Abstract
Background
Gingivitis and periodontitis are prevalent inflammatory diseases of the periodontal tissues. Current treatments are often ineffective or do not prevent disease recurrence. Uncontrolled complement activation and the resulting chronic gingival inflammation are hallmarks of periodontal diseases. We determined the efficacy and safety of a complement 3–targeted therapeutic, AMY-101, which was locally administered to adult patients with periodontal inflammation.
Methods
Thirty-two patients with gingival inflammation were enrolled in a randomized, placebo-controlled, double-blind, split-mouth phase IIa trial that followed a dose escalation study to select a safe and effective dose in an additional 8 patients. Half of the patient’s mouth was randomly assigned to AMY-101 (0.1 mg/site) or placebo injections at sites of inflammation, administered on days 0, 7, and 14, and then evaluated for safety and efficacy outcomes on days 28, 60, and 90. The primary efficacy outcome was a change in gingival inflammation, measured by a modified gingival index (MGI), and secondary outcomes included changes in bleeding on probing (BOP), the amount of plaque, pocket depth, clinical attachment level, and gingival crevicular fluid levels of matrix metalloproteinases (MMPs) over 90 days.
Results
A once-weekly intragingival injection of AMY-101 for 3 weeks was safe and well tolerated in all participants and resulted in significant (P < 0.001) reductions in clinical indices measuring gingival inflammation (MGI and BOP). AMY-101 significantly (P < 0.05) reduced MMP-8 and MMP-9 levels, indicators of inflammatory tissue destruction. These therapeutic effects persisted for at least 3 months after treatment.
Conclusion
AMY-101 treatment resulted in a significant and sustainable reduction in gingival inflammation without adverse events and, we believe, merits further investigation for the treatment of periodontitis and other oral or peri-implant inflammatory conditions.
Trial registration
ClinicalTrials.gov identifier NCT03694444.
Funding
Amyndas Pharmaceuticals.
Keywords: Clinical Trials, Immunology
Keywords: Complement, Drug therapy
Introduction
Periodontitis is a microbiome-driven chronic inflammatory disease of the tooth-supporting tissues, i.e., the gingiva, periodontal ligament, cementum, and alveolar bone (1). Nearly 50% of the global adult population is affected by periodontitis, with approximately 10% having severe disease (2, 3). Severe periodontitis is the sixth most prevalent inflammatory disease worldwide (4) and is associated with an increased risk of systemic comorbidities (e.g., cardiovascular disease, rheumatoid arthritis, and Alzheimer’s disease; refs. 5, 6). If not properly treated, periodontitis can lead to tooth loss, impaired mastication, and poorer esthetics and quality of life (7–9). Current standard-of-care periodontal therapy is often ineffective, particularly in highly susceptible patients, or it does not prevent disease recurrence; hence, periodontitis remains a serious public health and economic burden (8, 10). In 2018, the total cost (direct and indirect due to productivity losses) of periodontal disease in the United States and Europe was estimated to be $154.06 billion and €158.64 billion, respectively (11).
Periodontitis is often preceded by gingivitis, a relatively mild and reversible form of periodontal disease, in which inflammation is confined within the gingival epithelium and the underlying connective tissue, without involving the underlying bone (1). However, despite daily self-performed removal of the microbial biofilm (“dental plaque”) and periodic professional dental care, gingivitis often persists as a chronic condition that constitutes a constant risk for periodontitis.
Although the microbial biofilm is necessary, it is not sufficient by itself to cause gingivitis or periodontitis, in which tissue destruction is mediated predominantly by an overexuberant inflammatory response to the microbial challenge (12). Host modulation, the intentional alteration of the host response as an adjunctive therapy for periodontal diseases, may not only contribute to the management of gingivitis and periodontitis but may also mitigate the risk of periodontitis-associated systemic comorbidities (13, 14). Despite the attempts to develop and use host modulation therapies in the treatment of periodontal diseases (15), the concept of host modulation therapy in the clinical management of periodontal diseases has still remained underdeveloped and underutilized, with a limited number of safe and approved therapeutic agents.
The complement system is a central hub of innate immunity responsible for the induction and regulation of immune and inflammatory responses (16); yet, its dysregulation or overactivation can drive pathological inflammation (17). In this context, gingival inflammation in patients with periodontitis is associated with enhanced complement activity (18–20), whereas mice lacking the central complement component C3, or the receptor for the complement anaphylatoxin C3a, are protected from gingival inflammation and alveolar bone loss (21–23). Consistently, local pharmacological inhibition of C3 by AMY-101, a C3-targeted peptidic drug candidate based on the third-generation compstatin analog Cp40 (24, 25) inhibited periodontitis in nonhuman primates (NHPs) in both preventative and therapeutic settings (22, 26, 27). Importantly, the clinical host-modulatory effects of locally administered AMY-101 in NHPs with naturally occurring periodontitis persisted for at least 6 weeks after treatment completion (26).
The aforementioned preclinical study results (21, 22, 26, 27), in conjunction with the enhanced inhibitory potency and favorable pharmacologic and safety profile of AMY-101 (24), provided a strong rationale and support for the suitability of C3 as a treatment target and of AMY-101 as a potential therapeutic for human periodontal diseases. Of note, the PEGylated second-generation compstatin derivative pegcetacoplan (Empaveli, Apellis Pharmaceuticals) was recently approved by the FDA as a novel therapy for the rare hematologic disorder paroxysmal nocturnal hemoglobinuria (28), validating the broad activity profile and safety of C3 inhibitors in the clinic (29). Here, we performed a first-in-human phase IIa randomized, placebo-controlled, double-blind trial to evaluate the safety and efficacy of locally delivered AMY-101 in patients with periodontal inflammation.
Results
Participant population, dose selection phase, and interim analysis.
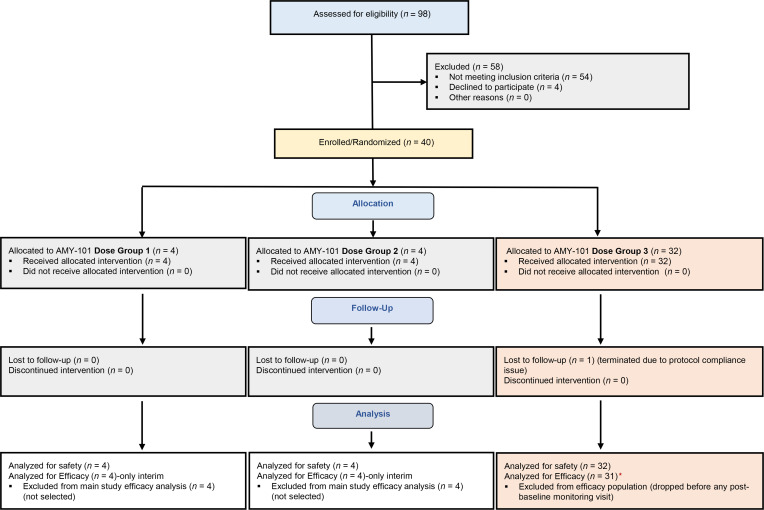
A total of 98 individuals were screened for study inclusion between July 2019 and August 2020. The first 12 individuals who were found eligible were randomly assigned to escalating dose groups: dose group 1, at 0.025 mg/interdental papilla (4 participants); dose group 2, at 0.5 mg/interdental papilla (4 participants); and dose group 3, at 0.1 mg/interdental papilla (4 participants) to determine a safe and effective dose in humans (dose selection phase) (Figure 1; see Supplemental Methods for details on the study design). On the basis of the safety and interim efficacy analysis of primary outcome measures (modified gingival index [MGI]), we chose the dose of 0.1 mg/interdental papilla for the main study (P = 0.08 on day 28 compared with placebo). Therefore, the remaining 28 patients, who were found to be eligible after screening, were enrolled in this dose group and included in the efficacy population (i.e., a total of 32 participants). The patients in the other 2 dose groups (n = 8) were monitored through day 90, like the main study patients, and were included in the safety population.
Figure 1. CONSORT diagram.
CONSORT subject flow diagram shows the number of subjects screened, enrolled/randomized, and included in the interim, primary safety, and secondary efficacy analyses. Of the 98 patients screened, 40 were found eligible, agreed to participate, and enrolled in the study. The first 12 subjects were randomized into 3 escalating dose groups (dose group 1, dose group 2, and dose group 3; 4 individuals/group). After selection of the dose of 0.1 mg/interdental papilla, 28 more individuals (total of 32) were randomized to the main study group (orange shaded boxes) and treated split-mouth with AMY-101 at 0.1 mg/interdental papilla and placebo (saline) injections at baseline, on day 7 and on day 14, as 3 once-weekly injections. The safety population included all participants (n = 40) treated with at least 1 dose of AMY-101 or placebo, including the participants from the dose selection phase. The efficacy population included those who completed at least 1 post-baseline visit (starting on day 21) for efficacy analysis in the selected dose group. One participant dropped out before completing the necessary day-21 visit and was thus replaced per protocol. Red asterisk indicate that of the 31 patients treated with AMY-101 at a dose of 0.1 mg/interdental papilla and placebo, 1 failed to complete the day-90 visit; dose group 1: 0.025 mg/interdental papilla; dose group 2: 0.05 mg/interdental papilla; dose group 3: 0.1 mg/interdental papilla.
Safety and efficacy populations: demographics and baseline characteristics.
As alluded to above, all randomized participants who received study treatments regardless of the AMY-101 dose (n = 40 participants) were included in the safety population. All study participants who received AMY-101 at the dose of 0.1 mg/interdental papilla and completed at least 1 post-baseline visit with efficacy measurements were included in the efficacy population. One patient was terminated from the study because of a protocol compliance violation before day 21 (an efficacy endpoint) and was replaced according to the protocol (Figure 1). In the safety and efficacy populations, 39 (97.5%) and 31 (96.9%) patients, respectively, were included in the analyses.
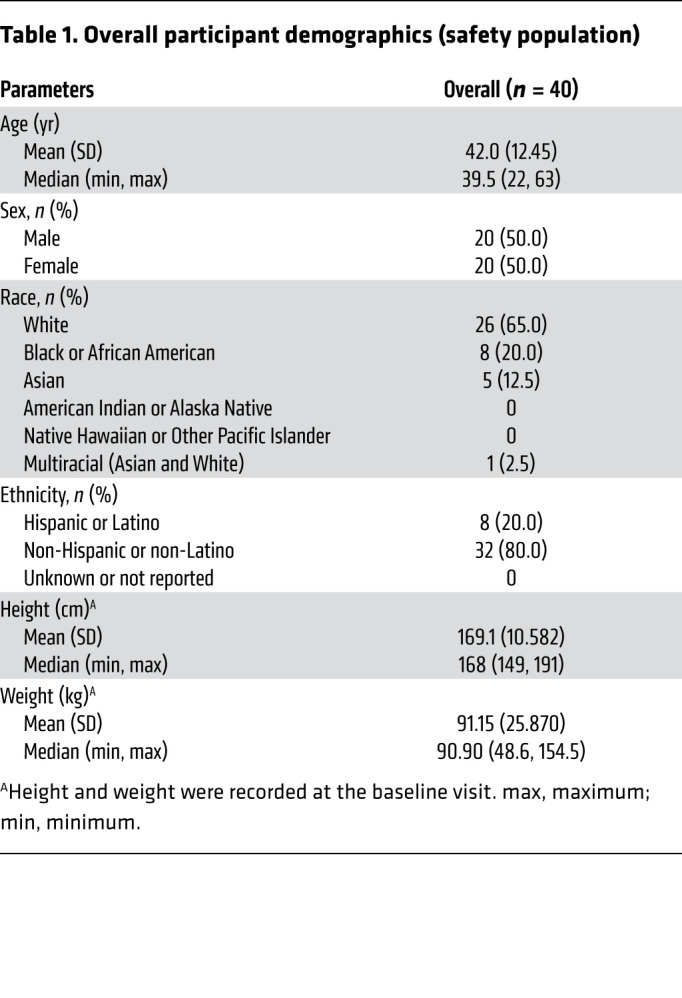
Baseline demographics for the safety population are shown in Table 1. The demographics of the study population are representative of individuals living in greater Boston and Cambridge, Massachusetts. The mean age of the safety population was 42 years, with a standard deviation of 12.4 years (median: 39.5; minimum: 22; maximum: 63). Although this was a cross-sectional study and enrollment was based on the level of gingival inflammation (determined by the MGI and bleeding on probing [BOP]) and other inclusion criteria, both sexes were equally represented in the study (50%). The diversity of the recruited individuals was representative of the overall population, with 65% being White, 20% Black or African American, and 5% Asian, whereas the majority of participants (80.0%) identifying their ethnicity as non-Hispanic or non-Latino. The study population consisted of medically healthy individuals with no condition reported (35%) as well as individuals with a stable condition such as hypertension, hypercholesterolemia, hypothyroidism, allergies, arthralgia, headache, or asthma (65%). Individuals with a medical condition or medication use known to affect periodontal disease were excluded from this study (exclusion criteria). The mean BMI for the study population was 31.9 (minimum: 21.9; maximum: 42.2).
Table 1. Overall participant demographics (safety population).
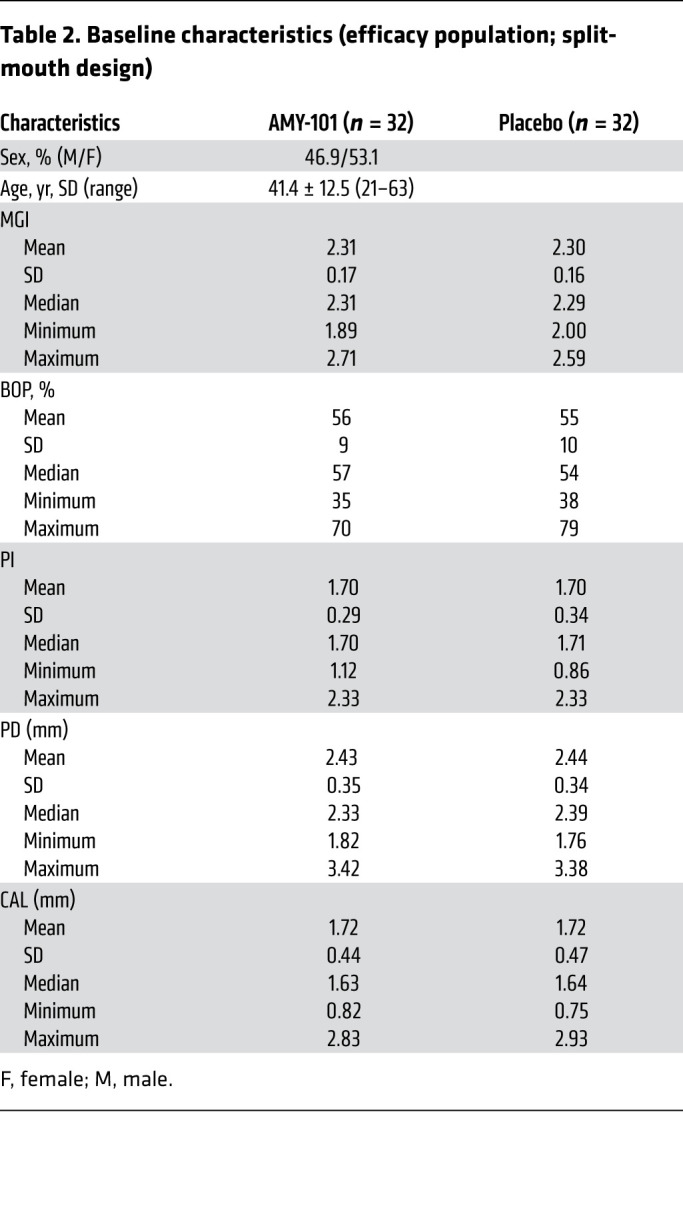
The baseline disease characteristics of the efficacy population per treatment site are listed in Table 2. Right and left sides of the mouth of 32 patients were randomly assigned to receive AMY-101 (0.1 mg/interdental papilla) or placebo (saline) injections (25 L/injection site) in a split-mouth design. The treatment sides were similar with regard to the baseline outcome measures of MGI, BOP percentage, plaque index (PI), pocket depth (PD), and clinical attachment level (CAL), with no significant differences. The mean MGI score and BOP percentage for AMY-101–treated quadrants were 2.3 ± 0.17 and 56% ± 9%, respectively, compared with 2.3 ± 0.16 and 55% ± 10%, respectively, for the placebo-treated sites. The participant retention and therefore compliance was high (93.8%), with only 1 individual who did not complete the day-90 visit.
Table 2. Baseline characteristics (efficacy population; split-mouth design).
AMY-101 significantly reduces gingival inflammation in humans.
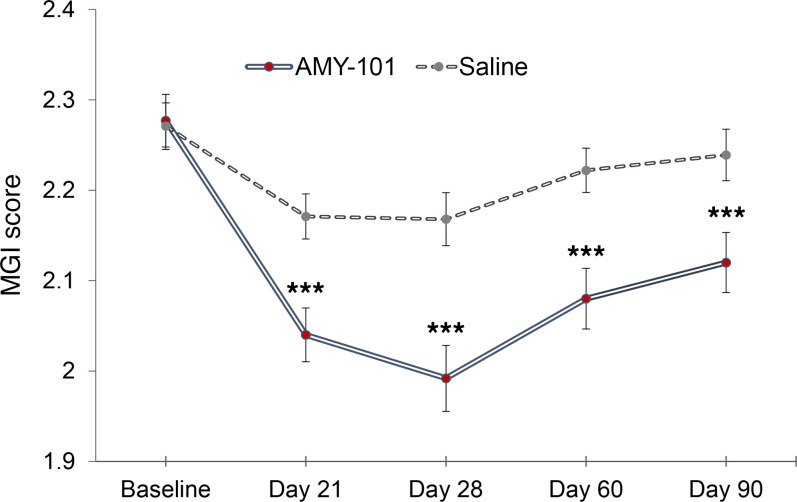
The primary efficacy endpoint of gingival inflammation, assessed by MGI at 6 sites per tooth (interproximal sites, facial and lingual/palatal sites), was significantly reduced by AMY-101 on day 28 compared with sites treated with placebo (least squares mean [LSM] difference of –0.181 ± 0.034, 95% CI: –0.248 to –0.114, P < 0.001). The mean reduction from baseline in AMY-101–treated sites was –0.29 ± 0.026 with a 95% CI of –0.336 to –0.234, whereas in placebo-treated sites the corresponding value was –0.10 ± 0.021 with a 95% CI of –0.147 to –0.062. The reduction in the primary outcome, the MGI, was significantly greater after AMY-101 therapy than after placebo on day 28 (LSM: –0.29 vs. –0.10, respectively; LSM difference: –0.181; P < 0.001; Supplemental Table 2; supplemental material available online with this article; https://doi.org/10.1172/JCI152973DS1). Most important, AMY-101 delivered through 3 weekly injections (days 0, 7, and 14) greatly reduced gingival inflammation (MGI) at all time points, starting as early as day 21, and maintained a continuous significant difference over 90 days compared with placebo-treated sites (P < 0.001, at all time points; Figure 2 and Supplemental Table 3). The sensitivity analysis results were consistent with those for the primary analysis.
Figure 2. Change in the gingival index over time (primary efficacy endpoint) following treatment with AMY-101.
The key clinical endpoint associated with gingival inflammation was measured using the MGI, scored as 0–4 (0 = healthy; 1 = localized mild; 2 = generalized mild; 3 = moderate; 4 = severe). The change from baseline on days 21, 28, 60, and 90 was compared between treatment groups. The LSM difference, along with its SE, 95% CI, and P value, was obtained through a GEE method with a normal distribution and identity link including treatment group, study visit (days 21, 28, 60, and 90), and interaction between the treatment group and study visit as fixed effects with the baseline as a covariate. Data are presented as the LSM ± SE (n = 31). ***P < 0.001 compared with saline (placebo).
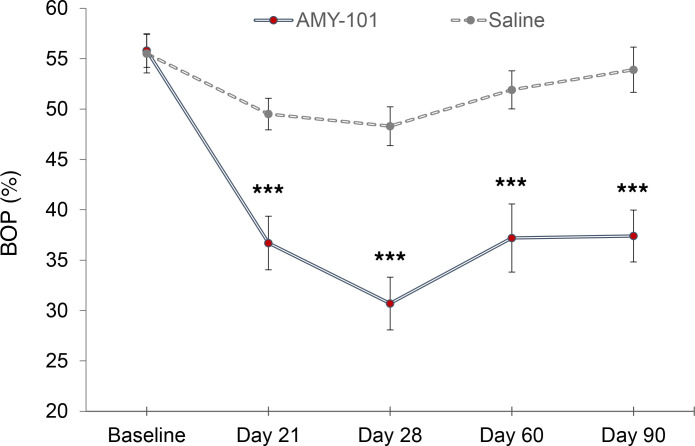
BOP, a key secondary endpoint closely related to the primary endpoint MGI, was also greatly reduced at all time points (days 21, 28, 60, and 90) compared with placebo (P < 0.001). The mean BOP reduction from baseline in AMY-101–treated sites was the highest on day 28 (–25% ± –0.07% with a 95% CI of –0.296 to –0.204), whereas in the placebo-treated sites, the corresponding value was –7% ± 1.5%, with a 95% CI of –0.103 to –0.044 (Figure 3 and Supplemental Table 4).
Figure 3. Change in the BOP percentage over time (secondary efficacy endpoint) following treatment with AMY-101.
Another key clinical endpoint associated with gingival inflammation, BOP, was assessed using a dichotomous measurement ( 1 = bleeding and 0 = no bleeding within 15 minutes of probing the site). Changes from baseline on days 21, 28, 60, and 90 were compared between the treatment groups. The LSM difference, along with its SE, 95% CI, and P value, was obtained using a GEE method with the normal distribution and identity link including treatment group, study visit (days 21, 28, 60, and 90), and interaction between the treatment group and study visit as fixed effects with the baseline as the covariate. Data are presented as the LSM ± SE (n = 31). ***P < 0.001 compared with saline (placebo).
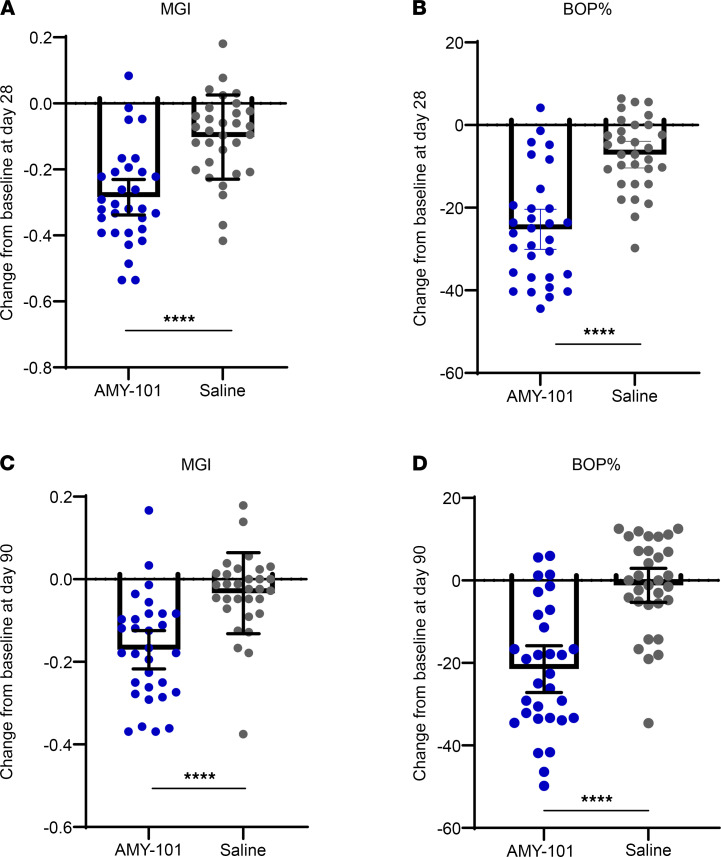
We also assessed the patients’ responses to AMY-101 treatment by plotting the changes in the MGI and BOP on both day 28 and day 90 compared with placebo treatment (Figure 4). Individual mean changes from baseline with 95% CIs were greater in the AMY-101-treated sites compared with the placebo-treated sites (P < 0.0001, unpaired, 2-tailed Student’s t test).
Figure 4. Change in gingival inflammation following treatment with AMY-101.
Scatter plots showing the individual’s response to each treatment on day 28 (A and B) and day 90 (C and D), as measured by the MGI and BOP percentage, respectively. Each dot represents each subject treated either with AMY-101 or saline, and the bar graphs show the mean with a 95% CI (n = 31). ****P < 0.0001, by 1-way ANOVA with 95% CIs.
We also evaluated the differences between AMY-101 and placebo treatments in the secondary efficacy endpoints of PI, PD, and CAL. The amount of plaque was only slightly reduced throughout the study in both the AMY-101– and placebo-treated sites (up to –6.2% for the AMY-101–treated sites and up to –4.5% for the placebo-treated sites), suggesting that the reduction in gingival inflammation observed was not due to plaque control but rather to modulation of the host-mediated inflammatory response by AMY-101 treatment. Moreover, since only a small number of patients had periodontal pockets of greater than 4 mm and a CAL of greater than 3 mm, the changes in the mean PD and CAL did not differ greatly from baseline with the treatments (P < 0.05). A subanalysis of sites with periodontitis (PD ≥5 mm) showed a continuous trend in PD reduction in the AMY-101–treated sites (–0.5 ± 0.79 mm, 95% CI of 0.8 to 0.3 on day 90 vs. –0.4 ± 0.7 mm, 95% CI of 0.6 to 0.2 in the placebo-treated sites), with a P value of 0.162, supporting further study of AMY-101 in the treatment of periodontitis as an adjunct to mechanical periodontal treatment in well-powered studies.
AMY-101 significantly reduces proinflammatory matrix metalloproteinase levels in the gingival crevicular fluid.
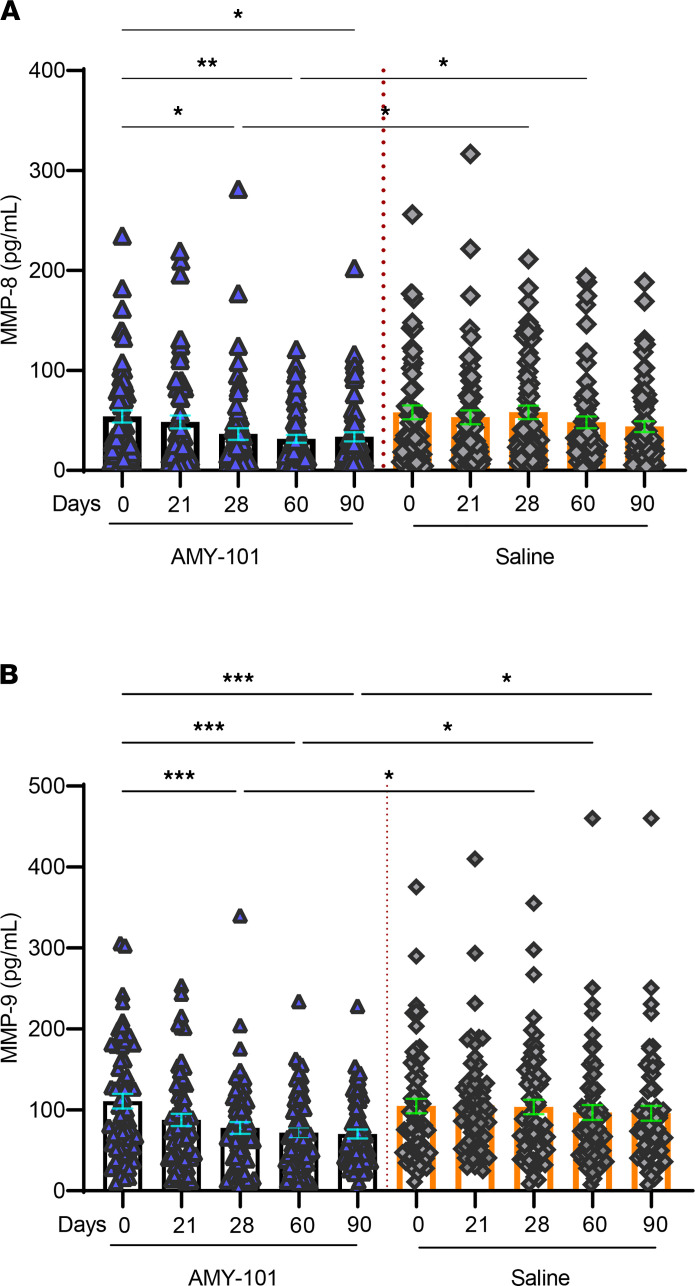
The matrix metalloproteinases MMP-8 and MMP-9 are key proteases associated with periodontal tissue destruction; moreover, they are abundantly found in the inflamed gingival tissue and are considered promising biomarkers for periodontitis (30–32). We therefore determined whether gingival crevicular fluid (GCF) concentrations of MMP-8 and MMP-9 were suppressed in sites treated with AMY-101 as compared with those treated with the placebo control (saline). To this end, we collected GCF at 2 interproximal sites (1 maxillary and 1 mandibular), with the highest gingival inflammation measured according to the MGI in each of the 2 sides of the mouth (AMY-101– and placebo-treated). We detected no statistically significant differences in the baseline GCF levels of MMPs between the AMY-101– and placebo-treated sites (MMP-8: 54 ± 6.1 pg/mL [AMY-101] vs. 58 ± 6.9 pg/mL [placebo], P > 0.05; MMP-9: 111 ± 9.1 pg/mL [AMY-101] vs. 105 ± 9.0 pg/mL [placebo], P > 0.05). However, in contrast to placebo treatment, AMY-101 treatment resulted in significantly decreased MMP-8 levels over time, specifically on day 60 (P < 0.01) and day 90 (P < 0.05) compared with baseline levels (Figure 5A). Moreover, GCF concentrations of MMP-8 in AMY-101–treated sites on day 28 were significantly different from those in placebo-treated sites at the same time point (P < 0.05; Figure 5A). AMY-101 treatment, but not treatment with placebo, also resulted in significantly decreased MMP-9 levels at all time points tested (except for day 21) as compared with baseline (P < 0.01 on day 28; P < 0.001 on days 60 and 90; Figure 5B). Moreover, the GCF levels of MMP-9 in AMY-101–treated sites on both day 28 and day 90 were significantly different from those in the placebo-treated sites at the same time points (P < 0.05; Figure 5B). These data show that local treatment with AMY-101 can reduce the GCF levels of MMPs involved in periodontal tissue destruction.
Figure 5. Changes in the GCF levels of MMP-8 and MMP-9 following treatment with AMY-101.
The levels of MMP-8 (A) and MMP-9 (B) were measured in the GCF from 2 separate sites on each treatment side (AMY-101 or saline) and were compared within groups (e.g., different time points after AMY-101 treatment) as well as between groups (i.e., AMY-101 vs. saline). The LSM, its 95 % CI, SE, and LSM difference, along with its SE, 95% CI, and the P value, were obtained through a GEE method with normal distribution and identity link including treatment group, study visit (day 21, day 28, day 60, and day 90), and interaction between the treatment group and study visit as fixed effects with the baseline as the covariate. Dot plots with whiskers represent individual values and the mean ± SE. Within-group comparisons were performed using repeated-measures 1-way ANOVA followed by Sidak’s multiple-comparison test. *P < 0.05, **P < 0.01, and ***P < 0.001. For baseline (day 0), day 21, and day 28, n = 62; for day 60, n = 56; and for day 90, n = 60.
AMY-101 is safe and well tolerated by the study participants.
There were no deaths reported during the study. One participant with a prior history of alcohol dependency experienced a non–study drug–related serious adverse event (AE) of alcohol withdrawal syndrome, which was diagnosed on the basis of a sequence of other nonserious treatment-emergent adverse events (TEAEs) of nausea, vomiting, increased heart rate, tremor, hyperhidrosis, and hypertension and was discontinued from the study.
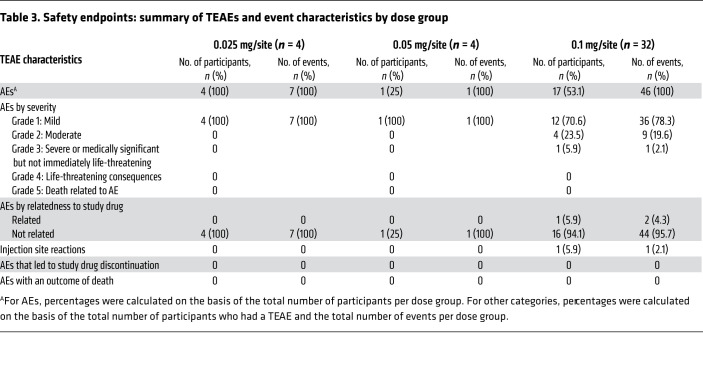
A total of 54 AEs were reported in 22 participants in the safety population during the course of the study that included the dose escalation phase (Table 3). All TEAEs were mild or moderate in severity and resolved without sequelae. No drug-related serious AEs or TEAEs leading to discontinuation were reported during the study. The reported non–drug-related TEAEs by preferred term included systolic blood pressure increase (3 participants), arthralgia (2 participants), thermal burn (2 participants), fatigue (2 participants), vomiting (2 participants), and tooth hypersensitivity to cold (2 participants). The only related TEAEs, lymph node pain, gingival erythema, and edema, were reported for the same subject and related to the same event.
Table 3. Safety endpoints: summary of TEAEs and event characteristics by dose group.
None of the participants had grade 2 or higher ulceration or erythema during the oral/dental examination. With the exception of 1 individual, who was noted to have grade 2 edema at the injection site in the buccal aspect of the left mandible on days 21 and 28, none of the participants who received AMY-101 or placebo had grade 2 or higher injection site reactions (Table 3 and Supplemental Table 5). None of the participants reported pain or discomfort, gingival bleeding, or irritation after the injections, except for the individual mentioned above, who had grade 2 edema. This reaction, which was reported after the last injection on day 14, was transient and resolved without concomitant treatment. No clinically important abnormal laboratory values of liver enzymes were reported during the study. Further, the exploratory analysis of anti-drug antibodies in plasma indicated that AMY-101 did not induce the generation of AMY-101–specific antibodies in any of the patients who received the drug.
None of the patients treated with AMY-101 were noted to have development or progression of gingivitis or existing periodontitis (≥3 mm increase in the mean PD or CAL from baseline or ≥3 mm increase in the mean PD from baseline in at least 3 sites). One participant on day 28 and 3 participants on day 90 had progression of gingivitis in placebo-treated sites, whereas none of the participants had progression of periodontitis.
Discussion
This phase IIa randomized, controlled clinical trial evaluated the safety and efficacy of the C3-targeted inhibitor AMY-101 in patients with existing gingivitis. Consistent with proof-of-concept studies of C3 inhibition in preclinical models of periodontitis (22, 26, 27), we found that AMY-101, locally delivered to the periodontal tissues, significantly dampened gingival inflammation, as evidenced by clinically meaningful reductions in indices related to inflammation (MGI and BOP) in a split-mouth design in patients. The drug produced a pronounced and sustainable resolution of gingival inflammation that was evident at least 3 months after the initiation of treatment. Consistent with the clinical findings, AMY-101 treatment also resulted in a significant reduction in the GCF levels of MMPs (MMP-8 and MMP-9) associated with inflammatory tissue destruction in periodontitis.
AMY-101 is a cyclic C3-inhibitory peptide based on the third-generation compstatin analog Cp40, which has been shown to exert effective and sustained C3 inhibition in many preclinical disease models, where C3 dysregulation or overactivation plays a pathogenic role (24). AMY-101 and earlier compstatin analogs (such as the recently FDA-approved C3 inhibitor Empaveli) bind C3 and block its cleavage by C3 convertases into the active fragments C3a and C3b (33). AMY-101 exhibits superior pharmacologic properties in terms of binding affinity for C3 (KD = 0.5 nM; 6000-fold higher vs. original compstatin), enhanced inhibitory potency, and a half-life in human plasma (>60 h) exceeding that of most peptidic drugs (24). By protecting the C3 substrate rather than interfering with a specific C3 convertase, AMY-101 can block the propagation and amplification of the complement cascade (including the generation of the proinflammatory effectors C3a and C5a), regardless of the complement-triggering mechanism (classical, lectin, or alternative) (24). Importantly, the complement activation products C3a and C5a mediate complement crosstalk signaling with innate and adaptive immune cells, leading to amplification of the host inflammatory response (34, 35). C3, therefore, appears to be an ideal target for therapeutic modulation not only of complement itself but also of downstream inflammatory pathways that are involved in periodontal disease pathogenesis (36, 37). These considerations explain why the blockade of a single complement molecule, C3, had such a pronounced and prolonged clinical therapeutic effect in a complex immune-driven disease in the present trial.
Although gingivitis can be resolved, at least in principle, with meticulous personal oral hygiene accompanied by professional plaque removal, in susceptible individuals, prolonged and unresolved gingivitis may eventually lead to periodontitis with alveolar bone destruction (38). In addition to strong independent contributors to increased susceptibility (e.g., smoking, diabetes mellitus, metabolic syndrome, and obesity), certain demographic characteristics such as, age, sex, ethnicity, and socioeconomic status further influence the severity and prevalence of periodontitis (39). Severe periodontitis, especially in the third and fourth decades of life, has a global prevalence of 10%–15% and is the major cause of tooth loss in adults (4, 40). Accumulating evidence from epidemiological, clinical, and preclinical mechanistic studies indicates that periodontitis (as well as tooth loss) can be linked to systemic diseases and other conditions affecting general health (5, 6). Therefore, prevention and timely and effective periodontal treatment are important for overall health (6, 8, 41). This phase IIb clinical trial is the first human study to our knowledge to evaluate the therapeutic opportunity provided by C3 blockade as an approach to treat gingival inflammation and prevent progression to a destructive form of the disease.
As a bacterially induced host response–mediated disease, periodontitis is currently being treated with mechanical therapies aimed at removal of the bacterial biofilm (“dental plaque”) and with adjunctive chemotherapeutic agents (42, 43). Because of the continuous accumulation of the microbial biofilm at the gingiva-tooth interface and eventual reemergence of the periodontal pathogens (44), these mechanical and antibacterial approaches alone have limited success in treating this host-mediated inflammatory disease. Thus, disease recurrence or progression to more advanced forms in susceptible individuals is an important clinical concern necessitating new and improved treatments. To this end, efficacious and sustainable host-modulating therapeutic interventions without substantial side effects are needed as adjunctive treatments (13), and AMY-101 appears to have the potential to fulfill these requirements.
Indeed, the results of this clinical trial provide evidence that AMY-101 is a promising host-modulatory therapy for the treatment of inflammatory periodontal diseases. AMY-101 delivered to the site of inflammation in well-tolerated doses by 3 weekly injections resulted in a significant reduction in gingival inflammation as early as 21 days after the first treatment, achieving continuous control of inflammation for at least 90 days. A smaller number of doses may also be effective (and this will be determined in future trials), given that less frequent local administration of AMY-101 (2 doses only, given 3 weeks apart) resulted in long-term improvement of clinical periodontal indices in naturally occurring NHP periodontitis (26). The improvements from baseline in both MGI and BOP were significantly greater in AMY-101–treated sites compared with placebo-treated sites in the same individual. The split-mouth design allowed for the elimination of any confounding factors that may influence the observed outcomes among different individuals, thereby revealing the actual drug-induced response on key clinical indices of gingival inflammation. We assessed safety using transient emerging AEs over the course of the trial. No adverse safety signals were noted throughout the trial. The only potentially related events seen at the site of injection in 1 participant were transient erythema and edema following the final injection (on day 14) that resolved within a couple of days without any treatment.
Prolonged systemic C3 inhibition with Empaveli in phase III trials did not compromise pathogen immune surveillance (i.e., no meningococcal infections were recorded), indicating a good safety profile of compstatins in the clinical setting (45). Nevertheless, like eculizumab, Empaveli was approved for paroxysmal nocturnal hemoglobinuria (PNH) with a risk evaluation and mitigation strategy in place requiring that patients be closely monitored during treatment to ensure effective mitigation of the risk of bacterial infection (46). However, in the context of periodontal disease, potential safety issues are further minimized not only because the treatment is local but also because the injected amount of AMY-101 is considerably smaller than that used in systemic administration protocols, thus precluding any considerable drug exposure in the circulation (47). Moreover, complement inhibition in preclinical models of periodontitis restrains rather than favors the expansion and persistence of periodontal pathogens (47).
We used the PI, a secondary efficacy endpoint, to assess potential changes in the amount of dental plaque at the gingival margin during the study. Although minor and nonsignificant reductions in the PI were observed in both treatment groups (AMY-101– vs. placebo-treated sites) compared with baseline, we found no substantial differences between the treatment groups. These findings suggest that the reduction of gingival inflammation in AMY-101–treated sites was not associated with a reduced amount of dental plaque biofilm but rather was due to the drug’s modulation of the host-mediated inflammatory response. Consistent with this notion, experimental periodontitis studies in mice have shown that destructive periodontal inflammation is not necessarily caused by a global reduction in microbial biomass but rather by specific alterations in the composition of the microbial community (36).
Although the majority of the study participants had gingivitis based on the main inclusion criterion (gingival inflammation confirmed by the MGI and BOP percentage scores), the study population also included a few patients with localized periodontitis as evidenced by a PD of greater than 4 mm and an interproximal CAL of greater than 3 mm. The mean changes in PD and CAL were not statistically different between sites treated with AMY-101 or placebo; however, we noted a trend toward slightly greater PD reductions in AMY-101–treated sites compared with placebo-treated sites. This study was not powered to detect any statistically significant differences in PD or CAL, thus further studies are warranted to investigate the impact of AMY-101 on pocket reduction and clinical attachment gain in moderate to severe periodontitis. Although AMY-101 was used as a standalone treatment in the current phase IIa trial, this C3-targeted drug is ultimately intended for use as an adjunct to mechanical debridement (scaling and root planing) that could facilitate an enhanced and sustainable host response to mechanical treatment and improve clinical outcomes. Specific to this trial, the standard of care for gingivitis is routine oral hygiene with twice-daily tooth brushing and professional dental prophylaxis at least once a year. Therefore, the patients in this study received AMY-101 and placebo in a split-mouth design in addition to (as an adjunct to) regular daily oral hygiene.
Another secondary endpoint, the levels of MMPs in the GCF, was also supportive of the primary endpoint, gingival inflammation. Indeed, the GCF levels of MMP-8 and MMP-9 were reduced in AMY-101–treated sites, but not in the placebo-treated sites, as compared with baseline values. The MMPs are involved in different stages of collagen remodeling and are considered to play an important role during tissue remodeling and extracellular matrix degradation. MMP-8 and MMP-9 represent the main collagenase and gelatinase, respectively, in the GCF of patients with adult periodontitis and are strongly associated with tissue breakdown in periodontitis (30, 48). MMP-8 in particular, which is used in point-of-care testing, is considered to be a biomarker for periodontitis based on studies that showed that this metalloproteinase is highly predictive of severe periodontitis and can discriminate between periodontal health and disease (31, 49–52). The significant reductions we observed in MMP-8 and MMP-9 therefore suggest that AMY-101 may act as a modulatory therapeutic agent that could offer promising benefits for the treatment of advanced periodontal disease.
This first-in-human gingivitis study presents some limitations as well. First, the study inclusion was based on the level of gingival inflammation, not on the severity of periodontal disease as indicated by PD and alveolar bone loss. Thus, the results of this study should be interpreted cautiously in terms of periodontitis. However, the significant reductions we observed in the MGI, an outcome measure for which it has been challenging to achieve significant favorable differences, clearly outweigh the limitations and, we believe, provide ample evidence that AMY-101 is a promising therapeutic agent for the treatment of inflammatory periodontal diseases. Future studies could expand on these observations by examining further improvements in drug dosage or the frequency of injections and route of administration.
Taken together, the results of this phase IIa clinical trial in patients with gingival inflammation provide strong evidence that locally delivered C3 inhibition with the compstatin-based drug candidate AMY-101 can elicit potent and durable antiinflammatory effects in humans. Thus, C3-targeted complement intervention may serve as a host-modulatory therapeutic approach for the treatment of oral inflammatory diseases including periodontal and perhaps peri-implant diseases, where complement has also been implicated (47, 53, 54). Other indications considered for therapeutic interventions using AMY-101 (via systemic administration) include hematopoietic stem cell transplantation–associated thrombotic microangiopathy, complications of ABO-incompatible kidney transplantation, hemodialysis-induced inflammation (24), as well as severe COVID-19, for which AMY-101 has already been tested in an exploratory study with promising results (55). The safety profile of the complement C3 inhibitor AMY-101 and its capacity to cause a pronounced and sustainable reduction of gingival inflammation are likely to enhance clinical outcomes beyond those achieved via conventional periodontal treatment, thus warranting further investigation in phase III clinical trials.
Methods
Study design and randomization
This phase IIa study included a dose selection phase (see details under “Route of administration and dose selection”) with escalating dose groups (3 dose groups, each consisting of 4 patients, i.e., 12 patients in total) and a subsequent main study phase testing AMY-101 at the selected dose compared with placebo per the estimated sample size. The main study phase comprised the test (AMY-101) and placebo groups in a randomized, double-blind, split-mouth design. After screening, qualified participants were enrolled in the study and received baseline evaluations within 30 days of screening. Treatment sites consisting of 1 maxillary and 1 mandibular quadrant were randomized into treatment groups as “Right” and “Left” of the mouth using a permuted block randomization schedule. The randomization sequence was generated by the study biostatistician with a random number generator. The randomization scheme was kept in the sole possession of the unblinded study staff, who were responsible for randomizing the treatment sites for each participant, preparing and providing masked study products to blinded study clinicians for injections, and maintaining the study product inventories throughout the study.
Participant selection, enrollment, and compliance assessment
Participants were recruited from the volunteer pool at The Forsyth Institute CCTR between July 2019 and October 2020, with a pause between March 12, 2020, and May 26, 2020, in new participant enrollment due to pandemic-related restrictions in the City of Cambridge and State of Massachusetts per CDC recommendations. Study participants (n = 39 total), aged 18 to 65 years, were generally healthy adults with at least 20 natural teeth and diagnosed with gingivitis/periodontitis presenting as generalized gingival inflammation, as defined by an MGI of 2.0 or higher and 40% or greater sites with BOP. The treatment sites in each participant were randomly assigned to receive AMY-101 at a dose of 0.1 mg/injection site in 25 μL water for the injection and saline solution (placebo) in a 1:1 randomization scheme. The sample size calculations were performed, and a group size of 30 was determined to allow for a statistical difference at P > 0.05 and 80% power with an assumption of a 10% dropout rate. Participants in all groups were instructed to maintain their regular brushing habits with twice-daily brushing.
Individuals with current medical conditions or on medications known to affect periodontal tissues or interfere with any of the study outcomes were excluded. In addition, individuals with orthodontic appliances, pregnant and nursing women, and current or former cigarette smokers within 1 year of enrollment (including e-cigarette and recreational cannabis use) were excluded, given the potential confounding effects on study outcomes. Further, participants who received periodontal therapy, except regular hygiene recall within a year, or who received systemic antibiotic therapy or used antiinflammatory drugs more than 3 times a week within 30 days of study initiation were not included, because they were potential confounders for efficacy outcomes.
After baseline, the participants were seen on days 7 and 14 for repeated treatments and safety evaluations and on days 21, 28, 60, and 90 for safety, compliance, and efficacy measurements. A follow-up phone call on the next day of each treatment visit on days 0, 7, and 14 was also performed for safety evaluation. Protocol compliance was assessed at all follow-up study visits by an exclusive interview of the participant regarding oral hygiene practices, dental or medical treatments received, and concomitant medication or other oral hygiene aids used. Deviations, if any, were recorded, and participants were reinstructed if needed.
Outcome measures
Clinical efficacy and safety endpoints were selected to determine the impact of AMY-101 on gingival inflammation and to assess its safety level.
Efficacy endpoints.
A primary efficacy endpoint, the MGI (56), and a secondary endpoint, BOP (57), were used as indices of gingival inflammation and evaluated at baseline and at all monitoring visits (days 21, 28, 60, and 90). The PI (58), which assesses the amount of plaque on tooth surfaces, PD, and CAL were measured as additional secondary efficacy endpoints at all monitoring visits. Moreover, MMP-8 and MMP-9 levels in the GCF were measured at baseline and at all post-treatment time points. GCF samples were collected from 2 sites per quadrant, with the highest MGI score determined using sterile PerioPaper strips (OraFlow) for 30 seconds. The samples were then snap-frozen in liquid nitrogen and stored at –80°C until analysis. On the day of analysis, the frozen GCF samples were thawed at room temperature, and proteins were eluted through 2 centrifugations at 13,000g at 4°C for 8 minutes in a total of 120 μL sterile PBS (pH 7.4). In all analyses, 100 μL eluted solution was used. GCF samples were analyzed for MMP-8 and MMP-9 using a 2-plex human MMP panel by multiplexed sandwich immunoassays, based on flowmetric multiplex technology at The Forsyth Institute Luminex Core as described previously (59).
Safety endpoints.
Safety evaluations were performed clinically at each visit and through phone calls to assess AEs. Assessments were made with regard to changes in medical history, concomitant medication use; vital signs (blood pressure, heart rate, and respiratory rate); oral soft and hard tissues, and injection sites (for ulceration, infection, or mucosal irritancy using an edema/erythema score; ref. 60); and the levels of liver enzymes, alkaline phosphatase (ALP), aspartate transaminase (AST), and alanine transaminase (ALT), as measured by a Clinical Laboratory Improvement Amendments (CLIA) laboratory (Quest Diagnostics). Physical assessments included the examination of the head and neck, lymph nodes, face, skin, and lips, in addition to the measurement of height and weight at baseline and on day 90. A urine pregnancy test using an over-the-counter rapid chromatographic immunoassay for the qualitative detection of human chorionic gonadotropin was performed for women of child-bearing potential at screening and at baseline prior to randomization. An independent medical monitor was assigned to review out-of-range laboratory values as well as AE assessment and to provide expert guidance on further actions, if needed, with the principal investigator and data and safety monitoring board (DSMB) members. Patient-related outcomes included pain/discomfort, bleeding, or gingival irritation, and information was collected by the study clinician immediately and 1 hour after injections. Patients were also followed up via phone call 24 hours after injections to assess patient-related outcomes.
As part of this study, the presence of anti-drug antibodies in the plasma of patients was evaluated as an exploratory endpoint. Blood samples were obtained and evaluated at baseline and on days 14 and 21. Immunogenicity analysis was performed using a direct ELISA with AMY-101 coated on the plate followed by serial dilution of patients’ plasma and anti-human IgG-HRP for the detection of any AMY-101–bound antibodies.
Examiner calibration and clinician and study team training
A single trained and calibrated examiner blinded to the study treatments was responsible for all clinical oral measurements and safety assessments throughout the course of the study. An intraexaminer calibration exercise was conducted prior initiation of the study to test the repeatability level (61) with a coefficient of 0.87. An experienced dental clinician blinded to treatment allocations was assigned to treatment injections after proper training. The study team received protocol-specific training prior to study initiation and throughout the conduct of the study under the supervision of the principal investigator and the independent study monitor.
Study products
The study product, AMY-101 acetate, which received an investigational new drug (IND) designation (sponsor: Amyndas Pharmaceuticals) from the FDA prior to study initiation, is a white to off-white lyophilized powder for injection after reconstitution. AMY-101 was supplied in single-use vials containing 50 mg of the drug substance AMY-101 acetate. For the injections in the study, the lyophilized product was reconstituted in 1 mL water for injections (WFI) to obtain an initial concentration of 50 mg/mL at pH 5.0. It was subsequently further diluted, using sterile saline, to a final concentration of 4 mg/mL. Saline was used for further dilutions as required. The diluents including WFI and saline were sterile and obtained from a commercial FDA-approved supplier (Hospira).
Storage and stability.
AMY-101 was stored at –20 ± 5 °C (protected from light) in a –20°C freezer located in a dedicated and secure room with limited access at The Forsyth Institute CCTR until use. Following reconstitution, the solutions were kept at 4°C and used within 1 day after further dilutions. Temperature logs were kept to document changes in temperature.
Route of administration and selection of dose
The route of administration and clinically effective dose were determined in preclinical NHP studies (22, 26, 27). In these studies, the effective therapeutic dose of locally administered AMY-101 was 0.1 mg/injection site in NHPs, with no local irritation and long-lasting antiinflammatory effects (sustained therapeutic efficacy). In addition, a phase I study of AMY-101 administered systemically through the subcutaneous and intravenous routes in the single ascending dose (SAD) part of the study, and as multiple doses subcutaneously, established a good safety profile for the drug (ClinicalTrials.gov ID: NCT03316521). Thus, this human clinical study was designed to use AMY-101 in a single dose of 0.1 mg/injection site in a split-mouth design compared with saline injection. Based on human PK studies, this locally administered dose (a “micro-dose” compared with the doses administered systemically in the phase I study of AMY-101) was not expected to result in any systemic drug exposure in the patient. Nevertheless, since this was a first-in-human gingival tissue use, a dose escalation phase was included at the beginning of the study to assess safety and local tissue parameters after injections and to select the safe and effective dose prior to enrollment in the main study (Supplemental Table 1). AMY-101, in escalating doses of 0.025 mg/injection site, 0.05 mg/injection site, and 0.1 mg/injection site, was randomly administered to 12 patients and followed the same treatment and monitoring schedule as the main study (split-mouth, placebo-controlled, 90 days). After treatment of the first group (once weekly over 3 weeks), the second dose group was added followed by the third dose group on the basis of safety evaluations by the principal investigator and the DSMB member.
AMY-101 was administered as a single injection into each papilla between all teeth present in the randomly assigned side of the mouth (left or right) at each treatment visit (3 weekly visits). For most of the study population, an equal number of sites were treated with AMY-101 and placebo, with only a small number of participants having a slight variation in the number of treated sites (22 versus 24, or 26 versus 28). Overall, in 31 patients included in the efficacy analysis, a total of 1076 sites received AMY-101 and the same number of sites (n = 1076) received placebo injections.
Data analyses and statistical methods
Analysis population.
The safety population consisted of all participants who were enrolled and received the study treatments at the baseline visit. The efficacy population consisted of all participants from the safety population who completed at least 1 post-baseline assessment of the primary and 1 or more of the secondary efficacy outcome measures. The per-protocol population consisted of all participants in the efficacy population who did not have any major protocol violations (deviation) during the study. Analyses were conducted on this population in support of the primary efficacy results.
Sample size determination and power.
As this was a phase II proof-of-concept study, exploratory statistical analysis including repeated measures and modeling approaches was performed. Sample size determination was based on the power to detect study arm differences with respect to the MGI, the primary efficacy outcome measure. Sample size calculations were made on the basis of a generalized estimating equation (GEE) model to detect a difference of a 0.2 MGI unit between the groups. With a SD of 0.3 MGI units and using (a) an overall 0.05 significance level; (b) a power of 78.9%; (c) a GEE model for repeated measurements; and (d) an exchangeable correlation structure with a correlation coefficient of ρ = 0.3 among repeated measurements, 27 individuals in the group were needed to detect an effect size of 0.20 (mean MGI change). Assuming a 10% participant attrition rate, we planned to enroll 30 participants who would receive the selected dose. Per the protocol, those who dropped out before the first post-baseline visit (day 21) on efficacy measurements were replaced.
Statistics
The primary efficacy analysis was based on the efficacy population and the per-protocol population. A secondary efficacy analysis of primary and secondary efficacy endpoints was based on the efficacy population. Descriptive statistics (including the number of participants and the mean, SD, median, minimum, and maximum) were calculated by treatment group and placebo for the means and mean change from baseline on day 28 for the primary efficacy endpoint and on days 21, 28, 60, and 90 for the secondary efficacy endpoints. A change in the mean value at a specified time point was analyzed using a repeated measures model and the GEE approach, with the treatment group, study visit, and interaction between treatment groups and study visit as fixed effects and the baseline as the covariate. The adjusted LSM and SEM from the model were presented for the treatment and placebo groups for each visit. The LSM estimates for the difference in treatment group versus placebo, SEM, 95% CI, and P values are also provided. The individual mean changes on day 28 and day 90 in the MGI and BOP as well as GCF levels of MMP-8 and MMP-9 were compared between treatments by unpaired, 2-tailed Student’s t test, while within-group analyses were performed by 1-way ANOVA with mixed effects followed by Tukey’s test. The safety analysis was based on the safety population. All TEAEs were summarized by the severity and relationship to the study drug. Descriptive statistics of the observed value and change from baseline were provided for vital signs and liver enzyme levels. Oral examination findings were summarized using the frequency count and percentages by treatment and placebo groups. The statistical programs SAS PROC GENMOD and GraphPad Prism 9.2.0 (GraphPad Software) were used for statistical analysis of the safety and efficacy data
Study approval
The protocol, screening, and study consent forms, recruitment materials, and all participant materials were reviewed and approved by the IRB of The Forsyth Institute before any participant was enrolled (IRB protocol no. 19-07). All amendments to the protocol were reviewed and approved by the IRB before changes in the study were implemented. The study was registered with ClinicalTrials.gov (NCT03316521).
Ethical conduct of the study.
This study was conducted in full conformity with the principles set forth in The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research, as drafted by the United States National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (April 18, 1979) and codified in 45 Code of Federal Regulations (CFR) Part 46 and/or the International Council for Harmonization (ICH) Guidelines for Good Clinical Practice E6. The study was conducted according to the principles expressed in the Declaration of Helsinki and policy of the International Committee of Medical Journal Editors was followed.
Participant information and consent.
The informed consent process was initiated prior to the individual agreeing to participate in study at the screening and enrollment visit and continued throughout study participation. An extensive explanation of the risks and possible benefits of study participation was provided, and informed consent was obtained from all participants prior to any study-related assessments or procedures.
Author contributions
HH and DY designed and conducted the clinical trial and wrote the manuscript. GH and JDL contributed to the writing and editing of the nonclinical part of the manuscript. DCM contributed to the study’s design, data analysis, and editing of the manuscript. The Forsyth Institute CCTR staff performed the clinical trial and data and sample collection. All authors read and approved the final version of the article.
Supplementary Material
Acknowledgments
This clinical trial was conducted at The Forsyth Institute CCTR. The authors would like to thank the CCTR’s clinical and administrative teams and laboratory staff members Danielle Stephens, Michele Patel, and Afsah Dean for their technical support and analysis of GCF samples (See Supplemental Acknowledgments for research staff consortium details). The services by Quartesian LLC for data entry and regulatory oversight, primary and post hoc statistical analyses for safety, and clinical endpoints are gratefully acknowledged. We also thank the study participants for their time and commitment during the study. The authors would like to thank Antonio Risitano for his expert medical guidance in the assessment of safety data, as well as J. Max Goodson, a DSMB member, for his independent statistical analysis on dose selection for AMY-101. The authors wish to thank Tomoki Maekawa, Tetsuhiro Kajikawa, Evlambia Hajishengallis, and Ranillo Resuello, whose contributions to the preclinical phase made this human trial possible. This study was supported by Amyndas Pharmaceuticals.
Version 1. 10/07/2021
In-Press Preview
Version 2. 12/01/2021
Electronic publication
Footnotes
Conflict of interest: JDL is the founder of Amyndas Pharmaceuticals, which develops complement inhibitors (including third-generation compstatin analogs such as AMY-101). JDL is an inventor on patents and patent applications that describe the use of complement inhibitors for therapeutic purposes, some of which were clinically developed by Amyndas Pharmaceuticals (“Compstatin Analogs with Increased Solubility and Improved Pharmacokinetic Properties,” patent no. 10,800,812; “Compstatin Analogs with Improved Pharmacokinetic Properties,” patent no. 10745442; “Modified Compstatin with Peptide Backbone and C-Terminal Modifications,” patent no. 8946145). JDL and GH are inventors on a joint patent that describes the use of complement inhibitors for therapeutic purposes in periodontitis (“Methods of Treating or Preventing Periodontitis and Diseases Associated with Periodontitis,” patent no. 10,668,135). JDL is also the inventor of the compstatin technology licensed to Apellis Pharmaceuticals [i.e., 4(1MeW)7W/POT-4/APL-1 and PEGylated derivatives such as APL-2/pegcetacoplan (Empaveli). “Compstatin Analogs with Improved Activity,” US patent no. 7989589; “Peptides which Inhibit Complement Activation,” patent no. 6319897; “Potent Compstatin Analogs,” patent no. 7888323]. DY is the managing director of Amyndas Pharmaceuticals, the sponsor of this study.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(23):e152973.https://doi.org/10.1172/JCI152973.
Contributor Information
Hatice Hasturk, Email: hhasturk@forsyth.org.
George Hajishengallis, Email: geoh@upenn.edu.
John D. Lambris, Email: lambris@pennmedicine.upenn.edu.
Dimitrios C. Mastellos, Email: mastellos@rrp.demokritos.gr.
Despina Yancopoulou, Email: dyancopoulou@amyndas.com.
References
- 1.Kinane DF, et al. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 2.Eke PI, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86(5):611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frencken JE, et al. Global epidemiology of dental caries and severe periodontitis - a comprehensive review. J Clin Periodontol. 2017;44(s18):S94–S105. doi: 10.1111/jcpe.12677. [DOI] [PubMed] [Google Scholar]
- 4.Kassebaum NJ, et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21(7):426–440. doi: 10.1038/s41577-020-00488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenkein HA, et al. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol 2000. 2020;83(1):90–106. doi: 10.1111/prd.12304. [DOI] [PubMed] [Google Scholar]
- 7.Chapple IL. Time to take periodontitis seriously. BMJ. 2014;348:g2645. doi: 10.1136/bmj.g2645. [DOI] [PubMed] [Google Scholar]
- 8.Peres MA, et al. Oral diseases: a global public health challenge. Lancet. 2019;394(10194):249–260. doi: 10.1016/S0140-6736(19)31146-8. [DOI] [PubMed] [Google Scholar]
- 9.Collaborators GDaIIaP. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Righolt AJ, et al. Global-, regional-, and country-level economic impacts of dental diseases in 2015. J Dent Res. 2018;97(5):501–507. doi: 10.1177/0022034517750572. [DOI] [PubMed] [Google Scholar]
- 11.Botelho J, et al. Economic burden of periodontitis in the United States of America and Europe – an updated estimation. J Periodontol. doi: 10.1002/jper.21-0111. [published online May 30, 2021]. [DOI] [PubMed] [Google Scholar]
- 12.Lamont RJ, et al. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balta MG, et al. Host modulation and treatment of periodontal disease. J Dent Res. 2021;100(8):798–809. doi: 10.1177/0022034521995157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasturk H, et al. Paradigm shift in the pharmacological management of periodontal diseases. Front Oral Biol. 2012;15:160–176. doi: 10.1159/000329678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golub LM, Lee HM. Periodontal therapeutics: current host-modulation agents and future directions. Periodontol 2000. 2020;82(1):186–204. doi: 10.1111/prd.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajishengallis G, et al. Novel mechanisms and functions of complement. Nat Immunol. 2017;18(12):1288–1298. doi: 10.1038/ni.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricklin D, et al. Complement in disease: a defence system turning offensive. Nat Rev Nephrol. 2016;12(7):383–401. doi: 10.1038/nrneph.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patters MR, et al. Assessment of complement cleavage in gingival fluid during experimental gingivitis in man. J Clin Periodontol. 1989;16(1):33–37. doi: 10.1111/j.1600-051X.1989.tb01609.x. [DOI] [PubMed] [Google Scholar]
- 19.Niekrash CE, Patters MR. Simultaneous assessment of complement components C3, C4, and B and their cleavage products in human gingival fluid. II. Longitudinal changes during periodontal therapy. J Periodontal Res. 1985;20(3):268–275. doi: 10.1111/j.1600-0765.1985.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 20.Grande MA, et al. Complement split product C3c in saliva as biomarker for periodontitis and response to periodontal treatment. J Periodontal Res. 2021;56(1):27–33. doi: 10.1111/jre.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajishengallis G, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10(5):497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maekawa T, et al. Genetic and intervention studies implicating complement C3 as a major target for the treatment of periodontitis. J Immunol. 2014;192(12):6020–6027. doi: 10.4049/jimmunol.1400569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajishengallis G, et al. Complement-dependent mechanisms and interventions in periodontal disease. Front Immunol. 2019;10:406. doi: 10.3389/fimmu.2019.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mastellos DC, et al. Clinical promise of next-generation complement therapeutics. Nat Rev Drug Discov. 2019;18(9):707–729. doi: 10.1038/s41573-019-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitz R, et al. C3 complement inhibition prevents antibody-mediated rejection and prolongs renal allograft survival in sensitized non-human primates. Nat Commun. 2021;12(1):5456. doi: 10.1038/s41467-021-25745-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kajikawa T, et al. Safety and efficacy of the complement inhibitor AMY-101 in a natural model of periodontitis in non-human primates. Mol Ther Methods Clin Dev. 2017;6:207–215. doi: 10.1016/j.omtm.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maekawa T, et al. Inhibition of pre-existing natural periodontitis in non-human primates by a locally administered peptide inhibitor of complement C3. J Clin Periodontol. 2016;43(3):238–249. doi: 10.1111/jcpe.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mastellos DC, et al. From discovery to approval: A brief history of the compstatin family of complement C3 inhibitors. Clin Immunol. doi: 10.1016/j.clim.2021.108785. [published online June 18, 2021]. [DOI] [PubMed] [Google Scholar]
- 29.Mullard A. First approval of a complement C3 inhibitor opens up autoimmune and inflammatory opportunities. Nat Rev Drug Discov. 2021;20(7):496. doi: 10.1038/d41573-021-00094-8. [DOI] [PubMed] [Google Scholar]
- 30.Cavalla F, et al. Matrix metalloproteinases as regulators of periodontal inflammation. Int J Mol Sci. 2017;18(2):440. doi: 10.3390/ijms18020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorsa T, et al. Active MMP-8 (aMMP-8) as a grading and staging biomarker in the periodontitis classification. Diagnostics (Basel) 2020;10(2):E61. doi: 10.3390/diagnostics10020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang S, et al. Meta-analysis of the association between serum and gingival crevicular fluid matrix metalloproteinase-9 and periodontitis. J Am Dent Assoc. 2019;150(1):34–41. doi: 10.1016/j.adaj.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Janssen BJ, et al. Structure of compstatin in complex with complement component C3c reveals a new mechanism of complement inhibition. J Biol Chem. 2007;282(40):29241–29247. doi: 10.1074/jbc.M704587200. [DOI] [PubMed] [Google Scholar]
- 34.Hajishengallis G, Lambris JD. More than complementing Tolls: complement-Toll-like receptor synergy and crosstalk in innate immunity and inflammation. Immunol Rev. 2016;274(1):233–244. doi: 10.1111/imr.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freeley S, et al. The “ins and outs” of complement-driven immune responses. Immunol Rev. 2016;274(1):16–32. doi: 10.1111/imr.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutzan N, et al. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med. 2018;10(463):eaat0797. doi: 10.1126/scitranslmed.aat0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garlet GP. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89(12):1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 38.Caton JG, et al. A new classification scheme for periodontal and peri-implant diseases and conditions — Introduction and key changes from the 1999 classification. J Periodontol. 2018;89 Suppl 1:S1–S8. doi: 10.1002/JPER.18-0157. [DOI] [PubMed] [Google Scholar]
- 39.Genco RJ, and Borgnakke WS. Risk factors for periodontal disease. Periodontol. 2013;62(1):59–94. doi: 10.1111/j.1600-0757.2012.00457.x. [DOI] [PubMed] [Google Scholar]
- 40.Thomson WM, et al. The natural history of periodontal attachment loss during the third and fourth decades of life. J Clin Periodontol. 2013;40(7):672–680. doi: 10.1111/jcpe.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albandar JM, Rams TE. Global epidemiology of periodontal diseases: an overview. Periodontol. 2002;29:7–10. doi: 10.1034/j.1600-0757.2002.290101.x. [DOI] [PubMed] [Google Scholar]
- 42.Sheridan RA, et al. Systemic chemotherapeutic agents as adjunctive periodontal therapy: a narrative review and suggested clinical recommendations. J Int Acad Periodontol. 2015;17(4):123–134. [PubMed] [Google Scholar]
- 43.Deas DE, et al. Scaling and root planing vs. conservative surgery in the treatment of chronic periodontitis. Periodontol. 2016;71(1):128–139. doi: 10.1111/prd.12114. [DOI] [PubMed] [Google Scholar]
- 44.Mombelli A, et al. Persistence patterns of Porphyromonas gingivalis, Prevotella intermedia/nigrescens, and Actinobacillus actinomyetemcomitans after mechanical therapy of periodontal disease. J Periodontol. 2000;71(1):14–21. doi: 10.1902/jop.2000.71.1.14. [DOI] [PubMed] [Google Scholar]
- 45.Hillmen P, et al. Pegcetacoplan versus Eculizumab in Paroxysmal Nocturnal Hemoglobinuria. N Engl J Med. 2021;384(11):1028–1037. doi: 10.1056/NEJMoa2029073. [DOI] [PubMed] [Google Scholar]
- 46. U.S. Food and Drug Administration. FDA approves new treatment for adults with serious rare blood disease. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-new-treatment-adults-serious-rare-blood-disease Updated May 18, 2021. Accessed October 13, 2021.
- 47.Hajishengallis G, et al. C3-targeted therapy in periodontal disease: moving closer to the clinic. Trends Immunol. 2021;42(10):856–864. doi: 10.1016/j.it.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ingman T, et al. Matrix metalloproteinases and their inhibitors in gingival crevicular fluid and saliva of periodontitis patients. J Clin Periodontol. 1996;23(12):1127–1132. doi: 10.1111/j.1600-051X.1996.tb01814.x. [DOI] [PubMed] [Google Scholar]
- 49.Katsiki P, et al. Comparing periodontitis biomarkers in saliva, oral rinse and gingival crevicular fluid: a pilot study. J Clin Periodontol. 2021;48(9):13479–1259. doi: 10.1111/jcpe.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Öztürk V, et al. Evaluation of active matrix metalloproteinase-8 (aMMP-8) chair-side test as a diagnostic biomarker in the staging of periodontal diseases. Arch Oral Biol. 2021;124:104955. doi: 10.1016/j.archoralbio.2020.104955. [DOI] [PubMed] [Google Scholar]
- 51.Hernandez M, et al. Active MMP-8 quantitative test as an adjunctive tool for early diagnosis of periodontitis. Diagnostics (Basel) 2021;11(8):Article 1503. doi: 10.3390/diagnostics11081503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorsa T, et al. Active matrix metalloproteinase-8 (aMMP-8) point-of-care test (POCT) in the COVID-19 pandemic. Expert Rev Proteomics. 2021;18(8):707–717. doi: 10.1080/14789450.2021.1976151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trindade R, et al. Osseointegration and foreign body reaction: Titanium implants activate the immune system and suppress bone resorption during the first 4 weeks after implantation. Clin Implant Dent Relat Res. 2018;20(1):82–91. doi: 10.1111/cid.12578. [DOI] [PubMed] [Google Scholar]
- 54.Liu X, et al. Osteoclast differentiation and formation induced by titanium implantation through complement C3a. Mater Sci Eng C Mater Biol Appl. 2021;122:111932. doi: 10.1016/j.msec.2021.111932. [DOI] [PubMed] [Google Scholar]
- 55.Mastellos DC, et al. Complement C3 vs C5 inhibition in severe COVID-19: Early clinical findings reveal differential biological efficacy. Clin Immunol. 2020;220:Article 108598. doi: 10.1016/j.clim.2020.108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lobene RR, et al. A modified gingival index for use in clinical trials. Clin Prev Dent. 1986;8(1):3–6. [PubMed] [Google Scholar]
- 57.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25(4):229–235. [PubMed] [Google Scholar]
- 58.Silness J, Loe H. Periodontal disease in pregnancy. Ii. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 59.Arvikar SL, et al. Periodontal inflammation and distinct inflammatory profiles in saliva and gingival crevicular fluid (GCF) compared with serum and joints in rheumatoid arthritis patients. J Periodontol. 2021;92(10):1379–1391. doi: 10.1002/JPER.20-0051. [DOI] [PubMed] [Google Scholar]
- 60.Sonis ST, et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group. Cancer. 1999;85(10):2103–2013. doi: 10.1002/(SICI)1097-0142(19990515)85:10<2103::AID-CNCR2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 61.Hefti AF, Preshaw PM. Examiner alignment and assessment in clinical periodontal research. Periodontol. 2012;59(1):41–60. doi: 10.1111/j.1600-0757.2011.00436.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.