and Michelle Fiander, Information Specialist, who provided editorial and administrative support.
The Methods section of this review is based on a standard template used by Cochrane Neonatal.
Carol Friesen, former Information Specialist, designed and ran the literature searches for the 2020 update, and Colleen Ovelman, former Managing Editor, peer reviewed the Ovid MEDLINE search strategy.
Bill McGuire and Nai Ming Lai have peer reviewed and offered feedback for this review.
Appendices
Appendix 1. 2020 Search methods
The RCT filters have been created using Cochrane's highly sensitive search strategies for identifying randomized trials (Higgins 2021). The neonatal filters were created and tested by the Cochrane Neonatal Information Specialist.
CENTRAL via CRS Web:
Date searched: 06 November 2020 Terms: 1 MESH DESCRIPTOR positive‐pressure respiration EXPLODE ALL AND CENTRAL:TARGET 2 MESH DESCRIPTOR Continuous Positive Airway Pressure EXPLODE ALL AND CENTRAL:TARGET 3 continuous positive airway pressure or continuous positive pressure or continuous distending airway pressure or continuous distending pressure or continuous positive transpulmonary pressure or continuous transpulmonary pressure or continuous inflating pressure or continuous negative distending pressure or continuous negative pressure or continuous airway pressure AND CENTRAL:TARGET 4 cpap or ncpap AND CENTRAL:TARGET 5 #1 OR #2 OR #3 OR #4 6 MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET 7 infant or infants or infant's or "infant s" or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET 8 #7 OR #6 AND CENTRAL:TARGET 9 #5 AND #8
MEDLINE via Ovid ‐ Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R):
Date ranges: 1946 to 06 November 2020 Terms: 1. exp positive‐pressure respiration/ or exp continuous positive airway pressure/ 2. (continuous positive airway pressure or continuous positive pressure or continuous distending airway pressure or continuous distending pressure or continuous positive transpulmonary pressure or continuous transpulmonary pressure or continuous inflating pressure or continuous negative distending pressure or continuous negative pressure or continuous airway pressure).mp. 3. (cpap or ncpap).mp. 4. 1 or 2 or 3 5. exp infant, newborn/ 6. (newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or 'infant s' or infant's or infantile or infancy or neonat*).ti,ab. 7. 5 or 6 8. randomized controlled trial.pt. 9. controlled clinical trial.pt. 10. randomized.ab. 11. placebo.ab. 12. drug therapy.fs. 13. randomly.ab. 14. trial.ab. 15. groups.ab. 16. or/8‐15 17. exp animals/ not humans.sh. 18. 16 not 17 19. 7 and 18 20. randomi?ed.ti,ab. 21. randomly.ti,ab. 22. trial.ti,ab. 23. groups.ti,ab. 24. ((single or doubl* or tripl* or treb*) and (blind* or mask*)).ti,ab. 25. placebo*.ti,ab. 26. 20 or 21 or 22 or 23 or 24 or 25 27. 6 and 26 28. limit 27 to yr="2019 ‐Current" 29. 19 or 28 30. 4 and 29
ISRCTN:
Date searched: 06 November 2020 Terms: Interventions: Continuous positive airway pressure AND Participant age range: Neonate Interventions: Cpap AND Participant age range: Neonate
Appendix 2. Previous search methods
We used the criteria and standard methods of Cochrane and Cochrane Neonatal.
We ran two separate searches for this review, one on 29 August 2017 and a top‐off search on 27 September 2019.
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 8) in The Cochrane Library; MEDLINE via PubMed (1966 to 29 August 2017); EMBASE (1980 to 29 August 2017); and CINAHL (1982 to 29 August 2017) using the following search terms: (noninvasive ventilation OR noninvasive respiratory support OR continuous positive airway pressure OR CPAP OR NCPAP) AND (low OR lower OR high OR higher OR level OR levels OR how much), plus database‐specific limiters for RCTs and neonates (see below for the full search strategies for each database). We did not apply language restrictions. We searched for clinical trials on the following platforms: clinicaltrials.gov, The World Health Organization’s International Clinical Trials Registry Platform (ICTRP), and the ISRCTN (at http://www.isrctn.com/, formerly Controlled‐trials.com).
We ran a top‐off search as follows: Cochrane Central Register of Controlled Trials (CENTRAL 2019, Issue 10) in The Cochrane Library; MEDLINE via PubMed (1 January 2017 to 27 September 2019); and CINAHL (1 January 2017 to 27 September 2019) with the same search terms.
For the top‐off search, we did not search on the following platforms: clinicaltrials.gov and the ICTRP, but rather we accessed these records via CENTRAL.
Neonatal standard search strategies prior to 2019:
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 3. Risk of bias tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality (to meet the validity criteria) of the trials. For each trial, we sought information regarding the method of randomization, and the blinding and reporting of all outcomes of all the infants enrolled in the trial. We assessed each criterion as low, high, or unclear risk. Two review authors separately assessed each study. Any disagreements were resolved by discussion. We added this information to the Characteristics of included studies table. We evaluated the following issues and entered the findings into the risk of bias table.
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomization; consecutively numbered, sealed, opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant had received. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk, high risk, or unclear risk for participants;
low risk, high risk, or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorized the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk for outcome assessors;
high risk for outcome assessors;
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data);
unclear risk.
Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all of the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; the study fails to include the results of a key outcome that would have been expected to have been reported);
unclear risk.
Other sources of bias. Did the study appear to be free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk.
If necessary, we explored the impact of the level of bias through the undertaking of sensitivity analyses.
Data and analyses
Comparison 1. Low versus moderate‐high NCPAP level, as initial respiratory support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Death or BPD at 36 weeks' PMA | 1 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.56, 1.85] |
| 1.2 Mortality, by hospital discharge | 1 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.51, 2.12] |
| 1.3 BPD, defined as supplemental oxygen use at 28 days of age | 1 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.56, 2.17] |
| 1.4 BPD, defined as supplemental oxygen or positive pressure support use at 36 weeks' PMA | 1 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.25, 2.57] |
| 1.5 Treatment failure or need for mechanical ventilation | 1 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.63, 1.57] |
| 1.6 Need for mechanical ventilation | 2 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.78, 1.70] |
| 1.7 Severe intraventricular hemorrhage | 1 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.21, 2.81] |
| 1.8 Severe retinopathy of prematurity | 1 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.11, 3.78] |
| 1.9 Pneumothorax | 1 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [0.48, 12.20] |
| 1.10 Necrotizing enterocolitis | 1 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.45, 2.55] |
| 1.11 Duration of positive pressure ventilation, days | 1 | 271 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.61, 1.21] |
| 1.12 Duration of oxygen supplementation, days | 1 | 271 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐2.13, 4.53] |
Comparison 2. Low versus moderate‐high NCPAP level, following endotracheal extubation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Death or BPD at 36 weeks' PMA | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.51, 1.49] |
| 2.2 Mortality, by hospital discharge | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [0.12, 70.30] |
| 2.3 BPD, defined as supplemental oxygen or positive pressure support use at 36 weeks' PMA | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.51, 1.49] |
| 2.4 Treatment failure or need for mechanical ventilation | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.92, 2.50] |
| 2.5 Need for mechanical ventilation | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.78, 2.05] |
| 2.6 Severe intraventricular hemorrhage | 2 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.23, 11.86] |
| 2.7 Severe retinopathy of prematurity | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.52, 7.36] |
| 2.8 Pneumothorax | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.63] |
| 2.9 Pulmonary air leak | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.25, 22.70] |
| 2.10 Necrotizing enterocolitis | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [0.32, 27.21] |
| 2.11 Any intraventricular hemorrhage | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.41, 1.51] |
| 2.12 Severe bronchopulmonary dysplasia | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.53, 2.82] |
| 2.13 Duration of positive pressure ventilation, days | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 5.00 [‐2.35, 12.35] |
| 2.14 Duration of oxygen supplementation, days | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐27.41, 25.41] |
| 2.15 Nasal injury | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.08, 16.78] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beker 2014.
| Study characteristics | ||
| Methods | Randomized cross‐over controlled trial | |
| Participants | Single center, 34 participants Inclusion criteria: gestational age 28 to 34 weeks, on nasal CPAP at 5 cm H2O with FiO2 of 0.21. Exclusion criteria: use of inotropic support; presence of significant structural anomalies; significant shunt including patent ductus arteriosus > 1.5 mm in diameter with a flow velocity of < 3 m/s and/or a pulsatile flow pattern, atrial septal defect/persistent foramen ovale > 3 mm in diameter, or any ventricular septal defect. Characteristics of enrolled participants: all median (IQR); gestational age: 28.8 (28.2 to 30.4) weeks, weight at intervention: 1300 (1000 to 1600) grams, chronologic age at intervention: 5 (2 to 11); 11 of 34 infants were receiving nasal CPAP as respiratory support following endotracheal extubation. |
|
| Interventions | Initial nasal CPAP level as initial respiratory support or following endotracheal extubation (both populations included). CPAP levels of 4, 6, and 8 cm H2O evaluated in random order. Echocardiographic outcome measures obtained 15 minutes after CPAP level change. Blood pressure, heart rate and oxygenation measured obtained 10 minutes after CPAP level changes. No wash‐out period between cross‐over periods described. |
|
| Outcomes |
|
|
| Notes |
Subgroup characteristics: Setting: high‐income country (Australia) Type of CPAP pressure generation: mixed, bubble CPAP (n = 30) and delivered by ventilator (n = 4) Type of CPAP delivery interface: short binasal prongs (presumed, based on: “the prongs remained in the nose during the study period.”) Antenatal steroid exposure: not described Methylxanthine exposure: not described Relevant methods details: “Prongs remained in the nose during the study period, and attention was paid to optimal positioning of the infant’s head and neck. No measures were taken to keep the mouth shut.” Oxygen saturations described as recorded in methods but not reported in results. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if known allocation could have biased performance of unmasked bedside nurse |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The bedside nurse changed the CPAP levels according to the order on the card, and echocardiographic measurements were taken by a single examiner (FB) blinded to the nCPAP level. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or missing data noted. |
| Selective reporting (reporting bias) | Low risk | Trial registered (ACTRN12611001057976), primary outcomes listed in registration reported in manuscript. |
Buzzella 2014.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | Single center, 93 participants Inclusion criteria: birth weight 500 to 1000 grams, gestational age 23 to 30 weeks, required mechanical ventilation within the first 7 days of life, had elective extubation during first 6 weeks of age while on FiO2 ≥ 0.25. Exclusion criteria: birth weight < 10th percentile for gestational age, major congenital anomalies, neuromuscular disease, upper airway obstruction. Characteristics of enrolled participants: gestational age, weeks, mean (SD): low 25.6 (1.2), moderate‐high 25.5 (1.3); birth weight, grams, mean (SD): low 776 (135), moderate‐high 736 (113); age at extubation, days, mean (SD): low 16.3 (14.7), moderate‐high 15.5 (12.4). |
|
| Interventions | Initial nasal CPAP level following endotracheal extubation: Low: 5 cm H2O; subsequent titration to range of 4 to 6 cm H2O allowed per protocol. Moderate‐high: 8 cm H2O; subsequent titration to range of 7 to 9 cm H2O allowed per protocol. Trial intervention period was 96 hours postextubation; beyond this period further respiratory management at discretion of clinical team. During intervention period, CPAP level titrations within range made at the discretion of the clinical team. CPAP level increases outside of range could occur for persistent apnea, symptomatic PDA with left to right shunting, or persistent hypoxemia with increased oxygen need secondary to low lung volume or atelectasis on chest radiograph. CPAP level decreases outside of range could occur for pulmonary interstitial emphysema, hemodynamic instability with decreased blood pressure, evidence of lung over distention on chest radiograph, or persistent gastric distention. |
|
| Outcomes |
|
|
| Notes |
Subgroup characteristics: Setting: high‐income country (USA) Type of CPAP pressure generation: CPAP delivered by ventilator Type of CPAP delivery interface: short binasal prongs Antenatal steroid exposure: yes (87%) Methylxanthine exposure: yes, all participants started on caffeine prior to extubation Relevant methods details: PDA reported, but not restricted to those requiring medical or surgical treatment as per review protocol, so not included. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list |
| Allocation concealment (selection bias) | Low risk | Sequential, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Caregivers not blinded to allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not registered as investigators’ understanding that not required at time of enrollment |
Dhir 2016.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | Single center, 45 participants Inclusion criteria: gestational age 28 to 35 weeks, birth weight less than 2000 grams, use of CPAP for respiratory distress within 24 hours of birth. Exclusion criteria: none specified. Characteristics of enrolled participants: not reported; “baseline parameters were similar in both groups.” |
|
| Interventions |
Initial nasal CPAP level for initial respiratory support following birth and neonatal resuscitation Low: 5 cm H2O Moderate‐high: 7 cm H2O Subsequent nasal CPAP level titrations based on Silverman Anderson Score, oxygenation, arterial blood gas analyses, and chest radiograph to a maximum of 8 cm H2O and FiO2 0.80 |
|
| Outcomes |
|
|
| Notes |
Subgroup characteristics: Setting: low‐/middle‐income country (India) Type of CPAP pressure generation: underwater bubble CPAP Type of CPAP delivery interface: unknown Antenatal steroid exposure: unknown Methylxanthine exposure: unknown Relevant methods details: Details limited to brief research letter |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomizer |
| Allocation concealment (selection bias) | Low risk | Serially numbered, opaque envelopes were used and opened just prior to intervention (via correspondence with study authors), though block size of 4 would have allowed allocation to be commonly inferred. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Brief reporting; completeness of data uncertain |
| Selective reporting (reporting bias) | Unclear risk | Protocol registration not specified. |
| Other bias | High risk | Published via: “Scientific Letter”, with limited study details provided |
Elgellab 2001.
| Study characteristics | ||
| Methods | Randomized cross‐over controlled trial | |
| Participants | Single center, 10 participants Inclusion criteria: gestational age 26 to 32 weeks, chronologic age < 7 days, moderate respiratory failure defined by FiO2 < 0.30 and PaCO2 < 50 mmHg, normal brain ultrasound. Exclusion criteria: more than 1 episode of apnea in a 2‐hour period, hemodynamic impairment or need for inotropic drugs, periventricular leukomalacia, or intraventricular haemorrhage. Characteristics of enrolled and evaluated participants: gestational age range: 27 to 32 weeks, birth weight mean (SD): 1300 (460) grams, postnatal age range: 1 to 21 days; 3 participants on nasal CPAP as primary respiratory support, 7 following endotracheal extubation. |
|
| Interventions | Nasal CPAP level as initial respiratory support or following endotracheal extubation (both populations included). CPAP level set to 2, 4, or 6 cm H2O in random order if baseline CPAP value < 6 cm H2O. CPAP level set to 2, 4, 6, or 8 cm H2O in random order if baseline CPAP value ≥ 6 cm H2O. CPAP levels maintained for 30‐minute intervals. Wash‐out period between cross‐over periods not described. |
|
| Outcomes |
|
|
| Notes |
Subgroup characteristics: Setting: high‐income country (France) Type of CPAP pressure generation: CPAP delivered by variable flow device Type of CPAP delivery interface: not described Antenatal steroid exposure: yes (80%) Methylxanthine exposure: not described Relevant methods details: Participants excluded from study if excessive motion artifact (n = 3) or clinical deterioration (n = 2) noted, deterioration defined as FiO2 > 0.35, transcutaneous pCO2 > 50 mmHg, recurrent apnea, drop in systemic arterial blood pressure. Unclear how many participants evaluated at 8 cm H2O. The outcomes of SpO2, transcutaneous pCO2, heart rate, and systemic blood pressure were measured but not reported in the published manuscript; authors state: “no significant changes in SpO2, TcPCO2, heart rate or systemic blood pressure was found during the study period.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants contributing to data at each nasal CPAP level unclear; authors describe availability in data at 8 cm H2O in restricted sample of participants (those with baseline nasal CPAP of > 6 cm H2O, but unclear how many participants evaluated at this level). |
| Selective reporting (reporting bias) | Unclear risk | No trial registration noted. |
Kitsommart 2013.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | Single center, 24 participants Inclusion criteria: birth weight 500 to 1250 grams, birth weight appropriate for gestational age, on mechanical ventilation and ready for endotracheal extubation before 14 days of age. Exclusion criteria: lethal abnormalities, upper airway abnormalities, grade III or IV intraventricular hemorrhage, neuromuscular disorders, congenital heart disease. Characteristics of enrolled participants: gestational age, weeks, mean (SD): low 27.0 (0.4), moderate‐high 26.1 (0.4); birth weight, grams, mean (SD): low 913 (71), moderate‐high 861 (49); age at randomized allocation not reported |
|
| Interventions | Initial nasal CPAP level following endotracheal extubation: Low: 5 cm H2O; subsequent titration to range of 4 to 6 cm H2O allowed per protocol Moderate‐high: 8 cm H2O; subsequent titration to range of 7 to 9 cm H2O allowed per protocol Interventional protocol ended 72 hours after extubation |
|
| Outcomes |
|
|
| Notes |
Subgroup characteristics: Setting: high‐income country (Canada) Type of CPAP pressure generation: CPAP delivered by ventilator Type of CPAP delivery interface: short binasal prongs Antenatal steroid exposure: yes (71%) Methylxanthine exposure: unknown, not reported Relevant methods details: In infants with birth weight ≤ 1000 grams, extubation considered when: mean airway pressure < 7 cm H2O and FiO2 ≤ 0.30. In infants with birth weight > 1000 grams, extubation considered when: mean airway pressure < 8 cm H2O and FiO2 ≤ 0.30. Use of moderate‐high levels (for low group) or nasal intermittent positive pressure following random allocation allowed but considered treatment failure. Blood gas analysis obtained 4 hours after extubation and then every 12 hours. Some of the reported outcomes were unpublished and obtained through correspondence with study authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomized list |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data analyst blind to allocation, but personnel obtaining outcomes unmasked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up noted. |
| Selective reporting (reporting bias) | Low risk | Trial registered (NCT00636324), and prespecified outcomes reported. |
Lavizzari 2014.
| Study characteristics | ||
| Methods | Randomized cross‐over controlled trial | |
| Participants | Single center, 7 participants* Inclusion criteria: gestational age 28 to 32 6/7 weeks, chronologic age < 96 hours, stable (per clinical team) on nasal CPAP or HFNC; institutional criteria to commence CPAP or HFNC were a Silverman score > 5 and/or FiO2 > 0.3 to maintain a target SpO2 of 88% to 92%. Exclusion criteria: major congenital anomalies, intraventricular hemorrhage. Characteristics of enrolled participants: birth gestational age, weeks, median (IQR): 31 (30 6/7 to 32); birth weight, grams, median (IQR): 1490 (1404 to 1657); chronologic age at intervention, hours, median (IQR): 49 (35 to 79); participants on either CPAP 4 to 6 cm H2O (n = 13) or HFNC 4 to 7 L/min (n = 7) at baseline. Characteristics of enrolled participants included in this review:* gestational age < 32 weeks, weight at study, grams, mean (SD): 1325 (303) |
|
| Interventions | Nasal CPAP as primary respiratory support. CPAP levels of 2, 4, and 6 cm H2O, in random order. Data included in review restricted to first cross‐over period.* CPAP levels maintained for 15‐minute intervals. Wash‐out period between cross‐over periods not described. |
|
| Outcomes |
|
|
| Notes |
Subgroup characteristics: Setting: high‐income country (Italy) Type of CPAP pressure generation: CPAP delivered by variable flow device Type of CPAP delivery interface: short binasal prongs Antenatal steroid exposure: not described Methylxanthine exposure: not described Relevant methods details: *Included data restricted to 7 participants of birth gestational age < 32 weeks obtained through correspondence with study authors; original study enrolled 20 participants, aggregate data eligible for inclusion in review unavailable in published manuscript. Purpose of study was to compare the impact of CPAP and HFNC at equal retropharyngeal pressure. Mouth air leaks were avoided by gently closing the mouth during evaluation. Participants also evaluated on various HFNC levels, order of CPAP vs HFNC evaluation determined randomly. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or missing data noted. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration described. |
Lomp 2010.
| Study characteristics | ||
| Methods | Randomized cross‐over controlled trial | |
| Participants | Single center, 37 participants Inclusion criteria: birth weight > 500 grams, use of nasal CPAP. Exclusion criteria: major congenital malformation, hemodynamic instability, necrotizing enterocolitis, pneumothorax, or FiO2 > 0.60. Characteristics of enrolled participants: “Median gestational age 30 ± 4.9 weeks, median birth weight 1234 ± 443 g, chronological age ≤ 24 hours” in 24/37 participants (65%) |
|
| Interventions | Initial nasal CPAP level as initial respiratory support or following endotracheal extubation (both populations included). CPAP levels of 2, 4, 6, and 8 cm H2O evaluated in random order. Adjustment period of 10 minutes or greater (until participant was “settled”) between CPAP level changes. No wash‐out period between cross‐over periods described. |
|
| Outcomes |
|
|
| Notes |
Subgroup characteristics: Setting: low‐/middle‐income country (South Africa) Type of CPAP pressure generation: delivered by variable flow device Type of CPAP delivery interface: short binasal prongs Antenatal steroid exposure: yes (65%) Methylxanthine exposure: not described Relevant methods details: Mouth closed with a soft elastic chin strap that allowed voluntary movements including yawning. Study interventions discontinued for increase in respiratory rate by 20%, any episode of apnea > 15 seconds, more than 1 episode of apnea with arterial desaturation, heart rate increase > 20% from baseline or persistent bradycardia (< 100/min) warranting intervention, need for urgent clinical intervention or any unexplained or any unexpected deterioration. FiO2 was adjusted to keep SpO2 between 85% to 90% for neonates < 1.5 kg or a gestational age of < 32 weeks, 88% to 92% for neonates > 32 weeks, and 90% to 95% for neonates ≥ 36 weeks of gestational age. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The randomisation list was kept in the research file. The subjects were allocated by the investigator according to the sequence of randomisation codes on the list.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysis complete in 37 of 56 enrolled participants. Measurements could not be completed due to clinical or technical limitations in 11 participants, and 8 participants excluded due to poor data quality. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration described. |
| Other bias | High risk | Not published in peer‐reviewed journal |
Magnenant 2004.
| Study characteristics | ||
| Methods | Randomized cross‐over controlled trial | |
| Participants | Single center, 11 participants Inclusion criteria: gestational age 26 to 32 weeks, moderate respiratory failure defined by FiO2 < 0.30, normal brain ultrasound. Exclusion criteria: more than 1 episode of apnea in a 2‐hour period, hemodynamic impairment or need for inotropic drugs, periventricular leukomalacia, or intraventricular hemorrhage. Characteristics of enrolled and evaluated participants: all median (range); gestational age: 28 (27 to 31) weeks, birth weight: 1060 (690 to 1650) grams, postnatal age median (range): 12 (1 to 35) days; 3 participants on nasal CPAP as primary respiratory support, 7 following endotracheal extubation. |
|
| Interventions | Nasal CPAP level following endotracheal extubation (presumed based on “treated with NCPAP for weaning from mechanical ventilation”). CPAP level set to 0, 2, 4, or 6 cm H2O in random order. CPAP levels maintained for 30‐minute intervals. Wash‐out period between cross‐over periods not described. |
|
| Outcomes |
|
|
| Notes |
Subgroup characteristics: Setting: high‐income country (France) Type of CPAP pressure generation: CPAP delivered by variable flow device Type of CPAP delivery interface: not described Antenatal steroid exposure: not described Methylxanthine exposure: not described Relevant methods details: Enrolled participants excluded from reporting for excessive motion artifact (7 of 18 excluded), or clinical deterioration was observed (FiO2 > 35%, transcutaneous pCO2 > 70 mmHg, recurrent apnea, and drop in systemic arterial pressure). FiO2 was set to maintain SpO2 between 92% and 96%. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or missing data (among those included) noted. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration noted. |
Miedema 2013.
| Study characteristics | ||
| Methods | Randomized cross‐over controlled trial | |
| Participants | Single center, 22 participants Inclusion criteria: gestational age < 32 weeks, chronologic age < 7 days, on nasal CPAP with FiO2 < 0.30 and on average < 1 apnea per every 2 hours. Exclusion criteria: congenital anomalies of the chest or abdomen, fragile skin. Characteristics of enrolled participants: all mean (SD): gestational age, weeks: 29.7 (1.5), birth weight, grams: 1318 (312), chronologic age at intervention, days: 3.2 (1.3); 20 of 22 participants on nasal CPAP as initial respiratory support, 2 of 22 following endotracheal extubation. |
|
| Interventions | Initial nasal CPAP level as initial respiratory support or following endotracheal extubation (both populations included). CPAP level first set to 2 cm H2O, then increased to 4 or 6 cm H2O in random order. CPAP levels maintained for 10‐minute intervals. Wash‐out period between cross‐over periods not described. |
|
| Outcomes |
|
|
| Notes |
Subgroup characteristics: Setting: high‐income country (the Netherlands) Type of CPAP pressure generation: CPAP delivered by variable flow device Type of CPAP delivery interface: not described Antenatal steroid exposure: yes (91%) Methylxanthine exposure: not described Relevant methods details: Participants also evaluated on nasal BiPAP following CPAP level evaluations. Fraction of inspired oxygen described as outcome in methods, but quantitative measures not reported in results (described as unchanged). Lung volume measurements (change in end‐expiratory lung volume as measured by electrical impedence tomography, tidal volume as measured by both electrical impedence tomography and respiratory inductance plethysmography) also obtained, but displayed in figures without tabular summaries and not included in tabular review summary; results described in review text. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up, no missing data |
| Selective reporting (reporting bias) | Unclear risk | Trial registration not described. |
Murki 2016.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | Single center, 271 participants Inclusion criteria: gestational age 27 to 34 weeks, development of respiratory distress within 24 hours of birth. Exclusion criteria: major congenital anomalies, poor respiratory drive, severe metabolic acidosis, active seizures, 5‐minute Apgar less than 4, severe respiratory distress requiring mechanical ventilation. Characteristics of enrolled participants: gestational age, weeks, mean (SD): low 30.2 (1.8), moderate‐high 30.2 (1.7); birth weight, grams, mean (SD): low 1250 (366), moderate‐high 1265 (302); age at randomized allocation, minutes of life, median (IQR): low 30 (26 to 30), moderate‐high 30 (30 to 30). |
|
| Interventions |
Initial nasal CPAP level for initial respiratory support following birth and neonatal resuscitation Low: 5 cm H2O Moderate‐high: 7 cm H2O Subsequent nasal CPAP level titrations based on a standardized protocol considering work of breathing, oxygenation, arterial blood gas analyses, and lung inflation of chest radiograph. |
|
| Outcomes |
|
|
| Notes |
Subgroup characteristics: Setting: low‐/middle‐income country (India) Type of CPAP pressure generation: underwater bubble CPAP Type of CPAP delivery interface: short binasal prongs Antenatal steroid exposure: yes (91%) Methylxanthine exposure: no, not reported quantitatively, but use described as “to treat apnea or facilitate extubation”. Given exclusion criteria of poor respiratory drive, and intervention as initial respiratory support, review authors' judgement of limited use at random allocation—confirmed via correspondence with study authors. Relevant methods details: Intubation, surfactant, and extubation administered as early rescue therapy if the chest radiograph suggested respiratory distress syndrome, and the FiO2 requirement was more than 0.30; this was not considered need for mechanical ventilation or failure of treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by web‐based computer program |
| Allocation concealment (selection bias) | Low risk | Serially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up noted. |
| Selective reporting (reporting bias) | Unclear risk | Trial registered (CTRI/2014/04/004539). Primary and all key outcomes consistent with registration, but a few secondary outcomes in registration (e.g. nasal injury, gastric perforation, time to reach full feeds) not reported in published outcomes. |
Rehan 2001.
| Study characteristics | ||
| Methods | Randomized cross‐over controlled trial | |
| Participants | Single center, 12 participants Inclusion criteria: “preterm infants who were clinically stable and receiving CPAP for either apnea of prematurity or postextubation respiratory support” Exclusion criteria: clinical instability (undefined), use of supplemental oxygen or ventilatory support, congenital malformations, and ≥ grade II intraventricular hemorrhage. Characteristics of enrolled participants: all mean (SD): gestational age: 29 (1) weeks, birth weight: 1120 (225) grams, chronologic age at intervention: 10 (8) days. |
|
| Interventions | Nasal CPAP level as initial respiratory support or following endotracheal extubation (both populations included). CPAP level increased or decreased from clinical baseline (presumably 4 to 6 cm H2O) by 3 cm H2O in random order, leading to measures at 3 CPAP level ranges: 1 to 3, 4 to 6, 7 to 9 cm H2O.* CPAP levels maintained for 10‐minute intervals. Wash‐out period between cross‐over periods not described. |
|
| Outcomes |
|
|
| Notes | *Results summarized in table at midpoint of each CPAP level range (i.e. 2, 5, 8 cm H2O). Subgroup characteristics: Setting: high‐income country (the USA) Type of CPAP pressure generation: unknown (“the conventional nasal prong system (Vesta, Franklin, WI)”) Type of CPAP delivery interface: short binasal prongs (presumed based on description of “nasal prong system”) Antenatal steroid exposure: not described Methylxanthine exposure: not described Relevant methods details: Primary objective of study was to assess impact of nasal CPAP levels on diaphragmatic thickness and excursion. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator recording outcomes blinded to allocations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or missing data |
| Selective reporting (reporting bias) | Unclear risk | Trial registration not described. |
BiPAP: biphasic positive airway pressure;BPD: bronchopulmonary dysplasia; CPAP: continuous positive airway pressure levels;FiO2: fraction of inspired oxygen; HFNC: high‐flow nasal cannula; IQR: interquartile range; nCPAP: nasal continuous positive airway pressure levels; pCO2: partial pressure of carbon dioxide; PaCO2:partial pressure of carbon dioxide; PDA: patent ductus arteriosus; SaO2:arterial oxygen saturation; SD: standard deviation; SpO2:peripheral capillary oxygen saturation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beker 2015 | Wrong patient population: median postnatal age at study 43 days |
| Courtney 2003 | Wrong study design: nasal CPAP levels not randomly allocated |
| Courtney 2011 | Wrong study design: nasal CPAP levels not randomly allocated |
| Dimitriou 1999 | Wrong intervention: invasive positive‐end expiratory pressure levels |
| Dinger 2001 | Wrong intervention: invasive positive‐end expiratory pressure levels |
| Lavizzari 2016 | Wrong comparator: nasal CPAP vs high‐flow nasal cannula |
| Locke 1991 | Wrong outcomes (no prespecified outcome reported) |
| Mukerji 2019 | Wrong study design: nasal CPAP levels not randomly allocated |
| Pandit 2001 | Wrong study design: nasal CPAP levels not randomly allocated |
| Pickerd 2014 | Wrong study design: nasal CPAP levels not randomly allocated |
| Veneroni 2014 | Wrong study design: nasal CPAP levels not randomly allocated |
| Zhou 2020 | Wrong study design: nasal CPAP levels not randomly allocated |
CPAP: continuous positive airway pressure; RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12618001638224.
| Study name | Extubation CPAP Level Assessment trial (ÉCLAT) |
| Methods | Randomized controlled trial |
| Participants | Multicenter, 200 participants targeted Inclusion criteria: born less than 28 weeks’ gestation, being extubated for the first time from mechanical ventilation to nasal CPAP, have received enteral or intravenous caffeine within 24 hours prior to the planned extubation, have received surfactant. Exclusion criteria: being extubated to any mode of non‐invasive respiratory support other than CPAP, or to no respiratory support; have a major congenital anomaly or condition that might have an adverse effect on breathing or ventilation: known upper airway obstruction or major airway abnormality, or major congenital heart disease; are 36 weeks or greater corrected age at time of extubation; are not receiving full intensive care after extubation |
| Interventions | Initial nasal CPAP level following endotracheal extubation:
Will not qualify for primary review intervention of low (≤ 5 cm H2O) vs moderate‐high (> 5 cm H2O) nasal CPAP levels, but may qualify for inclusion in planned sensitivity analysis comparing low‐moderate (≤ 8 cm H2O) vs high (> 8 cm H2O) nasal CPAP levels. |
| Outcomes |
|
| Starting date | 3 March 2019 |
| Contact information | Miss Anna Kidman; a.kidman@student.unimelb.edu.au |
| Notes | Characteristics obtained from clinical trial registration. Inclusion uncertain, conditional on age at randomization less than 28 days in enrolled participants. |
CPAP: continuous positive airway pressure
Differences between protocol and review
This review was updated in 2020/2021 at the request of the World Health Organization (WHO).
The Background was revised for increased clarity and to align with changes in the Methods, as detailed below.
The Methods have been updated as follows.
We expanded the included population to include all preterm infants; the title of the review has been modified to reflect this broader population.
We now rely on the central tendency of population characteristics (gestational age, postnatal age) to determine inclusion, rather than pursuing restricted data through author correspondence, to increase inclusion of publicly available data.
Modification of both primary and secondary outcomes measures in consultation with authorship team and WHO.
Modification of selected subgroup analyses in consultation with authorship team and WHO.
Modification of summary of findings table outcomes in consultation with authorship team and WHO.
Modification of planned sensitivity analyses to allow comparisons at an alternative pressure level threshold.
We now include aggregate data from cross‐over trials rather than pursuing data restricted to the first random nasal continuous positive airway pressure (CPAP) level period through author correspondence, to increase inclusion of publicly available data.
For the 2020 search, we ran searches in the following databases: CENTRAL via CRS Web and MEDLINE via Ovid. The search strategies are shown in Appendix 1. The previous search methods are shown in Appendix 2.
Contributions of authors
NB and HK conceived this review. NB, AM, and JF reviewed the search results to identify eligible studies and pertinent data. All review authors contributed to the development of the study protocol and editing of the review.
Sources of support
Internal sources
No sources of support provided
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH), USA
Nicolas Bamat received salary support through a career development grant during the conduct of this review (1K23HD101651‐01)
Declarations of interest
N Bamat is employed as a neonatologist, Children's Hospital of Philadelphia.
J Fierro has no interest to declare.
A Mukerji has no interest to declare.
CJ Wright is an Associate Professor, Section of Neonatology, University of Colorado.
D Millar has received honoraria teaching neonatal trainees from Chiesi Ltd. Dr Millar is employed as a Consultant Neonatologist, Belfast Health & Social Care Trust.
H Kirpalani has no interest to declare.
New
References
References to studies included in this review
Beker 2014 {published data only}
- Beker F, Rogerson SR, Hooper SB, Wong C, Davis PG. The effects of nasal continuous positive airway pressure on cardiac function in premature infants with minimal lung disease: a crossover randomized trial. Journal of Pediatrics 2014;164(4):726-9. [DOI: 10.1016/j.jpeds.2013.10.087] [PMID: ] [DOI] [PubMed] [Google Scholar]
Buzzella 2014 {published data only (unpublished sought but not used)}
- Buzzella B, Claure N, D’Ugard C, Bancalari E. A randomized controlled trial of two nasal continuous positive airway pressure levels after extubation in preterm infants. Journal of Pediatrics 2014;164(1):46-51. [DOI: 10.1016/j.jpeds.2013.08.040] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dhir 2016 {published and unpublished data}
- Dhir SK, Chawla D, Guglani V, Batta M. Comparison of seven and five centimetre of water as initiating continuous airway pressure levels in preterm neonates with respiratory distress. Indian Journal of Pediatrics 2016;83(9):1047-8. [DOI: 10.1007/s12098-016-2130-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Elgellab 2001 {published data only}
- Elgellab A, Riou Y, Abbazine A, Truffert P, Matran R, Lequien P, et al. Effects of nasal continuous positive airway pressure (NCPAP) on breathing pattern in spontaneously breathing premature newborn infants. Intensive Care Medicine 2001;27(11):1782-7. [DOI: 10.1007/s00134-001-1117-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kitsommart 2013 {published and unpublished data}
- Kitsommart R, Kalyn A, Al-Saleem N, Janes M, Sant'Anna GM. Levels of nasal CPAP applied during the immediate post-extubation phase: a randomized controlled pilot trial. e-Journal of Neonatology Research 2013;3(1):2-10. [Google Scholar]
Lavizzari 2014 {published and unpublished data}
- Lavizzari A, Veneroni C, Colnaghi M, Ciuffini F, Zannin E, Fumagalli M, et al. Respiratory mechanics during NCPAP and HHHFNC at equal distending pressures. Archives of Disease in Childhood, Fetal and Neonatal Edition 2014;99:F315-20. [DOI: 10.1136/archdischild-2013-305855] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lomp 2010 {unpublished data only}
- Lomp A. Measurement and assessment of work of breathing in neonates during nasal continuous positive airway pressure therapy. Thesis submitted for MDres degree, Department of Paediatrics, Imperial College London (spiral.imperial.ac.uk/bitstream/10044/1/7019/1/Lomp-A-2011-MD%28Res%29-Thesis.pdf) 2010.
Magnenant 2004 {published data only}
- Magnenant E, Rakza T, Riou Y, Elgellab A, Matran R, Lequien P, et al. Dynamic behavior of respiratory system during nasal continuous positive airway pressure in spontaneously breathing premature newborn infants. Pediatric Pulmonology 2004;37(6):485-91. [DOI: 10.1002/ppul.10445] [PMID: ] [DOI] [PubMed] [Google Scholar]
Miedema 2013 {published data only (unpublished sought but not used)}
- Miedema M, Burg PS, Beuger S, Jongh FH, Frerichs I, Kaam AH. Effect of nasal continuous and biphasic positive airway pressure on lung volume in preterm infants. Journal of Pediatrics 2013;162(4):691-7. [DOI: 10.1016/j.jpeds.2012.09.027] [PMID: ] [DOI] [PubMed] [Google Scholar]
Murki 2016 {published and unpublished data}
- Murki S, Nathani PP, Sharma D, Subramaniam S, Oleti TP, Chawla D. Initiating nasal continuous positive airway pressure in preterm neonates at 5 cm as against 7 cm did not decrease the need for mechanical ventilation. Acta Paediatrica 2016;105(8):e345-51. [DOI: 10.1111/apa.13385] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rehan 2001 {published data only}
- Rehan VK, Laiprasert J, Nakashima JM, Wallach JM, McCool FD. Effects of continuous positive airway pressure on diaphragm dimensions in preterm infants. Journal of Perinatology 2001;21(8):521-4. [DOI: 10.1038/sj.jp.7210587] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Beker 2015 {published data only}
- Beker F, Rogerson SR, Hooper SB, Sehgal A, Davis PG. Hemodynamic effects of nasal continuous positive airway pressure in preterm infants with evolving chronic lung disease, a crossover randomized trial. Journal of Pediatrics 2015;166(2):477-9. [DOI: 10.1016/j.jpeds.2014.10.019] [PMID: ] [DOI] [PubMed] [Google Scholar]
Courtney 2003 {published data only}
- Courtney SE, Aghai ZH, Saslow JG, Pyon KH, Habib RH. Changes in lung volume and work of breathing: a comparison of two variable-flow nasal continuous positive airway pressure devices in very low birth weight infants. Pediatric Pulmonology 2003;36(3):248-52. [DOI: 10.1002/ppul.10327] [PMID: ] [DOI] [PubMed] [Google Scholar]
Courtney 2011 {published data only}
- Courtney SE, Kahn DJ, Singh R, Habib RH. Bubble and ventilator-derived nasal continuous positive airway pressure in premature infants: work of breathing and gas exchange. Journal of Perinatology 2011;31(1):44-50. [DOI: 10.1038/jp.2010.55] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dimitriou 1999 {published data only}
- Dimitriou G, Greenough A, Laubscher B. Appropriate positive end expiratory pressure level in surfactant-treated preterm infants. European Journal of Pediatrics 1999;158(11):888-91. [DOI: 10.1007/s004310051235] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dinger 2001 {published data only}
- Dinger J, Topfer A, Schaller P, Schwarze R. Effect of positive end expiratory pressure on functional residual capacity and compliance in surfactant-treated preterm infants. Journal of Perinatal Medicine 2001;29(2):137-43. [DOI: 10.1515/JPM.2001.018] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lavizzari 2016 {published data only}
- Lavizzari A, Colnaghi M, Ciuffini F, Veneroni C, Musumeci S, Cortinovis I, et al. Heated, humidified high-flow nasal cannula vs nasal continuous positive airway pressure for respiratory distress syndrome of prematurity: a randomized clinical noninferiority trial. JAMA Pediatrics August 8, 2016:E1-E7. [DOI: 10.1001/jamapediatrics.2016.1243] [PMID: ] [DOI] [PubMed] [Google Scholar]
Locke 1991 {published data only}
- Locke R, Greenspan JS, Shaffer TH, Rubenstein SD, Wolfson MR. Effect of nasal CPAP on thoracoabdominal motion in neonates with respiratory insufficiency. Pediatric Pulmonology 1991;11(3):259-64. [DOI: 10.1002/ppul.1950110313] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mukerji 2019 {published data only}
- Mukerji A, Wahab MG, Mitra S, Mondal T, Paterson D, Beck J, et al. High continuous positive airway pressure in neonates: a physiological study. Pediatric Pulmonology 2019;54(7):1039-44. [DOI: 10.1002/ppul.24312] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pandit 2001 {published data only}
- Pandit PB, Courtney SE, Pyon KH, Saslow JG, Habib H. Work of breathing during constant- and variable-flow nasal continuous positive airway pressure in preterm neonates. Pediatrics 2001;108(3):682-5. [DOI: 10.1542/peds.108.3.682] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pickerd 2014 {published data only}
- Pickerd N, Williams EM, Watkins WJ, Kotecha S. Tidal breathing in preterm infants receiving and weaning from continuous positive airway pressure. Journal of Pediatrics 2014;164(5):1058-63.e1. [DOI: 10.1016/j.jpeds.2013.12.049] [PMID: ] [DOI] [PubMed] [Google Scholar]
Veneroni 2014 {published data only}
- Veneroni C, Zannin E, Ventura L, Colombo M, Tagliabue P, Suki B, et al. Effect of nasal continuous positive airways pressure (NCPAP) on variability in breathing pattern of preterm newborns. American Journal of Respiratory and Critical Care Medicine 2014;189:A2605. [Google Scholar]
Zhou 2020 {published data only}
- Zhou H, Hou X, Cheng R, Zhao Y, Qiu J. Effects of nasal continuous positive airway pressure on cerebral hemodynamics in preterm infants. Frontiers in Pediatrics 2020;8:487. [DOI: 10.3389/fped.2020.00487] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12618001638224 {published data only}
- ACTRN12618001638224. Extubation CPAP Level Assessment trial (ÉCLAT) [A multicentre randomised control trial comparing high (9-11 cmH2O) vs. standard (6-8 cmH2O) Continuous Positive Airway Pressures after Extubation in Extremely Preterm Infants to reduce extubation failure]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375912 (first received 25 September 2018).
Additional references
Abdel‐Hady 2008
- Abdel-Hady H, Matter M, Hammad A, El-Refaay A, Aly H. Hemodynamic changes during weaning from nasal continuous positive airway pressure. Pediatrics 2008;122(5):e1086-90. [DOI: 10.1542/peds.2008-1193] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bayley 1993
- Bayley N. Bayley Scales of Infant Development. 2nd edition. San Antonio (TX): The Psychological Corporation, 1993. [Google Scholar]
Bayley 2006
- Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio (TX): Harcourt Assessment, 2006. [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1-7. [DOI: 10.1097/00000658-197801000-00001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, 2021. Available at www.covidence.org.
Danan 2008
- Danan C, Durrmeyer X, Brochard L, Decobert F, Benani M, Dassieu G. A randomized trial of delayed extubation for the reduction of reintubation in extremely preterm infants. Pediatric Pulmonology 2008;43(2):117-24. [DOI: 10.1002/ppul.20726] [PMID: ] [DOI] [PubMed] [Google Scholar]
Davis 2003
- Davis P, Henderson-Smart DJ. Nasal continuous positive airway pressure immediately after extubation for preventing morbidity in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 2. Art. No: CD000143. [DOI: 10.1002/14651858.CD000143] [DOI] [PubMed] [Google Scholar]
De Paoli 2008
- De Paoli AG, Davis PG, Faber B, Morley CJ. Devices and pressure sources for administration of nasal continuous positive airway pressure (NCPAP) in preterm neonates. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD002977. [DOI: 10.1002/14651858.CD002977.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Vries 1992
- Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behavioural Brain Research 1992;49(1):1-6. [DOI: 10.1016/s0166-4328(05)80189-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ehrenkranz 2005
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al, National Institute of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116(6):1353-60. [DOI: 10.1542/peds.2005-0249] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fajardo 2014
- Fajardo MF, Claure N, Swaminathan S, Sattar S, Vazquez A, D'Ugard C, et al. Effect of positive end‐expiratory pressure on ductal shunting and systemic blood flow in preterm infants with patent ductus arteriosus. Neonatology 2014;105:9-13. [DOI: 10.1159/000355146] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fischer 2013
- Fischer HS, Bührer C. Avoiding endotracheal ventilation to prevent bronchopulmonary dysplasia: a meta-analysis. Pediatrics 2013;132(5):e1351-60. [DOI: 10.1542/peds.2013-1880] [PMID: ] [DOI] [PubMed] [Google Scholar]
Göpel 2011
- Göpel W, Kribs A, Ziegler A, Laux R, Hoehn T, Wieg C, et al, German Neonatal Network. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet 2011;378(9803):1627-34. [DOI: 10.1016/S0140-6736(11)60986-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 1 September 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2014. Available at gradepro.org.
Gregory 1971
- Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK. Treatment of the idiopathic respiratory-distress syndrome with continuous positive airway pressure. New England Journal of Medicine 1971;284(24):1333-40. [DOI: 10.1056/NEJM197106172842401] [PMID: ] [DOI] [PubMed] [Google Scholar]
Griffiths 1954
- Griffiths R. The Abilities of Babies: A Study in Mental Measurement. New York (NY): McGraw-Hill Book Co., Inc., 1954. [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA: on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Ho 2015
- Ho JJ, Subramaniam P, Davis PG. Continuous distending pressure for respiratory distress in preterm infants. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD002271. [DOI: 10.1002/14651858.CD002271.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICROP 2005
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991-9. [DOI: 10.1001/archopht.123.7.991] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD003311. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723-9. [DOI: 10.1164/ajrccm.163.7.2011060] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lemyre 2016
- Lemyre B, Laughon M, Bose C, Davis PG. Early nasal intermittent positive pressure ventilation (NIPPV) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No: CD005384. [DOI: 10.1002/14651858.CD005384.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lemyre 2017
- Lemyre B, Davis PG, De Paoli AG, Kirpalani H. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD003212. [DOI: 10.1002/14651858.CD003212.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 1977
- Martin RJ, Nearman HS, Katona PG, Klaus MH. Effect of a low continuous positive airway pressure on reflex control of respiration in preterm infant. Journal of Pediatrics 1977;90(6):976-81. [DOI: 10.1016/s0022-3476(77)80575-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Miller 1990
- Miller MJ, DiFiore JM, Strohl KP, Martin RJ. Effects of nasal CPAP on supraglottic and total pulmonary resistance in preterm infants. Journal of Applied Physiology 1990;68(1):141-6. [DOI: 10.1152/jappl.1990.68.1.141] [PMID: ] [DOI] [PubMed] [Google Scholar]
Milner 1977
- Milner AD, Saunders RA, Hopkin IE. Effects of continuous distending pressure on lung volumes and lung mechanics in the immediate neonatal period. Biology of the Neonate 1977;31(1-2):111-5. [DOI: 10.1159/000240950] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morley 2008
- Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB, COIN Trial Investigators. Nasal CPAP or intubation at birth for very preterm infants. New England Journal of Medicine 2008;358(7):700-8. [DOI: 10.1056/NEJMoa072788] [PMID: ] [DOI] [PubMed] [Google Scholar]
Muller 1979
- Muller NL, Bryan AC. Chest wall mechanics and respiratory muscles in infants. Pediatric Clinics of North America 1979;26(3):503-16. [DOI: 10.1016/s0031-3955(16)33745-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT04372953
- NCT04372953. Positive End-Expiratory Pressure (PEEP) Levels During Resuscitation of Preterm Infants at Birth (The POLAR Trial). clinicaltrials.gov/ct2/show/NCT04372953 (first received 4 May 2020).
NIH 1979
- National Institutes of Health. Report of Workshop on Bronchopulmonary Dysplasia. In: NIH Publication No. 80-1660. Washington, DC, 1979.
Palisano 1997
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental Medicine and Child Neurology 1997;39(4):214-23. [DOI: 10.1111/j.1469-8749.1997.tb07414.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529-34. [DOI: 10.1016/s0022-3476(78)80282-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Patel 2015
- Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Causes and timing of death in extremely premature infants from 2000 through 2011. New England Journal of Medicine 2015;372(4):331-40. [DOI: 10.1056/NEJMoa1403489] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2019 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019. Available at revman.cochrane.org.
Schmölzer 2013
- Schmölzer GM, Kumar M, Pichler G, Aziz K, O'Reilly M, Cheung PY. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ 2013;347:f5980. [DOI: 10.1136/bmj.f5980] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shennan 1988
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82(4):527-32. [PMID: ] [PubMed] [Google Scholar]
SUPPORT 2010
- SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network, Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, et al. Early CPAP versus surfactant in extremely preterm infants. New England Journal of Medicine 2010;362(21):1970-9. Erratum in N Engl J Med. 2010 Jun 10;362(23):2235. [DOI: 10.1056/NEJMoa0911783] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ueda 1994
- Ueda T, Ikegami M, Jobe AH. Developmental changes of sheep surfactant: in vivo function and in vitro subtype conversion. Journal of Applied Physiology 1994;76(6):2701-6. [DOI: 10.1152/jappl.1994.76.6.2701] [PMID: ] [DOI] [PubMed] [Google Scholar]
Waitz 2020
- Waitz M, Engel C, Schloesser R, Rochwalsky U, Meyer S, Zemlin M, et al. Application of two different nasal CPAP levels for the treatment of respiratory distress syndrome in preterm infants —“The OPTTIMMAL-Trial”—Optimizing PEEP To The IMMAture Lungs: study protocol of a randomized controlled trial. Trials 2020;21:822. [DOI: 10.1186/s13063-020-04660-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2004
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al, National Institute of Child Health and Human Development Neonatal Research Network. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114:1305-11. [DOI: 10.1542/peds.2004-0204] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wheeler 2011
- Wheeler KI, Klingenberg C, Morley CJ, Davis PG. Volume-targeted versus pressure-limited ventilation for preterm infants: a systematic review and meta-analysis. Neonatology 2011;100(3):219-27. [DOI: 10.1159/000326080] [PMID: ] [DOI] [PubMed] [Google Scholar]
World Bank 2021
- World Bank. World Bank Country and Lending Groups. datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed 4 May 2021).
Wu 2014
- Wu R, Li SB, Tian ZF, Li N, Zheng GF, Zhao YX, et al. Lung recruitment maneuver during proportional assist ventilation of preterm infants with acute respiratory distress syndrome. Journal of Perinatology 2014;34:5. [DOI: 10.1038/jp.2014.53] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bamat 2017
- Bamat N, Fierro J, Wright CJ, Millar D, Kirpalani H. Nasal continuous positive airway pressure levels for the prevention of morbidity and mortality in very low birth weight infants. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD012778. [DOI: 10.1002/14651858.CD012778] [DOI] [PMC free article] [PubMed] [Google Scholar]


