Abstract
The feasibility of using a capacitance method (CM) for direct antifungal susceptibility testing of yeasts in positive blood cultures was evaluated. The CM used the same test conditions as those recommended by the National Committee for Clinical Laboratory Standards. After direct inoculation of positive culture broths into module wells (Bactometer; bioMérieux, Inc., Hazelwood, Mo.), the end-point determination was made by monitoring the capacitance change in the culture broths with Bactometer. The MIC of amphotericin B was the lowest concentration at which yeast growth was completely inhibited, while the MICs of ketoconazole, flucytosine, and fluconazole were the concentrations at which a ≥80% reduction in capacitance change was observed. The MICs of the four drugs against each blood isolate obtained on subculture plates were also determined by the macrodilution method. For 51 positive blood cultures tested, the percent agreement (±2 log2 dilutions) between the CM and the macrodilution method were as follows: amphotericin B (98%), ketoconazole (92%), flucytosine (84%), and fluconazole (96%). The CM was further used for breakpoint susceptibility testing of fluconazole (8 and 64 μg/ml) and flucytosine (4 and 32 μg/ml) against yeasts in positive blood cultures. After testing of 74 specimens by the CM, flucytosine and fluconazole produced one (1.4%) major error and two (2.8%) minor errors, respectively. All yeasts that displayed resistance to flucytosine or fluconazole were detected within 24 h after direct inoculation of the positive broths into Bactometer. The CM may be useful for the rapid detection of antifungal resistance in positive blood cultures containing yeasts.
In the past few years, there has been a dramatic increase in the number of systemic fungal infections reported around the world. The incidence of nosocomial candidemia was estimated to rise fivefold in the past 10 years (3). In a recent report from Taiwan (4), fungal pathogens accounted for a higher proportion of nosocomial bloodstream infections than any single bacterial species. At the same time, there has been increasing concern about the emergence of resistance to antifungal agents among a variety of yeast species (15, 16, 22, 23, 26–28). Therefore, the development of a standardized method for antifungal susceptibility testing that can predict clinical outcome and response to therapy has assumed greater importance (11). The National Committee for Clinical Laboratory Standards (NCCLS) has developed a reference broth macrodilution method for susceptibility testing of yeasts (19). Several modifications of the reference method have been proposed; these techniques include flow cytofluorometric detection (21, 34), colorimetric microdilution (6, 24, 30), Etest (5, 25, 29), and a modified agar dilution method (37).
Candidemia has been shown to contribute to excess hospitalization stay and to be an independent determinant for death (1, 35). The mortality rates of fungemia are about two to three times those of bacteremia (33). A positive blood culture bottle containing yeasts, as revealed by the Gram stain, normally has been subcultured on agar plates for colony isolation followed by species identification and susceptibility testing, if necessary. Several studies (8, 17, 18) have demonstrated that direct antibacterial susceptibility testing, that is, performance of susceptibility testing without a prior isolation step, is feasible under most conditions for positive blood cultures containing bacteria. The benefits of rapid antimicrobial susceptibility testing for infectious disease outcome have been studied, and several advantages are recognized (7, 9, 31).
The activities of microbial growth will cause electrical changes (impedance, conductance, or capacitance) in the culture media. Measurement of an electrical signal is basically not prevented by the color or turbidity of the clinical specimens. The purpose of this study was to test the feasibility of direct antifungal susceptibility testing of positive blood cultures by a capacitance method (CM).
MATERIALS AND METHODS
Study design.
The objectives of this study were (i) to evaluate the reproducibility and correlation of the CM with the NCCLS macrodilution method for the determination of MICs for pure yeast strains, (ii) to evaluate the feasibility of the CM for direct measurement of the MICs of antifungal agents against yeasts in positive blood cultures, and (iii) to perform the CM in a breakpoint broth dilution format for direct determination of interpretive susceptibilities (resistant, susceptible-dose dependent [S-DD] or intermediate, or susceptible) of yeasts in positive blood cultures.
Yeast stock cultures.
A panel of 10 yeast strains was used to test the reliability of the CM for the determination of MICs. These yeasts included Candida albicans ATCC 18804, 24433, and 10231; C. tropicalis ATCC 750; C. parapsilosis C4-13 (a clinical isolate); C. glabrata ATCC 2001 and C3-2 (a clinical isolate); C. krusei ATCC 6258; and C. guilliermondii ATCC 9058. Among these 10 strains, C. krusei ATCC 6258 was a quality control strain, while C. albicans ATCC 24433 and C. tropicalis ATCC 750 were reference strains, as recommended by the NCCLS (19). All strains were subcultured at least twice on Sabouraud dextrose agar (Difco, Detroit, Mich.) and incubated at 35°C to ensure purity and viability.
Antifungal agents.
Amphotericin B (Sigma Chemical Co., St. Louis, Mo.), ketoconazole (U.S. Pharmacopoeia, Rockville, Md.), flucytosine (U.S. Pharmacopoeia), and fluconazole (Pfizer, New York, N.Y.) were used for MIC determinations. Stock solutions were prepared with the following solvents: dimethyl sulfoxide for amphotericin B and ketoconazole and sterile deionized water for flucytosine and fluconazole. The weight of each antifungal agent was adjusted according to the potency of each drug.
Clinical specimens.
The blood specimens were collected from the National Cheng Kung University Hospital (an 800-bed teaching hospital) and from the Kaoshung Chang Gung Memorial Hospital. BACTEC blood culture bottles (Becton Dickinson Microbiology Systems, Cockeysville, Md.) were normally inoculated with 3 to 10 ml of blood from patients, inserted into the BACTEC NR9240 instrument (Becton Dickinson Microbiology Systems), and incubated at 35°C. Positive bottles were automatically detected by the instrument, and smears were prepared to check the presence of yeasts in the positive bottles. Data for mixed cultures containing more than one strain of yeasts or containing yeasts and bacteria, as revealed on subculture plates, were not used for the calculation of agreement values.
Numbers of yeast cells in positive blood culture bottles.
To enumerate the numbers of yeast cells in positive bottles, serial 10-fold dilutions of the culture broths were made in sterile saline. The numbers of cells (CFU per milliliter) in the diluted suspensions were determined by the plate count method (10) with Sabouraud dextrose agar as the culture medium. Plates were incubated at 35°C and enumerated after 48 h of incubation. Fourteen randomly selected positive blood culture bottles were analyzed.
Determination of MICs.
For pure yeast strains, the CM used the same test conditions (medium, inoculum size, and incubation temperature) as those recommended by the NCCLS (19) for MIC determinations, except that the end point was determined by monitoring the capacitance change in the culture broth. To each module well (Bactometer; bioMérieux, Inc., Hazelwood, Mo.) containing 0.9 ml of the test organism (0.5 × 103 to 2.5 × 103 cells/ml), 0.1 ml of a dilution series of antifungal agent was added and mixed. The modules were incubated at 35°C for 48 h, and the capacitance change (i.e., capacitance growth curve) in each well was continuously monitored with Bactometer. Each strain was tested five times against each of the four drugs on different experimental days. A growth control (no antifungal agent in the well) and a negative control (culture broth only) were included for each strain tested. The MIC of amphotericin B was the lowest concentration at which yeast growth was completely inhibited; i.e., no change in capacitance was observed compared to the negative control. The MICs of flucytosine, fluconazole, and ketoconazole were the concentrations at which a ≥80% reduction in capacitance change (compared with the growth control) was observed. Detection time in hours for each module well was automatically determined by the instrument software when three consecutive readings of the signal change exceeded the default value in the instrument. At the detection time, the slope of the capacitance growth curve had an accelerating increase.
For direct determination of MICs of the four drugs against yeasts in positive blood cultures, serial log2 dilutions of each drug in 1 ml of RPMI 1640 broth were constructed in the module wells of Bactometer. Positive culture broths containing yeasts were diluted 1:10 with sterile saline, and 10 μl of the sample was inoculated into each of the module wells. The modules were incubated at 35°C for 48 h, and MICs were read by using the same criteria for end-point determination as those used with pure yeast cultures. A total of 51 positive blood cultures were analyzed by the CM for MIC determinations. Each blood isolate obtained on subculture plates was identified by conventional procedures (32), and the MICs of the four drugs against the blood yeast isolate were determined by the macrodilution method (19). Discrepancies (±1 log2 and ±2 log2 dilutions) in the MICs obtained by the CM and the macrodilution method were used for calculation of the percent agreement values.
Breakpoint susceptibility testing of yeasts in positive blood cultures.
Fluconazole and flucytosine were used for direct susceptibility testing, with each agent being tested at the two interpretive breakpoint concentrations (fluconazole, 8 and 64 μg/ml; flucytosine, 4 and 32 μg/ml), as defined by the NCCLS (19). Positive culture broths containing yeasts were diluted 1:10 with sterile saline, and 10 μl of the diluted sample was inoculated into each module well containing 1 ml of RPMI 1640 broth supplemented with an antifungal agent. The inoculated modules were incubated at 35°C, and the capacitance change in each module was monitored for 48 h. A growth control and a negative control were included for each blood specimen tested. A total of 75 positive blood cultures containing yeasts were analyzed. Interpretive categorization of the blood isolate by the direct method was based on the inhibition of the microorganism at the two breakpoint concentrations (19). All yeast isolates obtained on subculture plates were identified (32), and the MICs of fluconazole and flucytosine against each isolate were determined by the macrodilution method. The MIC data for each isolate were used for categorization of the interpretive susceptibilities (19).
Definitions of test errors.
The results of breakpoint susceptibility testing by the CM were compared with those obtained from the macrodilution method, and discrepancies were classified as very major, major, or minor errors (8). A very major error was a susceptible result by the CM and a resistant result by the standard method. A major error was a resistant result by the CM and a susceptible result by the macrodilution method. A minor error was any change involving an S-DD or intermediate result.
RESULTS
Reliability of the CM for MIC determination.
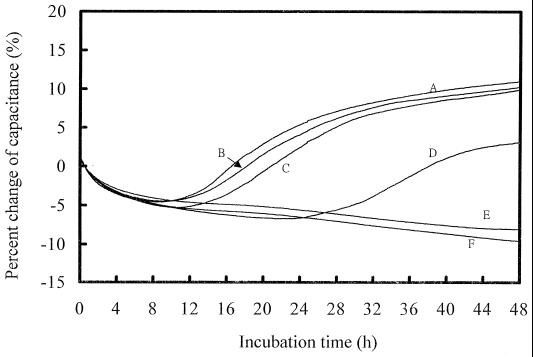
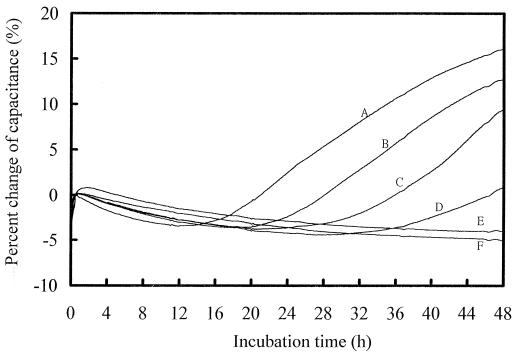
The MICs for 10 pure yeast cultures were determined by using four antifungal agents, with each strain being tested five times against each drug on different days. Figure 1 shows typical capacitance growth curves for C. krusei ATCC 6258 in the presence of different concentrations of fluconazole. The MIC of fluconazole was determined to be 64 μg/ml (Fig. 1, curve E); at this drug concentration, the reduction in capacitance change was ≥80% when a comparison of capacitance change against the growth control was made. The detection time of the growth control was about 10 h (Fig. 1). Figure 2 shows the capacitance growth curves for C. parapsilosis C4-13 in the presence of amphotericin B. The MIC was 0.5 μg/ml; at this drug concentration, yeast growth was completely inhibited and almost no capacitance change was observed when a comparison of capacitance change against the negative control was made. The detection time of C. parapsilosis C4-13 was longer (15 h) than that to detection of C. krusei ATCC 6258.
FIG. 1.
Capacitance growth curves for C. krusei ATCC 6258 in the presence of different concentrations of fluconazole. Curve A, growth control; curves B, C, D, and E, 8, 16, 32, and 64 μg/ml, respectively; curve F, negative control. The MIC was determined to be 64 μg/ml.
FIG. 2.
Capacitance growth curves for C. parapsilosis C4-13 in the presence of different concentrations of amphotericin B. Curve A, growth control; curves B, C, D, and E, 0.06, 0.12, 0.25, and 0.5 μg/ml, respectively; curve F, negative control. The MIC was determined to be 0.5 μg/ml.
Table 1 summarizes the median MICs of amphotericin B, ketoconazole, flucytosine, and fluconazole against each of the 10 yeast strains, as determined by the CM and the broth macrodilution technique. For the quality control strain (C. krusei ATCC 6258) tested by the CM, the median MICs of each of the four antifungal agents all fell within the reference ranges established by the NCCLS (19). The MICs for the two reference strains (C. albicans ATCC 24433 and C. tropicalis ATCC 750) also fell within the established ranges. In Table 1, a total of 40 pairs of data were obtained for method comparison. If a discrepancy of median MICs no greater than 2 log2 dilutions was allowed, the agreement rates between the CM and the reference method were 90% for ketoconazole and 100% for amphotericin B, flucytosine, and fluconazole.
TABLE 1.
Comparison of the MICs of amphotericin B, ketoconazole, flucytosine, and fluconazole determined by the CM and the broth macrodilution method (BMM) for 10 yeast strains
| Organism | Median MICa (μg/ml) of the following drug determined by the indicated method:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Amphotericin B
|
Ketoconazole
|
Flucytosine
|
Fluconazole
|
|||||
| CM | BMM | CM | BMM | CM | BMM | CM | BMM | |
| C. albicans ATCC 18804 | 0.25 | 0.25 | 0.12 | 0.12 | 0.12 | 0.06 | 0.25 | 0.5 |
| C. albicans ATCC 24433 | 0.25 | 0.5 | 0.06 | 0.06 | 1 | 1 | 0.25 | 0.5 |
| C. albicans ATCC 10231 | 0.25 | 0.25 | 0.03 | 0.12 | 0.25 | 0.12 | 0.5 | 2 |
| C. tropicalis ATCC 750 | 0.5 | 0.5 | 0.5 | 0.25 | 0.03 | 0.12 | 2 | 1 |
| C. tropicalis C2-1 | 0.25 | 0.25 | 2 | 0.25 | 0.25 | 0.06 | 1 | 1 |
| C. parapsilosis C4-13 | 0.5 | 0.25 | 0.06 | 0.06 | 0.25 | 0.5 | 0.5 | 0.5 |
| C. glabrata ATCC 2001 | 0.25 | 0.5 | 4 | 2 | 0.06 | 0.03 | 8 | 16 |
| C. glabrata C3-2 | 0.5 | 0.5 | 4 | 4 | 0.06 | 0.12 | 4 | 16 |
| C. krusei ATCC 6258 | 1 | 0.5 | 0.5 | 0.5 | 16 | 8 | 64 | 64 |
| C. guilliermondii ATCC 9058 | 0.25 | 0.5 | 0.03 | 0.12 | 0.06 | 0.03 | 8 | 8 |
Each experiment was performed five times.
Yeast cell numbers in positive blood cultures.
The counts of yeast cells in 14 randomly selected positive bottles ranged from 105 to 107 CFU/ml, with 13 bottles (93%) being in the range of 106 to 107 CFU/ml.
Direct MIC determination for yeasts in positive blood cultures. Fifty-two positive blood culture bottles containing yeasts were used for the direct determination of MICs of amphotericin B, ketonconazole, flucytosine, and fluconazole by the CM. The MICs for the blood isolates obtained on subculture plates were also determined by the reference macrodilution method. With one bottle being a mixed culture (C. albicans and C. pelliculosa), a total of 53 yeast strains were recovered from the 52 blood samples. The remaining 51 strains included C. albicans (30 strains), C. parapsilosis (8 strains), C. tropicalis (7 strains), C. glabrata (4 strains), C. guilliermondii (1 strain), and one unidentified species. For the mixed culture containing two strains of yeast, the CM detected the more resistant side of the mixed flora. For example, the respective MICs (in micrograms per milliliter) for the mixed culture (C. albicans and C. pelliculosa) determined by the reference method were as follows: amphotericin B (0.5 and 1), ketoconazole (0.06 and 2), flucytosine (0.12 and 0.25), and fluconazole (0.06 and 0.25). However, the MICs (in micrograms per milliliter) obtained by the direct CM were as follows: amphotericin B (1.0), ketoconazole (1.0), flucytosine (0.25), and fluconazole (0.25).
Table 2 shows the correlation of MICs obtained by the two methods. If a discrepancy of 1 log2 dilution was allowed, the percent agreement values between the CM and the macrodilution method ranged from 61% (flucytosine) to 84% (fluconazole). However, if a discrepancy of 2 log2 dilutions was allowed, the values increased to 84% (flucytosine), 92% (ketoconazole), 96% (fluconazole), and 98% (amphotericin B).
TABLE 2.
Correlation of MICs of four antifungal agents against yeasts in the 51 positive blood culturesa
| Antifungal agent | No. of isolates for which the log2 MIC ratiob was
|
% Agreement within the following log2 dilution(s):
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| <−2 | −2 | −1 | 0 | 1 | 2 | >2 | 1 | 2 | |
| Amphotericin B | 1 | 7 | 9 | 19 | 12 | 3 | 0 | 78 | 98 |
| Ketoconazole | 1 | 4 | 11 | 17 | 11 | 4 | 3 | 76 | 92 |
| Flucytosine | 1 | 1 | 0 | 14 | 17 | 11 | 7 | 61 | 84 |
| Fluconazole | 2 | 2 | 17 | 21 | 5 | 4 | 0 | 84 | 96 |
The MICs were determined by the direct CM and the macrodilution method.
MIC obtained by the direct CM divided by MIC obtained by the macrodilution method.
Direct breakpoint susceptibility testing of yeasts in positive blood cultures.
Fluconazole (8 and 64 μg/ml) and flucytosine (4 and 32 μg/ml) were used to perform the breakpoint susceptibility testing of yeasts in positive blood cultures by direct inoculation into Bactometer. After testing of 75 specimens, the CM produced one (1.4%) major error when a strain of C. tropicalis was tested against flucytosine. However, two minor errors (2.8%) were observed when fluconazole was tested against one strain each of C. albicans and C. glabrata. Therefore, the rates of agreement between the two methods for interpretive susceptibility testing were 98.6 and 97.2%, respectively, for flucytosine and fluconazole. Sixty-eight strains (92%) were susceptible to both flucytosine and fluconazole. The MIC range and MICs at which 10, 50, and 90% of these yeast blood isolates were inhibited by amphotericin B, flucytosine, fluconazole, and ketoconazole, as determined by the macrodilution method, are shown in Table 3.
TABLE 3.
MICs of amphotericin B, ketoconazole, flucytosine, and fluconazole for 74 blood yeast isolates as determined by the macrodilution method
| Antifungal agent | MIC (μg/ml)a
|
|||
|---|---|---|---|---|
| Range | 10% | 50% | 90% | |
| Amphotericin B | 0.063–2 | 0.125 | 0.25 | 0.5 |
| Ketoconazole | 0.016–16 | 0.016 | 0.063 | 2 |
| Flucytosine | 0.016–64 | 0.016 | 0.063 | 0.25 |
| Fluconazole | 0.063–64 | 0.25 | 0.5 | 8 |
10%, 50%, and 90%, MICs at which 10, 50, and 90% of isolates were inhibited, respectively.
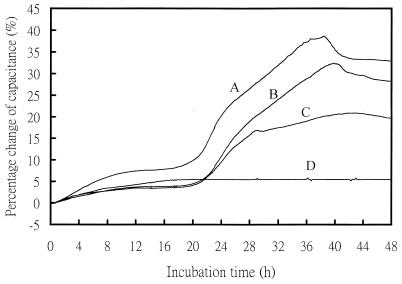
Among the 75 positive blood specimens, one was found to be a mixed culture containing C. albicans and Serratia marscens. Since the bacterium was resistant to the antifungal agents, the mixed culture produced false-resistant results (fluconazole MIC, >64 μg/ml; flucytosine MIC, >32 μg/ml) in the CM method. The sample was excluded from data analysis. Of the remaining 74 yeast strains, 2 strains of C. tropicalis were resistant to flucytosine, but other strains were susceptible to the drug. For fluconazole, one strain of C. albicans was resistant and three strains of C. glabrata were S-DD (Table 4). Table 4 shows the incubation time elapsed when resistance or S-DD was detected by the CM; in all situations, the time needed was less than 24 h after direct inoculation of the positive culture broths. Figure 3 shows the capacitance growth curves for a fluconazole-resistant blood isolate (C. albicans 5364524) grown with fluconazole concentrations of 8 and 64 μg/ml. The detection time (21 h) was determined with Bactometer when the increase in capacitance of three consecutive readings exceeded the default value of the instrument.
TABLE 4.
Incubation time elapsed when resistant or S-DD yeast strains in positive blood cultures were detected by the direct CM
| Blood sample | Antifungal agent | Microorganism | MICa (μg/ml) | Interpretive susceptibility | Time (h) elapsed when resistance or S-DD was detected |
|---|---|---|---|---|---|
| 0945 | Flucytosine | C. tropicalis | 64 | R | 12 |
| 1622 | Flucytosine | C. tropicalis | 64 | R | 15 |
| 5364524 | Fluconazole | C. albicans | 128 | R | 21 |
| 8498 | Fluconazole | C. glabrata | 16 | S-DD | 15 |
| 5436196 | Fluconazole | C. glabrata | 32 | S-DD | 17 |
| 6221651 | Fluconazole | C. glabrata | 16 | S-DD | 15 |
Determined by the macrodilution method.
FIG. 3.
Capacitance growth curves for a positive blood culture (C. albicans 5364524) directly inoculated into Bactometer and grown at the two breakpoint concentrations of fluconazole. Curve A, growth control; curves B and C, 8 and 64 μg/ml, respectively; curve D, negative control. The detection time for curve C was determined to be 21 h by Bactometer.
The species isolated from the 74 blood culture bottles were C. albicans (43 strains), C. tropicalis (12 strains), C. parapsilosis (8 strains), C. glabrata (6 strains), C. guilliermondii (1 strain), Cryptococcus neoformans (1 strain), C. famata (1 strain), and two unidentified yeast species. The C. neoformans strain failed to grow in RPMI 1640 broth after 48 to 72 h of incubation, and susceptibility results were not obtained by both the CM and the macrodilution method.
DISCUSSION
A direct antifungal susceptibility testing method based on the measurement of capacitance change was investigated for positive blood cultures containing yeasts. For the determination of MICs of four antifungal agents against yeasts in 51 positive blood culture bottles, the agreement rates (±2 log2 dilutions) between the CM and the NCCLS reference method were as follows: amphotericin B (98%), ketoconazole (92%), flucytosine (84%), and fluconazole (96%) (Table 2).
The CM was also performed in a format of breakpoint susceptibility testing of flucytosine and fluconazole against yeasts in positive blood cultures, with results being expressed as resistant, S-DD or intermediate, or susceptible. After testing of 74 blood specimens, a major error rate of 1.4% was found for flucytosine; however, fluconazole had a minor error rate of 2.8%. Under most conditions, the interpretive susceptibility results were available within 24 h (Table 4) with the direct CM and 72 h with routine procedures encompassing yeast isolation followed by susceptibility testing. That all resistant and S-DD strains were detected within 24 h after inoculation does not mean that strains causing no change in capacitance within 24 h should be susceptible to a test drug. Delayed resistance patterns may be encountered with slowly growing yeasts (e.g., C. parapsilosis). However, all eight strains of C. parapsilosis isolated from the 74 blood samples were susceptible to both flucytosine and fluconazole.
Although isolates of C. krusei are considered to be intrinsically resistant to fluconazole, the rate of isolation of this species in blood cultures is relatively low (4, 20), and strains of C. krusei were not isolated in this study. Although the breakpoints for itraconazole also have been defined (19, 27), the data for itraconazole were completely from studies of oropharyngeal candidiasis. For this reason, itraconazole was not included for direct interpretive susceptibility testing. For testing of pure yeast strains, the CM produced reproducible results (Table 1) and was comparable to the broth macrodilution method (19). The cost of one module of Bactometer was about $8 (U.S. dollars), and a test for one drug was estimated to cost $10. Bactometer was not designed for susceptibility testing but for the determination of total counts. Simpler equipment with the same function would be cost-effective for routine use.
There are three electrical signals (conductance, impedance, and capacitance) available for measurement of microbial growth in Bactometer. Our preliminary data showed that capacitance measurements had a greater response (data not shown) than the signals of impedance and conductance; therefore, this parameter was used throughout this study.
The rate of nosocomial candidemia increased by almost 500% from 1981 through 1989 (2, 3), particularly in large teaching hospitals. Therefore, rapid antifungal susceptibility testing of yeasts in blood cultures may have clinical importance. Through years of study, some alternative methods, including the colorimetric broth microdilution technique (6, 24, 30), flow cytometry (21, 34), and Etest (5, 25, 29), have been proposed for antifungal susceptibility testing. However, all of these procedures require isolated pure colonies for testing and do not seem feasible for direct susceptibility tests with positive culture broths.
Direct antimicrobial susceptibility testing, either by agar disk diffusion (8, 13, 17) or broth dilution (14), of positive blood cultures containing bacteria was found to be feasible under most conditions. In addition, an impedimetric method has been developed for direct antimicrobial susceptibility testing of gram-negative bacilli (12) and for detection of oxacillin-resistant Staphylococcus aureus in blood cultures (36). These results prompted us to use the CM for direct antifungal susceptibility testing of yeasts in positive blood cultures. Compared with the occurrence of bacteremia, the occurrence of fungemia caused by multiple yeast strains is very rare. Therefore, the difficulty of susceptibility interpretation in situations of polymicrobial infections is seldom encountered. Mixed cultures of bacteria and yeasts may be occasionally observed in positive blood culture bottles; however, the presence of the two completely different organisms normally can be detected by the Gram stain, which is a routine step when a positive blood culture bottle is found. The CM tended to detect the more resistant side if a mixed culture containing two strains of yeasts was encountered. In case of a mixed culture containing yeasts and bacteria, the CM might produce false-resistant results due to the resistance of bacteria to antifungal agents.
The numbers of cells in positive blood culture bottles containing yeasts were about 106 to 107 (CFU/ml), close to the cell density of a yeast cell suspension with a McFarland turbidity of 0.5. Therefore, an inoculum of 1 μl (i.e., 10 μl of a 1:10-diluted sample) of positive broth in 1 ml of RPMI 1640 medium would achieve a final cell density of about 1 × 103 to 10 × 103 CFU/ml. Although the inocula were somewhat larger than those (0.5 × 103 to 2.5 × 103 CFU/ml) recommended by the NCCLS (19), inoculum densities seem not to have caused significant deviations in the end-point determinations of the MICs (10). Direct susceptibility testing can test a broader representation of the yeast population present in blood cultures. Theoretically, about 103 cells were inoculated into each module well of Bactometer, whereas only several colonies on subculture plates were sampled for inoculum preparation in the conventional testing protocol (19).
In conclusion, the CM seems to be capable of earlier detection of resistant (or S-DD or intermediate) yeast isolates in positive blood cultures. The method would be simpler especially if performed in a format of breakpoint susceptibility testing. The signal detection in Bactometer is a continuous, real-time process, and susceptibility patterns can be obtained by a real-time comparison with the growth curve for a growth control.
ACKNOWLEDGMENTS
This project was supported by a grant (NSC 88-2314-B006-078) from the National Science Council, Taipei, Taiwan.
We thank Kaoshung Chang Gung Memorial Hospital for supplying some of the positive blood cultures.
REFERENCES
- 1.Anaissie E J, Bodey G P, Rinaldi M G. Emerging fungal pathogens. Eur J Clin Microbiol Infect Dis. 1989;8:323–330. doi: 10.1007/BF01963467. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee S N, Emori T G, Culver D H, Gaynes R P, Jarvis W R, Horan T, Edwards J R, Tolson J, Henderson T, Martone W J. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. National Nosocomial Infections Surveillance System. Am J Med. 1991;91(Suppl. 3B):86S–89S. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- 3.Beck-Sague C, Jarvis W R. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. National Nosocomial Infections Surveillance System. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y C, Chang S C, Sun C C, Yang L S, Hseih W C, Luh K T. Secular trends in the epidemiology of nosocomial fungal infections at a teaching hospital in Taiwan, 1981 to 1993. Infect Control Hosp Epidemiol. 1997;18:369–375. doi: 10.1086/647628. [DOI] [PubMed] [Google Scholar]
- 5.Colombo A L, Barchiesi F, McGough D A, Rinaldi M G. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for azole antifungal susceptibility testing. J Clin Microbiol. 1995;33:535–540. doi: 10.1128/jcm.33.3.535-540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davey K G, Szekely A, Johnson E M, Warnock D W. Comparison of a new commercial colorimetric microdilution method with a standard method for in-vitro susceptibility testing of Candida spp. and Cryptococcus neoformans. J Antimicrob Chemother. 1998;42:439–444. doi: 10.1093/jac/42.4.439. [DOI] [PubMed] [Google Scholar]
- 7.Doern G V, Scott D R, Rashad A L. Clinical impact of rapid antimicrobial susceptibility testing of blood culture isolates. Antimicrob Agents Chemother. 1982;21:1023–1024. doi: 10.1128/aac.21.6.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doern G V, Scott D R, Rashad A L, Kim K S. Evaluation of a direct blood culture disk diffusion antimicrobial susceptibility test. Antimicrob Agents Chemother. 1981;20:696–698. doi: 10.1128/aac.20.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doern G V, Vautour R, Gaudet M, Levy B. Clinical impact of rapid in vitro susceptibility testing and bacterial identification. J Clin Microbiol. 1994;32:1757–1762. doi: 10.1128/jcm.32.7.1757-1762.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinel-Ingroff A, Kish C W, Jr, Kerkering T M, Fromtling R A, Bartizal K, Galgiani J N, Villareal K, Pfaller M A, Gerarden T, Rinaldi M G, Fothergill A. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility tests. J Clin Microbiol. 1992;30:3138–3145. doi: 10.1128/jcm.30.12.3138-3145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghannoum M A, Rex J H, Galgiani J N. Susceptibility testing of fungi: current status of correlation of in vitro data with clinical outcome. J Clin Microbiol. 1996;34:489–495. doi: 10.1128/jcm.34.3.489-495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang A H, Wu J J, Weng Y M, Ding H C, Chang T C. Direct antimicrobial susceptibility testing of gram-negative bacilli in blood cultures by an electrochemical method. J Clin Microbiol. 1998;36:2882–2886. doi: 10.1128/jcm.36.10.2882-2886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson J E, Washington J A., II Comparison of direct and standardized antimicrobial susceptibility testing of positive blood cultures. Antimicrob Agents Chemother. 1976;10:211–214. doi: 10.1128/aac.10.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiehn T E, Capitolo C, Armstrong D. Comparison of direct and standard microtiter broth dilution susceptibility testing of blood culture isolates. J Clin Microbiol. 1982;16:96–98. doi: 10.1128/jcm.16.1.96-98.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Law D, Moore C B, Wardle H M, Ganguli L A, Keaney M G, Denning D W. High prevalence of antifungal resistance in Candida spp. from patients with AIDS. J Antimicrob Chemother. 1994;34:659–668. doi: 10.1093/jac/34.5.659. [DOI] [PubMed] [Google Scholar]
- 16.Lozano-Chiu M, Paetznick V L, Ghannoum M A, Rex J H. Detection of resistance to amphotericin B among Cryptococcus neoformans clinical isolates: performances of three different media assessed by using E-test and National Committee for Clinical Laboratory Standards M27-A methodologies. J Clin Microbiol. 1998;36:2817–2822. doi: 10.1128/jcm.36.10.2817-2822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirrett S, Reller L B. Comparison of direct and standard antimicrobial disk susceptibility testing for bacteria isolated from blood. J Clin Microbiol. 1979;10:482–487. doi: 10.1128/jcm.10.4.482-487.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore D F, Hamada S S, Marso E, Martin W J. Rapid identification and antimicrobial susceptibility testing of gram-negative bacilli from blood cultures by the AutoMicrobic system. J Clin Microbiol. 1981;13:934–939. doi: 10.1128/jcm.13.5.934-939.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 20.Nguyen M H, Peacock J E, Jr, Morris A J, Tanner D C, Nguyen M L, Snydman D R, Wagener M M, Rinaldi M G, Yu V L. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med. 1996;100:617–623. doi: 10.1016/s0002-9343(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 21.Peyron F, Favel A, Guiraud-Dauriac H, El Mzibri M, Chastin C, Dumenil G, Regli P. Evaluation of a flow cytofluorometric method for rapid determination of amphotericin B susceptibility of yeast isolates. Antimicrob Agents Chemother. 1997;41:1537–1540. doi: 10.1128/aac.41.7.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaller M, Wenzel R. Impact of the changing epidemiology of fungal infections in the 1990s. Eur J Clin Microbiol Infect Dis. 1992;11:287–291. doi: 10.1007/BF01962067. [DOI] [PubMed] [Google Scholar]
- 23.Pfaller M A. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin Infect Dis. 1996;22(Suppl. 2):S89–S94. doi: 10.1093/clinids/22.supplement_2.s89. [DOI] [PubMed] [Google Scholar]
- 24.Pfaller M A, Barry A L. Evaluation of a novel colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1994;32:1992–1996. doi: 10.1128/jcm.32.8.1992-1996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaller M A, Messer S A, Karlsson A, Bolmstrom A. Evaluation of the Etest method for determining fluconazole susceptibilities of 402 clinical yeast isolates by using three different agar media. J Clin Microbiol. 1998;36:2586–2589. doi: 10.1128/jcm.36.9.2586-2589.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rex J H, Pfaller M A, Barry A L, Nelson P W, Webb C D. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Antimicrob Agents Chemother. 1995;39:40–44. doi: 10.1128/aac.39.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 28.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simor A E, Goswell G, Louie L, Lee M, Louie M. Antifungal susceptibility testing of yeast isolates from blood cultures by microbroth dilution and the E test. Eur J Clin Microbiol Infect Dis. 1997;16:693–697. doi: 10.1007/BF01708563. [DOI] [PubMed] [Google Scholar]
- 30.Tiballi R N, He X, Zarins L T, Revankar S G, Kauffman C A. Use of a colorimetric system for yeast susceptibility testing. J Clin Microbiol. 1995;33:915–917. doi: 10.1128/jcm.33.4.915-917.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trenholme G M, Kaplan R L, Karakusis P H, Stine T, Fuhrer J, Landau W, Levin S. Clinical impact of rapid identification and susceptibility testing of bacterial blood culture isolates. J Clin Microbiol. 1989;27:1342–1345. doi: 10.1128/jcm.27.6.1342-1345.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warren N G, Hazen K C. Candida, Cryptococcus, and other yeasts of medical importance. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 723–737. [Google Scholar]
- 33.Weinstein M P, Towns M L, Quartey S M, Mirrett S, Reimer L G, Parmigiani G, Reller L B. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584–602. doi: 10.1093/clind/24.4.584. [DOI] [PubMed] [Google Scholar]
- 34.Wenisch C, Linnau K F, Parschalk B, Zedtwitz-Liebenstein K, Georgopoulos A. Rapid susceptibility testing of fungi by flow cytometry using vital staining. J Clin Microbiol. 1997;35:5–10. doi: 10.1128/jcm.35.1.5-10.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wey S B, Mori M, Pfaller M A, Woolson R F, Wenzel R P. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med. 1988;148:2642–2645. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]
- 36.Wu J J, Huang A H, Dai J H, Chang T C. Rapid detection of oxacillin-resistant Staphylococcus aureus in blood cultures by an impedance method. J Clin Microbiol. 1997;35:1460–1464. doi: 10.1128/jcm.35.6.1460-1464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida T, Jono K, Okonogi K. Modified agar dilution susceptibility testing method for determining in vitro activities of antifungal agents, including azole compounds. Antimicrob Agents Chemother. 1997;41:1349–1351. doi: 10.1128/aac.41.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]