Abstract
Objective:
To determine post-discharge patient-reported symptoms before and after implementation of restrictive opioid prescribing among women undergoing minimally invasive gynecologic surgery.
Methods:
We compared clinical outcomes and symptom burden among a cohort of 389 women undergoing minimally invasive gynecologic surgery at a single institution before and after implementation of a restrictive opioid prescribing quality improvement initiative in July 2018. Post-discharge symptom burdens were collected up to 42 days after discharge using the MD Anderson Symptom Inventory and analyzed using linear mixed effects models.
Results:
The majority of women included in this study were white non-smokers and the median age was 55 (range 23 – 83). Most women underwent hysterectomy (64%), had surgery for malignancy (71%), and were discharged from the hospital on the day of surgery (65%). Women in the restrictive opioid prescribing group had a median reduction in morphine equivalent dose prescribed at discharge of 83%, corresponding to a median reduction in 25 tablets of 5 mg oxycodone per person. There was no difference between opioid prescribing groups in either the rate of refill requests (P=1) or hospital re-admission (P=1) up to 30 days after discharge. After adjustment for covariates, there was no statistically significant difference in post-discharge symptom burden including patient-reported pain (P=.08), sleep (P=.30), walking interference (P=.64), activity interference (P=.12), or affective interference (P=.67). There was a trend toward less reported constiptation in the restrictive opioid prescribing group that did not reach statistical significance (P=.05).
Conclusion:
We found that restrictive post-operative opioid prescribing was not associated with differences in longitudinal symptom burden among women undergoing minimally invasive gynecologic surgery. These results provide the most comprehensive picture to date of post-operative symptom recovery under different opioid prescribing approaches, lending additional support for existing recommendations to reduce opioid prescribing following gynecologic surgery.
Precis:
Restrictive opioid prescribing after minimally invasive gynecologic surgery does not increase post-operative patient reported symptom burden.
Introduction
Premature mortality has increased in the United States over the last two decades, driven in large part by rising rates of accidental death from fatal drug overdoses.1 This mortality trend reflects a substantial contribution from prescription opioid overdose deaths, which have increased four-fold between 1999 and 2017 in tandem with even larger increases in overdose deaths related to illicit synthetic opioids such as fentanyl.2 The public health menace of prescription opioids is linked to physician prescribing patterns, as per capita daily consumption of prescription opioids in the United States is nearly double that of Canada and western Europe.3 Pain control after elective surgery is a common indication for new opioid prescriptions and thus post-operative pain management strategies have acquired urgent public health ramifications.
Both the rate of new opioid prescriptions as well as the average total morphine equivalent dose prescribed have increased between 2004 and 2014 among patients undergoing common general and gynecologic surgical procedures.4,5 Across surgical disciplines there is evidence that discharge opioid prescriptions are often in excess of what is needed for post-operative pain control, thus increasing the risk of opioid oversupply, addiction, and diversion.6–9 Indeed, epidemiologic data shows that rates of new persistent opioid after elective surgery are approximately 6%,10 and may be even higher in the population of patients undergoing surgery for the treatment of cancer.11 If considered as a post-operative complication, new persistent opioid use after surgery constitutes a common, hidden, and generally un-addressed source of serious post-operative morbidity.
Enhanced recovery after surgery is a multimodality approach to perioperative care that focuses on stress reduction, achievement of adequate pain control, return of bowel function, and early ambulation.12,13 Implementation of enhanced recovery after surgery has successfully reduced inpatient opioid usage among women undergoing open14 and minimally invasive gynecologic surgery in an enhanced recovery after surgery program.15 We therefore hypothesized that a restrictive opioid prescribing protocol could be used to safely reduce post-discharge opioid prescribing after minimally invasive gynecologic surgery in an enhanced recovery after surgery program. Our objective was to evaluate the safety and tolerability of an evidence-based restrictive opioid prescribing protocol following minimally invasive gynecologic surgery in an enhanced recovery after surgery program, using longitudinal post-discharge patient-reported outcomes.
Methods
Beginning on July 1, 2018 our department implemented a quality improvement initiative to reduce ongoing opioid prescriptions by at least 50% among patients undergoing minimally invasive gynecologic surgery within our established minimally invasive gynecologic surgery enhanced recovery after surgery program.16 At our institution all patients undergoing minimally invasive gynecologic surgery in our enhanced recovery after surgery program routinely receive discharge prescriptions for 800 mg ibuprofen three times daily with meals and 1 g acetaminophen every 6 hours as needed, unless these medications are otherwise contraindicated. Prior to this quality improvement initiative our group had no standardized approach to opioid prescribing after minimally invasive gynecologic surgery and historically most patients were prescribed between fifteen and thirty 5 mg oxycodone tablets, consistent with published approaches to opioid prescription after minimally invasive surgery.5,17
This quality improvement initiative specified that all minimally invasive surgery patients discharged on the day of surgery were to be prescribed five 5 mg oxycodone tablets. For patients admitted overnight, surgeons had discretion to prescribe fifteen 5 mg oxycodone tablets if pain control was deemed to be a contributing factor to the need for overnight admission. Patients were excluded from this initiative if they were admitted for more than two days or were chronic opioid users (defined as the use of a long-acting opioid at the time of the pre-operative visit). The development of this reduced opioid prescribing protocol also involved the creation of educational materials describing responsible opioid use and disposal, which were provided to patients at their pre-operative visit and reviewed again at the time of hospital discharge.
For the purpose of analyzing clinical outcomes, opioid refill rates, and patient reported outcomes, we identified a reduced opioid prescribing group comprised of all eligible patients who underwent minimally invasive surgery between August 1, 2018 and June 30, 2019. A historical group pre-restrictive opioid prescribing was also identified, which included all patients who underwent minimally invasive surgery between February 1, 2017 and December 31, 2017, a time period during which patient reported outcomes were previously collected.
Study data were collected and managed using Research Electronic Data Capture electronic data capture tools18 hosted at MD Anderson as part of an institutionally approved quality improvement study (protocol QI-6033). The analysis of clinical and demographic data related to this quality improvement project was approved by the institutional review board (protocol PA19–0642). Perioperative patient-reported symptom burden was collected on where patients gave written informed consent, as has been previously described.14 All patient reported outcomes for this study were collected under a separate institutional review board-approved protocol (BS99-094). Only patients who provided written, informed consent for patient reported outcome collection were included.
Perioperative patient-reported symptom burden for the pre-restrictive opioid prescribing group was evaluated with the MD Anderson Symptom Inventory-Ovarian Cancer module, a 27-item validated tool with two additional questions on diarrhea and heartburn.19 For each symptom component, individuals were asked to rank symptom severity during the previous 24 hours on a scale of 0–10, with 0 being “not present” and 10 being “as bad as you can imagine.” Symptom interference was also assessed on a 0–10 scale, with 0 being “did not interfere” and 10 being “interfered completely. Patients in the restrictive opioid prescribing group completed MD Anderson Symptom Inventory-Ovarian Cancer-PeriOP-GYN a recently validated tool for patients undergoing surgery for gynecologic cancer or benign conditions.20 The patient reported outcome questions in the MD Anderson Symptom Inventory-Ovarian Cancer module and MD Anderson Symptom Inventory-Ovarian Cancer-PeriOP-GYN that were utilized for this analysis are identical in both versions. The MD Anderson Symptom Inventory-Ovarian Cancer and MD Anderson Symptom Inventory-Ovarian Cancer-PeriOP-GYN were administered preoperatively, daily first week after surgery and weekly up to 42 days postoperatively.
Clinical outcomes and opioid refill rates were analyzed for all included patients in the pre-restrictive opioid prescribing and restrictive opioid prescribing groups. For patient reported outcome analysis, patients had to complete at least three assessments, which had to include the baseline preoperative MD Anderson Symptom Inventory with at least two subsequent assessments.
Clinical and demographic information collected from the medical record included age, body mass index (calculated as weight (kg)/[height (m)]2), ethnicity, race, tumor type (primary or recurrent disease and designation as malignant, benign, tumor of uncertain malignant potential) as well as surgical indication. Ovarian, fallopian tube, and primary peritoneal cancer were combined for the purpose of analysis. Other sites were cervix, uterine (non-sarcoma), and uterine sarcoma. American Society of Anesthesiologists classification of physical health and Charlson Comorbidity Index21 were used to assess comorbidities. The current malignancy was not included in the calculation of the Charlson Comorbidity Index to more accurately reflect other non-cancer comorbidities. The MD Anderson Symptom Inventory-Ovarian Cancer19 was administered as baseline during pre-operative visit, daily post-operatively for 7 days, and then weekly for up to 42 days after discharge. The MD Anderson Symptom Inventory-Ovarian Cancer was administered on paper, by phone, or electronically by email link.
Descriptive statistics were used to analyze the clinical and demographic characteristics of the study cohorts. Categorical variables were compared between the cohorts using a Fisher exact test or chi squared test, as appropriate. Two-sided t tests were used to compare symptom severity and symptom interference scores at the time of hospital discharge. Linear mixed-effects modeling was used to examine the longitudinal change of symptom burden from pain, drowsiness, constipation, appetite, activity, and walking after discharge. Models were adjusted for clinical and demographic characteristics including age, race, body mass index, American Society of Anesthesiologists classification, length of hospital stay, marital status, Charlson Comorbidity Index, education level, and Eastern Cooperative Oncology Group performance status. Statistical analyses were performed using R 3.5.1 for Mac OS X.22 The Revised Standards for Quality Improvement Reporting Excellence (also known as SQUIRE 2.0) guidelines were used in the planning of the quality improvement initiative and the preparation of this manuscript.23
This study was a retrospective analysis of a convenience sample. However, the rate of opioid refill requests was a key metric monitored as part of the quality improvement initiative. Based on this, we estimated that a sample size of 200 patients in each comparator group would be required to have 80% power to detect a change from 5% to 12% opioid refill request rate, using a one-sided Fisher exact test.
Results
A total of 357 patients underwent minimally invasive surgery with a gynecologic oncologist at our institution between August 2018 and June 2019, following implementation of the restrictive opioid prescribing. Of these, 192 patients provided written informed consent for participation in patient reported outcome collection and were included in the current study. Three patients were then excluded from analysis due to a history of chronic opioid use prior to surgery and 10 were excluded due to a length of stay >2 days, resulting in a final restrictive opioid prescribing group comprised of 179 patients. During a previous period of patient reported outcome collection between February 2017 and December 2017 prior to implementation of the restrictive opioid prescribing, 344 patients underwent minimally invasive surgery with a gynecologic oncologist and of these 221 provided written informed consent for patient reported outcome collection. One patient was excluded from analysis due to a history of chronic opioid use prior to surgery and 10 were excluded due to a length of stay >2 days leaving a final pre-restrictive opioid prescribing group comprised of 210 patients (Figure 1).
Figure 1.
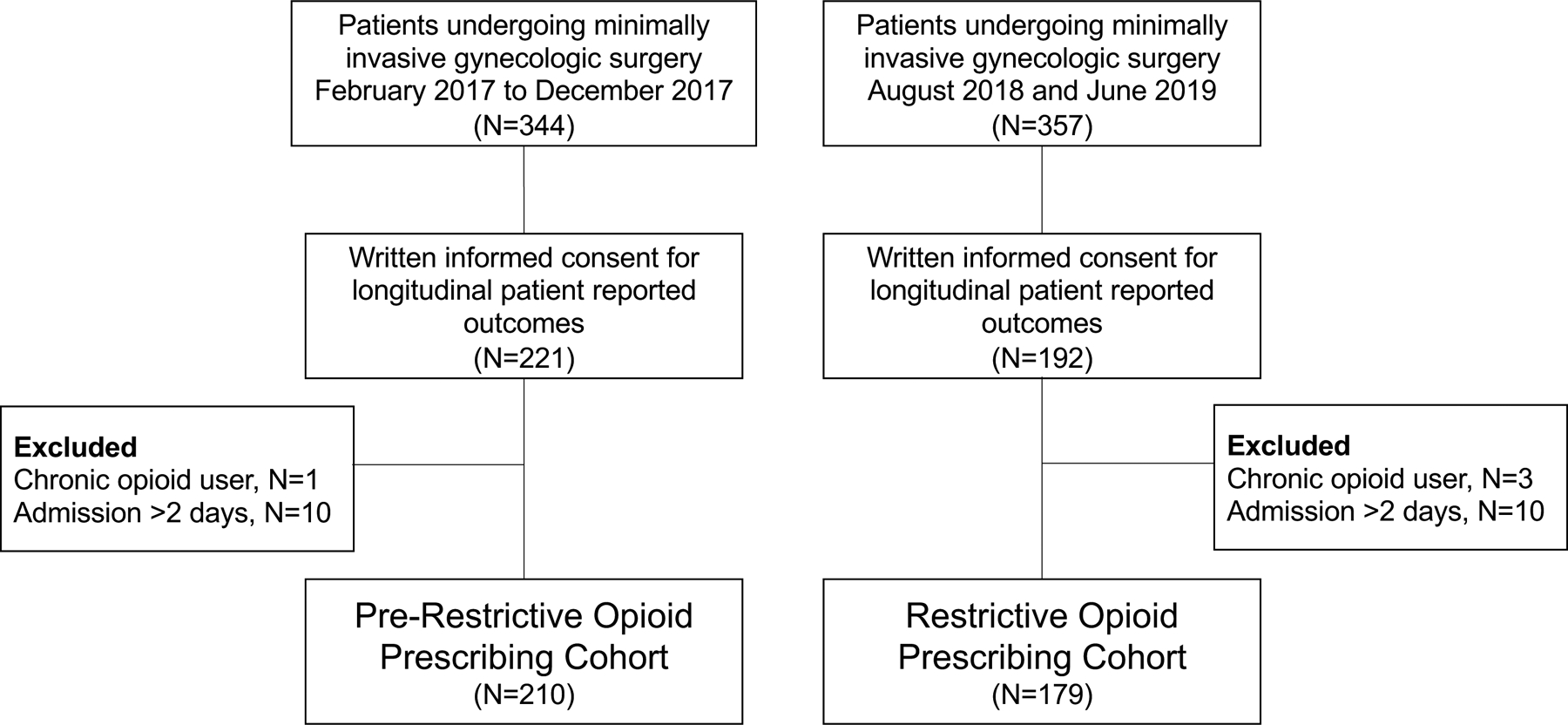
Overview of study groups.
Clinical and demographic characteristics of this cohort are summarized in Table 1 by opioid prescribing group. Overall clinical and demographic variables were similar between the pre-restrictive opioid prescribing and restrictive opioid prescribing groups. Specifically, the median age (pre-restrictive opioid prescribing: 55 years, range 24–80 years, restrictive opioid prescribing: 54 years, interquartile range 23–83 years; P=.57), rate of hysterectomy (pre-restrictive opioid prescribing 66.7% vs restrictive opioid prescribing 60.9%, P=.25) or rate of same-day discharge (pre-restrictive opioid prescribing 62.4% vs restrictive opioid prescribing 67.0%, P=.40) did not differ between groups. The proportion of hysterectomies that were classified as radical hysterectomies was significantly different between the cohorts (pre-restrictive opioid prescribing 8.6% vs restrictive opioid prescribing 0.9%, P=0.008) likely due to emerging data about the use of minimally invasive radical hysterectomy for the treatment of cervical cancer. Other aspects of surgical complexity including the performance of omentectomy, lymphadenectomy, bowel procedures, or genitourinary tract procedures did not differ between the cohorts (P>0.05 for all comparisons). Surgeon compliance with this quality improvement initiative was high, with 103 of 120 restrictive opioid prescribing patients (85.8%) discharged on the day of surgery received the per protocol amount of prescription opioids (five tablets of 5 mg oxycodone). For patients admitted overnight after minimally invasive surgery, surgeons were given discretion to prescribe additional opioids (fifteen tablets of 5 mg oxycodone) if pain control was felt to be a contributing factor to the need for an extended Post-operative stay. A total of 23 of 59 restrictive opioid prescribing patients (39.0%) admitted overnight were prescribed additional opioids.
Table 1.
Demographic, lifestyle, and clinical characteristics by opioid prescribing group.
| Pre-Restrictive Opioid Prescribing (n=210) | Restrictive Opioid Prescribing (n=179) | P | |
|---|---|---|---|
| Median age, y (range) | 55 (24 – 80) | 54 (23 – 83) | .57 |
| Body mass index (kg/m2), n (%) | |||
| Less than 25 | 54 (25.7) | 42 (23.5) | .03 |
| 25–39.9 | 129 (61.4) | 96 (53.6) | |
| 40 or greater | 27 (12.9) | 41 (22.9) | |
| Ethnicity, n (%)# | |||
| Not Hispanic or Latina | 175 (83.3) | 144 (80.4) | .43 |
| Hispanic or Latina | 30 (14.3) | 32 (17.9) | |
| Not reported | 5 (2.4) | 3 (1.7) | |
| Race, n (%)# | |||
| White or Caucasian | 147 (70.0) | 129 (72.1) | .20 |
| Black or African American | 27 (12.9) | 13 (7.3) | |
| Asian | 9 (4.3) | 13 (7.3) | |
| Other | 25 (11.9) | 19 (10.6) | |
| Not reported | 2 (1.0) | 5 (2.8) | |
| Marital status, n (%)# | |||
| Single | 28 (13.3) | 21 (11.7) | .18 |
| Married or Significant Other | 139 (66.2) | 132 (73.7) | |
| Separated/Divorced/Widowed | 42 (20.0) | 24 (13.4) | |
| Not reported | 1 (0.5) | 2 (1.1) | |
| Smoking status, n (%) | |||
| Never smoker | 140 (66.7) | 124 (69.3) | .78 |
| Former smoker | 64 (30.5) | 49 (27.4) | |
| Current smoker | 6 (2.9) | 6 (3.4) | |
| Charlson Comorbidity Index, n (%) | |||
| 0 | 62 (30.0) | 54 (30.2) | .16 |
| 1–2 | 95 (45.2) | 66 (36.9) | |
| 3 or greater | 53 (25.2) | 59 (33.0) | |
| American Society of Anesthesiologists classification, n (%) | |||
| I-II | 43 (20.5) | 20 (11.2) | .01 |
| III-IV | 167 (79.5) | 159 (88.8) | |
| Hysterectomy performed, n (%) | 140 (66.7) | 109 (60.9) | .25 |
| Surgical indication, n (%)* | |||
| Benign | 68 (31.9) | 44 (23.9) | <.001 |
| Cervical cancer† | 21 (9.9) | 2 (1.1) | |
| Ovarian, fallopian tube, primary peritoneal cancer | 10 (4.7) | 15 (8.2) | |
| Uterine cancer (non-sarcoma) | 64 (30.0) | 76 (41.3) | |
| Uterine sarcoma | 3 (1.4) | 4 (2.2) | |
| Other | 47 (22.1) | 43 (23.4) | |
| Same-day discharge, n (%) | 131 (62.4) | 120 (67.0) | .40 |
| Robotic-assisted approach, n (%) | 42 (20.0) | 26 (14.5) | .18 |
| Median morphine equivalent dose at discharge, mg (range) | 225 (0–520) | 37.5 (0–225) | <.001 |
Patients may have had more than one indication for surgery.
Statistical tests were performed after excluding “Not reported” category.
Due to emerging data, minimally invasive surgery was no longer performed for cervical cancer after October 2018.
Overall significantly fewer opioids were prescribed to patients in the restrictive opioid prescribing group (median 37.5 mg morphine equivalent dosage; range 0 – 225 mg) compared to the pre-restrictive opioid prescribing group (median 225 mg morphine equivalent dosage; range 0 – 520 mg; P<.001). Significantly less opioids were administered during surgery in the restrictive opioid prescribing group (median 37.5 mg morphine equivalent dosage; range 12.5 – 105 mg) compared to the pre-restrictive opioid prescribing group (median 50 mg morphine equivalent dosage; range 10 – 125 mg; P<.001). There was no significant difference in the use of local anesthetic wound infiltration between patients in the restrictive opioid prescribing group (96.6%) compared to the pre-restrictive opioid prescribing group (93.8%; P=.24). Similarly, there was no difference in the administration of opioids in the post-anesthesia recovery unit between patients in the restrictive opioid prescribing group (median 5 mg morphine equivalent dosage; range 0 – 50 mg) compared to the pre-restrictive opioid prescribing group (median 5 mg morphine equivalent dosage; range 0 – 60 mg; P=.58). Opioid refill requests made within 30 days of surgery were closely tracked following implementation of the restrictive opioid prescribing as part of the institutionally approved quality improvement initiative. Refill requests for the pre-restrictive opioid prescribing group were identified based on review of the medical record and electronic prescriptions. Overall 5.9% of patients requested an opioid refill within 30 days of surgery and this rate did not differ between pre-restrictive opioid prescribing (13/210 [6.2%]) and restrictive opioid prescribing (10/179 [5.6%], P=1) groups. The rate of hospital re-admission within 30 days also did not differ between cohorts (pre-restrictive opioid prescribing 8/210 [3.8%] vs restrictive opioid prescribing 4/179 [2.2%], P=1). In the restrictive opioid prescribing groups, the rate of opioid refill requests did not differ by length of stay (P=1) or the amount of oxycodone tablets prescribed at discharge (P=1).
Longitudinal assessments of patient-reported outcomes were analyzed after hospital discharge for both groups. On the day of hospital discharge, mean symptom severity did not differ between pre-restrictive opioid prescribing and restrictive opioid prescribing groups for any symptom including pain (5.67 ± 2.66 standard deviation vs 5.43 ± 2.78 standard deviation; P=.44), fatigue (5.06 ± 2.77 standard deviation vs 5.01 ± 2.89 standard deviation; P=.86), disturbed sleep (3.32 ± 3.06 standard deviation vs 2.99 ± 3.09 standard deviation; P=.34), or constipation (2.87 ± 3.32 standard deviation vs 2.39 ± 3.09 standard deviation; P=.18). Patient reported interference includes an affective (relations with others, enjoyment of life, and mood) and a physical activity component (walking, general activity, and work). The mean affective interference component at discharge did not differ between the pre-restrictive opioid prescribing group (2.25 ± 2.33 standard deviation) compared to the restrictive opioid prescribing group (2.10 ± 2.35 standard deviation; P=.57). The mean physical activity interference component at discharge was significantly higher in the pre-restrictive opioid prescribing group (5.46 ± 2.77 standard deviation) compared to the restrictive opioid prescribing group (4.77 ± 2.77 standard deviation; P=.03), although this fell below the half a standard deviation threshold conventionally used as a clinical minimally important difference for the MD Anderson Symptom Inventory.24
We next examined longitudinal change in symptom severity and interference out to 42 days after hospital discharge. For all symptoms, most patients had recovered to near baseline by 7 days after discharge so statistical comparisons were performed on this time period. After adjustment for clinical and demographic variables using mixed linear models, longitudinal pain intensity out to 7 days post-hospital discharge was not significantly different between the pre-restrictive opioid prescribing and restrictive opioid prescribing groups (P=.8; Figure 2A). Patient reported pain out to 42 days post-hospital discharge is shown in Figure 2B. There was no longitudinal difference between the pre-restrictive opioid prescribing and restrictive opioid prescribing groups in longitudinal patient reported fatigue (P=.43; Figure 3A), constipation (P=.05; Figure 3B), sleep (P=.30; Figure 3C), interference with walking (P=.64; Figure 3D), affective interference (P=.67; Figure 3E), or physical activity interference (P=.12; Figure 3F) between pre-restrictive opioid prescribing and restrictive opioid prescribing groups.
Figure 2.
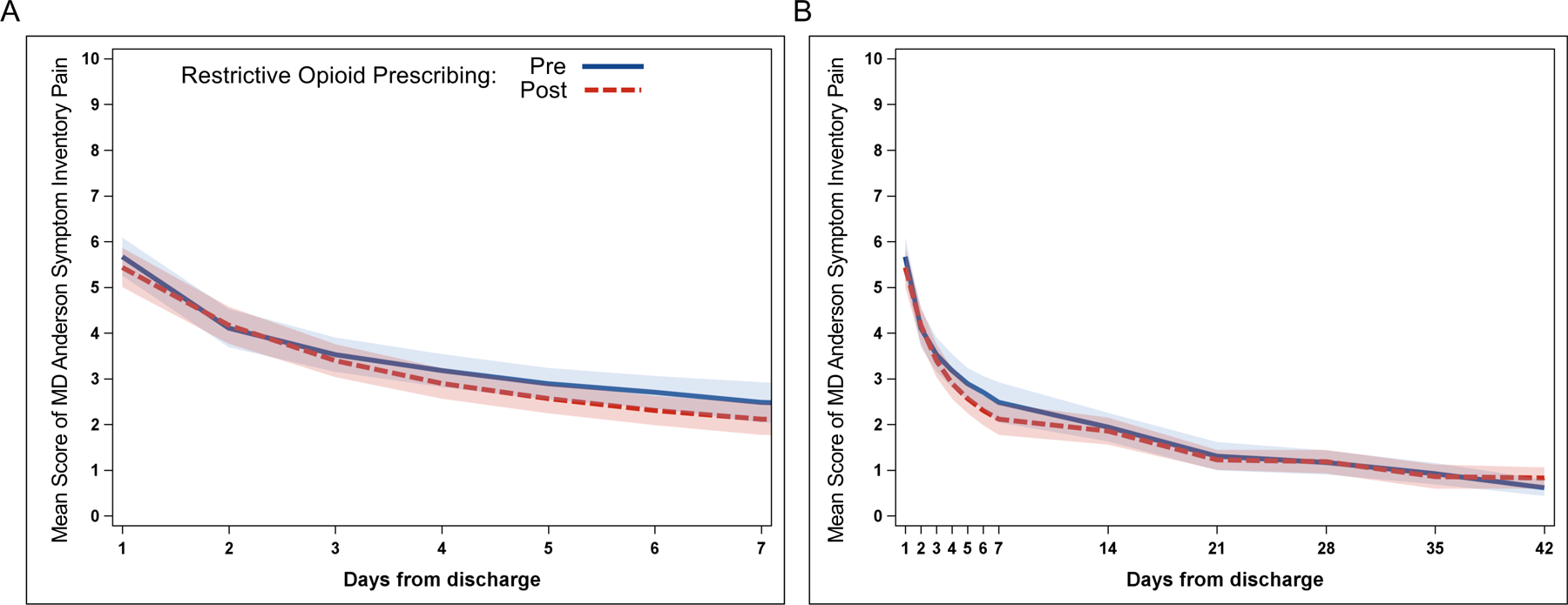
Longitudinal patient reported pain following minimally invasive gynecologic surgery. Mean longitudinal patient reported pain out to (A) 7 days and (B) 42 days for pre-restrictive opioid prescribing (blue solid) and post-restrictive opioid prescribing (red dotted) cohorts. Shading represents 95% confidence interval.
Figure 3.
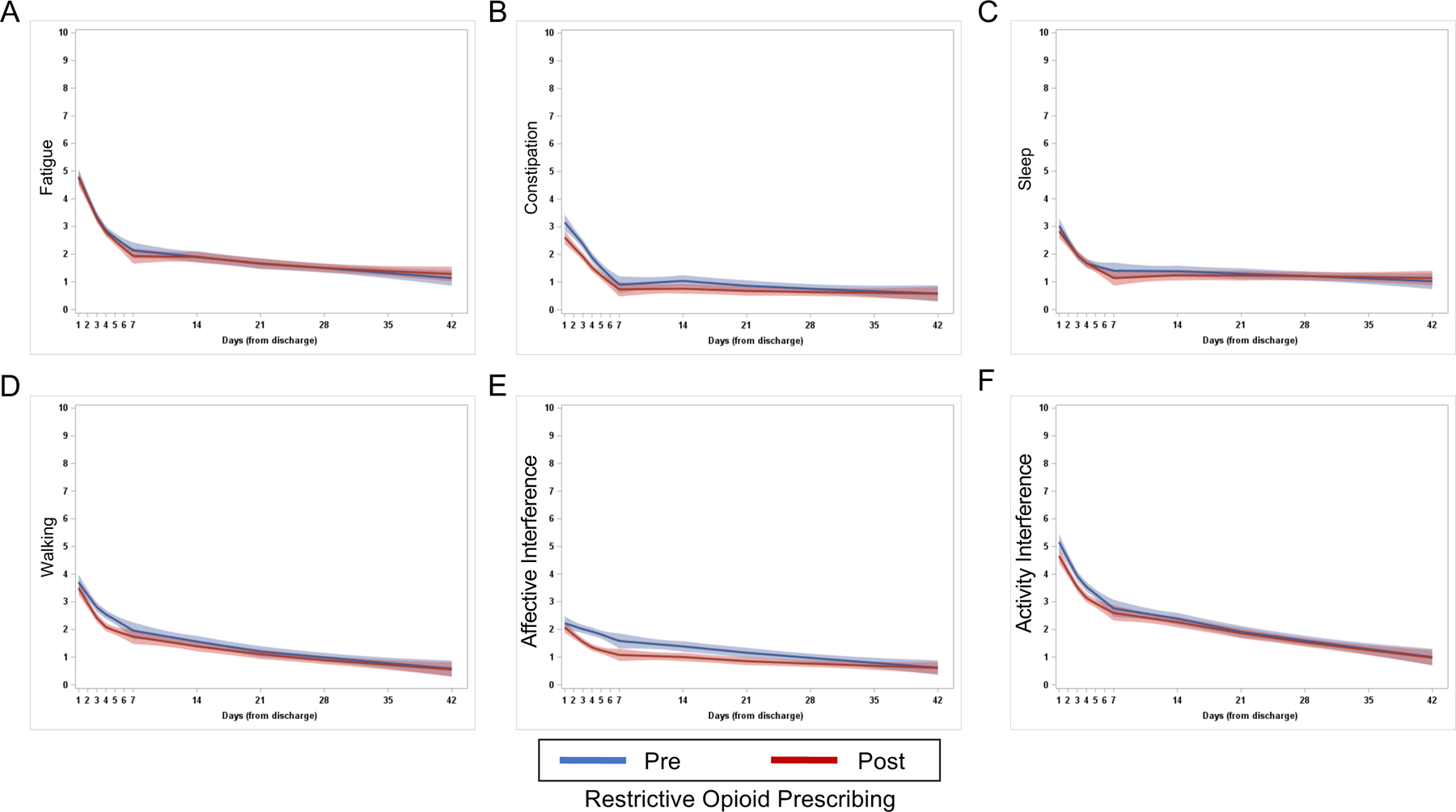
Longitudinal patient reported outcomes following minimally invasive gynecologic surgery. Mean longitudinal patient reported (A) fatigue, (B) constipation, (C) sleep, (D) interference with walking, (E) affective interference, and (F) activity interference out to 42 days post-discharge for pre-restrictive opioid prescribing (blue) and post-restrictive opioid prescribing (red) cohorts. Shading represents 95% confidence interval.
Discussion
The purpose of this study was to use detailed longitudinal patient-reported symptoms collected using a validated survey instrument to determine whether post-operative symptom burden and functional/affective interference are significantly affected by reduced opioid prescribing after minimally invasive surgery. We found that reduced opioid prescribing did not affect reported symptom burden up to 42 days after discharge, including symptoms such as pain, fatigue, constipation, or interference with sleep or walking. We did not find any difference in rate of opioid refill requests or 30-day re-admission rate among women who were prescribed a reduced amount of opioid pain medication after minimally invasive surgery.
These data confirm results from obstetrics/gynecology and other surgical disciplines showing that reducing post-operative opioid prescriptions does not affect broad metrics of quality care such as post-operative pain scores, opioid refill requests, the rate of 30-day re-admission.25–28 However, pain scores and serious complications requiring re-hospitalization are not the only adverse outcomes that could potentially be affected by inadequate pain control due to restrictive opioid prescribing. In fact, it is conceivable that pain scores reported by patients prescribed reduced amounts of opioid prescribing could be falsely reassuring if they in reality reflect compensatory reductions in patient ambulation or overall activity level. Our study provide important reassurance that additional, more nuanced aspects of the post-operative recovery patient experience are not adversely affected by a reduction in the amount of opioid pain medicine prescribed at hospital discharge.
In addition to short-term side effects and opioid toxicity, peri-operative opioid prescription can lead to rates of new, persistent opioid usage as high as 10% among patients undergoing surgical procedures.10,11 Hysterectomy with or without removal of the adnexa is among the most common surgical procedure performed on women in the United States, with approximately 100,000–200,000 minimally invasive hysterectomies being performed in this country every year.29,30 Minimally invasive surgery thus represents a substantial contribution to the national harms associated with post-operative opioid over-prescribing. Although the short- and long-term harms of opioid use and addiction are well documented, practicing surgeons require reassurance that post-operative opioid prescribing can safely be curtailed without adversely affecting patient experience.
The opioid prescribing protocol we introduced is simple in concept and was implemented among approximately two dozen minimally invasive surgeons at our institution with a high compliance rate. Not all patients who suffer from post-discharge pain may request opioid refills or require hospital re-admission. In addition, pain can interfere with other aspects of post-operative recovery and return to normal function. For these reasons, the comprehensive symptom burden data reported in the current study represent a detailed and holistic view of post-operative recovery under different opioid prescribing regimens. Interestingly, we found that patients in the restrictive opioid prescribing cohort were administered significantly less intra-operative opioids. On the whole, less intra operative opioid usage could be envisaged to result in a compensatory increase in opioid pill usage after discharge. If anything, this observation serves to strengthen our overall conclusion that room exists to safely reduce opioid prescribing after minimally invasive gynecologic surgery, even in a cohort given less intra operative opioids.
Studies from multiple surgical fields now indicate that historical post-operative opioid prescribing practices were often excessive and can lead to increased rates of opioid usage and addiction among surgical patients.27,31–33 These reports have spurred a broad effort to implement reduced opioid prescribing protocols across surgical disciplines. Successful implementation of such protocols has led to significant reductions in opioid prescribing for patients undergoing minimally invasive cholecystectomy27 as well as patients undergoing a wide range of open and minimally invasive gynecologic surgery.25,26 Although broad safety metrics such as refill requests and hospital re-admission do not appear to be affected by reduced opioid prescribing, to date there has been no systematic effort to assess the effect of restrictive post-operative opioid prescribing on patient symptom burden.
The current study provides important novel comparative information about symptom severity and interference under pre-restrictive opioid prescribing and restrictive opioid prescribing approaches. We did observe a non-significant trend towards reduced post-operative constipation reported by patients in the restrictive opioid prescribing group, an observation which may be related to reduced utilization of opioid pain medications in this group. However, this remains a hypothesis as we did not track actual opioid usage after hospital discharge in this study.
Further research efforts will examine whether patient reported symptoms are similarly unaffected by restrictive opioid prescribing following open gynecologic surgery, for which baseline pain control needs and post-operative opioid use would likely be higher. Prior work from our group and others have demonstrated that it may be possible to tailor opioid prescribing based on inpatient usage prior to hospital discharge.16,34,35 By tailoring opioid prescribing based on patient characteristics or inpatient usage, it may even be possible to identify a subset of patients who may not require any post-operative opioid prescriptions. Comprehensive data collection regarding multiple facets of patient symptom burden will be essential to assessing the safety and feasibility of further reductions in opioid prescribing.
This study has several strengths, including the relatively large size of the comparator groups and a broad range of benign and malignant minimally invasive surgery surgical indications. These strengths tend to increase the generalizability of these results, and it is likely that these conclusions would apply to similar patient populations undergoing non-gynecologic minimally invasive surgery. Another important strength of the current study is that patient symptoms were collected using a validated survey instrument that has been specifically adapted for use among gynecologic surgery patients.19,20
Limitations of this study include the single-institution design and the discontinuity between data collection for the pre-restrictive opioid prescribing and restrictive opioid prescribing groups, which increases the risk of changes in clinical practice over time contributing to measured and un-measured heterogeneity between the opioid prescribing groups. The temporal discontinuity between the groups occurred because patient symptoms were not continuously collected on minimally invasive surgery patients during the period between the two groups. It is also important to note that this restrictive opioid prescribing protocol was implemented as part of an existing peri-operative enhanced recovery after surgery framework. Multi-modal pain control as part of enhanced recovery after surgery is related to improvement in key patient symptoms such as pain and total interference with functioning while patients remain hospitalized14, and it is possible that these effects persist following hospital discharge. Therefore, these results may not be applicable to practice environments which have not yet implemented enhanced recovery after surgery protocols. Only patients provided written informed consent for longitudinal symptom collection were included in this analysis, and we cannot formally exclude the possibility that clinical or patient-reported outcomes may differ between the groups as a whole and those patients provided consent for study.
The provision of safe and high-quality surgical care includes striking an appropriate balance between post-operative analgesia and mitigation of the known harms of opioid pain medications. Based on these data we recommend that most women undergoing minimally invasive surgery be prescribed 5 tablets of 5 mg oxycodone (or equivalent) at discharge, along with non-opioid pain medications including nonsteroidal anti-inflammatory drugs and acetaminophen. Provision of excess opioid pain medication does not improve patient symptom burden and is associated with potential harms to both the individual and their community.
Highlights:
Post-operative restrictive opioid prescribing can be safely implemented without increasing adverse outcomes.
Longitudinal patient-reported pain for many weeks after surgery is not affected by restrictive opioid prescribing.
No difference in other aspects of symptom burden after surgery were observed.
Financial Support:
R. Tyler Hillman is supported by a CPRIT Scholars in Cancer Research grant RR200045. Larissa A. Meyer is supported by a NIH-NCIK07-CA201013 grant. This work was in part supported through a NIH-NCI P30 CA016672 core grant (Biostatistics Resource Group and Clinical Trials Support Resource). Funding sources had no role in study design or in the collection, analysis and interpretation of data.
Conflict of Interest:
Dr. Williams reports grants from AstraZeneca, Astellas, Bayer, Bristol Meyers Squibb, Genentech, Eli Lily, and Merck, all outside the submitted work. Dr Mena reports grants from Pacira Pharmaceuticals, outside of the submitted work.
References
- 1.Shiels MS, Chernyavskiy P, Anderson WF, et al. Trends in premature mortality in the USA by sex, race, and ethnicity from 1999 to 2014: an analysis of death certificate data. Lancet. 2017;389(10073):1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedegaard H, Warner M, Miniño AM. Drug Overdose Deaths in the United States, 1999–2016. NCHS Data Brief. 2017;(294):1–8. [PubMed] [Google Scholar]
- 3.Berterame S, Erthal J, Thomas J, et al. Use of and barriers to access to opioid analgesics: A worldwide, regional, and national study. Lancet. 2016;387(10028):1644–1656. [DOI] [PubMed] [Google Scholar]
- 4.Wunsch H, Wijeysundera DN, Passarella MA, Neuman MD. Opioids Prescribed After Low-Risk Surgical Procedures in the United States, 2004–2012. JAMA. 2016;315(15):1654–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson JC, Komesu YM, Qeadan F, et al. Trends in patient procurement of postoperative opioids and route of hysterectomy in the United States from 2004 through 2014. Am J Obstet Gynecol. 2018;219(5):484.e1–484.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiels CA, Anderson SS, Ubl DS, et al. Wide Variation and Overprescription of Opioids After Elective Surgery. Ann Surg. 2017;266(4):564–573. [DOI] [PubMed] [Google Scholar]
- 7.Bicket MC, White E, Pronovost PJ, Wu CL, Yaster M, Alexander GC. Opioid Oversupply After Joint and Spine Surgery: A Prospective Cohort Study. Anesth Analg. 2019;128(2):358–364. [DOI] [PubMed] [Google Scholar]
- 8.Fujii MH, Hodges AC, Russell RL, et al. Post-Discharge Opioid Prescribing and Use after Common Surgical Procedure. J Am Coll Surg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bateman BT, Cole NM, Maeda A, et al. Patterns of Opioid Prescription and Use After Cesarean Delivery. Obstet Gynecol. 2017;130(1):29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in us adults. JAMA Surg. 2017;152(6):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JSJ, Hu HM, Edelman AL, et al. New persistent opioid use Among patients with cancer after curative-intent surgery. J Clin Oncol. 2017;35(36):4042–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miralpeix E, Nick AM, Meyer LA, et al. A call for new standard of care in perioperative gynecologic oncology practice: Impact of enhanced recovery after surgery (ERAS) programs. Gynecol Oncol. 2016;141(2):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--Part I. Gynecol Oncol. 2016;140(2):323–32. [DOI] [PubMed] [Google Scholar]
- 14.Meyer LA, Lasala J, Iniesta MD, et al. Effect of an Enhanced Recovery After Surgery Program on Opioid Use and Patient-Reported Outcomes. Obstet Gynecol. 2018;132(2):281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weston E, Noel M, Douglas K, et al. The impact of an enhanced recovery after minimally invasive surgery program on opioid use in gynecologic oncology patients undergoing hysterectomy. Gynecol Oncol. 2020;157:469–75. [DOI] [PubMed] [Google Scholar]
- 16.Hillman RT, Sanchez-Migallon A, Meyer LA, et al. Patient characteristics and opioid use prior to discharge after open gynecologic surgery in an enhanced recovery after surgery (ERAS) program. Gynecol Oncol. 2019;153(3):604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffith KC, Clark NV, Zuckerman AL, Ferzandi TR, Wright KN. Opioid Prescription and Patient Use After Gynecologic Procedures: A Survey of Patients and Providers. J Minim Invasive Gynecol. 2017;25(4):684–688. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sailors MH, Bodurka DC, Gning I, et al. Validating the M. D. Anderson symptom inventory (MDASI) for use in patients with ovarian cancer. Gynecol Oncol. 2013;130(2):323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang XS, Shi Q, Williams LA, et al. Validation and application of a module of the MD Anderson Symptom Inventory for measuring perioperative symptom burden in patients with gynecologic cancer (the MDASI-PeriOp-GYN). Gynecol Oncol. 2019;152(3):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 22.R Core Team. R: A language and environment for statistical computing. 2016.
- 23.Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sloan JA, Dueck A. Issues for statisticians in conducting analyses and translating results for quality of life end points in clinical trials. J Biopharm Stat. 2004;14(1):73–96. [DOI] [PubMed] [Google Scholar]
- 25.Davidson ERW, Paraiso MFR, Walters MD, et al. A Randomized Controlled Non-Inferiority Trial of Reduced Versus Routine Opioid Prescription after Prolapse Repair. Am J Obstet Gynecol. 2020. [DOI] [PubMed] [Google Scholar]
- 26.Mark J, Argentieri DM, Gutierrez CA, et al. Ultrarestrictive Opioid Prescription Protocol for Pain Management After Gynecologic and Abdominal Surgery. JAMA Netw Open. 2018;1(8):e185452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasor SE, Evans TA, Cook JA, et al. Reduction in Opioid Prescribing Through Evidence-Based Prescribing Guidelines. JAMA Surg. 2018;153(3):285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osmundson SS, Raymond BL, Kook BT, et al. Individualized compared with standard postdischarge oxycodone prescribing after cesarean birth: A randomized controlled trial. Obstet Gynecol. 2018;132(3):624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fingar KR, Stocks C, Weiss AJ, Steiner CA. Most Frequent Operating Room Procedures Performed in U.S. Hospitals, 2003–2012: Statistical Brief #186; 2006. [PubMed]
- 30.Cohen SL, Ajao MO, Clark NV, Vitonis AF, Einarsson JI. Outpatient Hysterectomy Volume in the United States. Obstet Gynecol. 2017;130(1):130–137. [DOI] [PubMed] [Google Scholar]
- 31.Vogell A, Morris Stephanie, Isaacson K, Loring M, Wright K, Wong M. Opioid use after laparoscopic hysterectomy: prescriptions, patient use, and a predictive calculator. Am J Obstet Gynecol. 2018;220(3):259.e1–259.e11. [DOI] [PubMed] [Google Scholar]
- 32.Weston E, Raker C, Huang D, et al. Opioid use after minimally invasive hysterectomy in gynecologic oncology patients. Gynecol Oncol. 2019;155(1):119–125. [DOI] [PubMed] [Google Scholar]
- 33.Bates C, Laciak R, Southwick A, Bishoff J. Overprescription of postoperative narcotics: A look at postoperative pain medication delivery, consumption and disposal in urological practice. J Urol. 2011;185(2):551–555. [DOI] [PubMed] [Google Scholar]
- 34.Ramaseshan AS, Tunitsky-Bitton E, O’Sullivan DM, Reagan KML, Steinberg AC. Predictive Factors of Postdischarge Narcotic Use After Female Pelvic Reconstructive Surgery. Female Pelvic Med Reconstr Surg. 25(2):e18–e22. [DOI] [PubMed] [Google Scholar]
- 35.Glaser GE, Kalogera E, Kumar A, et al. Outcomes and patient perspectives following implementation of tiered opioid prescription guidelines in gynecologic surgery. Gynecol Oncol. 2020;157:476–81. 10.1016/j.ygyno.2020.02.025 [DOI] [PubMed] [Google Scholar]


